Abstract
Corresponding to the synchronized contraction of the myocardium and rhythmic pumping function of the heart, a single form of cardiac troponin T (cTnT) is present in the adult cardiac muscle of humans and most other vertebrate species. Alternative splicing variants of cTnT are found in failing human hearts and animal dilated cardiomyopathies. Biochemical analyses have shown that these cTnT variants are functional and produce shifted myofilament Ca2+ sensitivity. We proposed a hypothesis that the coexistence of two or more functionally distinct TnT variants in the adult ventricular muscle that is normally activated as a syncytium may decrease heart function and cause cardiomyopathy (Huang et al., Am J Physiol Cell Physiol 294: C213–C222, 2008). In the present study, we studied transgenic mouse hearts expressing one or two cTnT variants in addition to normal adult cTnT to investigate whether desynchronized myofilament activation decreases ventricular efficiency. The function of ex vivo working hearts was examined in the absence of systemic neurohumoral influence. The results showed that the transgenic mouse hearts produced lower maximum left ventricular pressure, slower contractile and relaxation velocities, and decreased stroke volume compared with wild-type controls. Ventricular pumping efficiency, calculated by the ejection integral versus total systolic integral and cardiac work versus oxygen consumption, was significantly lower in transgenic mouse hearts and corresponded to the number of cTnT variants present. The results indicated a pathogenic mechanism in which the coexistence of functionally different cTnT variants in cardiac muscle reduces myocardial efficiency due to desynchronized thin filament activation.
Keywords: cardiac function, muscle contraction, cardiomyopathy, heart failure
different from skeletal muscle, which functions with tetanic contraction fused from a series of twitches, cardiac muscle is activated as an electrical syncytium to produce synchronized contractions for rhythmic pumping. An effective generation of peak ventricular pressure over aortic pressure is essential for cardiac output and efficiency.
During the contraction of vertebrate cardiac myocytes, allosteric changes in troponin C (TnC) induced by Ca2+ binding are transmitted to other subunits of troponin and the actin filament (16). Troponin T (TnT) is the thin filament-anchoring subunit of the troponin complex (28). As an essential element in the Ca2+ signaling system in cardiac and skeletal muscles, TnT interacts with TnC, troponin I (TnI), and tropomyosin to regulate the actin thin filament during contraction and relaxation (16, 28). Three muscle type-specific TnT isoform genes [cardiac (cTnT), fast skeletal, and slow skeletal] have evolved in vertebrates, and each encodes multiple alternative splice forms (25). The structure of the NH2-terminal region of TnT is hypervariable, and variations in this region modulate the conformation and function of TnT and the contractility of muscle (3–5, 9, 22, 23, 30).
Skeletal muscle expresses both slow and fast TnT with multiple alternatively spliced variants. The coexistence of multiple functionally distinct TnT variants in the thin filament Ca2+-regulatory system might be favorable for the tetanic contractions of skeletal muscle by widening the twitches. In contrast, a single form of cardiac troponin is present in adult cardiac muscle of human and most other vertebrates after the alternative splicing-generated cTnT isoform switch during perinatal heart development (21). This feature corresponds to uniformed Ca2+ activation of the thin filaments suitable for generating rhythmic contractions. Abnormal cTnT splicing variants have been found in failing human hearts (1, 26). Abnormal splicing of cTnT in the NH2-terminal region has also been found in turkey and dog hearts with dilated cardiomyopathy (3, 4). Continuing expression of embryonic cTnT and/or deletion of exon 7 (exon 8 in turkey cTnT) generate the coexistence of two or more cTnT variants in adult cardiac muscle (3, 4). Previous studies (3, 15) have shown that these splice forms of cTnT cause changes in thin filament Ca2+ sensitivity, suggesting that the coexistence of two or more cTnT variants would result in a temporally split myofilament response to rising Ca2+ concentrations.
Based on these observations, we tested the hypothesis that the coexistence of two or more TnT variants in adult cardiac muscle would reduce ventricular efficiency, which, other than loss of function, would chronically cause cardiomyopathy and heart failure. This concept has been demonstrated by the negative consequences of transgenic overexpression of wild-type skeletal muscle TnT in the heart (19). To understand the mechanism by which the temporally split activation among troponin units due to the coexistence of two or more TnT variants impairs cardiac function, we investigated, in the present study, transgenic mouse hearts expressing one or two cTnT variants in addition to normal adult cTnT to examine the effects on ventricular pumping efficiency.
MATERIALS AND METHODS
Transgenic mice.
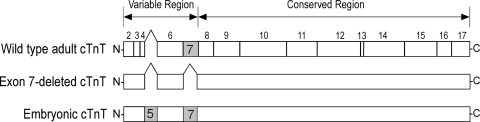
We (3) have previously generated two transgenic mouse lines that postnatally overexpress exon 7-deleted cTnT or embryonic cTnT (Fig. 1) under an α-myosin heavy chain (MHC) promoter (a gift from Dr. Jeffrey Robbins, University of Cincinnati).
Fig. 1.
Cardiac troponin T (cTnT) variants introduced into adult transgenic (TG) mouse hearts. TG mouse models were generated by the overexpression of exon 7-deleted cTnT and/or embryonic cTnT in adult hearts under the control of an α-myosin heavy chain (MHC) promoter. The structural alignment shows the NH2-terminal variations among wild-type (WT) adult cTnT, embryonic cTnT, and exon 7-deleted cTnT.
Double-transgenic mice were generated by crossing exon 7-deleted cTnT and embryonic cTnT mouse lines. These single- and double-transgenic mouse lines, which overexpress one or both of the two cTnT NH2-terminal splicing variants together with wild-type adult cTnT, provide an informative experimental system to examine the effects of the coexistence of two or more cTnT variants on cardiac function and ventricular pumping efficiency.
Mice were maintained on a 12:12-h light-dark cycle (6:00 AM/6:00 PM) and fed a standard pellet diet. Three- to five-month-old mice of both sexes were used for experiments. All protocols were approved by the Institutional Animal Care and Use Committee.
SDS-PAGE and Western blot analysis.
The transgenic overexpression of exon 7-deleted and/or embryonic cTnT in addition to endogenous adult cTnT was examined by SDS-PAGE and Western blot analysis. Total protein of muscle tissue was extracted by high-speed homogenization in SDS-PAGE sample buffer containing 50 mM Tris·HCl, 10% glycerol, 0.1% bromphenol blue, 2% SDS, and 1% 2-mercaptoethanol (pH 8.8). The protein extract was heated at 80°C for 5 min and then centrifuged at 14,000 rpm in a microcentrifuge (Beckman Coulter Microfuge 18) for 5 min to remove insoluble materials. Samples were resolved on 14% Laemmli gel with a acrylamide-to-bisacrylamide ratio of 180:1. The protein bands resolved were visualized with Coomassie blue R250 staining or electrically transferred to a nitrocellulose membrane using a semidry apparatus (Bio-Rad). Blotted membranes were blocked with Tris-buffered saline (TBS) containing 1% BSA and probed with anti-cTnT monoclonal antibody CT3 (22) or anti-TnI monoclonal antibody TnI-1 (24). The following washes and incubation with alkaline phosphatase-labeled anti-mouse IgG secondary antibody (Santa Cruz Biotechnology) and 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium substrate reaction were carried out as previously described (9).
As previously described (10), MHC isoforms in transgenic mouse hearts were examined using 8% SDS-PAGE with gels containing 30% glycerol and an acrylamide-to-bisacrylamide ratio of 50:1. The gel was run at 100 V in an ice box for 24 h and stained with Coomassie blue R250.
Histological examinations.
Hearts from 3- to 5-mo-old mice were isolated immediately after euthanasia and perfused with a relaxation saline containing 50 mM KCl. Hearts were then fixed in 3.7% formalin at 4°C for at least 24 h and processed for paraffin sectioning. Middle cross sections of the ventricles (5 μm thick) were cut, and hemotoxylin-eosin staining was done using Harris hematoxylin and eosin-phloxine Y solutions. After being mounted with no. 1 coverslips, the slides were examined and micrographs were taken under a Zeiss Axiovert 100 microscope.
Measurement of cardiac function using ex vivo working heart preparations.
Transgenic and wild-type mouse hearts were examined in isolated ex vivo working heart preparations as previously described (9). After being heparinized for 30 min, mice were anesthetized with pentobarbital (100 mg/kg body wt ip). Hearts were rapidly isolated and cannulated through the aorta with a modified 18-gauge needle to start Langendorff retrograde perfusion within 3 min after opening of the chest. The left atrium was then cannulated through a pulmonary vein with a 16-gauge needle for perfusion in the working mode. Beveled polyethylene-25 tubing was inserted into the main pulmonary artery to collect the coronary effluent and connected to an O2 sensor (Microelectrode) to measure the O2 concentration. A 1.2-Fr pressure-volume (P-V) catheter (Scisense, London, ON, Canada) was insert into the left ventricle (LV) through a path made in the apex by punctuation using a 30-gauge needle. A pressure sensor (MLT844 pressure transducer, Capto, Horten, Norway) was used to measure aortic pressure. Through a pair of platinum wires placed on the surface of the right atrium, heart rate was paced at 480 beats/min using an isolated constant current stimulator (A365, World Precision Instruments). After all the cannulations had been established, the heart was switched to working mode by turning on the left atrial perfusion. As previously described (2, 17), an air bubble of 0.5 ml was placed in the aortic trap to mimic in vivo arterial compliance. Baseline cardiac function was recorded at a preload of 10 mmHg and an afterload of 55 mmHg.
The perfusate used was Krebs buffer containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 2.25 mM MgSO4, 2.25 mM CaCl2, 0.32 mM EGTA, 2 mM pyruvate, and 15 mM d-glucose and was bubbled with 95% O2-5% CO2. NaHCO3 was added to adjust the pH to 7.4 at 37°C. The perfusion buffer was filtered with a 0.45-μm filter and not reused.
Aortic and coronary flows were recorded in real time by counting drops of the effluent. Pressure and volume development data were collected at a sampling rate of 1 kHz with a 100-Hz filter via a Powerlab 16-channel analog-to-digital interface using Chart 5.0 software (ADInstruments). LV end-systolic and end-diastolic pressure-volume relationships were recorded during afterload changes.
Immediately after functional measurements, LV muscle tissue was collected from each heart and stored at −80°C for SDS-PAGE and Western blot verification of the cTnT contents.
Calculation of cardiac efficiency from LV pressure traces.
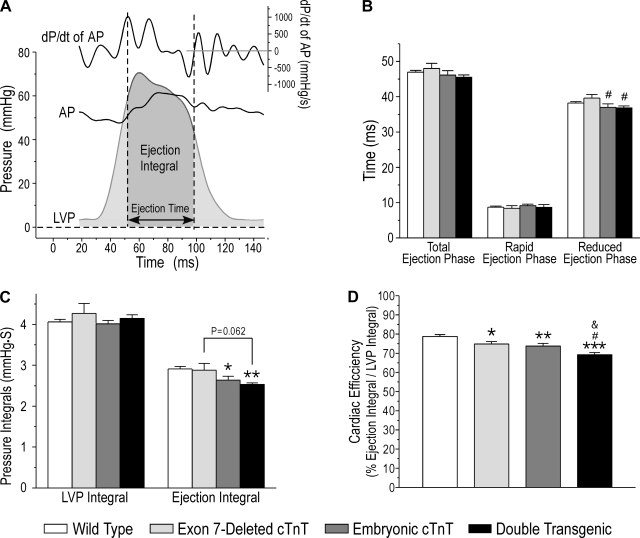
The total integral of the LV pressure (LVP) curve of each pumping cycle was used as an indictor for the total energy used in ventricular muscle contraction. The ejection time was determined from aortic pressure and pressure development traces (Fig. 5A). The portion of the LVP integral during the ejection phase was measured to indicate external work. Heart efficiency was calculated as the ratio between ejection and total integrals (Fig. 5A).
Fig. 5.
Decreased LV pumping efficiency in TG mouse hearts calculated by the ratio of the ejection integral to total systolic integral. A: determination of the ejection time from dP/dt of the AP trace. In the ex vivo working heart setup, an air bubble was set in the aortic path to mimic the compliance properties of the elastic vasculature. Under fixed afterload, this compliance element slightly delayed the AP changes during the ventricular pumping cycle. Therefore, the cross points of LVP and AP curves would not accurately represent the ejection time. However, the opening and closing of the aortic valve can be identified by analyzing the AP and dP/dt of the AP traces. The first and highest peak of +dP/dt of AP, indicating the full opening of the aortic valve, was used as the beginning of the rapid ejection phase. The lowest point of the AP curve at the end of the ejection phase, at which the dP/dt of AP = 0, was used as the closing of the aortic valve. The LV ejection time from the opening to the closing of the aortic valve defines the duration of ejection, and the enclosure area of the LVP wave reflects the ejection integral. B: time parameters of the total ejection phase, rapid ejection phase, and reduced ejection phase were unchanged in TG mouse hearts compared with WT controls. C: the whole LVP integral was the same in TG and WT mouse hearts, indicating similar total energy expenditures. Embryonic cTnT TG mouse hearts with two cTnT variants showed a reduced ejection integral compared with WT hearts. The ejection integral was further reduced in double-TG mouse hearts coexpressing three cTnT variants. D: LV pumping efficiency, calculated using the ratio of the ejection integral to total systolic integral, was decreased in both of the two-cTnT variant single-TG groups compared with the WT group. A further decrease in cardiac efficiency was found in double-TG mouse hearts coexpressing three cTnT variants. Data are presented as means ± SE; n = 15 in the WT group, 5 in the exon 7-deleted cTnT group, 8 in the embryonic cTnT group, and 6 in the double-TG group. Statistical analysis was performed using Fisher one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the WT group; &P < 0.05 vs. the embryonic cTnT group; #P < 0.05 vs. the exon 7-deleted cTnT group.
Calculation of cardiac efficiency from O2 consumption.
LV efficiency was also calculated from O2 consumption as previously described (13, 27). Briefly, heart efficiency (in %) was calculated as cardiac work/myocardial O2 consumption × 100. Myocardial O2 consumption was calculated by the difference between O2 concentrations in the perfusion influent and coronary effluent as follows:
where Po2a is Po2 in the left atrial influent (95%), Po2v is Po2 in the coronary effluent, and c is the solubility coefficient for O2 in Krebs buffer (22.7 ml O2·atm−1·ml−1 at 37°C).
Pressure work and kinetic work were calculated as follows:
where
Myocardial O2 consumption was converted into joules per minute per gram using a conversion factor of 20.054 J/ml O2 consumed (13). The ejection time was determined as described above.
Data analysis.
Two-dimensional densitometry was performed on 600 dpi images of the SDS-PAGe gels and Western blots done under nonsaturating conditions (33) to quantify the relative amounts of cTnT variants. All data are presented as means ± SE. Statistical analysis was performed using one-way ANOVA with a Fisher adjustment.
RESULTS
Transgenic overexpression to generate the coexistence of two or three cTnT variants in adult hearts.
Double-transgenic mice were successfully created by crossing the two single transgenic lines bearing α-MHC-directed transgene alleles encoding exon 7-deleted and embryonic cTnT. Independent and nonsex-linked Mendelian segregations were shown for the two transgene alleles, indicating their locations on different autosomes.
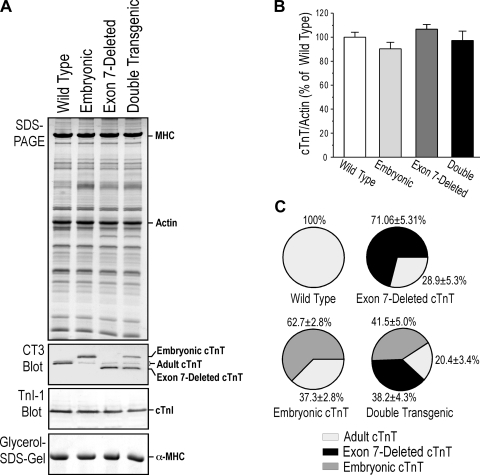
The expression of exon 7-deleted and embryonic mouse cTnT in the hearts of single- and double-transgenic mice was confirmed by Western blot analysis (Fig. 2A). The results demonstrated the coexistence of the transgene-encoded cTnT variants and endogenous wild-type adult cTnT. We (11) have previously shown that the capacity of myofilament incorporation determines the stoichiometry of TnI in transgenic mouse cardiac muscle, which remained constant when exogenous TnI was overexpressed with or without knockout of the endogenous cardiac TnI gene. Similarly, the total amount of cTnT in the single- and double-transgenic mouse hearts remained constant at the wild-type level as determined by densitometry quantification of the Western blots (Fig. 2B). Such effective replacement of endogenous troponin subunits via transgenic overexpression has been previously observed by us and other others (2, 3, 9–12, 19, 20). Saturated incorporation of TnT in the myofilaments and proteolytic removal of nonmyofilament-associated/surplus TnT in the myocytes (11, 32) form a foundation for the competitive replacement in transgenic mouse hearts overexpressing exogenous troponin subunits.
Fig. 2.
Coexpression of two or three cTnT variants in adult TG mouse cardiac muscle. A: TG expression of cTnT variants in the heart was examined with SDS-PAGE and Western blot analysis using monoclonal antibody CT3, which recognizes all three alternative splicing variants of cTnT studied. The results showed that cardiac muscles of the two single-TG mouse lines expressed the corresponding exogenous cTnT together with endogenous adult cTnT to produce two-variant cTnT heart models. Double-TG mouse cardiac muscle coexpressed two exogenous cTnT variants together with endogenous adult cTnT to produce a three-variant cTnT heart model. Monoclonal antibody TnI-1 Western blot analysis and glycerol-SDS-PAGE showed that cTnI and α-MHC were unchanged in TG mouse hearts compared with WT controls. B: the total amount of cTnT remained at the WT level in TG mouse hearts. Densitometry quantification of monoclonal antibody CT3 Western blots normalized for loading by the actin band in parallel SDS-PAGE gels showed no statistical differences among total cTnT levels in TG and WT hearts. C: densitometry quantification of CT3 Western blots further determined the proportions of cTnT variants in TG mouse hearts in contrast to 100% adult cTnT in WT adult mouse hearts. Data are presented as means ± SE; n = 3 in each group. Statistic analysis was performed using Fisher one-way ANOVA.
The Western blots further showed that the levels of cardiac TnI in all three types of cTnT transgenic mouse heats were the same as those in wild-type mouse hearts. This result indicates that the coexistence of two or three cTnT variants did not alter the normal expression and turnover of cardiac TnI in cardiac myofilaments. Quantification of the cTnT Western blots determined the proportion of each cTnT variant in the single- and double-transgenic mouse hearts (Fig. 2C). The results showed that these mouse lines can provide adult hearts containing two or three variants of cTnT for use in quantitative experiments of the effect of coexistence of TnT variants on cardiac function and efficiency.
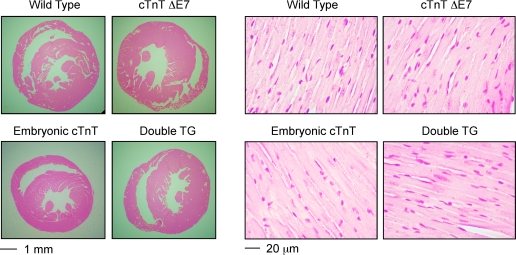
Glycerol SDS-PAGE detected only α-MHC in the adult hearts of all the transgenic mouse lines studied, the same as that in wild-type adult mouse hearts (Fig. 2A). No β-MHC was detected in the transgenic mouse hearts, indicating no signs of adaptive myocardial remodeling (10). Consistently, the 3- to 5-mo-old single- and double-transgenic mice showed no systemic signs of heart failure. Heart weight and the ratio of heart weight to body weight as an index of hypertrophy showed no differences between the transgenic and wild-type groups (Table 1). Within each experimental group, the heart weight-to-body weight ratio and functional parameters were not significantly different between male and female mice. Therefore, data from both sexes were combined for analysis. Histological examination found no hypertrophy or dilation and no myopathic changes compared with wild-type control (Fig. 3). The results support that the transgenic mouse hearts were in a state before the development of compensatory adaptation or dilated myopathic remodeling.
Table 1.
No hypertrophy in the transgenic mouse hearts
| Wild Type | Exon7-Deleted cTnT | Embryonic cTnT | Double Transgenic | |
|---|---|---|---|---|
| Body weight, g | 29.04 ± 1.26 | 24.92 ± 1.36* | 25.37 ± 0.90* | 27.71 ± 0.79 |
| Heart weight, mg | 145.09 ± 5.81 | 134.76 ± 11.37 | 130.13 ± 4.60 | 147.16 ± 10.48 |
| Heart weight/body weight, mg/g | 5.04 ± 0.15 | 5.38 ± 0.23 | 5.13 ± 0.10 | 5.28 ± 0.25 |
Values are presented as means ± SE; n = 15 wild-type hearts, 5 exon 7-deleted cardiac troponin T (cTnT) hearts, 8 embryonic cTnT hearts, and 6 double-transgenic hearts. Heart weight and body weight were measured from 3- to 5-mo-old mice from the four groups. Body weight was lower in exon 7-deleted cTnT and embryonic cTnT mice compared with wild-type mice. However, the ratio of heart weight to body weight as an index of hypertrophy showed no significant differences. Statistical analysis was performed using Fisher one-way ANOVA.
P < 0.05 vs. wild-type.
Fig. 3.
Absence of hypertrophy, dilation, or myopathy in 3- to 5-mo-old TG mouse hearts. Hematoxylin-eosin-stained ventricular cross sections of 3- to 5-mo-old TG mice did not detect hypertrophy or dilation compared with WT controls. The high-magnification micrographs found no myopathic changes in TG mouse cardiac muscle. ΔE7, exon 7 deleted.
Decreased myocardial contractility and ventricular function in transgenic mouse hearts.
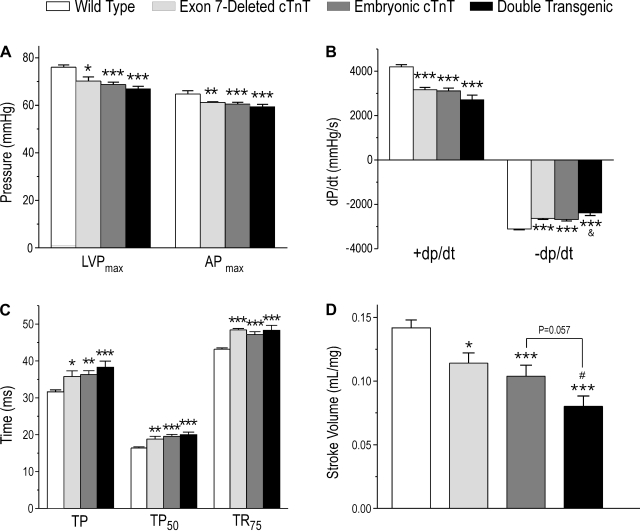
Myocardial function was measured in isolated ex vivo working heart preparations to exclude neurohumoral influences. At supraventricularly paced constant heart rate and baseline preload (10 mmHg) and afterload (55 mmHg), the results showed that maximal LVP and maximal aortic pressure were decreased in the three groups of mouse hearts coexpressing two or three cTnT variants compared with wild-type controls containing only normal adult cTnT (Fig. 4A). Contractile and relaxation velocities (+dP/dt and −dP/dt) were slower in hearts coexpressing two or three cTnT variants compared with wild-type hearts (Fig. 4B). Correspondingly, the times to develop peak pressure and 50% peak pressure and the time from peak pressure to 75% relaxation were elongated in the transgenic mouse hearts compared with wild type controls (Fig. 4C). The results indicate that the coexistence of two or three cTnT variants with different Ca2+ sensitivity decreased myocardial contractility.
Fig. 4.
Coexistence of two or three cTnT variants decreased cardiac function. A: maximal left ventricular (LV) pressure (LVPmax) and maximal aortic pressure (APmax) were decreased in all three groups of TG mouse hearts compared with WT hearts. B: contractile and relaxation velocities (+dP/dt and −dP/dt) were both decreased in hearts coexpressing two or three cTnT variants compared with WT controls. The relaxation velocity of double-TG mouse hearts was also lower than that of embryonic cTnT single-TG mouse hearts. C: times to develop peak pressure (TP) and 50% peak LV pressure (TP50) and time for 75% relaxation (TR75) were elongated in all three groups of TG mouse hearts. D: stroke volume normalized to heart weight was decreased in all three groups of TG mouse hearts and to the greatest degree in the double-TG group. Data are presented as means ± SE; n = 15 in the WT group, 5 in the exon 7-deleted cTnT group, 8 in the embryonic cTnT group, and 6 in the double-TG group. Statistical analysis was performed using Fisher one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the WT group; &P < 0.05 vs. the embryonic cTnT group; #P < 0.05 vs. the exon 7-deleted cTnT group.
Corresponding to the decreased myocardial contractility, the coexpression of two or three cTnT variants reduced ventricular pumping function. LV stroke volume significantly decreased in single-transgenic mouse hearts containing two cTnT variants (wild-type adult cTnT plus embryonic cTnT or exon 7-deleted cTnT) compared with that of wild-type hearts expressing only adult cTnT (Fig. 4D). Stroke volume was further decreased in double-transgenic mouse hearts containing three cTnT variants (P < 0.05 vs. the exon 7-deleted single-transgenic group and P = 0.057 vs. the embryonic cTnT single-transgenic group; Fig. 4D).
Coexistence of two or three cTnT variants decreased cardiac efficiency.
By relating the functional output of the contractile machinery to the energy cost, cardiac efficiency is a key factor for evaluating heart function. Measured at the intact organ level, the total LVP integral reflects the entire work of cardiac muscle contraction, whereas the LVP integral during the ejection period reflects the work that produced cardiac output. Therefore, the ratio of LVP ejection integral to whole LVP integral represents cardiac work efficiency.
Figure 5A shows the determination of the ejection phase using the aortic pressure and pressure development curves. The rapid ejection phase is from aortic valve opening to the peak of LVP and followed by the reduced ejection phase. The area under the LVP curve during the ejection phase of a pumping cycle (Fig. 5A) was used to determine the ejection integral.
LV ejection time parameters showed no differences among the four groups studied (Fig. 5B). Total LVP integrals were also similar in wild-type and transgenic mouse hearts coexpressing two or three cTnT variants. However, embryonic cTnT and double-transgenic mouse hearts showed statistically significant decreases in ejection integral compared with wild-type controls, in which the double-transgenic group was further down compared with that of the exon 7-deleted single-transgenic group (Fig. 5C). Cardiac efficiency, calculated as the ratio of the ejection integral versus total LVP integral, decreased in all three groups of transgenic mouse hearts compared with wild-type control (Fig. 5D). Double-transgenic mouse hearts containing three cTnT variants in cardiac muscle, corresponding to a higher degree of variation in thin filament activation, exhibited a more severe decrease in cardiac efficiency than single-transgenic mouse hearts containing two cTnT variants.
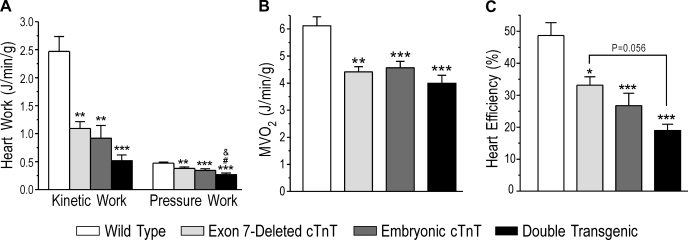
To verify the decreased cardiac efficiency calculated from the LVP integrals, we used a classic approach to measure cardiac output versus O2 consumption. Kinetic work and pressure work, the two parts of external work, both significantly decreased in all three transgenic groups compared with wild-type hearts (Fig. 6A). The external work of double-transgenic mouse hearts with a higher degree of cTnT variation decreased more than that of single-transgenic hearts. While O2 consumption was reduced in hearts coexpressing two or three cTnT variants relatively to that in wild-type hearts due to the decreased work (Fig. 6B), cardiac efficiency, calculated by the ratio of external work to O2 consumption, was significantly lower in single-transgenic mouse hearts bearing two cTnT variants than in wild-type controls, with a trend of further decreases in double-transgenic mouse hearts bearing three cTnT variants (Fig. 6C).
Fig. 6.
Decreased cardiac efficiency in TG mouse hearts calculated against O2 consumption. A: cardiac work, calculated from kinetic work and pressure work according to Neely et al. (27), was decreased in TG mouse hearts, with more severity in the three-cTnT variant hearts of double-TG mice. B: myocardial O2 consumption (MV̇o2) was decreased in TG mouse hearts, indicating less energy consumption due to the decreased cardiac work. C: cardiac efficiency, calculated using total cardiac work versus O2 consumption, was lower in all of the TG mouse hearts than in WT controls, and double-TG hearts showed the lowest efficiency. Data are presented as means ± SE; n = 15 in the WT group, 5 in the exon 7-deleted cTnT group, 8 in the embryonic cTnT group, and 6 in the double-TG group. Statistical analysis was performed using Fisher one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the WT group; #P < 0.05 vs. the exon 7-deleted cTnT group. &P < 0.05 vs. the embryonic cTnT group.
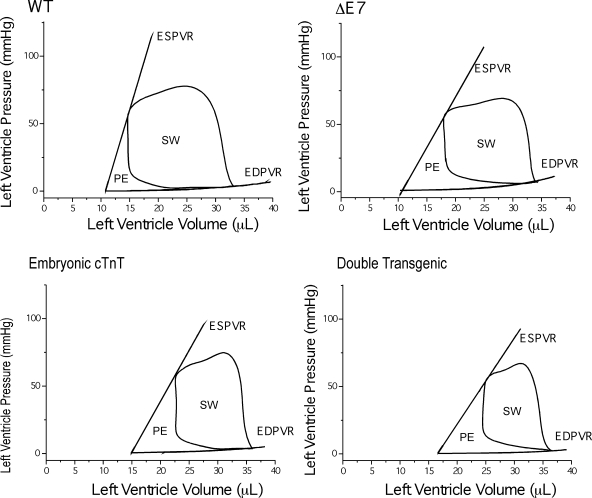
The negative effects of the coexistence of two or three cTnT variants on cardiac function and efficiency were further demonstrated by P-V loop analysis during changes in pressure and volume loads for the ratio between stroke work (the area of the P-V loop representing the energy converted into cardiac work) and the potential energy triangle (representing metabolic and other energy expenditures) (18). The representative P-V loops of wild-type and single- and double-transgenic mouse hearts shown in Fig. 7 demonstrate that transgenic hearts coexpressing two or three cTnT variants exhibited lower stroke work with a higher potential energy triangle compared with wild-type hearts. The decreased ratio between stroke work and the sum of potential energy and stroke work indicates impaired cardiac energetic efficiency due to the coexistence of two or three cTnT variants.
Fig. 7.
Decreased efficiency in TG mouse hearts illustrated by end-systolic pressure-volume (P-V) relationship (ESPVR) and end-diastolic P-V relationship (EDPVR) analysis. Representative P-V loops for WT, single-TG, and double-TG mouse hearts are shown. ESPVR was deduced from a series of P-V loops produced during temporary restriction in the path of aortic outflow to increase afterload. The EDPVR curve was obtained from the dilation effect of increasing afterload. The area of the P-V loops indicates stroke work (SW), and the triangle enclosed by the ESPVR and EDPVR lines indicates potential energy (PE) expenditure. Cardiac efficiency can be evaluated by the ratio between the areas of SW and PE. A comparison of the four experimental groups demonstrated decreased energetic efficiency in TG mouse hearts compared with WT mouse hearts.
DISCUSSION
The NH2-terminal variable region of TnT is a regulatory structure. Variations in the NH2-terminal region of TnT confer conformational changes in the middle and COOH-terminal regions to alter interactions with TnI, TnC, and tropomyosin as well as myofilament responses to Ca2+ activation (25). The present study used transgenic mouse hearts expressing multiple such cTnT variants as experimental models to test the hypothesis that the coexistence of two or three cTnT variants reduces cardiac function and efficiency. NH2-terminal alternatively spliced cTnT variant are known to produce different Ca2+ sensitivities (4, 15). In contrast to loss-of-function mutations, the moderate functional change resulting from the replacement of endogenous cTnT with an NH2-terminal alternatively spliced exogenous cTnT is consistent with the modulatory role of the NH2-terminal variable region. However, the coexistence of cTnT variants that differed in NH2-terminal structure generated differences in the myofilamental response to Ca2+, providing an informative experimental system for testing the effects on cardiac efficiency. The main findings are discussed below.
Vertebrate hearts have evolved to be an electrical syncytium at the organ level with corresponding expression of a single type of thin filament regulatory proteins, i.e., α-tropomyosin and the cardiac isoform of troponin subunits, for uniform activation to efficiently generate peak pumping pressure. In transgenic mouse hearts, the coexpression of cTnT variants with NH2-terminal differences would result in variable Ca2+ responses in the thin filament (4), which would broaden and lower the peak contractile force compared with the contraction induced by a single class of troponin in normal adult cardiac muscle, which contains only one adult cTnT. The lowered ventricular peak pressure would then decrease the ratio of the ejection integral to total contractile integral and cardiac efficiency (Fig. 5).
In contrast, skeletal muscles normally contain mixed slow and fast twitch fibers with multiple fast and slow TnT splicing forms. According to the above hypothesis, the blended activation in skeletal muscle thin filaments due to variable responses to Ca2+ activation would produce wider twitches that are more readily fused into functional tetanic contractions.
The altered responses to Ca2+ in myofilaments containing two or three cTnT variants decreased cardiac muscle contractility, as indicated by decreased contractile velocity (Fig. 4B). The corresponding reduction in LV stroke volume (Fig. 4D) resulted in decreases in cardiac efficiency of single- and double-transgenic mouse hearts. LVP integral calculation and cardiac work versus O2 consumption both demonstrated decreases in cardiac efficiency in transgenic mouse hearts proportional to the classes of cTnT variants present (Figs. 5 and 6).
Consistent with decreased energetic efficiency, the diastolic function of transgenic mouse hearts, as shown by the velocity and time of ventricular relaxation, was decreased proportionally to the classes of cTnT variants present (Fig. 4B). On the other hand, the decreased stroke volume of transgenic mouse hearts corresponded to a trend of increases in LV end-diastolic volume and pressure (Fig. 7). These results indicate that the cardiac muscle worked at a longer sarcomere length to compensate for the decreases in contractility through the Frank-Starling mechanism. This adaptation potential via relaxation was supported by the shape of the P-V loops, which reflected the critical importance of ventricular relaxation in a fast-beating heart, like in mouse hearts. During relaxation, the continuously decreasing LVP (Fig. 7) reflects that ventricular relaxation is leading ventricular filling.
This observation suggests that the 3- to 5-mo-old transgenic mouse hearts worked in vivo at a nonfailing state, consistent with the absence of gross myocardial remodeling such as hypertrophy or dilation. However, the maintenance of function was accompanied by increases in energetic expenditure in vivo with decreased energetic efficiency. Through mechanisms such as oxidative stress (6, 29), Ca2+ overload (6, 14), and matrix metalloproteinase activation (7, 8, 31), the decreased cardiac efficiency and higher energetic cost in maintaining cardiac output would chronically result in cardiac remodeling followed by decompensation and maladaptation, leading to dilated cardiomyopathy and heart failure.
We (19) have previously demonstrated the concept that the coexistence of two TnT variants generated by overexpression of a wild-type fast skeletal muscle TnT in the heart of transgenic mice decreased cardiac function and produced cardiomyopathy phenotypes. The present study further showed that instead of loss of function, relatively moderate differences in Ca2+ sensitivity due to cTnT NH2-terminal variation in adult ventricular muscle would chronically impair cardiac function by decreasing energetic efficiency. This mechanism may explain the phenotype that continuing expression of embryonic cTnT and/or aberrant splice out of exon 7 with normal adult cTnT in adult dog and turkey hearts caused dilated cardiomyopathy and heart failure (3, 4).
It is worth noting that this initial investigation focused on the contractile efficiency of ventricular muscle and studied transgenic mouse models at early adult age in the absence of cardiac hypertrophy and clinical heart failure. Although the energetic efficiency of the contractile machinery was significantly decreased, it was compensated by the capacity of energy supply in the cardiomyocyte in this early stage of the pathogenic process. Therefore, there was no detectable hypertrophy or cardiac remodeling at the tissue level. Long-term effects of pathogenic remodeling leading to a dilated phenotype may be anticipated based on the fact that these cTnT variants were originally discovered in dilated cardiomyopathic turkey and dog hearts (3, 4). Further investigations on the consequent myocardial remodeling will help to better understand this novel pathogenic mechanism. The expression of additional cTnT splicing variants has been reported for failing human hearts at low levels (1, 26). Although the transgenic mouse models tested in the present study are not direct replicas of the human disease, the experimental data lay a foundation for further investigating the effects of cTnT variation on cardiac efficiency and the development of human cardiomyopathy and heart failure.
In summary, our study found that the coexistence of two or three cTnT variants in adult mouse hearts decreased myocardial contractility, cardiac function, and ventricular pumping efficiency. The experimental data suggest a novel pathogenic mechanism in which the chronic coexistence of cTnT variants in cardiac muscle impairs heart efficiency due to variable activation of the thin filaments, which merits further studies.
GRANTS
This work was supported by National Institutes of Health Grants HL-078773 and AR-048816 (to J.-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Hui Wang for genotyping of the transgenic mice and Dr. Jeffrey Robbins for the α-MHC promoter used in the production of transgenic mice.
REFERENCES
- 1.Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76: 681–686, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Barbato JC, Huang QQ, Hossain MM, Bond M, Jin JP. Proteolytic N-terminal truncation of cardiac troponin I facilitates ventricular relaxation and enhances heart function. J Biol Chem 280: 6602–6609, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Biesiadecki BJ, Elder BD, Yu ZB, Jin JP. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem 277: 50275–50285, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Biesiadecki BJ, Jin JP. Exon skipping in cardiac troponin T of turkeys with inherited dilated cardiomyopathy. J Biol Chem 277: 18459–18468, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Biesiadecki BJ, Chong SM, Nosek TM, Jin JP. Troponin T core structure and the regulatory NH2-terminal variable region. Biochemistry 46: 1368–1379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101: 1833–1839, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res 89: 201–210, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM, Jin JP. Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis. J Physiol 586: 3537–3550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng HZ, Chen M, Weinstein LS, Jin JP. Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling. J Biol Chem 283: 33384–33393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng HZ, Hossain MM, Huang XP, Jin JP. Myofilament incorporation determines the stoichiometry of troponin I in transgenic expression and the rescue of a null mutation. Arch Biochem Biophys 487: 36–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517: 143–157, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier NS, Matherne GP, Morrison RR, Headrick JP. Determination of function in the isolated working mouse heart: issues in experimental design. J Mol Cell Cardiol 30: 453–461, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Goll DE, Thompson VF, Taylor RG, Christiansen JA. Role of the calpain system in muscle growth. Biochimie 74: 225–237, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem 279: 49579–49587, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000 [DOI] [PubMed] [Google Scholar]
- 17.How OJ, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS. Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol 288: H2979–H2985, 2005 [DOI] [PubMed] [Google Scholar]
- 18.How OJ, Aasum E, Larsen TS. Work-independent assessment of efficiency in ex vivo working rodent hearts within the PVA-MVO2 framework. Acta Physiol 190: 171–175, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Huang QQ, Feng HZ, Du Liu JJ, Stull LB, Moravec C, Huang X, Jin JP. Co-expression of skeletal and cardiac troponin T decreases mouse cardiac function. Am J Physiol Cell Physiol 294: C213–C222, 2008 [DOI] [PubMed] [Google Scholar]
- 20.James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, Robbins J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res 87: 805–811, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Jin JP, Lin JJC. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat heart during development. J Biol Chem 263: 7309–7315, 1988 [PubMed] [Google Scholar]
- 22.Jin JP, Chen A, Ogut O, Huang QQ. Conformational modulation of slow skeletal muscle troponin T by an NH2-terminal metal binding extension. Am J Physiol Cell Physiol 279: C1067–C1077, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Jin JP, Root DD. Modulation of troponin T molecular conformation and flexibility by metal ion binding to the NH2-terminal variable region. Biochemistry 39: 11702–11713, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Jin JP, Yang F, Yu ZB, Ruse C, Bond M, Chen A. The highly conserved COOH-terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40: 2623–2631, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mesnard-Rouiller L, Mercadier JJ, Butler-Browne G, Heimburger M, Logeart D, Allen PD, Samson F. Troponin T mRNA and protein isoforms in the human left ventricle: pattern of expression in failing and control hearts. J Mol Cell Cardiol 29: 3043–3055, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212: 804–814, 1967 [DOI] [PubMed] [Google Scholar]
- 28.Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui H. Novel pathophysiological insight and treatment strategies for heart failure. Circ J 68: 1095–1103, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Jin JP. Conformational modulation of troponin T by configuration of the N-terminal variable region and functional effects. Biochemistry 37: 14519–14528, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106: 1543–9 162–1642002 [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Huang QQ, Breckenridge MT, Chen A, Crawford TO, Morton DH, Jin JP. Cellular fate of truncated slow skeletal muscle troponin T produced by Glu180 nonsense mutation in Amish nemaline myopathy. J Biol Chem 280: 13241–13249, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]