Abstract
Babies are frequently exposed to cerebral hypoxia and ischemia (H/I) during the perinatal period as a result of stroke, problems with delivery, or postdelivery respiratory management. The sole approved treatment for acute stroke is tissue type plasminogen activator. H/I impairs pial artery dilation (PAD) induced by hypercapnia and hypotension, the impairment aggravated by type plasminogen activator and attenuated by the plasminogen activator inhibitor-1-derived peptide EEIIMD. Mitogen-activated protein kinase (MAPK), a family of at least three kinases, ERK, p38, and JNK, is upregulated after H/I and ERK contribute to impaired cerebrovasodilation. This study determined the roles of p38 and JNK MAPK in the impairment of dilation post-H/I in pigs equipped with a closed cranial window and the relationship between alterations in MAPK isoforms and EEIIMD-mediated cerebrovascular protection. Cerebrospinal fluid-phosphorylated (activated) p38 MAPK, but not JNK MAPK, was increased after H/I, an effect potentiated by intravenous EEIIMD administered 1 h postinjury. PAD in response to hypercapnia and hypotension was blunted by H/I, but dilation was maintained by EEIIMD. PAD was further impaired by the p38 antagonist SB-203580 but unchanged by the JNK antagonist SP-600125. Isoproterenol-induced PAD was unchanged by H/I, EEIIMD, SB-203580, and SP-600125. These data indicate that postinjury treatment with EEIIMD attenuated impaired cerebrovasodilation post-H/I by upregulating p38 but not JNK. These data suggest that plasminogen activator inhibitor-1-based peptides and other approaches to upregulate p38 may offer a novel approach to increase the benefit-to-risk ratio of thrombolytic therapy for diverse central nervous system disorders associated with H/I.
Keywords: cerebral circulation, newborn, plasminogen activator inhibitor-1, signal transduction, mitogen-activated protein kinase
perinatal cerebral hypoxia/ischemia (H/I) has many causes, unclear pathophysiology, no specific mechanism-related treatment, and poor outcome. Neonatal stroke may occur in as many as 1 in 4,000 births (22). In newborns with stroke, complications such as hypoxic/ischemic events are common (9). Maternal and perinatal coagulopathy predispose to perinatal stroke (10, 16) with 30% of neonatal strokes being due to thrombosis (8). A better understanding of the pathophysiological responses that occur in children after cerebral H/I is needed to develop mechanism-based approaches to therapy.
One contributor to neurological damage after H/I is thought to be cerebrovascular dysfunction. For example, hypotension leads to the loss of cerebrovascular regulation promoting tissue ischemia, whereas cerebrovasoconstriction associated with hypocapnia contributes to periventricular leukomalacia in the perinate (25). Using a piglet model, we have shown that pial artery dilation in response to hypotension and hypercapnia is blunted after cerebral H/I (4, 13, 19, 20).
Recombinant tissue type plasminogen activator (tPA) is the only Food and Drug Administration-approved treatment for stroke (15). However, tPA exhibits deleterious as well as beneficial effects that profoundly constrain its clinical utility. In addition to its salutary role in reperfusion, tPA contributes to excitotoxic neuronal cell death (23) and increases stroke infarct volume in mice (26). We have also observed that a topical administration of tPA or urokinase plasminogen activator (uPA) to the piglet cerebral cortex potentiates an impairment of pial artery dilation caused by H/I (5). The plasminogen activator inhibitor-1 (PAI-1)-derived peptide, EEIIMD, blocks tPA- and uPA-mediated effects on vascular contractility mediated by their interaction with the low-density lipoprotein receptor without inhibiting fibrinolytic activity (6, 21). uPA is upregulated after cerebral H/I in the piglet (4). Pretreatment with EEIIMD prevents the impairment of hypercapnic and hypotensive dilation after H/I (5), suggesting that endogenous plasminogen activators contribute to cerebral hemodynamic outcome postinsult.
Mitogen-activated protein kinase (MAPK), a family of at least three kinases [extracellular signal-related kinase (ERK), p38, and c-Jun-NH2-terminal kinase (JNK)], is upregulated after cerebral ischemia (2, 12, 18). Our recent studies show that uPA contributes to impaired stimulus-induced cerebrovascular dilation following cerebral H/I in the newborn pig through the upregulation of ERK MAPK, whereas the ERK MAPK antagonist U-0126 partially prevents vascular impairment (4). The upregulation of JNK MAPK and the protection against neuronal toxicity by antagonists of this MAPK isoform have been reported in rodent models of focal ischemia (7, 12). It has been speculated that JNK MAPK might contribute similarly to cerebrovasodilator outcome after cerebral H/I in the piglet. Using a combined biochemical and pharmacological approach, we investigated the roles of p38 and JNK MAPK in the H/I dilator impairment in piglets and the relationship between the changes in these MAPK isoforms and EEIIMD-mediated cerebrovascular protection postinsult.
MATERIALS AND METHODS
Closed cranial window technique and cerebral H/I.
Newborn pigs (1–5 days, and 1.2–1.6 kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were anesthetized with isoflurane (1 to 2 mean alveolar concentration), maintained with α-chloralose (30–50 mg/kg, supplemented with 5 mg·kg−1·h−1 iv). A catheter was inserted into a femoral artery to monitor blood pressure. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37–39°C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to cerebrospinal fluid (CSF), of the following composition: (in mM) 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37°C and had the following chemistry: pH 7.33, Pco2 of 46 mmHg, and Po2 of 43 mmHg, which was similar to that of endogenous CSF. The pial arterial vessel diameter was measured with a microscope, a camera, a video output screen, and a video microscaler.
Total cerebral ischemia was accomplished by infusing artificial CSF into a hollow bolt in the cranium to maintain an intracranial pressure 15 mmHg greater than the numerical mean of systolic and diastolic arterial blood pressure (19, 20). Intracranial pressure was monitored via a sidearm of the cranial window. To prevent the arterial pressure from rising inordinately (Cushing response), venous blood was withdrawn as necessary to maintain mean arterial blood pressure at no greater than 100 mmHg. As the cerebral ischemic response subsided, the shed blood was returned to the animal. Cerebral ischemia was maintained for 20 min. Hypoxia (Po2 of ∼35 mmHg) was produced for 10 min before ischemia by decreasing the inspired O2 via inhalation of N2, which was followed immediately by the total cerebral ischemia. Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 ml blood/kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25 and 45%, respectively) (3). Such drops in blood pressure were maintained constant for 10 min by a titration of additional blood withdrawal or blood reinfusion (2). Two levels of hypercapnia (low and high) were induced via the inhalation of graded levels of a 10% CO2-21% O2-balance N2 gas mixture for 10 min to produce levels of Pco2 of 50–60 mmHg for the low exposure and 70–80 mmHg for the high exposure (13).
Protocol.
Two types of pial vessels, small arteries (resting diameter, 120–160 μm) and arterioles (resting diameter, 50–70 μm) were examined to determine whether segmental differences in the effects of H/I could be identified. Typically, 2 to 3 ml of artificial CSF were flushed through the window over a 30-s period, and excess CSF was allowed to run off through one of the needle ports.
Thirteen experimental groups of animals were studied (all, n = 5): 1) sham-operated control, vehicle treated; 2) H/I, vehicle pretreated; 3) H/I, pretreated with EEIIMD (1 mg/kg iv); 4) H/I, pretreated with EEIIMR (1 mg/kg iv), the inactive analog of EEIIMD; 5) H/I, pretreated with the JNK MAPK antagonist, SP-600125 (1 mg/kg iv); 6) H/I, pretreated with the p38 MAPK antagonist SB-203580 (1 mg/kg iv); 7) H/I, pretreated with combined EEIIMD and SB-203580; 8) H/I, posttreated with vehicle; 9) H/I, posttreated with EEIIMD; 10) H/I, posttreated with EEIIMR; 11) H/I, posttreated with SP-600125; and 12) H/I posttreated with SB-203580 and H/I posttreated with combined EEIIMD and SB-203580. The vehicle for all agents was 0.9% saline, except for the MAPK antagonists, which was DMSO (stock) diluted with saline, with a maximal ratio of 1:1,000. These two types of vehicle-CSF controls had no significant effect on pial artery diameter. In sham-operated control animals, responses to hypercapnia, hypotension, and isoproterenol (10−8 and 10−6 M) were obtained initially and again 1 and 4 h later in the presence of agent vehicle. In H/I vehicle animals, responses to vasoactive stimuli were obtained initially and then again 1 and 4 h postinsult in the presence of vehicle. In drug-treated animals, the drugs were administered either 30 min before H/I (pretreatment) or 1 h after H/I (posttreatment).
ELISA.
Commercially available ELISA kits (Calbiochem) were used to quantity CSF p38, JNK, and ERK MAPK concentration. Phosphorylated MAPK enzyme values were normalized to total form and then expressed as percentages of the control condition. The lower limit of detection was 10 pg/ml after a subtraction of nonspecific background and cross-selectivity paradigms using respective MAPK isoform antagonists-confirmed specificity (e.g., p38 elevation after H/I unchanged by SP-600125).
Statistical analysis.
Pial artery diameter and CSF MAPK values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fisher protected least significant difference test. The sample populations studied were normally distributed, and the power was 0.86. An α level of P < 0.05 was considered significant in all statistical tests. Values are represented as means ± SE of the absolute values or as percent changes from control values.
RESULTS
H/I elevates CSF p38 MAPK, which is potentiated by EEIIMD, but has no effect on CSF JNK MAPK.
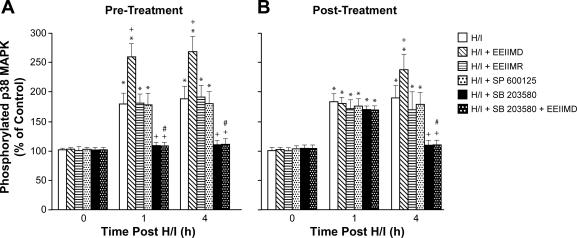
The activation (phosphorylation) state of the p38 and JNK MAPK isoforms was determined by expressing the data as a percentage of control (total). H/I induced a marked phosphorylation of p38 MAPK within 1 h postinjury (Fig. 1). EEIIMD (1 mg/kg iv) administered 30 min before or 1 h after H/I potentiated the phosphorylation of p38 MAPK (Fig. 1). In contrast, CSF p38 MAPK concentration was unchanged by the administration of the inactive analog EEIIMR (1 mg/kg iv) and the JNK MAPK antagonist SP-600125 (1 mg/kg iv) pre- or postinjury (Fig. 1). The purported p38 MAPK antagonist SB-230580 (1 mg/kg iv) and combined SB-203580 + EEIIMD blocked p38 MAPK phosphorylation (Fig. 1). CSF JNK MAPK was unchanged by H/I, EEIIMD, EEIIMR, SP-600125, and SB-203580 (data not shown). CSF ERK MAPK was upregulated by H/I and blunted by EEIIMD (102 ± 5, 289 ± 39, and 126 ± 10% for control, H/I, and H/I + EEIIMD, respectively).
Fig. 1.
Phosphorylation of p38 MAPK in cerebrospinal fluid before cerebral hypoxia/ischemia (H/I) (0 min) and as a function of time (in h) after H/I in vehicle or treated with EEIIMD, EEIIMR, SP-600125, SB-203580, or combined SB-203580 + EEIIMD (all, 1 mg/kg iv); n = 5 pigs. Data are expressed as percentages of control by ELISA determination of phospho-MAPK and total MAPK isoform and subsequent normalization to total form. A: pretreatment 30 min before H/I. B: posttreatment 1 h after H/I. *P < 0.05 vs. corresponding 0 time value; +P < 0.05 vs. corresponding H/I nontreated value; #P < 0.05 vs. corresponding EEIIMD alone value.
EEIIMD prevents, whereas the p38 MAPK antagonist SB-203580 aggravates, impairment of cerebrovasodilation after H/I.
Two levels of hypercapnia, hypotension, and isoproterenol elicited reproducible graded pial small artery (120 to 160 μm) and arteriole (50 to 70 μm) dilation in sham-operated control animals (data not shown). Pial small artery dilation in response to hypercapnia and hypotension was blunted 1 and 4 h after H/I, whereas responses to isoproterenol were unchanged (Figs. 2, 3, and 4). Similar reductions in responses were seen in arterioles (data not shown). Pre- and posttreatment with EEIIMD, but not EEIIMR, prevented the impairment of pial artery dilation in response to hypercapnia and hypotension, while having no effect on vasodilation in response to isoproterenol (Figs. 2–4).
Fig. 2.
Influence of hypotension (moderate and severe) on pial artery diameter in newborn pigs before (control) and after cerebral H/I or treated with EEIIMD, EEIIMR, SP-600125, SB-203580, or combined SB-203580 + EEIIMD (all, 1 mg/kg iv); n = 5 pigs. A: pretreatment 30 min before H/I. B: posttreatment 1 h after H/I. *P < 0.05 vs. corresponding control value; +P < 0.05 vs. corresponding nontreated H/I value; #P < 0.05 vs. corresponding EEIIMD alone value.
Fig. 3.
Influence of hypercapnia (low and high) on pial artery diameter in newborn pigs before (control) and after cerebral H/I or treated with EEIIMD, EEIIMR, SP-600125, SB-203580, or combined SB-203580 + EEIIMD (all, 1 mg/kg iv); n = 5 pigs. A: pretreatment 30 min before H/I. B: posttreatment 1 h after H/I. *P < 0.05 vs. corresponding control value; +P < 0.05 vs. corresponding nontreated H/I value; #P < 0.05 vs. corresponding EEIIMD alone value.
Fig. 4.
Influence of isoproterenol (10−8 and 10−6 M) on pial artery diameter in newborn pigs before (control) and after cerebral H/I or treated with EEIIMD, EEIIMR, SP-600125, SB-203580, or combined SB-203580 + EEIIMD (all, 1 mg/kg iv); n = 5 pigs. A: pretreatment 30 min before H/I. B: posttreatment 1 h after H/I.
However, vascular responses to hypercapnia and hypotension post-H/I were reversed to vasoconstriction by pre- and postinjury treatment with the p38 MAPK antagonist SB-203580 (Figs. 2 and 3). The coadministration of SB-203580 with EEIIMD blocked the protection of vascular responses to hypercapnia and hypotension post-H/I observed with EEIIMD alone, resulting in a vasoconstriction to these two stimuli (Figs. 2 and 3). Isoproterenol-induced pial artery vasodilation was unchanged by SB-203580 (Fig. 4). The JNK MAPK antagonist SP-600125 had no influence on vascular responses to hypercapnia, hypotension, or isoproterenol after H//I (Figs. 2–4). Similar observations were made in pial arterioles (data not shown).
Blood chemistry and mean arterial blood pressure.
Blood chemistry values were collected before and after all experiments. There were no statistical differences between sham-operated control, H/I, and H/I drug-treated animals. Hypoxia decreased Po2 to 34 ± 2 mmHg. Low levels of hypercapnia raised Pco2 to 56 ± 6 mmHg, and high levels of hypercapnia raised the Pco2 to 75 ± 8 mmHg. Carbon dioxide levels were kept constant during periods of hypoxia, and oxygen levels were kept constant during periods of hypercapnia. Mean arterial blood pressure was modestly decreased at 1 h post-H/I (64 ± 9 and 54 ± 9 mmHg for control and 1 h post H/I, respectively).
DISCUSSION
There are two principal new findings to be derived from this study. First, these data show that intravenous postinjury treatment with the PAI-1-derived peptide EEIIMD prevents the impairment of vasodilator responses to hypercapnia and hypotension after cerebral H/I. These data support the involvement of the upregulation of plasminogen activators in the impairment in vascular reactivity seen postinsult. In prior studies, we had shown that EEIIMD applied locally as a proof of principle preinjury provided protection (5). Here we show the comparable effectiveness when EEIIMD is given through the more clinically relevant intravenous route. The protection afforded by EEIIMD appears selective since the inactive analog EEIIMR failed to restore vasodilation in response to hypercapnia and hypotension postinsult. Because the vasodilation in response to isoproterenol was unchanged by cerebral H/I (3–5, 13, 14), these data also suggest that whereas the intrinsic reactivity of pial arteries was preserved, the activation of this reactivity was somehow inhibited by H/I. Mechanistically, we have previously observed that the ERK MAPK inhibitor U-0126 partially prevented the impaired reactivity to hypercapnia and hypotension (4, 13), indicating that the upregulation of this MAPK isoform (4) probably contributed to the H/I-induced impairment of the activation of reactivity.
In contrast, the results of the present study show that the p38 MAPK inhibitor SB-203580 aggravated the H/I-induced impairment of cerebrovasodilation, whereas the concentration of p38 MAPK in the CSF was elevated after injury. The coadministration of SB-203580 with EEIIMD blocked the protection of vascular responses to hypercapnia and hypotension after H/I. These data support the second key finding of this study, which indicate that p38 MAPK is protective in the setting of cerebral H/I. Because EEIIMD, but not EEIIMR, potentiated the elevation of CSF p38 MAPK concentration, these data suggest that this PAI-1-derived peptide produces protection, in part, in a p38 MAPK-dependent mechanism. The coadministration of SB-203580 with EEIIMD blocked the protection afforded by the former, strengthening the key role of p38 MAPK in responsiveness to vasodilator stimuli post H/I. The administration of SB-203580 also blocked p38 MAPK upregulation after H/I, indicating that the dose of this drug was efficacious and had crossed the blood brain barrier in sufficient concentration.
However, CSF JNK MAPK concentration was unchanged, and the respective antagonist, SP-600125, had no effect on cerebrovasodilator impairment post H/I. These data indicate that JNK MAPK appears to play a lesser, if any, role in the vascular impairment induced by global cerebral H/I in the piglet. These data are in contrast to the mechanism of injury caused by focal cerebral ischemia in the rat where JNK MAPK was upregulated and mediated neuronal cell toxicity (7, 12). It is presently uncertain whether these differences relate to model of cerebral ischemia (focal vs. global), age, and/or species but suggest the need for caution when extrapolating results across these parameters. A limitation of the closed cranial window technique to quantify CSF MAPK concentration is that the cellular site of origin cannot be determined. Potential sources include neurons, glia, vascular smooth muscle, and endothelial sources.
Our prior studies indicated that uPA was upregulated after H/I and contributed to an impaired cerebrovasodilation in an ERK MAPK-dependent mechanism (4). In the context of the present results, the dynamic interactive changes of ERK and p38 MAPK, the one opposing and the other promoting vasodilation, appear to yield the ultimate cerebrohemodynamic outcome after this central nervous system injury. Shifting the MAPK isoform profile with the administration of EEIIMD (blunting ERK and promoting p38 upregulation) yields a protection of cerebrovasodilation after H/I. A quantification of ERK MAPK in CSF appears to parallel changes in brain parenchyma under H/I conditions (4).
The PAI-1-derived peptide EEIIMD inhibits the vasoactivity of tPA and uPA without inhibiting their fibrinolytic activity (6, 21). Since EEIIMD binds to the docking site of tPA and uPA but does not inhibit their plasminogen activator activity, these data suggest that the effect of these plasminogen activators is mediated through their signal-transducing activities. We have previously shown that uPA binds directly to αVβ3 (17, 24) and that plasminogen activators can promote the formation of a signal-transducing complex between low-density lipoprotein receptor and αVβ3 (1). The results of a more recent study extend these initial observations and indicate that uPA released after cerebral H/I impairs pial artery dilation induced by hypercapnia and hypotension through an integrin αVβ3-dependent process (14). However, other as yet undefined mechanisms, which may include the opioid nociceptin/orphanin FQ and/or the activation of the N-methyl-d-aspartate receptor (2), may also contribute to the cerebrovascular derangement following cerebral H/I.
In conclusion, the data in the present study indicate that an intravenous administration of EEIIMD postinjury limits cerebrovasodilator impairment induced by H/I by upregulating p38 but not JNK. These data suggest that EEIIMD or other approaches to upregulate p38 may offer a novel approach to increase the benefit-to-risk ratio of thrombolytic therapy for diverse central nervous system disorders associated with H/I.
GRANTS
This research was funded by National Institutes of Health Grants NS-53410 and HD-57355 (to W. M. Armstead); HL-76406, CA-83121, HL-76206, HL-07971, and HL-81864 (to D. B. Cines); and HL-77760 and HL-82545 (to A. A-R. Higazi) and by the University of Pennsylvania Research Foundation (to W. M. Armstead), the University of Pennsylvania Institute for Translational Medicine and Therapeutics (to D. B. Cines), and the Israeli Science Foundation (to A. A-R. Higazi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA. LRP and avB3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol 291: H1351–H1359, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA 96: 12866–12869, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead WM. NOC/oFQ and NMDA contribute to piglet hypoxic ischemic hypotensive cerebrovasodilation impairment. Pediatr Res 51: 586–591, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Armstead WM, Cines DB, Bdeir K, Kulikovskaya I, Stein SC, Higazi A. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res 1231: 121–131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke 36: 2265–2269, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Dev Brain Res 156: 139–146, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Borsello T, Clarke PGH, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9: 1180–1186, 2003 [DOI] [PubMed] [Google Scholar]
- 8.DeVeber G, Andrew M. Cerebral sinovenous thrombosis in children. N Engl J Med 345: 417–423, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Ferriero DM. Neonatal brain injury. N Engl J Med 351: 1985–1995, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gunther G, Junker R, Strater R, Schobess R, Kurnik K, Kosch A, Nowak-Gottl U. Symptomatic ischemic stroke in full-term neonates. Stroke 31: 2437–2441, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Haj-Yehia A, Nassar T, Sachais BS, Kuo A, Bdeir K, Al Mehdi AB, Mazar A, Cines DB, Higazi AA. Urokinase-derived peptides regulate vascular smooth muscle contraction in vitro and in vivo. FASEB J 14: 1411–1422, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Sakai K, Sasaki C, Zhang WR, Warita H, Abe K. c-JUN N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neurosci Lett 284: 195–199, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Jagolino AL, Armstead WM. PTK MAPK, and NOC/oFQ impair hypercapnic cerebrovasodilation after hypoxia/ischemia. Am J Physiol Heart Circ Physiol 284: H101–H107, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kiessling JW, Cines DB, Higazi AA, Armstead WM. Inhibition of integrin αVβ3 prevents urokinase plasminogen activator-mediated impairment of cerebrovasodilation after cerebral hypoxia/ischemia. Am J Physiol Heart Circ Physiol 296: H862–H867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science 284: 647–650, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies, and cerebral palsy. Hum Pathol 30: 759–769, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Kwak SH, Mitra S, Bdeir K, Strassheim D, Park JS, Idell S, Cines DB, Abraham E. The kringle domain of urokinase-type plasminogen activator potentiates LPS induced neutrophil activation through interaction with αVβ3, integrins. J Leukoc Biol 78: 937–946, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cereb Blood Flow Metab 21: 887–906, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Leffler CW, Busija DW, Armstead WM, Mirro R, Beasley DG. Ischemia alters cerebral vascular responses to hypercapnia and acetylcholine in piglets. Pediatr Res 25: 180–183, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Leffler CW, Busija DW, Beasley DG, Armstead WM, Mirro R. Postischemic micro-vascular cerebral responses to norepinephrine and hypotension in newborn pigs. Stroke 20: 541–546, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi AA. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem 277: 40499–40504, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol 3: 150–158, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor mediated signaling. Nat Med 7: 59–64, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Tarui T, Nakakura J, Majumdar M, Andronicos N, Tagaki J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, Takada Y. Direct interaction of the kringle domain of urokinase type plasminogen activator with B1/3 integrins. Thromb Haemost 95: 524–534, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol 5: 135–151, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Wang YF, Tsirka SE, Strickland S, Stiege PE, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 4: 228–231, 1998 [DOI] [PubMed] [Google Scholar]