Abstract
Resistin is a newly discovered adipocyte-derived cytokine that may play an important role in insulin resistance, diabetes, adipogenesis, inflammation, and cardiovascular disease. However, it is largely unknown whether resistin impairs endothelial functions by affecting the endothelial nitric oxide synthase (eNOS) system. In this study, we determined the effect of human recombinant resistin protein on eNOS expression and regulation in human coronary artery endothelial cells (HCAECs). When cells were treated with clinically relevant concentrations of resistin (40 or 80 ng/ml) for 24 h, the levels of eNOS mRNA, protein, and activity and eNOS mRNA stability were significantly reduced. Cellular nitric oxide levels were also decreased. In addition, the cellular levels of reactive oxygen species (ROS), including superoxide anion, were significantly increased in resistin-treated HCAECs. Mitochondrial membrane potential and the activities of catalase and superoxide dismutase were reduced. Three antioxidants, seleno-l-methionine, ginsenoside Rb1, and MnTBAP (superoxide dismutase mimetic), effectively blocked resistin-induced eNOS downregulation. Meanwhile, resistin activated the mitogen-activated protein kinases p38 and c-Jun NH2-terminal kinase (JNK), and the specific p38 inhibitor SB-239063 effectively blocked resistin-induced ROS production and eNOS downregulation. Furthermore, immunoreactivity of resistin was increased in atherosclerotic regions of human aorta and carotid arteries. Thus resistin directly induces eNOS downregulation through overproduction of ROS and activation of p38 and JNK in HCAECs. Resistin-induced mitochondrial dysfunction and imbalance in cellular redox enzymes may be the underlying mechanisms of oxidative stress.
Keywords: oxidative stress, mitogen-activated protein kinase
numerous factors contribute to the risk for atherosclerotic vascular disease and subsequent cardiovascular events. Among them, obesity has become a major health concern bearing important social and economical impacts (5). It is associated with increases in all-cause mortality, including death from cardiovascular disease and heart failure (13, 51). Recent studies indicate that adipose tissue is not only an organ of energy storage but also an endocrine organ producing a number of bioactive molecules such as leptin, adiponectin, tumor necrosis factor-α (TNF-α), plasminogen activator inhibitor type 1, and resistin (21). Over the past several years, much effort has been made to explore the mechanisms linking these adipokines to insulin resistance, endothelial dysfunction, and vascular disease (21, 37).
Resistin is a newly described adipokine that has been suggested to play a role in the development of insulin resistance and obesity (31). In patients with type 1 and 2 diabetes, obesity, and inflammatory conditions, plasma levels of resistin can be elevated to >40 ng/ml (14, 16, 30). Chronic inflammation contributes to the formation of atherosclerosis (32). Clinical studies have indicated that high plasma levels of resistin are associated with the severity of coronary disease and hypertension (7, 8, 26, 33, 41, 50). Several types of cells can express resistin. In mice, adipocytes appear to be the major source of resistin (47). In humans, resistin may also come from monocytes and macrophages (42, 44). Macrophages in inflammatory sites such as obese adipose tissues and atherosclerotic lesions may secrete resistin (24). High levels of resistin can be detected at the atherosclerotic tissues (4). Although human vascular endothelial cells did not express resistin (44), high systemic and/or local concentrations of resistin could induce endothelial dysfunction. Indeed, resistin directly induces endothelin-1 production, upregulates adhesion molecules and chemokines, and downregulates TNF receptor-associated factor-3 (49). Our previous reports and other studies also showed that resistin can impair endothelium-dependent vasorelaxation (18, 29) and induce angiogenesis (38).
However, the precise mechanisms of resistin-mediated effects on vascular cells are largely unknown. In the present study, we hypothesized that resistin may affect endothelial nitric oxide synthase (eNOS) expression and regulation through oxidative stress and mitogen-activated protein kinase (MAPK) activation. Specifically, the effects of resistin on eNOS mRNA, protein levels, and activity were determined in human coronary artery endothelial cells (HCAECs). Reactive oxygen species (ROS) production, mitochondrial membrane potential, and internal antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) activities, as well as MAPK phosphorylation were investigated. Expression levels of resistin in human atherosclerotic tissues were also determined. This study provides new insights into the mechanisms by which resistin interacts with endothelial cells, which may contribute to cardiovascular disease.
MATERIALS AND METHODS
Cell culture.
HCAECs and endothelial growth medium-2 were purchased from Cambrex BioWhittaker (Walkersville, MD). Cells were used at passage 4 to 6. Human recombinant resistin was obtained from Phoenix Pharmaceuticals (Belmont, CA). In the culture condition, we confirmed that almost all HCAECs had positive immunostaining for eNOS, one of the most important functional markers for mature endothelial cells [Supplemental Fig. S1 (Supplemental data for this article may be found on the American Journal of Physiology: Heart and Circulatory Physiology website.)].
Real-time PCR.
Total RNA from HCAECs was isolated. cDNA was generated by reverse transcription from mRNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control to account for variations in mRNA loading. Real-time PCR was performed in an iCycler iQ real-time PCR detection system (Bio-Rad). To assess the mRNA stability or half-life of eNOS mRNA, HCAECs were treated with 5 μg/ml actinomycin D [a direct inhibitor of RNA polymerase II (RNAPII)] in the presence or absence of resistin (40 ng/ml). Total cellular RNA was isolated at multiple time points (0, 0.5, 1, 3, 6, and 12 h) and analyzed for eNOS mRNA levels by real-time PCR. In separate experiments, HCAECs, human aorta smooth muscle cells (AoSMCs), and human THP-1-derived macrophages were treated with or without TNF-α (10 ng/ml) for 24 h, and resistin mRNA levels were determined by real-time PCR.
Western blot.
Equal amounts of endothelial proteins (6 μg) were resolved electrophoretically by one-dimensional SDS-PAGE (10% polyacrylamide). Subsequently, the proteins were electrophoretically transferred to nitrocellulose. eNOS was detected using a mouse anti-human eNOS monoclonal antibody (BD Biosciences, San Jose, CA), and β-actin was detected using a mouse anti-human β-actin monoclonal antibody (Sigma). The eNOS and β-actin primary antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody.
NOS activity assay.
A fluorometric cell-associated NOS detection system (Sigma) was used to measure intracellular production of nitric oxide (NO) from supplemented l-arginine by a nonradiometric method.
Nitrite detection.
NO levels released from HCAECs were determined by measuring the accumulation of its stable degradation products, nitrite and nitrate (Griess reaction NO assay kit; Calbiochem). Total nitrite levels in cell culture supernatants were measured and normalized to total proteins of HCAECs (pmol/mg protein).
Flow cytometry analyses.
Cells were harvested with 0.025% Trypsin/EDTA and adjusted to 1 × 106 cells per each FACS tube. For ROS and NO staining, respectively, dihydroethidium (DHE, 3 μM; Molecular Probes) and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF, 10 μM; Molecular Probes) were added and incubated at 37°C for 30 min. DHE is often used for the detection of ROS production because it can be oxidized by ROS such as superoxide anion (O2−) into two red fluorescent molecules [2-hydroxyethidium (2-EOH, excitation wavelength 490 and emission wavelength 590 nm) and ethidium (excitation wavelength 480 and emission wavelength 580 nm)] (35). Samples were analyzed using FACScan and Cell Quest software (Becton Dickinson, Franklin Lakes, NJ). Loss of mitochondrial membrane potential was assessed using flow cytometry analysis of cells stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide iodine (JC-1, MitoScreen kit; BD Biosciences).
HPLC analysis for O2−.
We confirmed the increased production of O2− in the resistin-treated HCAECs by DHE staining and HPLC analysis as previously described, which is able to separate 2-EOH and ethidium (15, 53, 55). DHE was added in the cell culture medium with a final concentration of 25 μM and incubated for 30 min. The cells were harvested in 200 μl methanol and sonicated for 10 s, and the cell lysates were subjected to HPLC analysis.
Cellular glutathione assay.
GSH-Glo Glutathione assay (Promega, Madison, WI) measures a change in the redox state of the cell due to oxidants, which are downstream metabolites of O2−.
Measurement of CAT and SOD activity.
CAT and SOD enzyme activities were measured with commercial enzyme assay kits (Cayman Chemical, Ann Arbor, MI) following the manufacturer's protocols. The CAT and SOD activity was represented as nanomoles per minute per milliliter and units per milliliter, respectively.
BioPlex immunoassay.
HCAECs were cultured with 40 ng/ml of resistin for 0, 5, 10, 20, 30, 45, 60, or 90 min. Cell lysate was prepared. Detection of phospho- and total extracellular signal-regulated kinase (ERK) 1/2, c-Jun NH2-terminal kinase (JNK), and p38 was performed by the BioPlex Luminex system 2200 (Bio-Rad).
Immunohistochemical analysis.
Full-thickness arterial wall specimens of aorta and carotid arteries were obtained from five patients with or without atherosclerosis undergoing autopsy [National Disease Research Interchange (NDRI), Philadelphia, PA]. Immunohistochemistry was done using anti-resistin antibody (1:200) (Phoenix Pharmaceuticals, Burlingame, CA), biotinylated secondary antibody, and avidin-biotin reaction using peroxidase enzyme (ABC kit; Vector Laboratories, Burlingame, CA). The protocol of use of human tissues obtained from NDRI was approved by the Institutional Review Board at the Baylor College of Medicine. The investigation conformed to the principles outlined in the Declaration of Helsinki.
Statistical analysis.
Data from the different treatment groups and control groups were compared with a paired Student's t-test (two tails). Significance was considered if P < 0.05. Data are reported as means ± SE.
Detailed materials and methods are provided in the online supplementary materials.
RESULTS
Resistin decreases eNOS expression and NO levels in HCAECs.
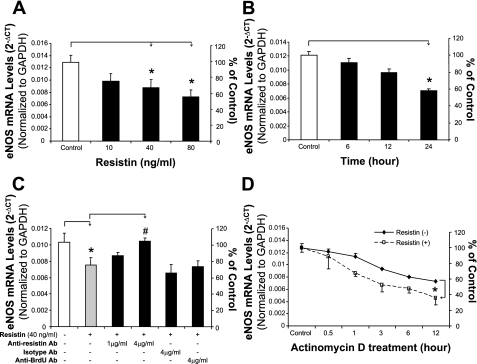
HCAECs were treated with resistin in a concentration- and time-dependent manner. eNOS mRNA and protein levels were detected using real-time PCR (n = 3) and Western blot (n = 3), respectively. When cells were treated with resistin (40 or 80 ng/ml) for 24 h, eNOS mRNA levels were decreased by 32 and 44%, respectively, compared with controls (P < 0.05, Fig. 1A). When cells were cultured with resistin (40 ng/ml) for different times, only the 24-h treatment group showed a significant reduction of eNOS mRNA levels compared with controls (P < 0.01, Fig. 1B). To determine the specific effect of resistin on eNOS expression, HCAECs were treated with resistin (40 ng/ml) and anti-resistin antibody (1 or 4 μg/ml), isotype IgG (4 μg/ml), or anti-bromodeoxyuridine antibody (4 μg/ml) for 24 h. Anti-resistin antibody at a concentration of 4 μg/ml significantly blocked the decrease in eNOS induced by resistin (P < 0.05, Fig. 1C). Isotype and anti-bromodeoxyuridine antibodies as negative controls at the same concentration showed no effect on the resistin-induced eNOS mRNA decrease (Fig. 1C). In addition, by using actinomycin D, a direct inhibitor of RNA polymerase II, we showed that eNOS mRNA stability was significantly decreased in cells treated with resistin (40 ng/ml) compared with control cells (P < 0.05, Fig. 1D). The half-life of eNOS mRNA decreased from >12 h in control cells to <6 h in resistin-treated HCAECs.
Fig. 1.
Effect of resistin on endothelial nitric oxide synthase (eNOS) mRNA levels in human coronary artery endothelial cells (HCAECs). HCAECs were cultured with different concentrations of resistin for different periods of time. The mRNA levels of eNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by real-time PCR analysis. A: cells were treated with different concentrations of resistin (10, 40, or 80 ng/ml) for 24 h. *P < 0.05, n = 3 experiments. B: cells were treated with resistin (40 ng/ml) for different times (6, 12, and 24 h). *P < 0.05, n = 3. C: cells were treated with 40 ng/ml resistin and different antibodies (Ab) for 24 h. *P < 0.05, n = 3 (compared with untreated controls). #P < 0.05, n = 3 (compared with resistin treatment alone). D: HCAEC eNOS mRNA stability was determined by real-time PCR after cells were treated with 5 μg/ml actinomycin D in the presence or absence of resistin (40 ng/ml) for indicated time points (0, 0.5, 1, 3, 6, or 12 h). *P < 0.05, n = 3 experiments.
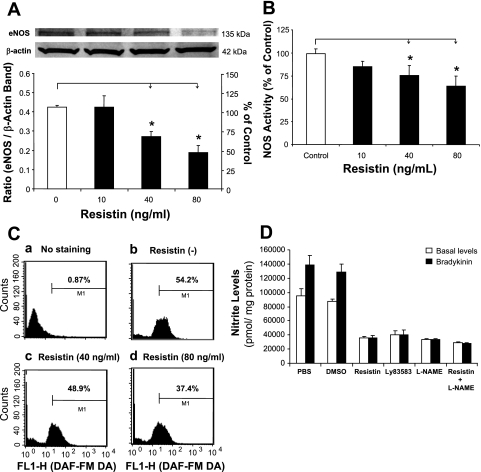
Western blot showed significant decreases in eNOS protein levels after treatment with resistin (40 or 80 ng/ml) for 24 h, compared with controls (P < 0.05, Fig. 2A). NOS activities were also studied using a NOS detection system (n = 3). Consistent with the real-time PCR and Western blot data, resistin at 40 and 80 ng/ml significantly decreased NOS activity by 25 and 36%, respectively, compared with controls (P < 0.05, Fig. 2B).
Fig. 2.
Effects of resistin on eNOS protein levels and NOS activity in HCAECs. A: Western blot analysis. HCAECs were treated with 40 or 80 ng/ml resistin for 24 h. Representative bands of eNOS and β-actin staining and quantitation of band density ratios (eNOS and β-actin). *P < 0.05, n = 3. B: NOS activity assay by fluorescence microplate reader. *P < 0.05, n = 3. C: flow cytometry (cellular NO levels). Treated HCAECs were stained with 10 μM of 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) for 30 min. D: nitric oxide (NO) levels in the supernatant of cell culture (Griess assay). HCAECs were treated with resistin (40 ng/ml) and/or other molecules [3 μM LY-83583 and 100 μM NG-nitro-l-arginine methyl ester (l-NAME)] for 24 h. Basal and bradykinin-stimulated levels of NO-derived nitrite in the culture supernatant were detected. *P < 0.05, n = 3.
We also measured cellular NO levels in HCAECs after resistin treatment using the fluorescent dye DAF. DAF staining is a unique method measuring NO production in living cells or solutions (28). Treated HCAECs were incubated with 10 μM of DAF for 30 min and then washed. The stained cells were studied using flow cytometry assay. Resistin at 40 and 80 ng/ml decreased the NO positive cell number by 10 and 31%, respectively, compared with controls (Fig. 2C). Furthermore, NO-derived nitrite levels in the supernatants of HCAEC cultures were determined by Griess assay. Resistin treatment at 40 ng/ml for 24 h significantly reduced both basal and bradykinin-stimulated levels of NO. Meanwhile, the combination of specific eNOS inhibitor l-NAME (100 μM) with resistin did not cause further reduction in NO levels compared with resistin treatment alone in HCAECs (Fig. 2D). These data indicate that resistin specifically inhibits eNOS.
Resistin increases ROS production in HCAECs.
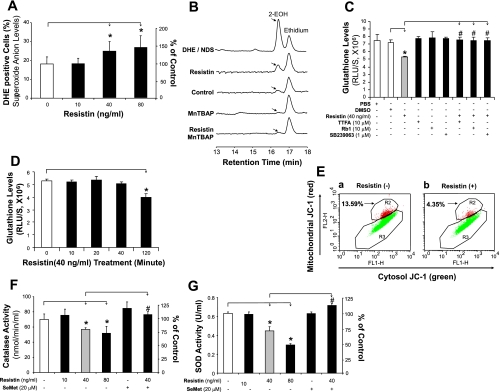
To study whether oxidative stress plays a role in resistin-induced endothelial dysfunction, ROS production in HCAECs was measured using fluorescence dye DHE staining and flow cytometry analysis (n = 3). In HCAECs that were cultured with 40 and 80 ng/ml of resistin for 24 h, ROS production increased substantially by 39 and 56%, respectively, compared with controls (P < 0.05, Fig. 3A). Resistin at 10 ng/ml, however, did not lead to any change in DHE staining. DHE staining with current flow cytometry analysis is correlated to the ROS production, while it cannot separate O2− and other free radicals. Thus we confirmed the increased production of O2− in the resistin-treated HCAECs by HPLC analysis as recently described (15, 53, 55). HCAECs were treated with resistin (40 ng/ml) for 3 h, and then DHE (25 μM) was added to the cells for 30 min. Cellular levels of 2-EOH and ethidium were determined by HPLC analysis. As shown in Fig. 3B, resistin-treated cells increased 2-EOH levels, indicating increased levels of O2− compared with untreated control cells. Positive control of 2-EOH was prepared by using nitrosodisulfonate radical dianion with DHE in the aqueous phosphate buffer, pH 7.4, containing diethylene triamine pentaacetic acid (55). In addition, the specific O2− scavenger manganese [III] tetrakis(4-benzoic acid)porphyrin (MnTBAP; SOD mimetic) completely blocked the effect of resistin on 2-EOH increase. These data indicate that resistin increases cellular O2− levels in human endothelial cells.
Fig. 3.
Effect of resistin on oxidative stress in HCAECs. A: reactive oxygen species (ROS) production [dihydroethidium (DHE) staining and flow cytometry analysis]. HCAECs were treated with 40 or 80 ng/ml of resistin for 24 h. *P < 0.05, n = 3. B: superoxide anion (O2−) levels (DHE staining and HPLC analysis). HCAECs were treated with resistin (40 ng/ml) for 3 h, and then DHE (25 μM) was added to the cells for 30 min. Cellular levels of 2-hydroxyethidium (2-EOH) and ethidium were determined by HPLC analysis. Positive control of 2-EOH was prepared by using nitrosodisulfonate radical dianion (NDS) with DHE in the aqueous phosphate buffer, pH 7.4, containing diethylene triamine pentaacetic acid (DTPA). Specific O2− scavenger manganese [III] tetrakis(4-benzoic acid)porphyrin [MnTBAP, superoxide dismutase (SOD) mimetic] was used. C: ROS production (glutathione assay). HCAECs were treated with resistin and/or other molecules for 24 h. RLU, relative light units; TTFA, thenoyltrifluoroacetone. *P < 0.05, n = 3 [compared with dimethyl sulfoxide (DMSO) controls]. #P < 0.05, n = 3 (compared with resistin treatment alone). D: time course of ROS production (glutathione assay). HCAECs were treated with resistin (40 μg/ml) for different time points. *P < 0.05, n = 3 [compared with dimethyl sulfoxide (DMSO) controls]. #P < 0.05, n = 3 (compared with resistin treatment alone). E: mitochondrial membrane potential (JC-1 staining and flow cytometry). F: catalase activity assay. HCAECs were treated with resistin (40 or 80 ng/ml) and/or selenomethionine (SeMet, 20 μM) for 24 h. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). G: SOD activity assay. HCAECs were treated with resistin (40 or 80 ng/ml) and/or SeMet (20 μM) for 24 h. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone).
Furthermore, the redox state of the cell was detected using GSH assay. Resistin treatment at 40 ng/ml for 24 h led to significantly decreased GSH levels compared with controls (n = 3, P < 0.05, Fig. 3C). HCAECs were also treated with resistin for different time points (0, 10, 20, 40, and 120 min), and reduced GSH levels were seen after treatment for 120 min (Fig. 3D), indicating oxidative stress.
Resistin decreases mitochondrial membrane potential and ATP production in HCAECs.
Mitochondria are known to be a major source of ROS, and they are also particularly susceptible to oxidative damage produced by the action of ROS on lipids, proteins, and DNA (17). For this reason, the membrane potential can serve as an indicator of mitochondrial respiratory chain function. HCAECs were seeded on six-well plates and cultured with or without resistin (40 ng/ml) for 24 h. Treatment with resistin substantially reduced mitochondrial membrane potential by 68% compared with controls (Fig. 3E).
Energy produced by mitochondrial respiration is used for ATP synthesis by a complex mechanism referred to as “oxidative phosphorylation.” Impairment in mitochondrial respiration can lead to a decrease in ATP production. We determined ATP levels in HCAECs by using the ATPLite kit. Treatment with resistin (40 ng/ml) significantly reduced ATP levels by 29% compared with controls (P < 0.05, n = 3, Supplemental Fig. S2).
To further confirm the mitochondrial source of ROS, HCAECs were treated with resistin, the mitochondrial inhibitor thenoyltrifluoroacetone (TTFA), or a combination of both for 24 h, and ROS production was detected by GSH assay. Resistin reduced GSH levels, whereas TTFA effectively blocked the effect of resistin in HCAECs (Fig. 3C). This indicates that mitochondria contribute to resistin-induced ROS production in HCAECs.
To determine whether resistin causes cell death in the current experimental conditions, HCAECs were treated with resistin (80 μg/ml) for 24 and 48 h, and cell viability was studied with a MTS assay. There was no significant difference of cell viability between control and resistin-treated cells at either the 24- or 48-h time points (Supplemental Fig. S3). Thus resistin used in the current study did not affect cell viability or apoptosis in HCAECs.
Resistin decreases CAT and SOD activities in HCAECs.
A variety of intrinsic antioxidants, including CAT and SOD, are present in organisms and protect them from oxidative stress (39). The enzyme activities of CAT and SOD were studied with commercial assay kits (n = 3 for each). Treatment with resistin at 40 or 80 ng/ml significantly reduced CAT activities by 19 and 26%, respectively, compared with controls (P < 0.05, Fig. 3F). Similarly, SOD activities were also reduced by 30 and 53%, respectively (P < 0.05, Fig. 3G).
LY-83583 induces ROS overproduction and eNOS mRNA reduction.
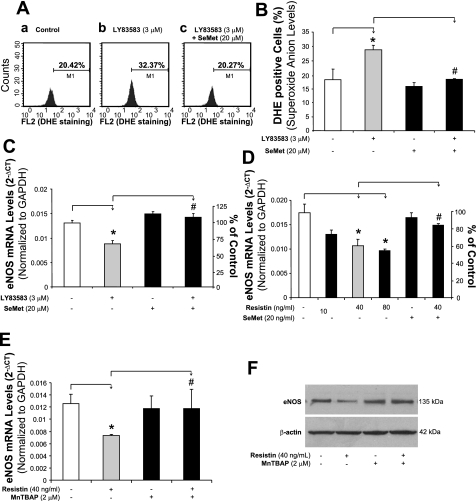
To demonstrate the direct link between increased ROS and decreased eNOS mRNA expression levels, we included LY-83583, a known superoxide generator (22), in our experiments as a positive control. HCAECs were treated with LY-83583 (3 μM) for 24 h. DHE staining showed an increase in ROS production by 61% compared with controls (P < 0.05, n = 3, Fig. 4, A and B). Meanwhile, in HCAECs treated with LY-83583, eNOS mRNA levels showed a decrease (33%), which was similar to that seen in resistin-treated cells. Coculture with the antioxidant selenomethionine (SeMet) reduced ROS production and increased eNOS mRNA expression to the level of untreated controls (Fig. 4C). Furthermore, NO released from HCAECs was detected by Griess assay. LY-83583 significantly reduced both basal and bradykinin-stimulated levels of NO (Fig. 2D).
Fig. 4.
Effects of superoxide donor LY-83583, resistin, SeMet, and MnTBAP on eNOS mRNA expression in HCAECs. A: ROS production (DHE staining and flow cytometry analysis). HCAECs were treated with LY-83583 (3 μM) and/or SeMet (20 μM) for 24 h. B: quantitative data of LY-83583-induced ROS overproduction. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). C: eNOS mRNA (real-time PCR assay) in LY-83583-treated HCAECs. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). D: effect of SeMet on eNOS mRNA levels (real-time PCR assay) in HCAECs. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). E: effect of MnTBAP on eNOS mRNA levels (real-time PCR assay) in HCAECs. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). F: effect of MnTBAP on eNOS protein levels (Western blot analysis) in HCAECs.
Antioxidants SeMet, ginsenoside rb1, and SOD mimetic MnTBAP block resistin-induced eNOS downregulation in HCAECs.
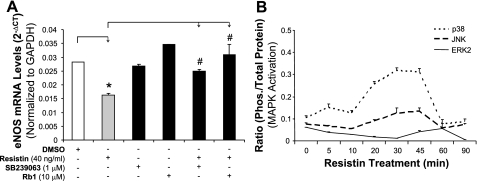
Because oxidative stress is involved in the action of resistin, we further tested whether antioxidants could block the reduction in eNOS mRNA induced by resistin. HCAECs were cocultured with resistin (40 ng/ml) and antioxidant SeMet (20 μM) for 24 h. The eNOS mRNA decrease seen in cells treated with resistin alone was significantly blocked by the addition of SeMet (P < 0.05, n = 3, Fig. 4D). SeMet alone showed no effect on eNOS expression. In addition, resistin-induced decreases in cellular CAT and SOD activities were also blocked by SeMet (P < 0.05, n = 3, Fig. 3, F and G). To determine the effect of the specific O2− scavenger MnTBAP on resistin-induced eNOS downregulation in HCAECs, the cells were treated with resistin (40 ng/ml) and/or MnTBAP (2 μM) for 24 h. The eNOS mRNA and protein levels were determined by real-time PCR and Western blot analysis, respectively. As shown in Fig. 4, E and F, MnTBAP effectively blocked resistin-induced eNOS downregulation at both mRNA and protein levels. In addition, the action of ginsenoside Rb1 on these resistin-mediated events was examined in HCAECs. Adding ginsenoside Rb1 effectively blocked resistin-induced eNOS downregulation (P < 0.05, n = 3, Fig. 5A). Also, ginsenoside Rb1 was able to reduce resistin-induced ROS production (Fig. 3C).
Fig. 5.
Role of mitogen-activated protein kinases (MAPKs) and ginsenoside Rb1 on resistin-induced eNOS downregulation. A: eNOS mRNA levels (real-time PCR assay). HCAECs were treated with resistin and/or other molecules (p38 inhibitor SB-239063 and ginsenoside Rb1) for 24 h. *P < 0.05, n = 3 (compared with DMSO controls). #P < 0.05, n = 3 (compared with resistin treatment alone). B: phosphorylation of MAPKs [p38, c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) 2] by using Bio-Rad Bioplex luminex immunoassay. HCAECs were treated with resistin (40 ng/ml) for different time points.
Resistin induces MAPK p38 and JNK activation.
Phosphorylation of MAPKs was investigated with a BioPlex immunoassay. After resistin treatment (40 ng/ml), there were an early p38 phosphorylation peak at 5–10 min and a second peak at 30–45 min. JNK activation only showed a single peak at 45 min after the treatment. However, ERK1/2 did not show any significant changes at any time points (Fig. 5B). To confirm the functional role of p38 activation in the action of resistin, a p38 inhibitor (SB-239036, 1 μM) was used to pretreat HCAECs for 1 h. Next, the cells were cultured with resistin (40 ng/ml) for 24 h, and eNOS mRNA levels were detected using real-time PCR. Pretreatment with SB-239036 effectively blocked the resistin-induced eNOS decrease (P < 0.05, n = 3, Fig. 5A). Meanwhile, treatment with SB-239036 alone did not show any effect on eNOS expression. Also, in the assay for ROS production, SB-239036 effectively blocked resistin-induced ROS production in HCAECs (Fig. 3B).
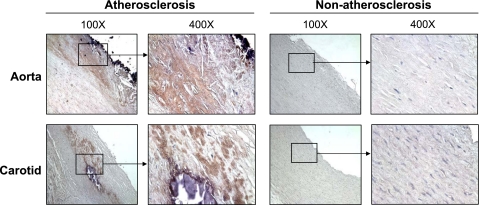
Level of human resistin is increased in human atherosclerotic vessels.
Because resistin can be expressed by human macrophages (42), we performed immunostaining to determine resistin levels in human arteries. Nonatherosclerotic vessels, including human aorta and carotid arteries, showed no or very limited resistin immunoreactivity. In contrast, atherosclerotic regions of these arteries displayed strong resistin immunoreactivity. The increased signal of resistin was mainly located in the regions of the intima and media of atherosclerotic plaques (Fig. 6). To confirm the capability of three cell types of vascular tissues (macrophages, smooth muscle cells, and endothelial cells) to express resistin in response to TNF-α, the cells were treated with or without TNF-α (10 ng/ml) for 24 h, and resistin mRNA levels were determined by real-time PCR. As shown in Supplemental Fig. S4, macrophages expressed much higher levels of resistin mRNA than AoSMCs and HCAECs, which had very low levels of resistin mRNA; and TNF-α had no effect on resistin expression in all cell types.
Fig. 6.
Resistin immunoreactivity of human atherosclerotic and nonatherosclerotic arteries. Human aorta and carotid arteries were fixed in formalin and embedded in paraffin. Immunostaining was performed by using the anti-human resistin antibody (1:200), biotinylated secondary antibody, and avidin-biotin reaction using peroxidase enzyme. Brown color represents positive staining of resistin.
DISCUSSION
In this study, we present evidence that human recombinant resistin is able to induce endothelial dysfunction in HCAECs. Specifically, resistin significantly reduces eNOS mRNA, protein levels, and NOS activity as well as NO bioavailability in HCAECs. In addition, resistin directly induces oxidative stress and activation of p38 and JNK MAPKs in HCAECs. This study provides insight into the biological functions and molecular mechanisms of resistin in the vascular system.
Consistent with previous reports (4), we observed increased immunochemistry reactivity of resistin in human atherosclerotic lesions; however, we did not know which cell types in the vascular wall could express resistin. Our in vitro experiments showed that macrophages expressed much higher levels of resistin mRNA than AoSMCs and HCAECs, which had very low levels of resistin mRNA; and TNF-α had no effect on resistin expression in all cell types. Thus we speculate that resistin in the vascular lesion might be produced by vascular macrophages. It is also possible that plasma resistin could enter the vascular wall. Further studies of the source of resistin in the vascular wall are warranted.
In the endothelium, NO is constitutively generated from the conversion of l-arginine to l-citrulline by the enzymatic action of eNOS, which is considered to be the most important factor in maintaining normal vascular function. However, under various pathological conditions, eNOS may become dysfunctional or its expression may be decreased (25). Dysfunctional eNOS or low levels of eNOS not only impair endothelium-dependent vasorelaxation but also accelerate atherosclerotic lesion formation (45). In the present study, we demonstrated a direct effect of resistin on cultured HCAECs. Using real-time PCR analysis, we showed that resistin at concentrations relevant to plasma concentrations of obese individuals (14, 16) can decrease eNOS mRNA levels in a time- and concentration-dependent manner. Western blot analysis also confirmed that eNOS protein levels were decreased after resistin treatment. This effect is specific because anti-resistin antibody effectively blocked resistin-induced eNOS downregulation. Resistin also reduced NOS activity and NO levels in cultured HCAECs. These data significantly extend our previous observations that resistin decreased endothelium-dependent vasorelaxation in porcine coronary arteries (29). Furthermore, we found that resistin significantly decreased the stability of eNOS mRNA in HCAECs. However, the mechanisms behind this observation are not clear.
Cardiovascular disease is a multifactorial disease, but ROS have been shown to be key mediators for vascular inflammation and atherogenesis (36). Human investigations support the oxidative stress hypothesis of atherogenesis (2, 36). This is further supported by impaired vascular function and enhanced atherogenesis in animal models that have deficiencies in internal antioxidant enzymes (12). In the present study, we stained cells with a fluorescent DHE dye to detect ROS in HCAECs using flow cytometry assay. We found that resistin significantly induced an increase in ROS levels in HCAECs. DHE is often used for the detection of ROS production because it can be oxidized by ROS such as O2− into two red fluorescent molecules (2-EOH and ethidium) (35). 2-EOH is generated specifically by O2− oxidation of DHE, whereas ethidium is associated mainly with pathways involving H2O2 and metal-based oxidizing systems, including heme proteins and peroxidases. Flow cytometry analysis at the current condition can detect both 2-EOH and ethidium but cannot separate 2-EOH and ethidum. Recent studies showed that HPLC analysis can separate 2-EOH and ethidium after DHE staining (15, 54, 55). In the current study, we confirmed the increased production of O2− in the resistin-treated HCAECs by HPLC analysis. Specific O2− scavenger MnTBAP (SOD mimetic) is also able to effectively block the effect of resistin on 2-EOH increase. The data obtained from DHE staining were confirmed using an assay of cellular GSH levels, which are negatively correlated to oxidative stress. Meanwhile, LY-83583 (O2− generating molecule) was used in this study to confirm the role of O2− in eNOS expression. Indeed, treatment with LY-83583 led to a decrease in eNOS expression in HCAECs. These data are consistent with our previous publication in which we showed, using the lucigenin-enhanced chemiluminescence method, that resistin increased O2− production in porcine coronary artery rings; this effect was inhibited by the antioxidant seleno-l-methionine (29). Thus resistin has a strong impact on ROS production, which is likely one of the mechanisms for eNOS downregulation and dysfunction. Dick et al. (12), however, did not observe ROS production in resistin-treated canine coronary arteries by DHE tissue staining; there were, however, no quantitative data presented. The reason for this discrepancy is not clear. It could be because of experimental conditions, different species, and/or sensitivity of detection methods.
O2− is produced by a variety of sources, including the mitochondrial respiratory chain (17), NADH/NADPH oxidase (20), xanthine oxidase (46), and lipoxygenases (9). In addition, there are several cellular mechanisms that counterbalance the production of O2−, including enzymatic and nonenzymatic pathways (40). Among them, the best-characterized enzymatic pathways are SOD, which facilitates the formation of H2O2 from O2− (19), CAT (27), and glutathione peroxidase (GPX) (10), which coordinates the conversion of H2O2 to water. To study potential sources of increased O2− in resistin-treated cells, we tested mitochondrial membrane potential and ATP production in resistin-treated cells. In both areas, we found a significant decrease after treatment with resistin; this suggests that resistin induces mitochondrial dysfunction. This, in turn, may be partially responsible for the increase in O2− production detected in resistin-treated cells. TTFA, a mitochondrial inhibitor, effectively blocked the resistin-induced ROS production in HCAECs. In addition, we tested enzyme activities of CAT and SOD in HCAECs. Clearly, treatment with resistin substantially decreased the activities of both enzymes; this may impair the internal cellular response to oxidative stress in resistin-treated cells. Thus resistin-increased O2− production may result from mitochondrial dysfunction and compromised cellular redox enzymes.
Understanding the underlying molecular mechanisms of the action of resistin can help us to develop new and effective strategies to control the detrimental effects of resistin on the vascular system. Because the action of resistin may be mediated by oxidative stress, we have tested the ability of the antioxidants SeMet, ginsenoside Rb1, and SOD mimetic MnTBAP to block resistin's effects on resistin-treated cells. Indeed, all three antioxidants effectively blocked resistin-induced eNOS downregulation and ROS production. SeMet participates in an intramolecular transsulfuration reaction, forming selenocystein, which, in turn, increases the activity of internal antioxidant enzymes GPX and thioredoxin reductase (1). In addition, SeMet is able to directly interact with some oxidant molecules or oxidant-generating ions (3, 43). Ginsenosides act as antioxidants by increasing internal antioxidant enzymes and acting as free-radical scavengers (11, 34, 48, 52). Previously, we have successfully used ginsenoside Rb1 to block homocysteine- and HIV protease inhibitor-induced oxidative stress and endothelial dysfunction in porcine coronary arteries (6, 54). Overall, antioxidant therapy may provide an effective strategy to prevent vascular diseases mediated by these specific risk factors, such as resistin, as well as others.
MAPKs are important in regulating cell growth, migration, and differentiation in response to various extracellular stimuli (23). Three major subfamilies of structurally related MAPKs have been identified in mammalian cells: p44/42 MAPK (ERKl/2), p38 MAPK, and JNK/stress-activated protein kinases. The pattern of MAPK activation in response to oxidative stress varies depending on the oxidant strength and cell type. Detailed regulation pathways for MAPKs are still unknown. In the present study, we demonstrated that p38 and JNK were activated in response to resistin stimulation, and we also demonstrated that the p38 inhibitor SB-239063 effectively blocked resistin-induced eNOS downregulation and ROS production in HCAECs. Because resistin increased ROS production at 120 min and induced p38 phosphorylation at 40 min, and p38 inhibitor SB-239063 effectively blocked resistin-induced ROS production, we conclude that MAPK activation may occur upstream of oxidative stress in resistin-treated HCAECs.
In conclusion, our study demonstrates that resistin, at concentrations found in obese individuals, significantly decreases eNOS expression and NO production through oxidative stress and p38 and JNK MAPK activation in HCAECs. Resistin-induced mitochondrial dysfunction and unbalanced cellular redox enzymes may be the underlying mechanisms of increased ROS production. These data, combined with the finding that resistin expression is increased in human atherosclerotic tissues, support the hypothesis that resistin may contribute to vascular disease through endothelial dysfunction. Antioxidants SeMet, ginsenoside Rb1, and MnTBAP potentially hold clinical applications in blocking resistin-induced endothelial dysfunction, thereby preventing vascular disease.
GRANTS
This work was partially supported by a research Grant from the National Heart, Lung, and Blood Institute (HL-083471 to C. Chen) and by the Baylor College of Medicine, Houston, Texas.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Sarah W. Weakley for technique assistance.
REFERENCES
- 1.Allan CB, Lacourciere GM, Stadtman TC. Responsiveness of selenoproteins to dietary selenium. Annu Rev Nutr 19: 1–16, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, Shinke T, Kobayashi S, Hirata K, Kawashima S, Itabe H, Hayashi Y, Imajoh-Ohmi S, Itoh H, Yokoyama M. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 22: 1838–1844, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Battin EE, Perron NR, Brumaghim JL. The central role of metal coordination in selenium antioxidant activity. Inorg Chem 45: 499–501, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, Zbinden S, Clavijo LC, Jang GJ, Andrews JA, Zhu J, Epstein SE. The potential role of resistin in atherogenesis. Atherosclerosis 182: 241–248, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep 10: 32–38, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol 288: H2965–H2971, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chu S, Ding W, Li K, Pang Y, Tang C. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circ J 72: 1249–1253, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cohen G, Hörl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Semin Dial 22: 373–377, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest 103: 1597–1604, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, Hertzog P, Kola I. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med 36: 53–64, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Deng HL, Zhang JT. Anti-lipid peroxilative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl) 104: 395–398, 1991 [PubMed] [Google Scholar]
- 12.Dick GM, Katz PS, Farias M, 3rd, Morris M, James J, Knudson JD, Tune JD. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol 291: H2997–H3002, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 27: 996–1003, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fehmann HC, Heyn J. Plasma resistin levels in patients with type 1 and type 2 diabetes mellitus and in healthy controls. Horm Metab Res 34: 671–673, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Forsblad d'Elia H, Pullerits R, Carlsten H, Bokarewa M. Resistin in serum is associated with higher levels of IL-1Ra in post-menopausal women with rheumatoid arthritis. Rheumatology 47: 1082–1087, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Castelli GP, Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci 1011: 86–100, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Gentile MT, Vecchione C, Marino G, Aretini A, Di Pardo A, Antenucci G, Maffei A, Cifelli G, Iorio L, Landolfi A, Frati G, Lembo G. Resistin impairs insulin-evoked vasodilation. Diabetes 57: 577–583, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gualillo O, González-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med 17: 275–283, 2007 [DOI] [PubMed] [Google Scholar]
- 22.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol 24: 2021–2027, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Inagaki N, Ito M, Nakano T, Inagaki M. Spatiotemporal distribution of protein kinase and phosphatase activities. Trends Biochem Sci 19: 448–452, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Jung HS, Park KH, Cho YM, Sung Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res 69: 76–85, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol 24: 998–1005, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Krecki R, Drozdz J, Szcześniak P, Orszulak-Michalak D, Krzemińska-Pakuła M. Novel atherogenesis markers for identification of patients with a multivessel coronary artery disease. Kardiol Pol 66: 1173–1780, 2008 [PubMed] [Google Scholar]
- 27.Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem 274: 13908–13914, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38: 3209–3212, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg 41: 691–698, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, Rappl G, Abken H, Hahn M, Schulz O, Krone W. Visfatin/PBEF/Nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm Metab Res 42: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Lazar MA. Resistin- and obesity-associated metabolic diseases. Horm Metab Res 39: 710–716, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis (Abstract). Circulation 105: 1135, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Ha JW, Kim JS, Choi EY, Park S, Kang SM, Choi D, Jang Y, Chung N. Plasma adiponectin and resistin levels as predictors of mortality in patients with acute myocardial infarction: data from infarction prognosis study registry. Coron Artery Dis 20: 33–39, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 28: 191–200, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J Cell Mol Med Sep In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost 3: 254–267, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes 14: 365–369, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res 70: 146–157, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release 71: 1–21, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31: 1287–1312, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulos DP, Perrea D, Thomopoulos C, Sanidas E, Daskalaki M, Papazachou U, Votteas V, Makris T. Masked hypertension and atherogenesis: the impact on adiponectin and resistin plasma levels. J Clin Hypertens 11: 61–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun 300: 472–476, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Ramoutar RR, Brumaghim JL. Effects of inorganic selenium compounds on oxidative DNA damage. J Inorg Biochem 101: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes 50: 2199–2202, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Sessa WC. eNOS at a glance. J Cell Sci 117: 2427–2429, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschläger N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 107: 1383–1389, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Tian J, Fu F, Geng M, Jiang Y, Yang J, Jiang W, Wang C, Liu K. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett 374: 92–97, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation 108: 36–740, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J 24: 161–166, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med 4: 436–443, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan CS. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol 532: 201–207, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA 102: 5727–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg 41: 861–868, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.