Abstract
Poor lung function and respiratory disorders like asthma have a positive correlation with the development of adverse cardiovascular events. Increased adenosine levels are associated with lung inflammation that could lead to altered vascular responses and systemic inflammation. We hypothesized that asthmatic lung inflammation has systemic effects through A1 adenosine receptors (A1AR) and investigated the effects of aerosolized adenosine on vascular reactivity and inflammation, using A1AR knockout (A1KO) and corresponding wild-type (A1WT) mice that were divided into three experimental groups each: control (CON), allergen sensitized and challenged (SEN), and SEN + aerosolized adenosine (SEN + AD). Animals were sensitized with ragweed (200 μg ip; days 1 and 6), followed by 1% ragweed aerosol challenges (days 11 to 13). On day 14, the SEN + AD groups received one adenosine aerosol challenge (6 mg/ml) for 2 min, and aortae were collected on day 15. 5′-N-ethylcarboxamidoadenosine (NECA; nonselective adenosine analog) induced concentration-dependent aortic relaxation in the A1WT CON group, which was impaired in the A1WT SEN and SEN + AD groups. All groups of A1KO mice showed similar (no significant difference) concentration-dependent relaxation to NECA. The A1WT SEN and SEN + AD groups had a significantly higher contraction to selective A1 agonist 2-chloro-N6-cyclopentyladenosine (CCPA) compared with the CON group. Western blot data showed that aortic A1AR expression was significantly increased in WT SEN and SEN + AD mice compared with CON mice. Gene expression of ICAM-1 and IL-5 was significantly increased in allergic A1WT aorta and were undetected in the A1KO groups. A1WT allergic mice had significantly higher airway hyperresponsiveness (enhanced pause) to NECA, with adenosine aerosol further enhancing it. In conclusion, allergic A1WT mice showed altered vascular reactivity, increased airway hyperresponsiveness, and systemic inflammation. These data suggest that A1AR is proinflammatory systemically in this model of allergic asthma.
Keywords: vascular reactivity, airway responsiveness, adenosine
asthma is a chronic lung disease characterized by airway hyperreactivity, inflammation, and poor ventilation. Evidence has increasingly implicated adenosine in the pathophysiology of asthma (53). Increases in adenosine levels correspond to increased airway inflammation and tissue damage (14). The sources of adenosine include platelets, neurons, parenchymal lung cells and endothelial cells, and also inflammatory cells, i.e., mast cells, neutrophils, and eosinophils (32, 35, 48). A hallmark study done in the early 1980s showed inhaled adenosine to be a potent bronchoconstrictor in patients with asthma as opposed to normal subjects who showed no response (10). Genetically modified mice lacking adenosine deaminase (ADA), the enzyme that breaks down adenosine, had severe pulmonary inflammation, airway hyperreactivity, and airway remodeling (5, 9), indicating a strong correlation between chronic elevation of adenosine levels and increased lung inflammation.
Impaired lung function is a risk factor for cardiovascular disease (57). Many epidemiological studies have reported that people suffering from chronic respiratory diseases, including asthma, have a higher incidence of cardiovascular diseases (13, 27, 30, 50, 58). There is an association between atherosclerosis and stroke with reactive airway diseases. Adult-onset asthma is associated with increased carotid atherosclerosis in women (46), and subjects who had bronchial hyperresponsiveness to methacholine (MCh) demonstrated increased carotid intima-media thickness (60). Systemic inflammation in these patients, believed to be a consequence of airway inflammation (28, 39), may be one of the reasons for altered cardiovascular parameters (21). Studies done in animal models of allergic asthma have also reported enhanced systemic inflammation, myocardial ischemia-reperfusion injury, and neutrophil recruitment to the myocardium (23, 24). A recent study from our laboratory (47) has also shown that allergic mice have poor vascular responses and systemic inflammation, with adenosine aerosol exacerbating theses effects.
The four adenosine receptor subtypes, viz., the A1, A2A, A2B, and, A3, have different modulatory roles in asthma and on the cardiovascular system (4, 37, 52). Many reports suggest that the A1 adenosine receptor (A1AR) is involved in the direct bronchoconstrictor effects of adenosine. A1AR expression was found to be elevated in a rabbit model of asthma, and the use of an A1 antisense nucleotide to inhibit this upregulation resulted in blunted bronchoconstriction to adenosine (1, 43). The use of a selective A1 antagonist produced a significant reduction of airway hyperresponsiveness in allergic rabbits by blocking these receptors (44). A very recent study reported that the expression of A1AR is significantly elevated in airway smooth muscle in asthmatic patients (7). Other than the effects on airway smooth muscle, the A1AR has been implicated in increased mucin production in human tracheal cells (36) and in mediating monocyte phagocytosis (49). All these studies suggest a strong role for the A1AR in asthma, both in airway obstruction and inflammation.
A1ARs also have systemic effects and are involved in blood pressure regulation (8) and vasoconstriction (22, 51, 54, 55). However, the role of A1ARs in the control of vascular tone and systemic inflammation in an allergic model of asthma has not been characterized. In addition, the vascular effects of A1ARs in response to aerosolized adenosine challenge as an added insult in a murine model of asthma have not been studied previously. Therefore, this study was undertaken to investigate the effects of allergen challenge and inhaled adenosine on vascular reactivity and systemic inflammation using our murine model of asthma in A1AR wild-type (A1WT) and knockout (A1KO) mice. We also determined the role of the A1AR in airway responsiveness by whole body plethysmography. Our data suggest that allergic A1WT mice have altered peripheral vascular reactivity, increased bronchoconstriction, increased aortic expressions of inflammatory cytokine IL-5, and vascular adhesion molecule ICAM-1, with inhaled adenosine further exacerbating these effects. These effects were not observed in A1KO mice.
MATERIALS AND METHODS
Animals.
A1KO mice on an inbred BALB/c background were generated by backcrossing A1 heterozygotes (originally 129/OlaHsd background provided by Dr. Bertil Fredholm) with 12 generations to the BALB/c strain. BALB/c control mice (A1WT) were obtained from Harlan Sprague-Dawley (Indianapolis, IN). A1KO and A1WT males 8–10 wk of age were used in all experiments. The animals were maintained on a ragweed-free diet. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of West Virginia University.
Animal sensitization.
Allergen (ragweed) sensitization and challenge was performed according to the protocol described earlier from this laboratory (16–18, 38, 45). This model of allergic asthma has been shown to develop airway inflammation and airway hyperreactivity to MCh. The study comprised six groups of animals: 1) A1WT control (A1WT CON); 2) A1WT allergen sensitized and challenged (A1WT SEN); 3) A1WT allergen sensitized and challenged and further challenged with 6 mg/ml of adenosine aerosol for 2 min on day 14 (A1WT SEN + AD); 4) A1KO control (A1KO CON); 5) A1KO allergen sensitized and challenged (A1KO SEN); and 6) A1KO allergen sensitized and challenged and further challenged with 6 mg/ml of adenosine aerosol for 2 min on day 14 (A1KO SEN + AD). Experiments were also carried with A1WT CON mice subject to adenosine challenge (A1WT CON + AD), and these had no differences from A1WT CON for vascular reactivity in this study (data not shown) as well as in a previous study from our laboratory (47); hence, this group was not included for further experiments. Mice were sensitized on days 1 and 6 with intraperitoneal injections of ragweed allergen (200 μg/dose; Greer, Lenoir, NC) with 200 μl Imject alum (Pierce; Rockford, IL). Nonsensitized control animals received only the Imject alum with the same volumes. Ten days after sensitization, the mice were placed in a Plexiglas chamber and challenged with 1% aerosolized ragweed or with 0.9% saline as a control, using an ultrasonic nebulizer (DeVilbiss; Somerset, PA) for 20 min both in the morning and afternoon for 3 consecutive days. The aerosolization of allergen was performed at a flow rate of 2 ml/min, and the aerosol particles had a median aerodynamic diameter of <4 μm (DeVilbiss).
Acute elevations in lung adenosine levels were produced experimentally by adenosine inhalation (6 mg/ml for 2 min on day 14) to the SEN + AD group to further enhance the allergen-induced effects. This dose was chosen based on our previous studies in this model (16, 17). Adenosine inhalation in this model has been shown to enhance allergen-induced airway inflammation and airway hyperreactivity to adenosine [or its analog 5′-N-ethylcarboxamidoadenosine (NECA)] (16–18). ADA-deficient mice having sustained and chronic elevations in lung adenosine levels have also been shown to have similar features. The CON and SEN groups received only saline on day 14. Twenty-four hours after the last challenge, animals were euthanized for the collection of aorta for further experiments.
Preparation of isolated mouse aorta and isometric force measurement.
Mice were euthanized by anesthesia with pentobarbital sodium (65 mg/kg ip), followed by thoracotomy and removal of aorta that was then cut transversely into 3- to 4-mm rings. The rings were mounted vertically between two stainless steel wire hooks and then suspended in 10-ml organ baths containing Krebs-Henseleit buffer. The Krebs-Henseleit buffer (pH 7.4) containing (in mM) 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2 was maintained at 37°C with continuous bubbling of 95% O2 and 5% CO2. For the measurement of isometric force response, the aortic rings were equilibrated for 90 min with a resting force of 1 g and a change of the bathing solution at 15-min intervals. The resting force of 1 g has been used earlier in our laboratory (2, 55). At the end of equilibration period, tissues were contracted with 50 mM KCl twice to check the contractility of individual aortic rings that were washed out with Krebs-Henseleit buffer. The aortic rings were then preconstricted with phenylephrine (PE, 10−7 M) to obtain a steady contraction, and changes in tension were continuously monitored with a fixed range precision force transducer (TSD, 125 C, Biopac system) connected to the differential amplifier (DA 100B, Biopac system). The data were recorded using MP100, Biopac digital acquisition system, and analyzed using Acknowledge 3.5.7 software (Biopac system).
Contraction/relaxation experiments.
After equilibration, the responsiveness and stability of individual rings were checked by successive administration of submaximally effective concentration of PE (10−7 M). The integrity of the vascular endothelium was assessed pharmacologically by acetylcholine (10−7 M) to produce the relaxation of PE-precontracted rings. The aortic rings were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began.
Experimental protocol.
The concentration-response curves for NECA (10−11-10−5 M) and 2-chloro-N6-cyclopentyladenosine (CCPA; 10−11-10−5 M), respectively, were run parallel in aortic rings from all the groups. In all cases, drug was added to yield the next higher concentration only when the response to the earlier dose reached a steady state. Contraction/relaxation responses were expressed as a percent increase/decrease in the contraction with respect to PE (alone) in response to each concentration of agonist used.
Western blot analysis for aortic A1AR expression.
Aorta from the CON, SEN, and SEN + AD A1WT mice were isolated, and each sample was treated with 1 ml of lysis buffer containing 50 mM Tris·HCl (pH 7.4), 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, 0.25% sodium deoxycholate, 1 μg/ml aprotinin, 1 μg /ml pepstatin, 1 μg/ml leupeptin, 1 mM Na3VO4, and 1 mM NaF and homogenized on wet ice. The samples were transferred to dry ice for 5 min and then thawed on wet ice. After being thawed, the samples were vortexed and centrifuged for 5 min at 12,000 rpm at 4°C. The supernatants were sonicated and stored at −80°C. Protein was measured using Bio-Rad assay based on the Bradford dye-binding procedure with bovine serum albumin as a standard. The protein mixture was divided into aliquots and stored at −80°C. At the time of analysis, the samples were thawed and ∼40 μg of total protein per lane were loaded on a slab gel. Proteins were separated by SDS-PAGE using 10% acrylamide gels (1 mm thick). After electrophoresis, the proteins on the gel were transferred to nitrocellulose membrane (Hybond-ECL) by electroelution. Protein transfer was confirmed by employing prestained molecular weight markers (Bio-Rad; Hercules, CA). Following the blocking with nonfat dry milk, the nitrocellulose membranes were incubated with polyclonal antibody cross-reacting with primary antibody for A1AR (Sigma; St. Louis, MO); β-actin antibody (Santa Cruz Biotechnology; Santa Cruz, CA) was used as an internal control to normalize the target protein expression in each lane. The secondary antibody was a horseradish peroxidase-conjugated anti-rabbit IgG. The membranes were developed using enhanced chemiluminescence (Amersham BioSciences) and exposed to X-ray film for the appropriate time. The data were presented as the ratio of A1AR expression to β-actin.
Real-time PCR for expression of ICAM-1, VCAM-1, and IL-5 genes.
The aortic tissues from all experimental groups were processed for total RNA isolation using the TRIzol reagent from Life Technologies/Invitrogen followed by DNase treatment to eliminate potential genomic DNA contamination as described recently by us (2, 56). This was followed by the conversion of 0.5 μg of total RNA into cDNA using High-Capacity cDNA archive kit (Applied Biosystems; Foster City, CA) in a total volume of 100 μl. Real-time PCR was then performed using an ABI PRISM 7300 Detection System (Applied Biosystems) using Taqman Universal Mastermix (Applied Biosystems; Branchburg, NJ) according to the instructions of the manufacturer. The reaction volume (25 μl) consisted of 12.5 μl of 2× Taqman Universal Mastermix, 1 μl of cDNA, and 1.25 μl of 20× FAM-labeled Taqman gene expression assay master mix solution. For the real-time PCR of the concerned genes (ICAM-1, VCAM-1, and IL-5), the Taqman inventoried assays-on-demand gene expression products were purchased from Applied Biosystems (Foster City, CA). 18S rRNA (ribosomal RNA) was used as an endogenous control. The fold difference in expression of target cDNA was determined using the comparative CT method as described earlier (31).
Airway responsiveness to MCh and NECA.
The airway responsiveness was assessed by whole body plethysmography using the chambers obtained from Buxco (Max II; Buxco Systems; Troy, NY) as described previously by our laboratory (40). This system enables the measurement of airway obstruction using a dimensionless parameter called enhanced pause (Penh). The higher the Penh values, the greater is the airway obstruction. Penh has been shown to correlate with airway resistance and dynamic compliance (29). Unrestrained mice were placed in individual Plexiglas chambers 24 h after the last allergen challenge and subjected to increasing concentrations of MCh (1.5–48.0 mg/ml) to obtain a dose response. NECA dose response was performed 24 h after the MCh experiment. After the baseline and vehicle Penh values were obtained, the mice were exposed to increasing concentrations of NECA (23.44–750 μg/ml) via the Buxco aerosol delivery system (version 1.5; Buxco; Sharon, CT). Each concentration of NECA was aerosolized for 2 min, and the Penh readings were recorded for 5 min to establish a dose-response relationship. The next dose was not administered until the mice had returned to baseline Penh levels. Airway responsiveness was normalized to the vehicle control and expressed as a percent increase in Penh.
Drugs.
Acetylcholine, adenosine, and PE were dissolved in distilled water. NECA and CCPA were dissolved in 100% DMSO as a 10 mM stock solution for vascular reactivity experiments. The final concentration of DMSO in organ bath (10 ml) had no effect by itself on the aortic rings (2). For Penh responses, MCh was dissolved in 0.9% saline and NECA was dissolved in 100% ethanol to create a stock solution from which subsequent dilutions were made with 0.9% saline. Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals.
Statistical analysis.
The data were expressed as means ± SE. Comparisons among different groups were analyzed by ANOVA, followed by Tukey's multiple comparison tests. Comparison between two groups was assessed by unpaired t-test. A P value of <0.05 was considered as the level of significance. All statistical analyses were performed using GraphPad Prism statistical package.
RESULTS
Vascular reactivity in CON, allergic, and allergic + AD groups.
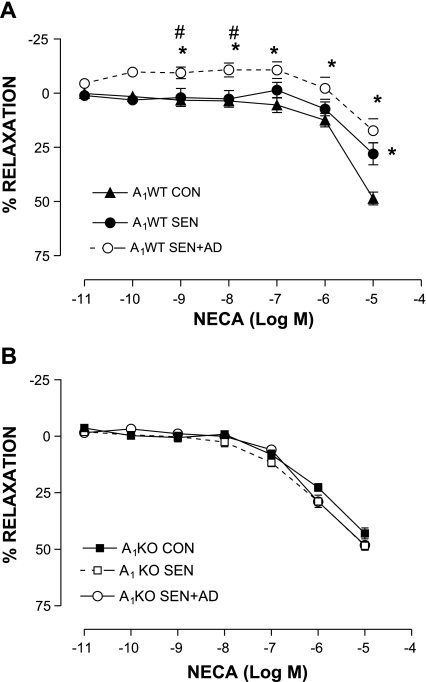
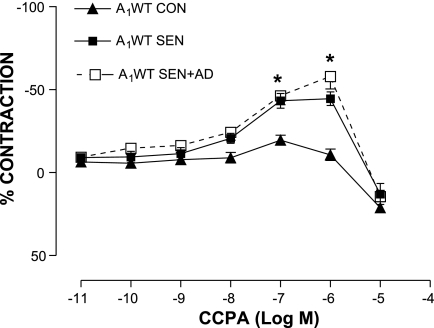
The aortic vascular response to NECA was studied in all experimental groups. Organ bath data showed a concentration-dependent relaxation response to NECA in the A1WT CON group (48.64 ± 1.93% at 10−5 M; n = 10), whereas relaxation was significantly lower in A1WT SEN aorta (maximum relaxation of 22.8 ± 4.9% at 10−5 M; n = 10). NECA failed to elicit any relaxation response in the A1WT SEN + AD group except at the highest concentration (17.4 ± 5.53% at 10−5 M; n = 10, Fig. 1A). In contrast, all three groups of KO mice showed a concentration-dependent relaxation (maximum, ∼50% at 10−5 M), and there was no difference in the response seen among the groups (Fig. 1B). CCPA, a selective A1 agonist, produced significantly higher contraction in the A1WT SEN and SEN + AD groups (44.44 ± 4.12 and 46.2 ± 5.4%, respectively, vs. 10.62 ± 3.39% in A1WT CON at 10−6 M, Fig. 2; n = 6). These data show that allergen sensitization and challenge leads to altered vascular reactivity in allergic A1WT mice, which suggests that the A1AR is responsible for the contractile responses in aorta from allergic mice and those challenged with adenosine aerosol.
Fig. 1.
Concentration-response curves for 5′-N-ethylcarboxamidoadenosine (NECA)-mediated aortic relaxation/contraction in A1 adenosine receptor (A1AR) wild-type (A1WT; A) and A1AR knockout (A1KO; B) with control (CON), allergic (SEN), and adenosine aerosolized allergic (SEN + AD) groups. Values are expressed as means ± SE; n = 10 mice. On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with A1WT CON; #P < 0.05 compared with A1WT SEN.
Fig. 2.
Concentration-response curves for 2-chloro-N6-cyclopentyladenosine (CCPA)-mediated aortic contraction in A1WT CON, SEN, and SEN + AD groups. Values are expressed as means ± SE; n = 6 mice. On y-axis, positive and negative values indicate relaxation and contraction, respectively. *P < 0.05 compared with A1WT CON.
Western blot analysis for aortic A1AR expression.
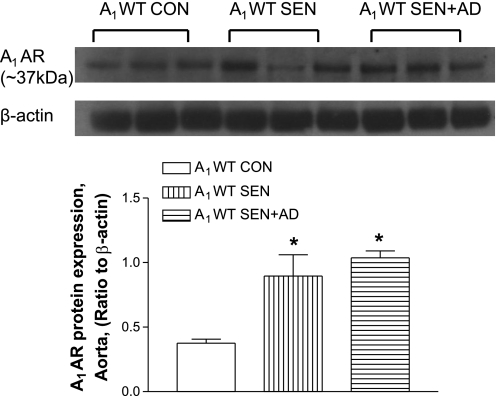
Protein expression of A1AR (∼37 kDa) was determined by Western blot analysis in all three groups (CON, SEN, and SEN + AD) of A1WT mice. Densitometric analysis revealed that the SEN (P < 0.05; n = 3) and SEN + AD (n = 3; P < 0.05) mice had a significantly higher expression of A1AR compared with CON mice (Fig. 3). These data suggest that the upregulation of the A1AR may be responsible for the altered vascular responses observed in allergic mice and those challenged with adenosine aerosol.
Fig. 3.
Protein expression of A1AR in mice aorta from A1WT CON, A1WT SEN, and A1WT SEN + AD groups. Values are expressed as means ± SE; n = 3 mice after normalizing A1AR protein expression with β-actin in each lane. *P < 0.05 compared with A1WT CON.
Gene expression of vascular adhesion molecules ICAM-1 and VCAM-1, and inflammatory cytokine IL-5 in aorta.
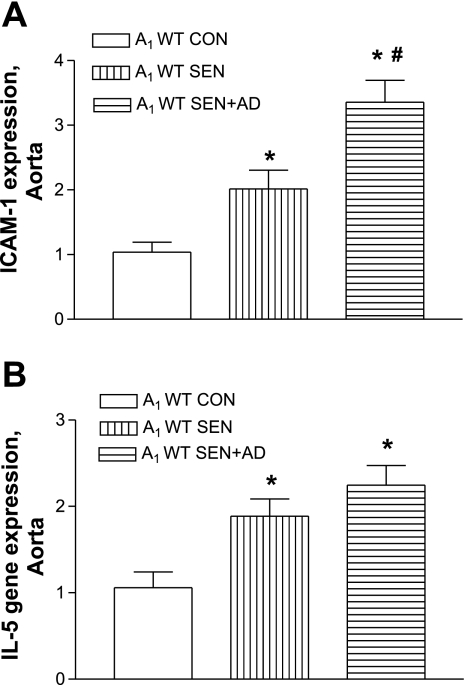
Allergen challenge significantly increased levels of vascular adhesion molecule ICAM-1 in A1WT SEN compared with A1WT CON mice while being undetected in all A1KO groups (Fig. 3). Allergen-sensitized and -challenged A1WT mice exposed to adenosine aerosol (SEN + AD) had significantly higher levels of ICAM-1 (P < 0.05, Fig. 4A) compared with the A1WT SEN and A1WT CON groups. There was no induction of VCAM-1 gene expression in the A1WT and A1KO groups in allergic mice and those exposed to adenosine aerosol (data not shown). Inflammatory cytokine IL-5 was significantly higher in the A1WT SEN and SEN + AD groups compared with A1WT controls (Fig. 4B), whereas IL-5 was not induced in any A1KO group (data not shown). These data suggest the A1AR may have a proinflammatory role in vascular tissue.
Fig. 4.
Expression of ICAM-1 (A) and IL-5 (B) by real-time PCR in A1WT mouse aorta from CON, SEN, and SEN + AD groups. For gene expression by comparative CT method using real-time PCR, first column was made as the calibrator against which all other groups were compared. Values are expressed as means ± SE; n = 4 mice. *P < 0.05 compared with A1WT CON; #P < 0.05 compared with A1WT SEN.
Airway responsiveness to MCh and NECA.
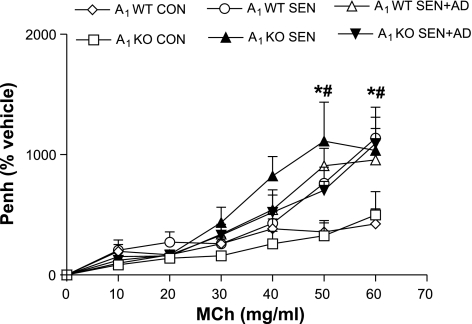
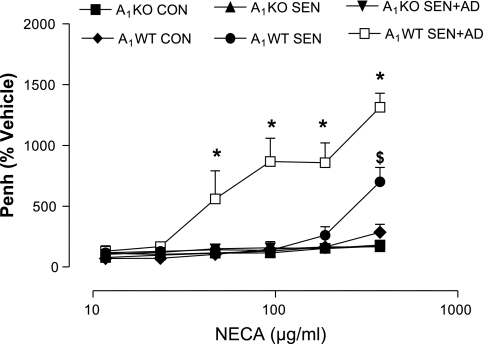
The airway responsiveness to MCh (Fig. 5) was assessed, and there was no difference between control A1WT and A1KO mice. All groups of sensitized and challenged A1WT and KO mice showed significantly increased airway response to MCh compared with respective controls, but there was no difference between them. The response to NECA was next assessed in all groups (Fig. 6). A1WT SEN mice had significantly higher airway responsiveness at the maximum dose of NECA (750 μg/ml) compared with A1WT CON and all A1KO groups. The A1WT SEN + AD group had the highest Penh values compared with the A1WT SEN, A1WT CON, and all A1KO groups. In contrast, the A1KO SEN, A1KO SEN + AD, and A1KO CON groups had similar airway responsiveness to NECA with no differences among them. These data indicate that adenosine-mediated hyperresponsiveness is mediated through A1AR.
Fig. 5.
Airway responsiveness [enhanced pause (Penh)] to methacholine (MCh) in A1KO and A1WT mice. Values are expressed as means ± SE; n = 4–8 mice. On y-axis, Penh is expressed as percentage of the vehicle. *P < 0.05, A1WT SEN and SEN (S) + AD compared with A1WT CON; #P < 0.05, A1KO SEN and SEN + AD compared with A1KO CON.
Fig. 6.
Airway responsiveness (Penh) to adenosine analog NECA in A1KO and A1WT mice. Values are expressed as means ± SE; n = 4–8 mice. On y-axis, Penh is expressed as percentage of the vehicle. *P < 0.05 compared with all other groups; $P < 0.05 compared with A1WT CON and all A1KO groups.
DISCUSSION
The principal finding of the present study is that allergic A1WT mice had vascular tissue inflammation, increased A1AR expression, increased airway hyperresponsiveness, impaired vasorelaxation to NECA, a nonselective adenosine analog, and a greater contraction to A1 selective agonist CCPA. These outcomes were further aggravated after acute adenosine aerosol challenge in these mice. Allergen- and adenosine-induced effects seen in A1WT SEN and A1WT SEN + AD mice were absent in the A1KO groups, indicating that A1AR is responsible for altered vascular reactivity, increased airway hyperresponsiveness, and increased systemic inflammation.
Adenosine is chronically elevated in asthmatic lungs (14, 33, 34). Evidence indicates that adenosine may be involved in the release of inflammatory mediators in the lung and systemic circulation by acting on its receptors present on different cells including mast cells, eosinophils, neutrophils, and other inflammatory cells (15, 16, 41, 53) and can thus contribute to pulmonary and systemic inflammation. Adenosine is a known bronchoprovocant (11), and experimentally induced temporary elevations in lung adenosine levels through the inhalation of adenosine (resulting from the breakdown of adenosine 5′-monophosphate) cause an increase in the infiltration of eosinophils in patients with asthma (59). A recent study from our laboratory has also shown an increased release of inflammatory cell markers in the lung and plasma after inhalation of adenosine aerosol in allergic mice (17). Adenosine aerosol in humans with asthma also causes an increased release of neutrophil chemotactic factor in serum (15). These studies suggest that adenosine enhances the release of systemic inflammatory mediators from sensitized inflammatory cells. However, the systemic effects of such an inhalation on vascular tissue inflammation with respect to the involvement of A1ARs have not been studied previously.
Systemic inflammation has been reported previously in human and animal models of asthma (23, 28). Neutrophils and eosinophils were increased in bronchoalveolar lavage and blood in a murine model of asthma (24). Systemic inflammatory markers have also been shown to be elevated in patients with asthma and in allergic mouse model of asthma after adenosine aerosol challenge (15, 16). Our earlier study (47) has also shown that allergic mice had significantly elevated systemic levels of proinflammatory cytokines, poor endothelial function, and lower aortic relaxation, with acute adenosine aerosol further causing exacerbation of systemic inflammation and producing an impairment of aortic relaxation. The use of A1 antagonist DPCPX increased aortic relaxation in both sets of experimental groups. These data suggested a possible involvement of A1ARs in alteration of vascular reactivity and inflammation. The next logical step was to determine whether inflammation was present in aortic tissue of allergic mice and whether A1AR had any role in this.
We found that the gene expression of ICAM-1 was significantly higher in both groups of allergic A1WT mice (SEN and SEN + AD) compared with controls. There was no difference in ICAM-1 expression in A1KO groups (data not shown). Several lines of evidence indicate that a crucial development observed in atherosclerosis is the increased expression of vascular adhesion molecules in the endothelium (6, 20). In the present study, we have observed an increased expression of ICAM-1 correlating well with poor endothelial relaxation reported in our earlier study (42) in allergic A1WT mice. For the first time, a link has been found among the presence of A1AR, increased expression of ICAM-1, and endothelial dysfunction in aorta from allergic mice. While both ICAM-1 and VCAM-1 are proinflammatory and controlled by the NF-κB pathway, evidence suggests that their expression may be differentially regulated (26, 42). Higher levels of ICAM-1 were also observed in obese children with asthma, compared with VCAM-1 levels (25). This could explain why differences in VCAM-1 were not seen in our study.
Gene expression of inflammatory cytokine IL-5 was significantly higher in the A1WT SEN and A1WT SEN + AD groups compared with the A1WT CON group, whereas IL-5 was undetected in all the A1KO groups. IL-5 is a very important mediator in allergic asthma (19) and is a key player in the induction of eosinophilia (3). Though eosinophilia is considered to be a characteristic hallmark of asthmatic inflammation, there is no previous evidence suggesting a link between asthma and IL-5 in vascular tissue inflammation. In fact, it is likely that several inflammatory cytokines induced by allergen challenge may be responsible for altered vascular responses and may play a part in systemic and vascular inflammation. More studies are needed to determine the signaling pathway responsible for the upregulation of IL-5 through A1AR in the vasculature of allergic mice.
These findings on ICAM-1 and IL-5 gene expression suggest that the absence of the A1AR appears to limit adenosine-mediated inflammation in the vasculature. Further studies are required to delineate the exact mechanism for the role of the A1AR in the development of these adverse vascular events observed in our model of asthma.
Asthma has been associated with an increased incidence of negative cardiovascular outcomes (13, 27, 30, 58). While asthmatic inflammation is believed to be responsible for the development of these cardiovascular effects, there is little information linking the two, especially in relation to the vascular effects of A1AR. In the present study, NECA, a nonselective adenosine analog, produced a concentration-dependent vasorelaxation in A1WT CON mice, which was impaired in allergic A1WT mice. Vascular reactivity to NECA was further altered in aorta of allergic A1WT mice that had received aerosolized adenosine. On the other hand, NECA produced a concentration-dependent vasorelaxation response in allergic and nonallergic A1KO groups. We also used the selective A1 agonist CCPA to further confirm the role of A1AR in altered vascular reactivity. CCPA elicited a significantly higher contraction in allergic A1WT mice, thus underscoring the involvement of A1AR. Western blot analysis showed that the expression of A1AR was significantly higher in the WT SEN and SEN + AD groups compared with CON. These findings strongly suggest that the A1AR indeed is responsible for the altered vascular responses in allergic and adenosine aerosolized allergic WT mice. Whereas adenosine aerosol exacerbated the NECA response in A1WT SEN + AD compared with A1WT SEN mice, no difference was observed in the CCPA response. Previously, we found that the aortic expression of A2A and A2B receptors, which are involved in vasorelaxation, was lower in the SEN + AD group (47), and this could possibly explain the difference in the NECA response in the present study.
Poor lung function is associated with an increased risk of cardiovascular disease (51). Whole body plethysmography has been used previously to determine the extent of airway hyperresponsiveness and as a measure of airway obstruction (12, 17, 18, 45). We used this method to determine the differences between A1WT and A1KO responses to MCh and NECA. MCh produced higher airway responsiveness in both A1WT- and A1KO-sensitized and -challenged animals compared with the respective controls. No difference was observed between different groups of allergic A1WT and A1KO mice. However, airway responses to the nonselective adenosine analog NECA were different between A1KO and A1WT mice, suggesting the involvement of A1AR. There was no difference in NECA-mediated airway responsiveness in all three groups of A1KO mice; on the other hand, the A1WT SEN group had significantly higher bronchoconstriction (Penh) compared with the A1WT CON group. The highest bronchoconstrictor response was observed in the A1WT SEN + AD group, which was significantly different from all other study groups. A1AR has been implicated in the adenosine-mediated bronchoconstrictor response earlier (1, 43), and selective A1AR antagonist has been shown to produce a significant reduction of airway hyperresponsiveness in allergic rabbits by blocking these receptors (44). Our data support a role for A1AR-mediated airway hyperresponsiveness and also show that adenosine aerosol challenge exacerbates this response in allergic mice.
In conclusion, our findings provide evidence for the first time that the A1AR is involved in altered vascular tissue parameters (aortic inflammation, impaired vasorelaxation, and increased A1AR expression) and airway hyperresponsiveness as a result of allergen exposure and adenosine aerosol challenge in sensitized mice. Understanding the exact mechanism of A1AR-mediated signaling would provide an important tool in the prevention of systemic inflammation and impaired vascular responses observed in people with asthma. Ultimately, this could be beneficial in lowering cardiovascular events in patients with asthma.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-027339, HL-094447, and HL-071802.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Bertil B. Fredholm for contribution to this study and Kevin Roush for help with the Western blots for A1 adenosine receptors.
REFERENCES
- 1.Abebe W, Mustafa SJ. A1 adenosine receptor-mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 275: L990–L997, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292: H719–H725, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Asquith KL, Ramshaw HS, Hansbro PM, Beagley KW, Lopez AF, Foster PS. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J Immunol 180: 1199–1206, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis 32: 73–97, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Blackburn MR, Kellems RE. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv Immunol 86: 1–41, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 170: 191–203, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brown RA, Clarke GW, Ledbetter CL, Hurle MJ, Denyer JC, Simcock DE, Coote JE, Savage TJ, Murdoch RD, Page CP, Spina D, O'Connor BJ. Elevated expression of adenosine A1 receptor in bronchial biopsy specimens from asthmatic subjects. Eur Respir J 31: 311–319, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol 290: R1324–R1329, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol 167: 4676–4685, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cushley MJ, Tattersfield AE, Holgate ST. Adenosine-induced bronchoconstriction in asthma. Antagonism by inhaled theophylline. Am Rev Respir Dis 129: 380–384, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol 15: 161–165, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drazen JM, Finn PW, De Sanctis GT. Mouse models of airway responsiveness: physiological basis of observed outcomes and analysis of selected examples using these outcome indicators. Annu Rev Physiol 61: 593–625, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Drislane FW, Samuels MA, Kozakewich H, Schoen FJ, Strunk RC. Myocardial contraction band lesions in patients with fatal asthma: possible neurocardiologic mechanisms. Am Rev Respir Dis 135: 498–501, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 148: 91–97, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Driver AG, Kukoly CA, Metzger WJ, Mustafa SJ. Bronchial challenge with adenosine causes the release of serum neutrophil chemotactic factor in asthma. Am Rev Respir Dis 143: 1002–1007, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Fan M, Mustafa SJ. Role of adenosine in airway inflammation in an allergic mouse model of asthma. Int Immunopharmacol 6: 36–45, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fan M, Mustafa SJ. Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pulm Pharmacol Ther 15: 147–155, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Fan M, Qin W, Mustafa SJ. Characterization of adenosine receptor(s) involved in adenosine-induced bronchoconstriction in an allergic mouse model. Am J Physiol Lung Cell Mol Physiol 284: L1012–L1019, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 183: 195–201, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2292–2301, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59: 574–580, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hazarika S, Van Scott MR, Lust RM. Myocardial ischemia-reperfusion injury is enhanced in a model of systemic allergy and asthma. Am J Physiol Heart Circ Physiol 286: H1720–H1725, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hazarika S, Van Scott MR, Lust RM. Severity of myocardial injury following ischemia-reperfusion is increased in a mouse model of allergic asthma. Am J Physiol Heart Circ Physiol 292: H572–H579, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Huang F, del-Río-Navarro BE, Monge JJ, Torres Alcántara S, Ontiveros JA, Olivos EN, Martínez de Castro NG, Zhao L, Hong E. Endothelial activation and systemic inflammation in obese asthmatic children. Allergy Asthma Proc 29: 453–460, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85: 199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol 33: 743–748, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol 89: 381–385, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol 280: L363–L368, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med 165: 2521–2526, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-Adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest 91: 2320–2325, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann JS, Holgate ST, Renwick AG, Cushley MJ. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol 61: 1667–1676, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Mann JS, Renwick AG, Holgate ST. Release of adenosine and its metabolites from activated human leucocytes. Clin Sci (Lond) 70: 461–468, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Marquardt DL, Gruber HE, Wasserman SI. Adenosine release from stimulated mast cells. Proc Natl Acad Sci USA 81: 6192–6196, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNamara N, Gallup M, Khong A, Sucher A, Maltseva I, Fahy J, Basbaum C. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J 18: 1770–1772, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Mubagwa K, Mullane K, Flameng W. Role of adenosine in the heart and circulation. Cardiovasc Res 32: 797–813, 1996 [PubMed] [Google Scholar]
- 38.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A2B adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther 320: 1246–1251, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 111: 72–78, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Nadeem A, Fan M, Ansari HR, Ledent C, Jamal Mustafa S. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol 292: L1335–L1344, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Nadeem A, Mustafa SJ. Adenosine receptor antagonists and asthma. Drug Disc Today: Therapeutic Strategies 3: 269–275, 2006 [Google Scholar]
- 42.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 18: 842–851, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Nyce JW, Metzger WJ. DNA antisense therapy for asthma in an animal model. Nature 385: 721–725, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, Mustafa SJ. A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther 315: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Oldenburg PJ, Mustafa SJ. Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther 313: 319–324, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Onufrak S, Abramson J, Vaccarino V. Adult-onset asthma is associated with increased carotid atherosclerosis among women in the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 195: 129–137, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponnoth DS, Nadeem A, Mustafa SJ. Adenosine-mediated alteration of vascular reactivity and inflammation in a murine model of asthma. Am J Physiol Heart Circ Physiol 294: H2158–H2165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resnick MB, Colgan SP, Patapoff TW, Mrsny RJ, Awtrey CS, Delp-Archer C, Weller PF, Madara JL. Activated eosinophils evoke chloride secretion in model intestinal epithelia primarily via regulated release of 5′-AMP. J Immunol 151: 5716–5723, 1993 [PubMed] [Google Scholar]
- 49.Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen BX, Erlanger BF. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J Immunol 151: 2775–2785, 1993 [PubMed] [Google Scholar]
- 50.Schanen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, Folsom AR. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax 60: 633–638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepherd RK, Linden J, Duling BR. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res 78: 627–634, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 79: 2–10, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol 533: 77–88, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Talukder MA, Morrison RR, Jacobson MA, Jacobson KA, Ledent C, Mustafa SJ. Targeted deletion of adenosine A3 receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol 282: H2183–H2189, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288: H1411–H1416, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tockman MS, Pearson JD, Fleg JL, Metter EJ, Kao SY, Rampal KG, Cruise LJ, Fozard JL. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med 151: 390–398, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol 25: 617–620, 1996 [DOI] [PubMed] [Google Scholar]
- 59.van den Berge M, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Provocation with adenosine 5′-monophosphate, but not methacholine, induces sputum eosinophilia. Clin Exp Allergy 34: 71–76, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Zureik M, Kony S, Neukirch C, Courbon D, Leynaert B, Vervloet D, Ducimetiere P, Neukirch F. Bronchial hyperresponsiveness to methacholine is associated with increased common carotid intima-media thickness in men. Arterioscler Thromb Vasc Biol 24: 1098–1103, 2004 [DOI] [PubMed] [Google Scholar]