Abstract
Vascular endothelial growth factor (VEGF)-C is necessary for lymphangiogenesis, and excess VEGF-C has been shown to be ameliorative for edema produced by lymphatic obstruction in experimental models. However, it has recently been shown that edema can resolve in the mouse tail even in the complete absence of capillary lymphangiogenesis when distal lymph fluid crosses the regenerating wound site interstitially. This finding has raised questions about the action of VEGF-C/VEGF receptor (VEGFR) signaling during the resolution of experimental edema. Here, the roles of VEGFR-2 and VEGFR-3 signaling in edema resolution were explored. It was found that edema resolved following neutralization of either VEGFR-2 or VEGFR-3 in the mouse tail skin, which inhibited lymphangiogenesis. Neutralization of either VEGFR-2 or VEGFR-3 reduced angiogenesis at the site of obstruction at day 10 (9.2 ± 1.2% and 11.5 ± 1.0% blood capillary coverage, respectively) relative to controls (14.3 ± 1.5% blood capillary coverage). Combined VEGFR-2/-3 neutralization more strongly inhibited angiogenesis (6.9 ± 1.5% blood capillary coverage), leading to a reduced wound repair of the lymphatic obstruction and extended edema in the tail skin. In contrast, improved tissue repair of the obstruction site increased edema resolution. Macrophages in the swollen tissue were excluded as contributing factors in the VEGFR-dependent extended edema. These results support a role for VEGFR-2/-3-combined signaling in the resolution of experimental edema that is lymphangiogenesis independent.
Keywords: vascular endothelial growth factor receptor, lymphatic capillary, angiogenesis, macrophages
secondary lymphedema is often a consequence of axillary clearance (12, 52), a standard surgical treatment for breast cancer that excises large collecting vessels and associated lymph nodes that drain fluid from the arm. This procedure disrupts the path of lymphatic drainage from the arm (46). A recent innovation that restricts the dissection to the sentinal lymph nodes has been found to reduce the incidence of arm swelling early on, but by 12 mo, the swelling between the two treatments is no longer statistically different (29). Breast cancer surgery produces two types of iatrogenic damages: 1) axillary tissue wound with disrupted lymphatic capillaries (draining arm skin and subcutis) and 2) transection of large lymphatic vessels (draining muscles, bones, and associated tissues) (39, 42, 43). Following the surgery, a scar develops that obstructs lymph flow at the site of surgical intervention (44). Because the surgical removal of collecting lymphatics across the axilla is coincident with reduced lymphatic drainage from the arm when assessed at later times during lymphedema (6, 7, 31, 41), it has been widely hypothesized that the poor regeneration of lymphatic function across the regenerating axillary cavity during the postsurgical wound healing process may increase the potential for lymphedema to develop. For this reason, research efforts have recently focused on promoting lymphatic growth (lymphangiogenesis) at the site of injury to achieve the amelioration of secondary lymphedema (11, 38, 47, 48, 55).
It has been shown that vascular endothelial growth factor (VEGF)-C can promote lymphangiogenesis through activation of VEGF receptor (VEGFR)-2 and VEGFR-3 on lymphatic endothelial cells (1–3, 13, 21, 22, 24, 26), and several studies have demonstrated the potential of excess VEGF-C therapy for increasing the growth of lymphatic capillaries and vessels across a surgically produced lymphatic obstruction and hastening the natural resolution of acute tissue swelling in experimental models (11, 20, 38, 47, 48, 55). These findings have raised hopes that excess VEGF-C therapy may be beneficial for restoring a functional lymphatic network in humans with secondary lymphedema. However, other studies have found that endogenous VEGF-C levels are already significantly elevated during experimental edema (35, 50) and that excess VEGF-C may promote lymphatic hyperplasia (14, 19, 28) without increasing lymphatic function (18, 35), suggesting that supraphysiological levels of VEGF-C may not necessarily promote functional lymphangiogenesis. Therefore, the mechanism by which VEGF-C reduces edema in acute experimental models remains unclear.
The present study was intended to clarify the role of VEGFR signaling during edema resolution to clarify the mechanism by which VEGF-C may work to hasten the natural resolution of edema in acute experimental models. We have previously found that the inhibition of VEGFR-3, which completely prevents lymphangiogenesis, does not impair the natural resolution of tissue swelling in the mouse tail, since fluid is able to spread interstitially across the regenerating wound site (50). Therefore, we asked whether VEGFR-2 signaling may be more important than VEGFR-3 signaling or whether VEGFR-2 and VEGFR-3 may work synergistically to resolve the swelling. Here, we employ a model of acute edema in mouse tail skin (45) to clarify the dependence of edema resolution on VEGFR signaling. The mouse tail model uniquely allows for lymphatic flow to be traced across the obstruction site, for new lymphatic growth to be distinguished from existing lymphatics, and for lymphatic function to be measured via microlymphangiography.
MATERIALS AND METHODS
Female wild-type C57BL/6 or balb/c mice (Harlan; 6–8 wk) were used. Mice were anesthetized with 2.5% isoflurane mixed with oxygen gas for surgical procedures and were euthanized at experimental end points by CO2 asphyxiation. All protocols were approved by the Animal Care and Use Committee of Michigan Technological University.
Neutralizing antibodies.
Signaling via VEGFR-2 and VEGFR-3 is critical for lymphangiogenesis (16). Antagonist antibodies against mouse VEGFR-3 (mF4–31C1) and mouse VEGFR-2 (DC101) were provided by ImClone Systems (New York, NY). Continuous inhibition of VEGFR-3 or VEGFR-2 with 150-μl intraperitoneal injections of mF4–31C1 and DC101, respectively, at 0.625 mg per dose has been shown to completely inhibit lymphangiogenesis in vivo (15, 16, 33). Successful lymphangiogenesis suppression in the present study was confirmed in lymph vessel hyaluronan receptor-1 (LYVE-1)-stained tail skin sections (see supplemental Fig. 1; note: supplemental material may be found with the online version of this article). One hundred fifty microliters of saline or isotype-matched control antibody were used for control injections.
Model 1—tail edema: normal wound healing.
Tail skin edema was created in C57BL/6 mice by excising a 1-mm circular band of dermis (which contained the lymphatic capillary network) 1 cm from the base of the tail, leaving the underlying bone, muscle, tendons, and major blood vessels intact, similar to previous studies (11, 35, 45). Mice were divided into four groups and received intraperitoneal injections of saline or blocking antibodies (for VEGFR-3, VEGFR-2, or combined receptor blocking) 1 day before surgery and then every 5 days until euthanasia.
Model 2—tail edema: covered wound healing.
A scar-free/enhanced tissue repair model of tail skin edema was created in C57BL/6 mice by the removal of dermis as described for model 1. However, following the injury, the wound region was covered with a close-fitting, gas-permeable silicone sleeve, similar to previous studies (10, 14–16, 33, 34). The sleeve protected the injury site, allowing the tissue to repair more rapidly and in a relatively scar-free manner. The placement of a circular wound 1 cm from the tail base in the present model resulted in edema of the distal tail skin, whereas the placement of a circular wound midway up the tail in previous studies did not produce skin swelling (10, 14–16, 33, 34). This may be due to the lower volume of capillary filtrate that must traverse the wound site to maintain fluid balance when the injury is placed farther down the tail.
Detection of functional lymphatics via microlymphangiography.
Visualization of lymph flow through tail lymphatics and across the obstruction site was performed as previously described (10). Briefly, mice were anesthetized with a subcutaneous injection of ketamine (65 mg/kg), xylazine (13 mg/kg), and morphine (2 mg/kg). In order for the location of lymph within the tissue to be determined later in skin sections, lysine fixable tetramethylrhodamine-conjugated dextran (2,000 kDa; Invitrogen, Carlsbad, CA) was injected intradermally into the tail tip under constant pressure. The fluid tracer becomes immobilized in the tissue following fixation with 4% formaldehyde. The fluid tracer was taken up by the tail lymphatics in the live mouse and transported across the wound region to the proximal side. Fluorescence images of the microlymphangiographies were captured with a DP71 color camera on a BX51 Olympus fluorescence microscope.
Immunofluorescence and immunohistochemistry.
Following microlymphangiography, the tail specimens were cut into 10-μm longitudinal cryosections and immunostained. Immunostaining of tail cryosections proceeded with antibodies against LYVE-1 and podoplanin to detect lymphatic endothelial cells. A rabbit polyclonal antibody against the lymphatic-specific hyaluronan receptor LYVE-1 (Upstate, Charlottesville, VA) was used along with an Alexa Fluor 488 goat anti-rabbit secondary antibody (Invitrogen). A hamster monoclonal antibody against podoplanin (AngioBio, DelMar, CA) was used with an Alexa Fluor 647 goat anti-hamster secondary antibody (Invitrogen). The path taken by lymph through the tail was identified in situ by the immobilized tetramethylrhodamine-conjugated dextran fluid tracer. Macrophages were detected in 10-μm cryosections of mouse tail skin by labeling the F4/80 macrophage-specific marker (5). A biotinylated anti-F4/80 antibody (Serotec) was used along with Alexa Fluor 555-conjugated streptavidin (Invitrogen). Blood endothelial cells (BECs) were detected in 10-μm cryosections of mouse tail skin by labeling the CD31 BEC marker. A biotinylated anti-CD31 antibody (BD Bioscience) was used along with Alexa Fluor 555-conjugated streptavidin (Invitrogen). Cell nuclei in LYVE-1-, podoplanin-, F4/80-, and CD31-labeled sections were counterstained with 4′,6-diamino-2-phenylindole (DAPI; Vector, Burlingame, CA).
Physiological measurements.
Tail swelling in both the uncovered and covered regenerating skin edema models was determined by measuring tail diameters in Photoshop from digital images of the tail distal to the wound site. Two images of the tail at a 90° rotation from each other were captured for each mouse tail with a DP71 color camera mounted to a stereo microscope, and the two diameters were averaged together. Wound closure was measured along the longitudinal axis of the tail as the distance between the distal and proximal regenerating tissue across the wound site. Tracer clearance (or transit time) of the lymphatic dye was defined as the time for the tracer leading edge to flow from the injection site (tail tip) to the proximal side of the regenerating wound. Because the tracer becomes taken up by lymphatics and is then transported toward the proximal direction across the wound, the time for the tracer to cross the wound site is indicative of lymphatic function. Blood capillary percent coverage was determined within 500 μm of the wound edge in CD31-labeled tissue sections with a threshold analysis using Metamorph software.
Statistical methods.
At least five animals were used for each data point. Data are presented as means ± SE. P values were calculated using Student's t-test, ANOVA, or Tukey-Kramer honestly significant difference (HSD) as indicated.
RESULTS
Edema resolution is dependent on combined VEGFR-2 and VEGFR-3 signaling.
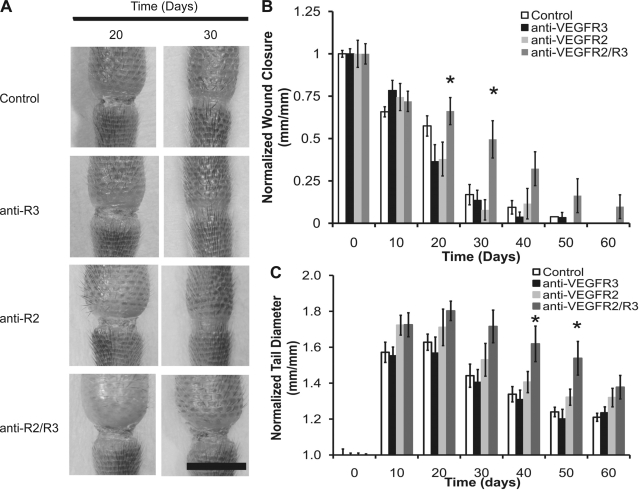
Recently, we found that the neutralization of VEGFR-3 and the complete prevention of lymphangiogenesis did not impede acute edema resolution of the mouse tail since lymph was able to spread interstitially across a regenerated obstruction site that was protected by a silicone cuff (50). Because exogenously administered VEGF-C, which signals through VEGFR-2 and VEGFR-3, has been shown to ameliorate acute edema, we asked whether VEGFR-2 neutralization or combined VEGFR-2 and VEGFR-3 neutralization may inhibit the resolution of skin swelling. Lymphatic obstructions were surgically prepared according to model 1, and wound repair of the obstruction site was allowed to proceed in the presence of saline or neutralizing antibodies against VEGFR-2 and VEGFR-3, either alone or in combination (Fig. 1A). In the mouse tail model, edema was seen to develop within several days postsurgery and to resolve without any intervention within 2 mo. It was found, based on the measurement of wound closure across the obstruction site, that the combined VEGFR-2/-3 neutralization impaired wound repair across the obstruction relative to what occurred following saline or individual receptor blocking treatments (Fig. 1B, P = 0.02 and 0.003 by ANOVA at days 20 and 30, respectively). Tissue repair proceeded normally following the individual receptor blocking treatments (Fig. 1), which we have shown inhibits lymphangiogenesis (see supplemental Fig. 1 and Ref. 16). An analysis of the tail diameters showed that the inhibition of either VEGFR-3 or VEGFR-2 alone did not significantly change the evolution of tail swelling relative to the controls. However, the combined receptor neutralization inhibited the resolution of edema relative to the controls at days 40 and 50 (Fig. 1C, P < 0.02 by ANOVA). Therefore, the combined VEGFR-2/-3 neutralization correlated with reduced tissue repair at the site of obstruction and reduced the resolution of the tail swelling, whereas the lack of lymphangiogenesis at the wound site, obtained following individual neutralization of either VEGFR-2 or VEGFR-3, did not reduce the resolution of the tail skin swelling.
Fig. 1.
Edema resolution may be dependent on combined VEGF receptor (VEGFR)-2/-3 signaling. Edema in the mouse tail skin was induced over a period of 60 days by a 1-mm-wide surgical excision of the skin that was left unprotected. Injury sites at days 20 and 30 for control, VEGFR-3 neutralization, VEGFR-2 neutralization, and combined receptor neutralization conditions are shown from top to bottom, respectively. Scale bar (A, bottom, right) = 5 mm. The evolution of wound closure (B) and tail swelling (C) over the 60-day period for the different conditions is graphically depicted. For the wound closure data, wound lengths are normalized to the initial 1-mm-long wound. For the swelling data, tail diameters are normalized to the average initial (preswelling) diameter of the tails within each group. n = 10 mice per group. *Statistical significance.
Enhanced tissue repair improves edema.
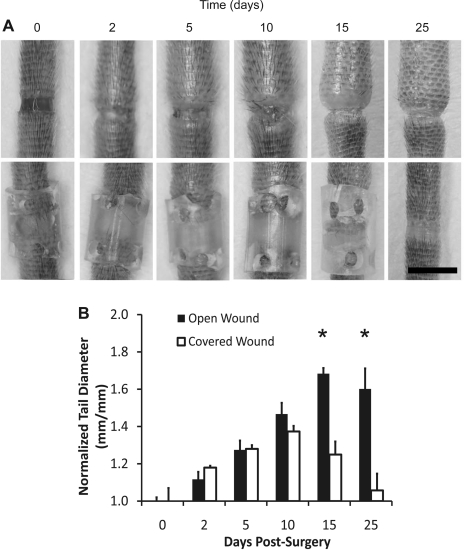
We observed that the combined VEGFR-2 and VEGFR-3 neutralization correlated with impaired tissue repair across the dermal obstruction and worsened the edema relative to the controls. However, it was unclear whether the extended tail swelling that we saw with the combined receptor blocking condition was caused by the impaired wound repair process locally at the obstruction site or by other more widespread effects of the inhibited VEGFR signaling elsewhere in the tail, such as possible alterations in inflammation. To help clarify this issue, we produced 1-mm skin injuries in the mouse tail and either protected the injury site to promote tissue repair locally at the obstruction site (model 2) or left the injury site unprotected to allow the wound to heal normally (model 1) as before. It was found that protecting the injury site from the outside environment promoted tissue repair (Fig. 2A) and reduced skin swelling relative to the unprotected site (Fig. 2B, P < 0.001 by ANOVA at days 15 and 25). Because we have previously reported that edema of the mouse tail resolves naturally as fluid drains across a similarly protected wound site even when lymphangiogenesis is completely blocked with VEGFR-3 neutralizing antibodies (50), these results suggest that edema may resolve when a path across the obstruction site is present for fluid to drain from the tail interstitially.
Fig. 2.
Edema resolution in the mouse tail skin is enhanced by accelerated tissue repair at the site of obstruction. Edema in the mouse tail skin was induced by a 1-mm-wide surgical excision of the skin that was left unprotected or protected with a silicone cuff. Images are shown at 0, 2, 5, 10, 15, and 25 days postsurgery (A). Scale bar (A, bottom, right) = 5 mm. B: tail diameters in the normal and protected tissue regeneration conditions. *P < 0.001, differences at day 15 and day 25 between these groups were highly significant. Tail diameters were normalized to their average initial (day 0) value. n = 10 mice.
Enhanced tissue repair at the site of injury improves long-term regeneration of lymphatic function.
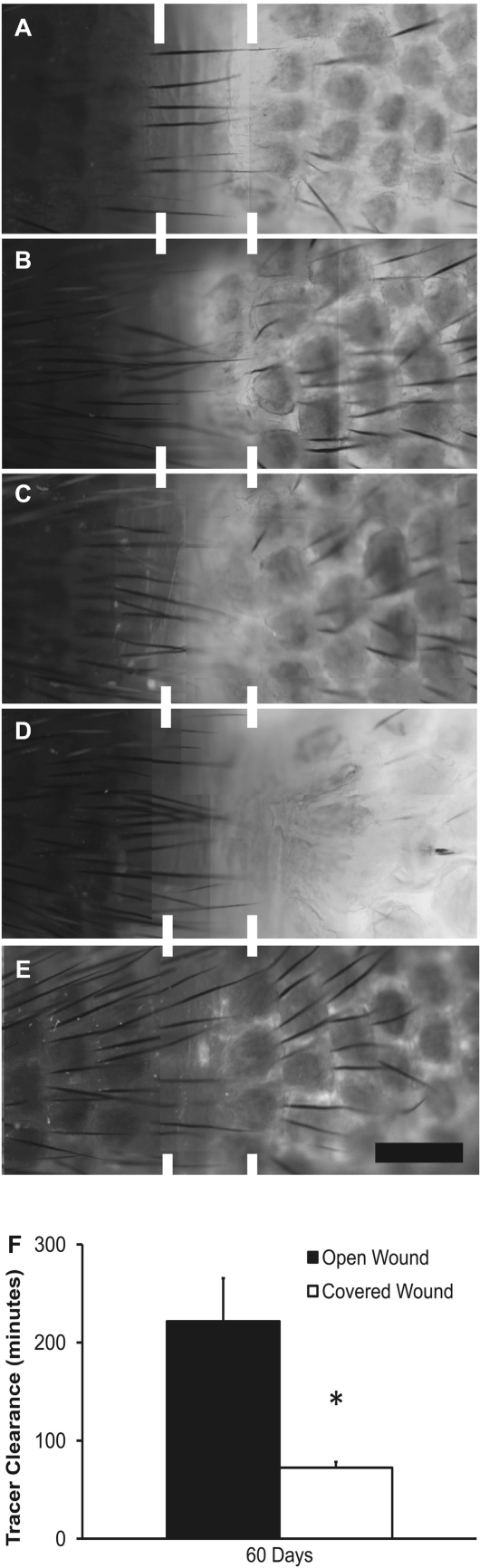
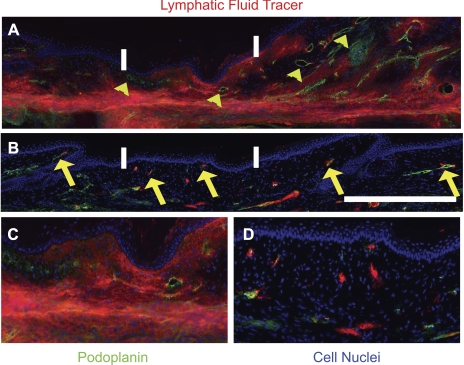
We have previously demonstrated that interstitial flow may promote functional lymphangiogenesis (15). We have also shown that protecting the wound site resulted in a significant reduction of the swelling at day 15, which is before functional recovery of the regenerating lymphatics (10, 34, 50), suggesting that interstitial flow was increased across the obstruction site. Therefore, we asked whether the functional regeneration of the lymphatic capillaries was increased in the covered wound relative to the uncovered wound. Microlymphangiographies of the mouse tails were conducted at day 60 postsurgery to determine the speed and path of fluid tracer transport from the tail tip across the site of injury. It was found that the fluid tracer diffused across the uncovered obstruction sites of model 1 (Fig. 3, A–D), whereas the fluid tracer was transported across the covered obstruction site of model 2 inside the regenerated lymphatic capillaries (Fig. 3E). A comparison of the time for the fluid tracer to transport from the tail tip to the proximal edge of the regenerating regions in the two models was undertaken. It was found that the fluid drainage of the injected dye was significantly improved across the covered obstruction site relative to the uncovered obstruction site (P < 0.02 by Student's t-test, 72 ± 6 vs. 221 ± 44 min, respectively). Observations in podoplanin-labeled skin sections showed that some fluid marker overlapped in some cases with regenerated lymphatics in the covered regenerating region. In some cases, small focal regions of fluid tracer did not overlap with podoplanin label, suggesting that some transport of lymph in the covered wound site may be through interstitial fluid channels rather than capillaries. In contrast to the covered wound, the transport of fluid tracer across the normal regenerating region demonstrated a prominent overlap of fluid tracer with the interstitium (Fig. 4, A–D). These findings demonstrate that fluid transport was increased across the covered wound site (model 2) relative to the uncovered wound site (model 1). This also provides additional evidence that the reduced wound repair that followed combined VEGFR-2/-3 neutralization may have reduced interstitial flow and caused the extended swelling.
Fig. 3.
Tissue repair promotes fluid drainage across the obstruction site. Edema in the mouse tail was induced by a 1-mm-wide surgical incision, and microlymphangiographies were conducted at 60 days in tails that were left uncovered (A) and treated with anti-VEGFR-3 (B), anti-VEGFR-2 (C), or combined anti-VEGFR-2/-3 blocking antibodies (D) or covered with a cuff that enhanced wound repair (E). Distal to proximal direction is right to left in all images. Bar (in E) = 1 mm. It was seen that fluid tracer diffused slowly across the wound site in A–D and was transported within regenerated lymphatics (identified by unique hexagonal architecture) in E. Regenerated lymphatic function was assessed at day 60 by measuring fluid tracer clearance across the obstruction via microlymphangiography in the covered and uncovered tails (F). *Statistical significance. A–E: panels consist of multiple images at different locations of the mouse tail that were collected under the fluorescence microscope and then assembled in Photoshop to form a complete representation of the respective tails.
Fig. 4.
Tissue repair improves regeneration of lymphatic function. Sections (10 μm thin) of the uncovered (A) and covered (B) tail skin were immunostained against podoplanin (distal to proximal direction shown right to left), showing fluid tracer (red) overlap with interstitium (blue, cell nuclei) and lymphatic capillaries (green). Distal to proximal direction is right to left in the images. Several hyperplastic and poorly functioning lymphatics are identified by yellow arrowheads in A, and several lymphatics colocalizing with tracer are identified by yellow arrows in B. Bar (in H) = 1 mm. Regenerated region is located between the white bars for each image. C and D: enlargements of the 1-mm-long regenerating regions from images in A and B, respectively. n = 10 mice per group. A–D: panels consist of multiple images at different locations of the tissue section that were collected under the fluorescence microscope and then assembled in Photoshop to form a complete representation of the respective tissue.
VEGFR signaling regulates angiogenesis but not macrophage infiltration during experimental edema.
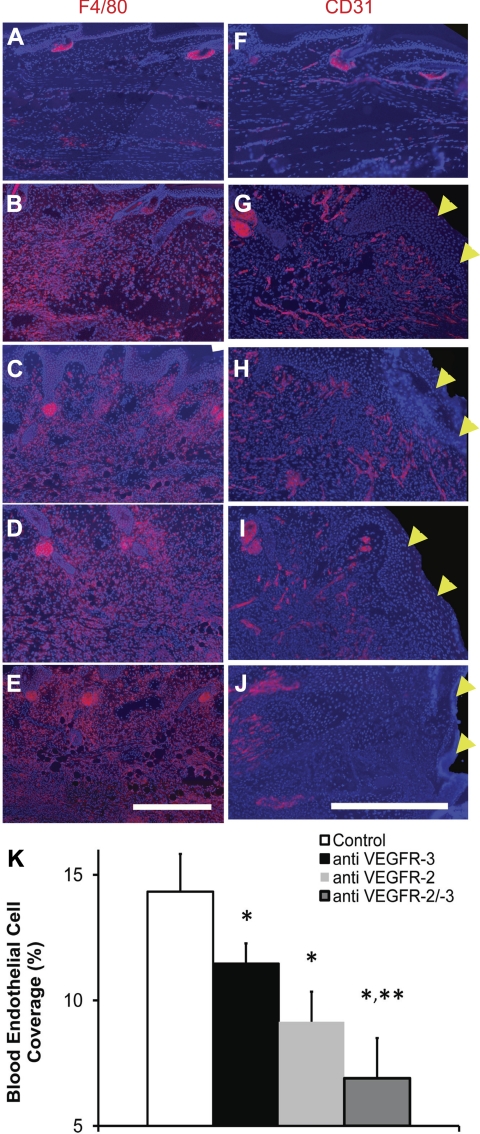
Combined VEGFR-2/-3 neutralization reduced swelling resolution, possibly by reducing tissue repair and interstitial flow at the site of obstruction.tk;1 However, interference with the inflammatory response to injury by the combined VEGFR-2/-3 neutralization may also potentially reduce swelling. Indeed, VEGF-C has been shown to chemoattract macrophages, which express VEGFR-2 and VEGFR-3 (40, 54). Furthermore, macrophage involvement in inflammation (25) and lymphangiogenesis (30, 51) may be regulated in part by VEGFR-2 and VEGFR-3 signaling. Thus it is possible that VEGFR neutralization in the swollen mouse tail may have decreased lymphangiogenesis (via blocking VEGFR-2 or VEGFR-3 signaling individually) or worsened the swelling (via blocking VEGFR-2 and VEGFR-3 signaling together) by reducing macrophage infiltration into the swollen skin. To clarify the roles of VEGFR-2 and VEGFR-3 in macrophage infiltration during tissue edema, we assessed the presence of macrophages in the edematous tail skin via immunostaining of the F4/80 macrophage marker. It was found that macrophage residence at day 10 became sharply increased in edematous skin relative to normal skin but was unaffected by VEGFR blocking since F4/80 antigen was present throughout the swollen skin under all treatments (Fig. 5, A–E). Thus VEGFR-2 and VEGFR-3 neutralization, either alone or in combination, failed to restrain macrophage accumulation into the edematous skin.
Fig. 5.
Dependence of macrophage infiltration and angiogenesis during experimental edema on VEGFR signaling. Edema in the mouse tail skin was induced by a 1-mm-wide surgical excision of the skin that was placed 10 mm from the tail base and left unprotected. Shown are fluorescence images of the F4/80 macrophage marker (A–E) and CD31 blood endothelial cell marker (F–J) (immune-detected antigen in each image is shown in red). Macrophages were detected in tissue sections of normal mouse tail skin (A), in edematous mouse tail skin (B), and in edematous mouse tail skin treated with anti-VEGFR-3 neutralizing antibodies (C), anti-VEGFR-2 neutralizing antibodies (D), and combined receptor signaling neutralization (E). In B–E, which depict macrophages in the swollen skin, the skin epidermis is located at the top of each image. Scale bar (in E) = 0.5 mm. Blood endothelial cells were detected in tissue sections of normal mouse tail skin (F) or the distal wound margin in edematous mouse tail skin treated with isotype matched control antibody (G) or in edematous mouse tail skin treated with anti-VEGFR neutralizing antibodies (H), anti-VEGFR-2 neutralizing antibodies (I), and combined receptor signaling neutralizing antibodies (J). Wound margin is shown on the right side in G–J, indicated by yellow arrowheads. Blue color in A–J is 4′,6-diamino-2-phenylindole (DAPI)-labeled cell nuclei. Scale bar (in J) = 0.5 mm. Measurement of blood capillary percent coverage for the different treatments within 500 μm of the wound edge (K) demonstrated inhibition of angiogenesis in the regenerating tissue by the receptor blocking antibodies. n = 5 mice per group. *Significant difference relative to the control condition. **Significant difference relative to VEGFR-3 blocking. A–J: panels consist of multiple images at different locations of the tissue section that were collected under the fluorescence microscope and then assembled in Photoshop to form a complete representation of the respective tissue.
VEGF-C activates VEGFR-2 and -3, both of which can be expressed in a wound healing environment by BECs in addition to lymphatic endothelial cells (21, 27) and macrophages. Furthermore, it has recently been demonstrated that VEGF-C can accelerate wound healing (36) and increase angiogenesis (4, 9) as VEGFR-3 becomes upregulated on blood endothelium during normal wound repair (49). Because we found that combined VEGFR-2/-3 signaling neutralization had reduced wound repair of the lymphatic obstruction and reduced swelling resolution, we probed for angiogenesis at the wound margins to determine whether angiogenesis was reduced by the combined VEGFR-2/-3 neutralization. We assessed the presence of blood capillaries in the edematous tail skin wound margins via immunostaining of the CD31 BEC marker (Fig. 5, F–J). It was apparent that blood capillary regeneration at the wound site at day 10 was reduced in the VEGFR antibody-treated edematous skin relative to the control edematous skin. A measurement of the coverage of regenerating blood capillaries demonstrated a statistically significant reduction in blood capillary coverage near the edge of the wound in the mice treated with the blocking antibodies relative to isotype-matched control antibody treatment (P < 0.05, by Tukey-Kramer HSD), falling from 14.3 ± 1.5% coverage in the control antibody-treated condition to 11.5 ± 1.0%, 9.2 ± 1.2%, and 6.9 ± 1.5% for VEGFR-3, VEGFR-2, and combined VEGFR-2/-3 blocking antibody-treated edematous skin, respectively (Fig. 5K). Blood capillary coverage following combined receptor neutralization, but not following VEGFR-2 neutralization, was significantly lower than in the VEGFR-3 neutralized condition (P < 0.05, by Tukey-Kramer HSD). Thus the combined VEGFR-2 and VEGFR-3 neutralization impaired angiogenesis more potently than the individual receptor neutralizations at the site of the lymphatic obstruction.
DISCUSSION
Lymphedema of the human arm is a consequence of a surgical procedure that removes lymph nodes, lymphatic collecting vessels, and surrounding tissue from the axilla in an effort to treat breast cancer. It has been hypothesized that lymphedema arises because of poor lymphatic regeneration at the axilla and that increased lymphangiogenesis with excess VEGF-C may improve lymphatic drainage (20, 32). Indeed, excess VEGF-C has been shown to increase lymphangiogenesis and improve edema in several acute experimental models (11, 38, 47, 48, 55). The results of these studies suggest that a resolution of experimental edema may be dependent on lymphangiogenesis. However, we found that blocking either VEGFR-2 or VEGFR-3, which prevents lymphatic growth (17, 33), after a disruption of lymphatic capillaries in the mouse tail does not impair the resolution of the edema. These results demonstrate that edema resolution in the mouse tail is not dependent on new lymphatic growth across the surgical obstruction. They also indicate that the balance of interstitial forces may be more important than lymphangiogenesis for maintaining fluid drainage and tissue volume during acute edema.
VEGF-C has been shown to promote the growth of lymphatic capillaries and lymphatic collecting vessels in various experimental models of lymphatic injury and tissue repair (11, 17, 37, 38, 47, 48, 55). In these studies, VEGF-C was found to promote lymphatic capillary regeneration in the context of wound repair in the rabbit ear (47), mouse tail (11, 55), and across incision wounds in the mouse (37). VEGF-C was also found to promote the regeneration of lymph vessels in the axilla following lymph node excision (48) and the reconnection of severed popliteal prenodal lymph vessels in the mouse (17), although the regeneration of lymphatic vessels has not yet been observed in humans. We have found that a lack of lymphangiogenesis of lymphatic capillaries or collecting vessels does not affect edema resolution, whereas a combined VEGFR-2/-3 neutralization was found to reduce wound repair at the site of obstruction and inhibit the resolution of tail skin swelling. The reduced skin swelling resolution may be causally related to the impaired wound repair process. Indeed, we found that the enhanced tissue repair with a protective silicone cuff had an ameliorative effect on the edema. Tissue repair at the obstruction site may contribute to the resolution by restoring a matrix bridge across the obstruction and thereby reducing interstitial resistance to interstitial fluid flow, in addition to potentially reducing inflammation. These results demonstrate that a combined VEGFR-3 and VEGFR-2 signaling may promote the resolution of experimental edema by inducing angiogenesis and wound repair, thereby restoring a matrix bridge for fluid drainage. Therefore, edema resolution in the mouse may be VEGFR signaling dependent but lymphangiogenesis independent. However, our data do not rule out the possibility that the increased lymphatic growth obtained with excess VEGF-C during wound repair may reduce the predisposition for developing secondary lymphedema some time following the resolution of the initial edema.
VEGF-C activates VEGFR-2 and -3 (3, 23), both of which can be expressed in a normal wound healing environment by BECs and macrophages (40, 54) in addition to lymphatic endothelial cells (21). We found that macrophage infiltration into the swollen skin was independent of VEGFR signaling. Therefore, an altered infiltration of macrophages is unlikely to have caused the worsened skin swelling that was found following the combined VEGFR blockade. It has been recently reported that VEGF-C can accelerate wound healing (36) and increase angiogenesis (4, 8, 9). VEGFR-3 neutralization was found to reduce angiogenesis (49), as VEGFR-3 becomes upregulated on blood endothelium during normal wound repair (53). We have found that the inhibition of VEGFR-2 and VEGFR-3 signaling in combination impaired angiogenesis and wound healing. Thus we speculate that the reported therapeutic effects of excess VEGF-C treatments during experimental edema may have occurred in part by promoting wound repair by inducing angiogenesis at the site of obstruction. By the reduction of interstitial resistance to flow across the obstruction, VEGF-C and its receptors VEGFR-2 and VEGFR-3 may promote lymphangiogenesis-independent edema resolution in animal models.
It is important to note that the development of human secondary lymphedema is a much slower process (sometimes several years) than can be recapitulated in the mouse model. Additionally, tissue edema in these mouse models will, to some degree, resolve itself over time, which is not the case for the human disease. This and similar models are more reflective of the resolution of acute tissue swelling that occurs in humans immediately following lymphatic disruption rather than of secondary lymphedema. In contrast to acute edema, secondary lymphedema usually becomes clinically evident several months or years after the healing of the original injury with lymphatic-related damage but following some initiating insult. Thus the present data suggest the importance of interstitial forces in the resolution of acute tissue swelling induced by lymphatic disruption and not the resolution of secondary lymphedema. This and similar models could be used to investigate the importance of wound healing for the resolution of acute tissue edema, but not secondary lymphedema. The extent to which conclusions about VEGF-C effectiveness to treat chronic secondary lymphedema can be made after experiments in models of acute tissue edema is not clear.
Although most human patients do not develop secondary lymphedema immediately after surgery (as occurs in experimental models), a cavity remains at the surgical site following axillary dissection. This cavity undergoes a normal wound healing process that parallels the poor regeneration of lymphatic function at the wound site, similar to what we have found following a normal regeneration of the mouse skin. We speculate that the poor regeneration of lymphatic function in the human arm may have been caused by a normal wound healing process that impedes interstitial flow and lymphatic flow through the obstructive tissue, similar to what we have found in the mouse. Thus, while lymphangiogenesis may not be necessary for the resolution of acute tissue swelling following lymphatic disruption, a lack of adequate lymphangiogenesis (perhaps in the context of wound healing as our data suggest) may predispose the tissue to swelling in the future. We have found an increased fluid drainage and functional lymphangiogenesis following augmented tissue repair. This raises the possibility that improvements to the natural healing of the axillary wound may reduce interstitial resistance to fluid flow in humans and increase lymphatic regeneration. Taken together, these data suggest that therapy with VEGFs may be more successful if administered during the wound repair process that follows axillary dissection in humans rather than subsequent to the onset of secondary lymphedema, which occurs after wound repair has largely subsided. The data also suggest that VEGFR neutralization therapy to reduce tumor size or metastatic spread may increase the susceptibility for lymphedema following axillary dissection.
GRANTS
This work was funded by National Institutes of Health Grants R15-HL-081102, R21-AR-053094, and R15-HL-093705.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Alitalo K. Growth factors controlling angiogenesis and lymphangiogenesis. Ugeskr Laeger 164: 3170–3172, 2002 [PubMed] [Google Scholar]
- 2.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 438: 946–953, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Anisimov A, Alitalo A, Korpisalo P, Soronen J, Kaijalainen S, Leppanen VM, Jeltsch M, Yla-Herttuala S, Alitalo K. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ Res 104: 1302–1312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Bates DO, Levick JR, Mortimer PS. Starling pressures in the human arm and their alteration in postmastectomy oedema. J Physiol 477: 355–363, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates DO, Levick JR, Mortimer PS. Subcutaneous interstitial fluid pressure and arm volume in lymphoedema. Int J Microcirc Clin Exp 11: 359–373, 1992 [PubMed] [Google Scholar]
- 8.Bauer SM, Bauer RJ, Liu ZJ, Chen H, Goldstein L, Velazquez OC. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. J Vasc Surg 41: 699–707, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res 78: 315–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res 92: 801–808, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cheung L, Han J, Beilhack A, Joshi S, Wilburn P, Dua A, An A, Rockson SG. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. BioDrugs 20: 363–370, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM 98: 343–348, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol 126: 2167–2177, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res 96: 1193–1199, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowksi JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol 292: H2176–H2183, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 21: 1003–1012, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ikomi F, Kawai Y, Nakayama J, Ogiwara N, Sasaki K, Mizuno R, Ohhashi T. Critical roles of VEGF-C-VEGF receptor 3 in reconnection of the collecting lymph vessels in mice. Microcirculation 1–13, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res 64: 4400–4404, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumara D, Jain R, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276: 1423–1425, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jin da P, An A, Liu J, Nakamura K, Rockson SG. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol 7: 47–57, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15: 290–298, 1996 [PMC free article] [PubMed] [Google Scholar]
- 22.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson WL, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16: 3898–3911, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev 82: 673–700, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kaipainen A, Korhonen J, Mustonen T, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 92: 3566–3570, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113: 5650–5659, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 122: 3829–3837, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21: 154–165, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol 173: 1891–1901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 98: 599–609, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170: 1178–1191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modi S, Stanton AW, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol 5: 183–202, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Rockson SG. Molecular targets for therapeutic lymphangiogenesis in lymphatic dysfunction and disease. Lymphat Res Biol 6: 181–189, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst 97: 14–21, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol 291: H1402–H1410, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 72: 161–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol 169: 1080–1087, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saaristo A, Tammela T, Timonen J, Yla-Herttuala S, Tukiainen E, Asko-Seljavaara S, Alitalo K. Vascular endothelial growth factor-C gene therapy restores lymphatic flow across incision wounds. FASEB J 18: 1707–1709, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 196: 719–730, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol 15: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 159: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanton AW, Mellor RH, Cook GJ, Svensson WE, Peters AM, Levick JR, Mortimer PS. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat Res Biol 1: 121–132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanton AW, Modi S, Bennett Britton TM, Purushotham AD, Peters AM, Levick JR, Mortimer PS. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat 117: 549–557, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol 7: 29–45, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Suami H, Pan WR, Taylor GI. The lymphatics of the skin filled by a dermal backflow: an observation in a scarred cadaver leg. Lymphology 40: 122–126, 2007 [PubMed] [Google Scholar]
- 45.Swartz MA, Kaipainen A, Netti PA, Brekken C, Boucher Y, Grodzinsky AJ, Jain RK. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech 32: 1297–1301, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Szuba A, Rockson SG. Lymphedema: anatomy, physiology and pathogenesis. Vasc Med 2: 321–326, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458–1466, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454: 656–660, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 294: H1326–H1334, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Watari K, Nakao S, Fotovati A, Basaki Y, Hosoi F, Bereczky B, Higuchi R, Miyamoto T, Kuwano M, Ono M. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun 377: 826–831, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Williams AF, Franks PJ, Moffatt CJ. Lymphoedema: estimating the size of the problem. Palliat Med 19: 300–313, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol 195: 490–497, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, Fan ST. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol 173: 2507–2515, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111: 717–725, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.