Abstract
Neurons in the ventrolateral medulla (VLM) and in the nucleus tractus solitarius (NTS) play important roles in the regulation of cardiovascular and other autonomic functions. In the present study, we demonstrate an inhibition of brown adipose tissue (BAT) thermogenesis evoked by activation of neurons in the VLM, as well as by neurons in the intermediate NTS, of chloralose/urethane-anesthetized, artificially ventilated rats. Activation of neurons in either rostral VLM or caudal VLM with N-methyl-d-aspartate (12 nmol) reversed the cold-evoked increase in BAT sympathetic nerve activity (SNA), BAT temperature, and end-expired CO2. Disinhibition of neurons in either VLM or NTS with the GABAA receptor antagonist, bicuculline (30 pmol), reversed the increases in BAT SNA, BAT temperature, and end-expired CO2 that were elicited 1) by cold defense; 2) during the febrile model of nanoinjection of prostaglandin E2 into the medial preoptic area; 3) by activation of neurons in the dorsomedial hypothalamus or in the rostral raphe pallidus (rRPa); or 4) by the μ-opioid receptor agonist fentanyl. Combined, but not separate, inhibitions of neurons in the VLM and in the NTS, with the GABAA receptor agonist, muscimol (120 pmol/site), produced increases in BAT SNA, BAT temperature, and expired CO2, which were reversed by nanoinjection of glycine (30 nmol) into the rRPa. These findings suggest that VLM and NTS contain neurons whose activation inhibits BAT thermogenesis, that these neurons receive GABAergic inputs that are active under these experimental conditions, and that neurons in both sites contribute to the tonic inhibition of sympathetic premotor neuronal activity in the rRPa that maintains a low level of BAT thermogenesis in normothermic conditions.
Keywords: chemoreceptor, fentanyl, raphe
in mediating nonshivering thermogenesis, brown adipose tissue (BAT) is a site of glucose utilization, lipid oxidation, and energy expenditure that plays an important role in the cold defense response of small mammals and infant primates, but which also contributes to diet-induced thermogenesis (47), and whose reduced function may be a factor in the overweight phenotype of obesity (59). The recent demonstration of significant, metabolically active BAT depots in adult humans (14, 43, 59) and the potential for dysregulation of BAT energy expenditure in human obesity (14, 59) and in models of the wastage syndrome in cachexia (5) have focused interest on the central neural mechanism controlling BAT sympathetic outflow, the primary determinant of BAT thermogenesis and energy consumption (8). The principal pathways for the regulation of BAT thermogenic responses during cold defense and in response to central prostaglandin (PG) E2 administration have been described (36). In particular, the rostral raphe pallidus (rRPa) is the principal site of BAT sympathetic premotor neurons, and BAT sympathetic outflow is markedly increased by antagonism of GABAA receptors in rRPa (37), presumably mediated by a disinhibition of BAT sympathetic premotor neurons in rRPa. This response reveals a potent, tonic, GABAergic inhibitory influence, mediated within the rRPa, on BAT thermogenesis. In addition, a tonically active inhibition of the spinal circuits mediating BAT sympathetic outflow has been postulated to explain the increased excitability of BAT sympathetic preganglionic circuits following activation of the inhibitory 5-HT1A receptor in the intermediolateral nucleus of the upper thoracic spinal cord (29).
The ventrolateral medulla (VLM) plays a major role in the control of cardiovascular function, including cardiac output, vasomotor tone, arterial pressure (AP), and venous return. The rostral VLM is the site of sympathetic premotor neurons, providing the glutamatergic excitatory drive to spinal sympathetic circuits determining vasoconstrictor and cardiac sympathetic outflows. In contrast, although neurons in the rostral VLM are retrogradely infected following viral injections into BAT, the spinal sympathetic circuits controlling BAT sympathetic outflow do not appear to be influenced by an excitatory drive from the VLM (35, 44). The caudal VLM contains GABAergic, sympathoinhibitory neurons projecting to the rostral VLM to mediate a tonic, as well as the baroreceptor-driven (i.e., phasic) inhibitory, influence on cardiovascular sympathetic premotor neurons (1, 13, 26, 58). Although evidence from an early study (35) suggested an inhibitory effect on BAT sympathetic nerve activity (SNA) from activation of neurons in the rostral VLM, neither the extent of the VLM region producing this effect, nor the potential capacity of VLM neurons to inhibit the increases in BAT SNA from different sources was determined.
The intermediate subnucleus of the nucleus tractus solitarius (NTS) is the site of both second-order sensory neurons receiving the primary baroreceptor afferents and projection neurons that transmit baroreceptor and other cardiovascular sensory information, in particular to the caudal VLM and to the paraventricular nucleus of the hypothalamus (3, 6). BAT SNA, however, is not influenced by the baroreceptor reflex (35). Regarding thermoregulatory responses, interruption of the activity of NTS neurons attenuates the PGE1-evoked increases in core and BAT temperatures (19) and the fever produced by IL-1β (20). In metabolic regulation, the NTS contains proopiomelanocortin neurons that receive visceral afferent inputs (4), neurons in the medial NTS are activated by leptin (25), and fourth ventricular administration of leptin produces hyperthermia (23) and body weight loss that is dependent on brainstem melanocortin receptor activation (53). The effect of intermediate/medial NTS neuronal activation on BAT SNA and BAT energy expenditure remains to be explored.
The present study was undertaken to determine whether neurons in the VLM and in the intermediate NTS can influence the level of BAT SNA and BAT thermogenesis and energy expenditure. The results indicate that neurons throughout the VLM and within the intermediate NTS can elicit a potent inhibitory regulation of BAT SNA, and that, in the anesthetized rat, the BAT sympathoinhibitory neurons in both of these regions contribute an active inhibitory input, determining the level of BAT SNA and energy expenditure.
METHODS
General procedures.
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University. Animal numbers and experimental procedures selected minimized the number of animals used and minimized animal discomfort. Male Sprague-Dawley rats (300–450 g, Charles River, Indianapolis, IN) were initially anesthetized with 3% isoflurane in 100% O2, allowing cannulation of the femoral artery for monitoring AP and the femoral vein for drug administration. Subsequently, the animals were transitioned to urethane (750 mg/kg iv) and α-chloralose (60 mg/kg iv). The trachea was cannulated for artificial ventilation. The animals were paralyzed with d-tubocurarine (initially 0.6 mg/rat iv, thereafter 0.3 mg/h iv) and artificially ventilated with 100% O2 at a minute volume of 180–300 ml. End-expired CO2 was continuously monitored and maintained during the control condition between 3.5 and 5.0% by adjusting ventilation volume and rate. The rats were positioned prone in a stereotaxic frame with the incisor bar −12 mm below the interaural line. Colonic temperature was maintained between 36.5 and 37.5°C with a thermostatically controlled heating pad and lamp or a water-perfused thermal blanket, except as noted for cold-evoked increases in BAT SNA. BAT temperature was continuously monitored (TC-1000 thermocouple reader, Sable Systems, Las Vegas, NV) with a thermocouple (Physitemp, Clifton, NJ) inserted into the left interscapular BAT pad.
Sympathetic nerve recording.
Right postganglionic BAT SNA was recorded from a small nerve bundle dissected from the ventral surface of the right interscapular BAT pad. Left renal SNA (RSNA) was recorded from a renal sympathetic nerve strand isolated from the renal plexus located near the renal artery and vein after retroperitoneal exposure and lateral retraction of the left kidney. The central cut ends of the nerves were placed on a bipolar hook recording electrode under mineral oil. Nerve activity was differentially amplified (10,000 to 20,000 times; CyberAmp 380, Axon Instruments, Union City, CA), filtered (1–300 Hz for BAT SNA, 100–3,000 Hz for RSNA), digitized, and recorded onto a hard drive using Spike 2 software (Cambridge Electronic Design).
Administration of drugs.
After appropriate craniotomies, pipettes (∼20-μm tip diameter) were stereotaxically positioned into selected brain sites, and drug nanoinjections (30–60 nl) were accomplished with a pressure-injection apparatus (Toohey, Fairfield, NJ) by estimating the injection volume using a calibrated microscope reticule to observe the displacement of the fluid meniscus in the glass pipette. All drugs were obtained from Sigma-Aldrich and diluted in 0.9% saline. In addition to the cold-evoked increase in BAT SNA (30), BAT SNA and BAT thermogenesis were activated with each of the following: injection of PGE2 (60 pmol, 1 mM in 60 nl) into the right medial preoptic nucleus (MPO; 1.0–1.5 mm posterior and 0.7 mm lateral to the midline, 8.2 mm below the dural surface) (32); injection of bicuculline (Bic; 15 pmol, 0.5 mM in 30 nl) into the right dorsomedial hypothalamus (DMH; 4.5 mm posterior to bregma, 0.5 mm lateral to the midline, 8.3 mm below the dural surface) (10); injection of Bic (15 pmol) into the rRPa (3.1 mm anterior, 0.0 mm lateral, −2.7 to −3.0 mm ventral to calamus scriptorius) (35, 37); or intravenous (iv) administration of fentanyl [100 μg (300 nmol)/kg] (11). The specificity of each of these neurochemical stimuli vs. their vehicles was determined in the referenced studies. To activate local neurons through disinhibition, Bic (30 pmol, 0.5 mM in 60 nl) was injected into the VLM (2.6 mm anterior, 2.0 mm lateral, −2.7 mm ventral to calamus scriptorius) or into the intermediate NTS (0.5 mm anterior, 0.5 mm lateral, −0.5 mm ventral to calamus scriptorius). Saline (vehicle) nanoinjections (60 nl) into the VLM or into the intermediate NTS were without effect (P > 0.05) on the elevated levels of BAT SNA or BAT temperature elicited by any of the above-mentioned stimuli. To map the extent of the BAT sympathoinhibitory area in the VLM, local neurons were briefly activated through stimulation of their glutamate receptors with nanoinjections of N-methyl-d-aspartate (NMDA) (6 or 12 pmol, 0.2 mM in 30 or 60 nl) in the region of 3.2 to −0.4 anterior, 1.0 to 1.9 lateral, and 2.0 to 3.5 ventral to the calamus scriptorius. Brief chemical inactivation of neurons in rRPa was accomplished with nanoinjection of glycine (30 nmol, 0.5 M in 60 nl). The optimal site for nanoinjection into rRPa was that yielding the lowest microstimulation threshold (<10 μA) for evoking an excitatory BAT SNA potential with twin pulses (1-ms duration, 6-ms interpulse interval, 0.4 Hz) applied to rRPa (35). Longer inhibitions of local neurons in the VLM and in the NTS were effected with nanoinjections of the GABAA receptor agonist, muscimol (Musc; 120 pmol, 2.0 mM in 60 nl). The arterial baroreceptor reflex was stimulated by inducing a pressor response with an injection of phenylephrine (PE; 10 μg/kg iv).
Histological localization of injection sites.
At the end of each experiment, the nanoinjection pipettes were retracted vertically, refilled with a saturated (∼2%) solution of fast green dye, and stereotaxically repositioned at the sites of the nanoinjections, and the dye was electrophoretically deposited (−20 μA direct current for 5–10 min). Alternatively, fluorescent polystyrene microspheres (FluoSpheres, F8797, F8801, or F8803; Molecular Probes, Eugene, OR) were included in the injectate (1:200 dilution of FluoSpheres in the injectate). Following transcardial perfusion with normal saline followed by 10% formalin solution, brains were stored in 30% sucrose overnight, 60-μm coronal sections of the brain stem and hypothalamus containing the dye spots or microsphere deposits were identified, and the nanoinjection site locations were plotted on representative drawings from a rat stereotaxic atlas (45).
Data analysis.
For analysis of SNA, Spike 2 software was used to obtain a continuous measure (4-s bins) of SNA amplitude by calculating the root-mean-square amplitude of the SNA (square root of the total power in the 0- to 20-Hz band) from the autospectra of sequential 4-s segments of SNA. Measurements of SNA were made after subtracting noise levels determined after intravenous ganglionic blockade with trimethaphan (1.0 mg in 0.1 ml saline). Control values of mean AP (MAP), heart rate (HR), BAT temperature, expired CO2, BAT SNA, and RSNA were calculated as the averages of these variables during either the 32- or 60-s periods immediately before drug injections. Response values were calculated as the averages of the variables during the 32- or 60-s period of their maximal change following a drug treatment. Data are expressed as the means ± SE. Responses were compared with Student's t-test, with P < 0.05 considered significant.
RESULTS
Excitation of neurons in VLM reverses cold-evoked BAT thermogenic responses.
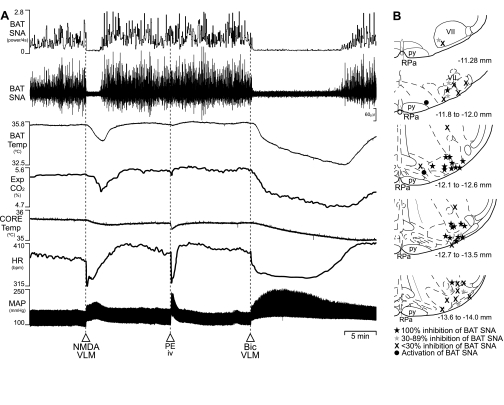
We tested the hypothesis that activation of neurons in the VLM would inhibit the increase in BAT SNA and BAT thermogenesis activated by cold exposure. As illustrated in Fig. 1A, at a reduced core temperature, BAT SNA is characterized by large bursts reflecting the summed action potentials of the postganglionic axons in the recorded BAT nerve bundle (33, 40). Unilateral nanoinjection of the glutamate receptor agonist, NMDA (12 nmol), into the VLM (1.9 mm lateral to the midline, 2.3–2.8 mm ventral to the calamus scriptorius) between 0.8 and 2.6 mm rostral to the calamus scriptorius [corresponding approximately to atlas levels −13.7 to −11.9 from bregma (45)] consistently elicited prompt and complete inhibitions of cold-evoked increases in BAT SNA and lowered BAT temperature, expired CO2, and HR from their cold-evoked levels (Fig. 1A, Table 1). Nanoinjections of NMDA into the VLM between 2.6 and 3.2 mm rostral or between 0.8 mm rostral and 0.4 mm caudal to the calamus scriptorius usually elicited less robust inhibitions of BAT SNA (Fig. 1B), and these regions were considered to represent the rostral and caudal borders of the most responsive VLM region for BAT sympathoinhibition. The most responsive VLM region included sites producing either decreases or increases in MAP (Table 1), corresponding closely to the caudal VLM and the rostral VLM (Fig. 1B). Saline (vehicle) nanoinjections (60 nl) into sites throughout the most responsive BAT sympathoinhibitory region of the VLM or into the intermediate NTS (see below) were without effect (P > 0.05) on the elevated levels of BAT SNA or BAT temperature elicited by skin and core cooling or by any of the treatments described below that stimulated BAT SNA and increased BAT thermogenesis.
Fig. 1.
Effects of activation of neurons in the ventrolateral medulla (VLM) on cold-evoked BAT thermogenesis. A: the stimulation of brown adipose tissue (BAT) sympathetic nerve activity (SNA) (to 467% of the control level at 36.8°C) and BAT thermogenesis by a reduced core temperature (to 35.6°C at trace onset) was reversed by nanoinjection of N-methyl-d-aspartate (NMDA) into the right VLM [BAT SNA to 94% of control, BAT temperature (Temp): −1.1°C from 35.5°C], which also lowered expired (Exp) CO2 and heart rate (HR) and increased mean arterial pressure (MAP). A similar pressor response, elicited by phenylephrine (PE) [intravenous (iv)], reduced HR, but did not change BAT SNA (95% of pre-PE control). Nanoinjection of bicuculline (Bic; 30 pmol) into the right VLM also reversed cooling-stimulated BAT thermogenesis (BAT SNA: to 106% of the control level at 36.8°C; BAT Temp: −3.2°C), as well as Exp CO2 and HR, while increasing MAP. B: the anatomical distribution and relative effects on cold-evoked BAT SNA of NMDA nanoinjection sites in the VLM. Schematic anatomical drawings adapted from Ref. 45. py, pyramidal tract; RPa, raphé pallidus; VII, facial nucleus; bpm, beats/min.
Table 1.
Effects on cold-evoked BAT thermogenic and cardiovascular parameters of nanoinjection of NMDA or of Bic into the VLM or PE iv
| Cold Evoked | NMDA in Rostral VLM | Cold Evoked | NMDA in Caudal VLM | Cold Evoked | PE iv | Cold Evoked | Bic in Rostral VLM | |
|---|---|---|---|---|---|---|---|---|
| n | 10 | 10 | 12 | 12 | 4 | 4 | 5 | 5 |
| BAT SNA, %control | 488 ± 69 | 92 ± 3† | 834 ± 255 | 111 ± 11* | 780 ± 193 | 913 ± 239 | 755 ± 229 | 92 ± 4* |
| BAT Temp, °C | 34.1 ± 0.2 | 33.0 ± 0.2† | 35.1 ± 0.3 | 34.2 ± 0.3† | 34.2 ± 0.6 | 34.5 ± 0.5 | 34.1 ± 0.5 | 31.8 ± 0.3† |
| DBAT Temp, °C | −1.1 ± 0.1 | −0.9 ± 0.2 | +0.3 ± 0.1 | −2.3 ± 0.3 | ||||
| Exp CO2, % | 5.1 ± 0.2 | 4.6 ± 0.2† | 5.2 ± 0.1 | 4.7 ± 0.1† | 5.0 ± 0.4 | 5.2 ± 0.4 | 5.0 ± 0.4 | 4.3 ± 0.3† |
| ΔExp CO2, % | −0.5 ± 0.1 | −0.5 ± 0.1 | +0.2 ± 0.1 | −0.7 ± 0.1 | ||||
| HR, beats/min | 370 ± 13 | 358 ± 15 | 387 ± 15 | 330 ± 16† | 417 ± 18 | 378 ± 13* | 417 ± 19 | 347 ± 10* |
| ΔHR, beats/min | −12 ± 16 | −57 ± 11 | −39 ± 10 | −70 ± 22 | ||||
| MAP, mmHg | 118 ± 4 | 139 ± 4† | 126 ± 6 | 106 ± 6† | 129 ± 4 | 164 ± 6† | 121 ± 7 | 166 ± 8† |
| ΔMAP, mmHg | +21 ± 4 | −20 ± 4 | +35 ± 4 | +45 ± 3 |
Values are means ± SE; n, no. of rats. NMDA, N-methyl-d-aspartate; VLM, ventrolateral medulla; PE, phenylephrine; iv, intravenous; Bic, bicuculline; Δ, change; BAT, brown adipose tissue; SNA, sympathetic nerve activity; Temp, temperature; Exp, expired; HR, heart rate; MAP, mean arterial pressure. Mean parameter values for “Cold Evoked” are the group means of the parameter values during the 32-s interval before the subsequent nanoinjection into VLM or administration of PE. Mean parameter values for “NMDA in VLM” or for “PE iv” are the group means of the parameter values during the 32-s maximum response within 5 min of the nanoinjection or iv injection (or within 10 min for BAT Temp only). Mean parameter values for “Bic in VLM” are the group means of the parameter values during the 32-s maximum response within 10 min of the nanoinjection (or within 15 min for BAT Temp only). Statistically different compared with the immediately preceding cold-evoked value:
P< 0.05,
P< 0.01 (paired t-test).
In subsequent experiments to determine the effectiveness of VLM neuronal activation in inhibiting increases in BAT SNA elicited by stimuli other than cooling, we elected to make nanoinjections of Bic only into the rostral portion of the most responsive VLM region (i.e., ∼2.4 to 2.6 mm rostral to the calamus scriptorius). Since activation of neurons in the rostral VLM produces an increase in AP (Fig. 1, Table 1), likely due to stimulation of the vasoconstrictor sympathetic premotor neurons that reside there (15, 46), we tested whether the activation of the baroreceptor reflex, which would occur with an increase in AP, might influence the level of BAT SNA. Consistent with earlier results (37), injection of PE (10 μg/kg iv), which elicited a pressor response of similar magnitude to that during activation of neurons in the rostral VLM and which reduced HR (Fig. 1, Table 1), did not change BAT SNA from the levels before the PE injection (Fig. 1, Table 1). Disinhibition of local neurons with a unilateral nanoinjection of the GABAA antagonist, Bic (30 pmol), into the VLM also reversed the cold-stimulated increases in BAT SNA, BAT thermogenesis, HR, and expired CO2 (Fig. 1, Table 1), each of which recovered following the waning of the pharmacological actions of Bic.
Disinhibition of neurons in either VLM or intermediate NTS reversed the increases in BAT SNA and BAT temperature in response to PGE2 into the MPO.
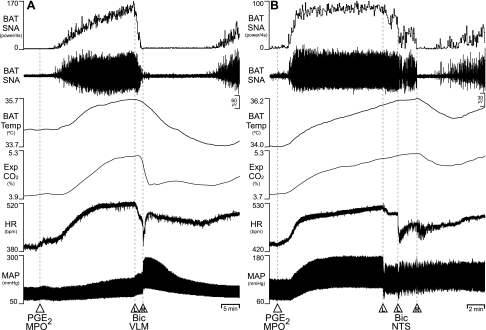
We sought to determine whether neurons in the VLM or in the NTS can inhibit the BAT thermogenic responses to PGE2 into the MPO (32). As illustrated in Fig. 2, nanoinjection of PGE2 (60 pmol) into the MPO increased BAT SNA, BAT temperature, expired CO2, HR, and MAP (Table 2). Subsequent nanoinjections of Bic (30 pmol/site) into the VLM or into the NTS promptly reversed (P < 0.05) the PGE2-evoked increases in BAT SNA, which resulted in marked reductions (P < 0.05) in the elevated levels of BAT temperature and expired CO2. The BAT sympathoinhibitory effects of Bic nanoinjections into the VLM and into the NTS were relatively short-lived, likely due to the sustained stimulation of BAT thermogenesis by nanoinjection of PGE2 into MPO. The levels of HR following Bic nanoinjections into either the VLM or the NTS were not different from those following nanoinjection of PGE2 into MPO (Table 2). Nanoinjection of Bic into the VLM elicited the large pressor responses expected from disinhibition of the vasoconstrictor sympathetic premotor neurons located there (57), and, conversely, nanoinjection of Bic into the NTS produced a modest decrease in MAP, potentially due to activation of baroreceptor reflex pathways within NTS (12) (Table 2).
Fig. 2.
Effects of disinhibition of neurons in the VLM or in the nucleus tractus solitarius (NTS) on BAT thermogenesis evoked by nanoinjection of prostaglandin (PG) E2 into medial preoptic nucleus (MPO). A: nanoinjection of PGE2 into MPO increased BAT SNA (peak: +7,009% of control), BAT Temp, Exp CO2, HR, and MAP. Bilateral nanoinjections of Bic into the VLM (L: left side, R: right side) reversed the increases in BAT SNA (to 212% of pre-Bic control), BAT Temp, and Exp CO2. B: nanoinjection of PGE2 into the MPO increased BAT SNA (peak: +2,164% of control), BAT Temp, Exp CO2, HR, and MAP. Bilateral nanoinjection of Bic into the NTS (the left-side injection was repeated in this example) transiently reversed the increase in BAT SNA (to 50% of pre-Bic control) and began to reduce BAT Temp and Exp CO2, although the effects of Bic in NTS were short-lived, potentially due to the sustained BAT thermogenic stimulatory effect of PGE2 into MPO.
Table 2.
Effects on BAT thermogenic and cardiovascular parameters of nanoinjection of PGE2 into the MPO and of subsequent nanoinjection of Bic into either VLM or NTS
| PGE2 in MPO | Bic in VLM | PGE2 in MPO | Bic in NTS | |
|---|---|---|---|---|
| n | 5 | 5 | 4 | 4 |
| BAT SNA, %control | 5,850 ± 2,347 | 298 ± 80* | 6,986 ± 1,858 | 146 ± 43.5* |
| BAT Temp, °C | 35.1 ± 0.5 | 34.1 ± 0.3* | 36.2 ± 0.7 | 35.3 ± 0.4* |
| ΔBAT Temp, °C | +1.3 ± 0.4 | −1.1 ± 0.2 | +1.8 ± 0.4 | −0.8 ± 0.4 |
| Exp CO2, % | 4.6 ± 0.3 | 4.2 ± 0.3* | 5.0 ± 0.3 | 4.7 ± 0.2* |
| ΔExp CO2, % | +0.8 ± 0.2 | −0.4 ± 0.1 | +1.1 ± 0.2 | −0.4 ± 0.1 |
| HR, beats/min | 494 ± 16 | 458 ± 36 | 495 ± 15 | 422 ± 46 |
| ΔHR, beats/min | +63 ± 20 | −35 ± 22 | +84 ± 21 | −73 ± 34 |
| MAP, mmHg | 103 ± 4.8 | 163 ± 5.6† | 117 ± 5.9 | 105 ± 9.0* |
| ΔMAP, mmHg | +12 ± 4.7 | +59 ± 3.3 | +20 ± 4.1 | −12 ± 3.9 |
| RSNA, %control | 151 ± 18.5 | 211 ± 30* | 132 ± 13.1 | 103 ± 15.9* |
Values are means ± SE; n, no. of rats. PGE2, prostaglandin E2; MPO, medial preoptic area; NTS, nucleus tractus solitarius; RSNA, renal SNA. Mean parameter values for “PGE2 in MPO” are the group means for the parameter values during the 1-min interval before the subsequent Bic nanoinjections into either VLM or NTS, and the corresponding changes for “PGE2 in MPO” are relative to the mean parameter values during the 1 min before nanoinjection of PGE2 into the MPO. Mean parameter values for “Bic in VLM” and “Bic in NTS” are the group means for the parameter values during the 5th min (or 8th min for BAT Temp only) following the Bic nanoinjections. The values for BAT SNA and RSNA are expressed as percentage of pre-“PGE2 in MPO” control levels. For other parameters, the changes for “Bic in VLM” or “Bic in NTS” are relative to the mean “PGE2 in MPO” parameter values (i.e., during the 1 min before nanoinjection of Bic into VLM or NTS). Core (rectal) temperatures were maintained between 36.5 and 37.5°C with a servo-controlled heating lamp. Statistically significant compared with control value following saline into VLM or NTS:
P< 0.05,
P< 0.01 (paired t-test).
Disinhibition of neurons in either VLM or intermediate NTS reversed the BAT thermogenic responses evoked by disinhibition of neurons in DMH.
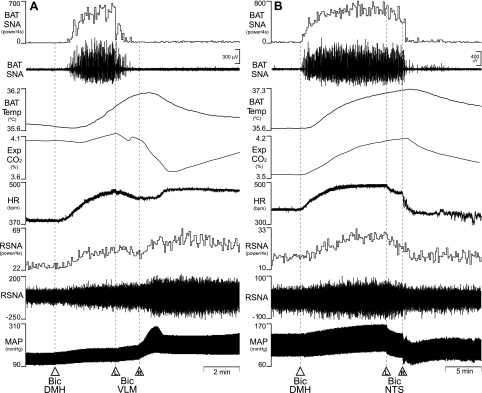
Since neurons in DMH are required for the BAT thermogenic component of the cold defense response to skin cooling (40) and of the febrile response to nanoinjection of PGE2 into the MPO (32, 41, 60), we tested the ability of disinhibitions of neurons in the VLM and in the NTS to inhibit the increases in BAT SNA and BAT thermogenesis evoked by disinhibition of DMH neurons. As illustrated in Fig. 3, nanoinjection of Bic (15 pmol) into the DMH increased BAT SNA, BAT temperature, expired CO2, RSNA, HR, and MAP, paralleling the BAT thermogenic and cardiovascular responses already described following disinhibition of neurons in the DMH (10, 24). Subsequent nanoinjections of Bic into the VLM or into the NTS promptly reversed (P < 0.05) the evoked increases in BAT SNA (Fig. 3, Table 3), as well as those in BAT temperature and expired CO2, although with the slower time course (Fig. 3) expected for metabolic variables. Nanoinjection of Bic into the VLM had no effect on the increase in HR evoked by nanoinjection of Bic into DMH, but produced further increases in RSNA and MAP (Fig. 3A, Table. 3). Nanoinjection of Bic into the NTS reversed not only the increase in HR evoked by nanoinjection of Bic into DMH, but also the accompanying increases in RSNA and MAP (Fig. 3B, Table 3).
Fig. 3.
Effects of nanoinjection of Bic in the VLM or the NTS on BAT thermogenic responses evoked by disinhibition of dorsomedial hypothalamus (DMH) neurons. A: nanoinjection of Bic into DMH increased BAT SNA (peak: +4,799% of control), BAT Temp, Exp CO2, HR, renal SNA (RSNA) (peak: +65% of control), and MAP. Bilateral nanoinjections of Bic into VLM (L and R) reversed the increases in BAT SNA (to 159% of pre-Bic in DMH control), BAT Temp, and Exp CO2, and produced increases in RSNA (to 254% of pre-Bic in DMH control) and MAP, but did not affect the HR increase. B: Nanoinjection of Bic into DMH evoked increases in BAT SNA (peak: +9,622% of control), BAT Temp, Exp CO2, HR, RNSA (peak: +65% of control), and MAP. Bilateral nanoinjections of Bic into the NTS reversed the increases in BAT SNA (to 126% of pre-Bic in DMH control) and those in BAT Temp, Exp CO2, HR, RSNA (to 102% of pre-Bic in DMH control), and MAP.
Table 3.
Effects on BAT thermogenic and cardiovascular parameters of nanoinjection of Bic into the DMH and of subsequent nanoinjection of Bic into either VLM or NTS
| Bic in DMH | Bic in VLM | Bic in DMH | Bic in NTS | |
|---|---|---|---|---|
| n | 6 | 6 | 7 | 7 |
| BAT SNA, %control | 5,097 ± 1,131 | 184 ± 16* | 6,016 ± 1,272 | 121 ± 28.3† |
| BAT Temp, °C | 36.1 ± 0.3 | 35.6 ± 0.3 | 35.8 ± 0.5 | 35.0 ± 0.4 |
| ΔBAT Temp, °C | +0.8 ± 0.2 | −0.5 ± 0.1* | +1.3 ± 0.2 | −0.7 ± 0.2† |
| Exp CO2, % | 4.2 ± 0.1 | 3.8 ± 0.0 | 4.4 ± 0.1 | 4.2 ± 0.1 |
| ΔExp CO2, % | +0.4 ± 0.1 | −0.4 ± 0.1* | +0.5 ± 0.1 | −0.2 ± 0.1† |
| HR, beats/min | 465 ± 16 | 458 ± 18 | 467 ± 13 | 357 ± 29 |
| ΔHR, beats/min | +70 ± 8 | −7 ± 15 | +68 ± 14 | −110 ± 28† |
| MAP, mmHg | 131 ± 4.5 | 186 ± 6.7 | 128 ± 5.4 | 98 ± 7.3 |
| ΔMAP, mmHg | +24 ± 10.2 | +55 ± 5.3* | +16 ± 2.5 | −30 ± 2.6† |
| RSNA, %control | 161 ± 15.6 | 242 ± 36* | 175 ± 11.2 | 94 ± 3.9† |
Values are means ± SE; n, no. of rats. DMH, dorsomedial hypothalamus. Mean parameter values for “Bic in DMH” are the group means for the parameter values during the 1-min interval before the subsequent Bic nanoinjections into either VLM or NTS, and the corresponding changes for “Bic in DMH” are relative to the mean parameter values during the 1 min before nanoinjection of Bic into the DMH. Mean parameter values for “Bic in VLM” and “Bic in NTS” are the group means for the parameter values during the 5th min (or 8th min for BAT Temp only) following nanoinjections of Bic into the VLM or NTS. The values for BAT SNA and RSNA are expressed as percentage of pre-“Bic in DMH” control levels. For other parameters, the changes for “Bic in VLM” or “Bic in NTS” are relative to the mean “Bic in DMH” parameter values (i.e., during the 1 min before nanoinjections of Bic into VLM or NTS). Core (rectal) temperatures were maintained between 36.5 and 37.5°C with a servo-controlled heating lamp. Statistically significant compared with control value following saline into VLM or NTS:
P< 0.05;
P< 0.01 (paired t-test).
Disinhibition of neurons in VLM or in NTS reversed the increases in BAT SNA and BAT thermogenesis induced by disinhibition of neurons in rRPa.
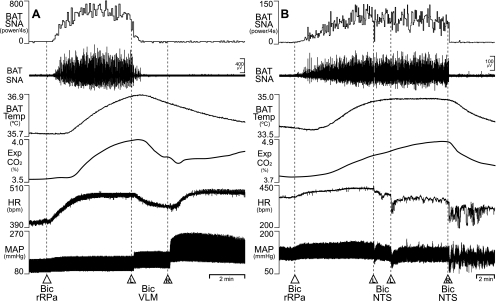
Since neurons in rRPa are required for the increase in BAT thermogenesis during the cold-defense response to skin cooling (40) and during the febrile response to nanoinjection of PGE2 into the MPO (31, 39), we tested the ability of disinhibitions of neurons in the VLM and in the NTS to inhibit the BAT thermogenesis evoked by disinhibition of neurons in rRPa. We reasoned that, if the inhibition elicited from VLM or from NTS is mediated by GABAA receptors in rRPa, activation of these inhibitions should fail to reduce the increase in BAT thermogenesis elicited by blockade of GABAA receptors in the rRPa. Nanoinjection of Bic (15 pmol) into the rRPa increased BAT SNA, BAT temperature, expired CO2, HR, and MAP (Fig. 4, Table 4), and subsequent Bic nanoinjections into either the VLM or into the NTS promptly reversed the responses evoked by disinhibition of rRPa neurons (Fig. 4; Table 4). Nanoinjection of Bic into the VLM reduced (P < 0.05) the increase in HR evoked by nanoinjection of Bic into rRPa (Table 4), but produced further increases in MAP (Fig. 4A, Table 4). Nanoinjection of Bic into the NTS reduced (P < 0.05) the increases in both HR and MAP evoked by nanoinjection of Bic into rRPa (Fig. 4B, Table 4).
Fig. 4.
Effects of nanoinjections of Bic into the VLM or into the intermediate NTS on BAT thermogenesis evoked by disinhibition of neurons in the rostral raphe pallidus (rRPa). A: nanoinjection of Bic into rRPa increased BAT SNA (peak: +5,186% of control), BAT Temp, Exp CO2, HR, and MAP. Bilateral nanoinjections of Bic into VLM (L and R) reversed the increases in BAT SNA (to 61% of pre-Bic in rRPa control), BAT Temp, and Exp CO2, while increasing MAP. HR was transiently reduced after the nanoinjection of Bic into the L VLM, but this was reversed following R VLM nanoinjection of Bic. B: nanoinjection of Bic into rRPa increased BAT SNA (peak: +8,457% of control), BAT Temp, Exp CO2, MAP, and HR. Bilateral nanoinjections of Bic into NTS (L nanoinjection was repeated in this example) reduced BAT SNA (to 121% of pre-Bic in rRPa control), BAT Temp, Exp CO2, HR, and MAP.
Table 4.
Effects on BAT thermogenic and cardiovascular parameters of nanoinjection of Bic in rRPa and of subsequent disinhibition of neurons in either VLM or NTS
| Bic in RPa | Bic in VLM | Bic in RPa | Bic in NTS | |
|---|---|---|---|---|
| n | 6 | 6 | 6 | 6 |
| BAT SNA, %control | 4,919 ± 826 | 364 ± 33† | 6,993 ± 1,478 | 189 ± 26† |
| BAT Temp, °C | 35.7 ± 0.4 | 35.1 ± 0.4 | 35.5 ± 0.4 | 34.7 ± 0.4 |
| ΔBAT Temp, °C | +1.1 ± 0.2 | −0.6 ± 0.2* | +1.3 ± 0.2 | −0.8 ± 0.2† |
| Exp CO2, % | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.6 ± 0.1 | 4.4 ± 0.1 |
| ΔExp CO2, % | +0.3 ± 0.0 | −0.1 ± 0.1* | +0.5 ± 0.1 | −0.2 ± 0.0† |
| HR, beats/min | 483 ± 19 | 455 ± 17 | 463 ± 14 | 359 ± 30 |
| ΔHR, beats/min | +51 ± 11 | −80 ± 22* | +28 ± 10 | −104 ± 21† |
| MAP, mmHg | 97 ± 6.7 | 161 ± 9.2 | 109 ± 10.8 | 102 ± 9.8 |
| ΔMAP, mmHg | +10 ± 3.2 | +64 ± 9.1† | +7 ± 2.6 | −7 ± 2.8* |
Values are means ± SE; n, no. of rats. rRPa, rostral raphe pallidus. Mean parameter values for “Bic in rRPa” are group means of the parameter values during the 1-min interval before the subsequent Bic nanoinjections into either VLM or NTS, and the corresponding changes for “Bic in rRPa” are relative to the mean parameter values during the 1 min before nanoinjection of “Bic into the rRPa”. Mean parameter values for “Bic in VLM” or “Bic in NTS” are group means of the parameter values during the 5th min (or 8th min for BAT Temp only) following nanoinjections of Bic into the VLM or NTS. The values for BAT SNA are expressed as percentage of pre-“Bic in rRPa” control levels. For other parameters, the changes for “Bic in VLM” or “Bic in NTS” are relative to the mean “Bic in rRPa” parameter values (i.e., during the 1 min before nanoinjections of Bic into VLM or NTS). Core (rectal) temperatures were maintained between 36.5 and 37.5°C with a servo-controlled heating lamp. Statistically significant compared with control value following saline into VLM or NTS:
P< 0.05;
P< 0.01 (paired t-test).
Disinhibition of neurons in the VLM reversed the fentanyl-evoked increases in BAT SNA and BAT temperature.
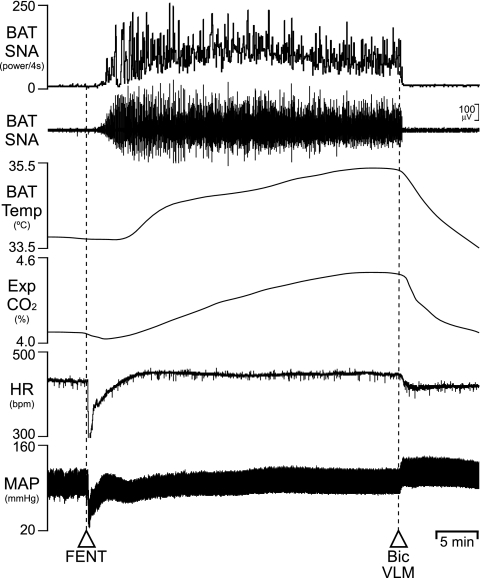
Intravenous fentanyl increases BAT SNA and BAT temperature (11). Here, we tested the potential for activation of neurons in the VLM to affect the fentanyl-evoked BAT thermogenic responses. Injection of fentanyl (100 μg/kg iv) increased BAT SNA, BAT temperature, and expired CO2 (Fig. 5). Subsequent unilateral nanoinjection of Bic (30 pmol) into the VLM promptly reversed the fentanyl-induced BAT thermogenic responses. In five animals, fentanyl produced a significant (P < 0.05) mean peak increase in BAT SNA of +3,256 ± 243% of control, which increased BAT temperature by +1.2 ± 0.2°C (P < 0.05) and expired CO2 by + 0.3 ± 0.1% (P < 0.05). These were accompanied by a tachycardia of 41 ± 12 beats/min (P < 0.05) and a pressor response of +11 ± 3 mmHg (P < 0.05). Nanoinjection of Bic into the VLM resulted in a fall (P < 0.05) in BAT SNA to +311 ± 287% of control, which decreased BAT temperature by −0.9 ± 0.3°C (P < 0.05) and expired CO2 by −0.9 ± 0.3°C (P < 0.05) from the peak levels evoked by fentanyl.
Fig. 5.
Effects of disinhibition of neurons in the VLM on the increases in BAT thermogenic variables evoked by fentanyl (FENT). FENT evoked an increase in BAT SNA (peak: +2,561% of control), BAT Temp, Exp CO2, and HR. Nanoinjection of Bic into the L VLM reversed these increases.
Disinhibition of neurons in the VLM reversed the increases in BAT SNA and BAT thermogenesis elicited by pontomedullary transection.
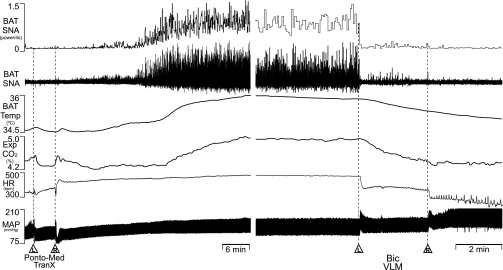
Pontomedullary, but not postmammillary, transections of the neuraxis increase BAT temperature and core temperature (48, 51), consistent with the existence of a tonically active inhibition of BAT thermogenesis located in the pontine retrorubral field (52). We sought to establish that pontomedullary transections increase BAT thermogenesis through stimulation of BAT SNA and to determine if the putative BAT sympathoinhibitory mechanism in the VLM would influence the activation of BAT SNA elicited by pontomedullary transection. Vertical transections of the neuraxis, confirmed at the end of each experiment to lie between −7 to −9 mm caudal to bregma and to include the region within 3 mm on each side of the midline, increased BAT SNA, BAT temperature, expired CO2, HR, and MAP (Fig. 6, Table 5). Unilateral nanoinjection of Bic into the VLM reduced the transection-evoked increases in BAT SNA, BAT temperature, expired CO2, and HR (Fig. 6, Table 5). Bilateral nanoinjections of Bic into VLM elicited a large pressor response (Fig. 6, Table 5).
Fig. 6.
Effects of nanoinjections of Bic into the VLM on BAT thermogenesis elicited by pontomedullary transection (Ponto-Med TranX). Ponto-Med TranX (L and R) evoked an increase in BAT SNA (peak: +1,318% of intact control), BAT Temp, Exp CO2, HR, and MAP. Bilateral nanoinjections of Bic into the VLM reversed the increases in BAT SNA (to 109% of pretransection control), BAT Temp, Exp CO2, and HR and increased MAP.
Table 5.
Effects on sympathetic BAT thermogenic and cardiovascular parameters of Ponto-Med TranX and of subsequent nanoinjection of Bic into VLM
| Intact Control | Ponto-Med TranX | Bic in VLM | |
|---|---|---|---|
| n | 6 | 6 | 6 |
| BAT SNA, %control | 100 | 776 ± 197* | 162 ± 39‡ |
| BAT Temp, °C | 34.1 ± 0.5 | 36.2 ± 0.4† | 35.0 ± 0.3§ |
| ΔBAT Temp, °C | +2.0 ± 0.3 | −1.2 ± 0.2 | |
| Exp CO2, % | 3.8 ± 0.4 | 4.3 ± 0.4* | 3.8 ± 0.4‡ |
| ΔExp CO2, % | +0.5 ± 0.1 | −0.5 ± 0.1 | |
| HR, beats/min | 424 ± 26 | 495 ± 14† | 360 ± 47‡ |
| ΔHR, beats/min | +71 ± 18 | −135 ± 43 | |
| MAP, mmHg | 94 ± 4 | 111 ± 5* | 145 ± 15§ |
| ΔMAP, mmHg | +16 ± 5 | +34 ± 7 |
Values are means ± SE; n, no. of rats. Ponto-Med TranX, pontomedullary transection. The values for BAT SNA are expressed as percentage of intact, pretransection control levels. Mean parameter values for “Ponto-Med TranX” are the group means of the parameter values during the 1-min interval representing the peak response within 15 min of completing the transection, and the corresponding changes for “Ponto-Med TranX” are relative to the mean control parameter values during a 1-min interval just before transection. Mean parameter values for “Bic in VLM” are the group means of the parameter values during the 1-min interval representing the peak response within 5 min of the nanoinjection of Bic into VLM, and the corresponding changes for “Bic in VLM” are relative to the “Ponto-Med TranX” parameter values. Core (rectal) temperatures were maintained between 36.5 and 37.5°C with a servo-controlled heating lamp. Statistically significant compared with intact control value:
P< 0.05,
P< 0.01; and from “Ponto-Med TranX” value:
P< 0.05;
P< 0.01.
Simultaneous inhibition of neurons in the VLM and in the NTS increases BAT SNA and BAT thermogenesis.
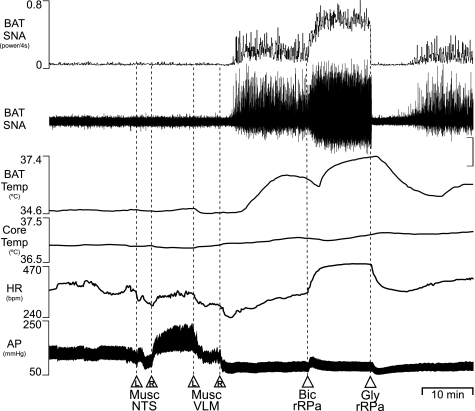
Inhibition of neurons in the intermediate NTS with bilateral nanoinjections of Musc (120 pmol/side) did not change BAT SNA or BAT temperature, but markedly increased MAP (Fig. 7), consistent with an interruption of the baroreceptor reflex afferent pathway. Subsequent bilateral nanoinjections of Musc into the VLM (120 pmol/side) not only decreased AP (Fig. 7, Table 6, P < 0.01), consistent with inhibition of vasoconstrictor sympathetic premotor neurons, but also produced a significant increase in BAT SNA, resulting in a rise in BAT temperature (Fig. 7, Table 6, P < 0.01). Although inhibition of cutaneous vasoconstrictor sympathetic premotor neurons in the rostral VLM would have increased heat loss, core body temperature never fell below 36.5°C due to servo-controlled external heating and, as in the example in Fig. 7, was often slightly increased, likely due to the contribution of elevated BAT thermogenesis. To determine whether the NTS and the VLM constitute the principal sources of GABAA-receptor-mediated inhibition of putative BAT sympathetic premotor neurons in the rRPa, we determined the effect of GABAA receptor blockade in the rRPa following combined inhibition of neurons in the NTS and in the VLM. Nanoinjection of Bic (15 pmol) into rRPa, following nanoinjections of Musc into NTS and VLM, increased HR and resulted in a further increase (P < 0.05) in BAT SNA (to 341 ± 136% of that following combined inhibition of neurons in NTS and VLM) and a rise in BAT temperature (Fig. 7, Table 6). To determine whether the activity of neurons in rRPa is required for the increase in BAT SNA arising from inhibition of neurons in NTS and VLM, we determined the effect of inhibition of neurons in the rRPa subsequent to the inhibition of neurons in NTS and VLM and nanoinjection of Bic into rRPa (Fig. 7). Nanoinjection of glycine (30 nmol) into rRPa completely inhibited BAT SNA (to 33 ± 14% of the control level before nanoinjection of Musc into NTS and VLM) (Fig. 7, Table 6), suggesting that there was no component of the increased BAT SNA that was independent of the activity of rRPa neurons. In two additional rats, reversing the order of inhibition of neurons in NTS and in VLM had no effect on the marked increase in BAT SNA (data not shown), suggesting that the BAT sympathoexcitatory response to combined inhibition of neurons in the NTS and VLM does not result from a serial interaction between putative BAT sympathoinhibitory neurons in the NTS and in the VLM.
Fig. 7.
Combined inhibition of neurons in the NTS and in the VLM increases BAT thermogenesis. Bilateral nanoinjections of muscimol (Musc) in the intermediate NTS increased MAP without changing BAT SNA or BAT Temp. Subsequent nanoinjections of Musc into the rostral VLM, which reduced MAP, initiated an increase in BAT SNA (peak: +530% of the control value before nanoinjection of Musc into the NTS). Subsequent nanoinjection of Bic into the rRPa increased BAT SNA (+1,292% of control), BAT Temp, and HR. Subsequent inhibition of neurons in the rRPa with nanoinjection of glycine (Gly) completely reversed these increases in BAT SNA (to 95% of control). Vertical scale bar is 20 μV for BAT SNA. AP, arterial pressure.
Table 6.
Effects on sympathetic BAT thermogenic and cardiovascular parameters of combined inhibition of neurons in rostral VLM and in NTS, of subsequent disinhibition of neurons in rRPa and of subsequent inhibition of neurons in rRPa
| Control | Musc in NTS and VLM | Bic in rRPa | Gly in rRPa | |
|---|---|---|---|---|
| n | 14 | 14 | 8 | 11 |
| BAT SNA, %control | 100 | 4,001 ± 1,035† | 12,090 ± 2,645‡ | 67 ± 14§ |
| BAT Temp, °C | 33.6 ± 0.2 | 34.0 ± 0.3† | 34.6 ± 0.3‡ | 33.8 ± 0.2§ |
| ΔBAT Temp, °C | +0.5 ± 0.1 | +0.8 ± 0.1 | −1.0 ± 0.2 | |
| Exp CO2, % | 4.0 ± 0.1 | 3.7 ± 0.2† | 4.0 ± 0.2‡ | 3.6 ± 0.2§ |
| ΔExp CO2, % | −0.3 ± 0.1 | +0.3 ± 0.1 | −0.4 ± 0.1 | |
| HR, beats/min | 463 ± 8 | 400 ± 18† | 463 ± 12‡ | 375 ± 21§ |
| ΔHR, beats/min | −64 ± 13 | +61 ± 16 | −94 ± 17 | |
| MAP, mmHg | 80 ± 6 | 46 ± 3† | 44 ± 4 | 37 ± 3§ |
| ΔMAP, mmHg | −35 ± 5 | +1 ± 1 | −5 ± 1 | |
| RSNA, %control | 54 ± 5.8† | 53 ± 5.8 | 48 ± 3.7 |
Values are means ± SE; n, no. of rats. Musc, muscimol; Gly, glycine. The values for BAT SNA and RSNA are expressed as percentage of pre-“Musc in NTS and VLM” control levels. Mean parameter values for “Musc in NTS and VLM” are the group means of the parameter values during the 1-min interval representing the peak response within 15 min of the final Musc nanoinjection, and the corresponding changes for “Musc in NTS and VLM” are relative to the mean parameter values during the 1 min before the first nanoinjection of Musc into NTS. Mean parameter values for “Bic in rRPa” and for “Gly in rRPa” are the group means of the parameter values during the 1-min interval representing the peak response within 5 min of the nanoinjection of Bic into rRPa and of Gly into rRPa, respectively, and the corresponding changes for “Bic in rRPa” and for “Gly into rRPa” are relative to the “Musc in NTS and VLM” and “Bic in rRPa” parameter values, respectively. Core (rectal) temperatures were maintained between 36.5 and 37.5°C with a servo-controlled heating lamp. Statistically significant compared with control value following saline into NTS and VLM:
P< 0.01 (paired t-test); from “Musc in NTS and VLM” value:
P< 0.01; and from “Bic in rRPa” value:
P< 0.01.
Histological localization of nanoinjection sites.
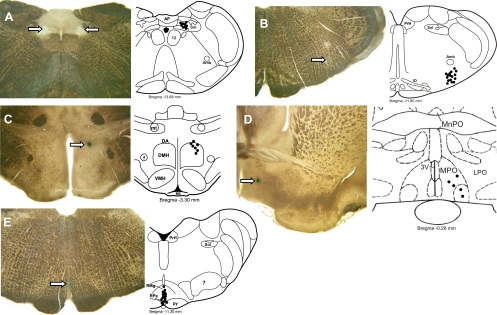
As shown in the representative histological sections containing dye deposits (Fig. 8), the micropipette tip positions indicating the approximate centers of the nanoinjection sites were within the targeted regions. Figure 8A illustrates recovered Bic and Musc nanoinjection sites (n = 9) in the medial NTS. The Bic and Musc nanoinjection sites in the rostral VLM (Fig. 8B, n = 16) were caudal to the facial nucleus and ventral to the nucleus ambiguus, in the region containing sympathetic premotor neurons, whose activity is essential for the maintenance of normal vasoconstrictor sympathetic tone and AP (7, 15, 46). The Bic nanoinjection sites in the DMH (Fig. 8C, n = 7) were localized in the dorsal aspect of the DMH and in the dorsal hypothalamic area, rostral to the compact division of the DMH. These sites were within the region of DMH from which activation of BAT, renal, and cardiac sympathetic activities can be elicited (10, 11, 18, 24, 61), which contains neurons necessary for the BAT thermogenic responses to application of PGE2 into the MPO (32, 60), and which contains neurons with direct projections to the rRPa (41, 49) and the VLM (18). The PGE2 nanoinjection sites in the MPO (Fig. 8D, n = 5) were similar to those in our laboratory's previous studies at which nanoinjection of PGE2 produced increases in BAT thermogenesis (31, 32, 39, 41), to those containing EP3 receptor immunoreactivity (39, 41) and to those with neurons projecting to DMH and to rRPa (39, 41, 42). The Bic and glycine nanoinjection sites in the rRPa (Fig. 8E; n = 16) were in the ventral midline medulla at a level of the rRPa extending from the rostral VLM to the middle of the facial motor nucleus and correspond to the locations of putative sympathetic premotor neurons controlling thermogenesis in BAT (9, 37).
Fig. 8.
Histological localization of nanoinjection sites in NTS, VLM, RPa, DMH, and MPO. Left panels: histological sections with dye deposits (arrows) marking typical nanoinjection sites in the medial NTS (A), the VLM (B), the rRPa (C), the DMH (D), and the MPO (E). Right panels: solid circles indicate the centers of nanoinjection sites plotted on atlas drawings (adapted from Ref. 43). A: right-side muscimol or Bic nanoinjection sites made into the medial NTS in 9 rats at atlas level bregma −13.68 mm. B: right-side muscimol or Bic nanoinjection sites made into the VLM of 16 rats at atlas level bregma −11.80 mm. C: centers of the muscimol or Bic nanoinjections made into the rRPa of 6 rats plotted on a drawing of the brainstem at bregma −11.30 mm. D: Bic nanoinjection sites made into the right DMH of 7 rats at atlas level bregma −3.30 mm. E: PGE2 nanoinjection sites made into the right MPO of 5 rats at atlas level bregma −0.26 mm. Amb, nucleus ambiguus; DA, DMH area; f, fornix; IO, inferior olivary nucleus; LPO, lateral preoptic area; ME, median eminence; MnPO, median preoptic nucleus; mt, mamillothalamic tract; PrH, nucleus prepositus hypoglossal; RMg, nucleus raphe magnus; Sol, nucleus of the solitary tract; VMH, ventromedial hypothalamic nucleus; 3V, third ventricle; 4V, fourth ventricle; 7, facial motor nucleus.
DISCUSSION
The major finding of this study is that a population of neurons in the VLM can exert a potent inhibitory influence on the sympathetic outflow to BAT, capable of reversing the increases in BAT SNA and BAT thermogenesis induced during cold defense or during the febrile response. This inhibition of the sympathetic outflow to BAT and of BAT energy expenditure and thermogenesis can be elicited not only by activation of neurons in the VLM with local application of the glutamate receptor agonist, NMDA, but also by disinhibition of VLM neurons with the local application of the GABAA receptor antagonist, bicuculline. The latter is consistent with these BAT sympathoinhibitory neurons in the VLM being regulated by a GABAA-receptor-mediated inhibitory input that is tonically active under the conditions of our experiments. Despite the tonically active inhibitory input to the BAT sympathoinhibitory neurons in the VLM, they, nonetheless, do provide a moderate level of inhibition of BAT sympathetic outflow and BAT thermogenesis under the conditions of our experiment, as shown by the increase in BAT SNA and BAT temperature following inhibition of neuronal activity in the VLM. The sources of the excitatory inputs that drive the BAT sympathoinhibitory neurons in the VLM under basal conditions and during local application of Bic remain unknown. These studies also reveal another source of tonically active BAT sympathoinhibition located in the medial NTS, whose activation is equally capable to that of VLM neurons of reversing the excitations of BAT sympathetic outflow during pyrogenic stimulation of the MPO or in response to disinhibitions of the DMH or the rRPa. A hypoxia-driven inhibition of BAT SNA and BAT thermogenesis, mediated by neurons in the commissural NTS (comm-NTS), has been described (33), but the relationship between the medial NTS inhibitory area studied here and the inhibitory effect of hypoxia on BAT thermogenesis remains to be determined.
The VLM has received considerable attention in the regulation of autonomic function following the demonstration that the rostral VLM is the primary source of the excitatory sympathetic premotor input to vasoconstrictor and cardiac sympathetic preganglionic neurons that maintains the basal cardiovascular sympathetic tone supporting arterial blood pressure, and that neurons in the caudal VLM mediate both a baroreceptor-mediated (phasic) and a non-baroreceptor-mediated (tonic) GABAergic inhibition of the cardiovascular sympathetic premotor neurons in the rostral VLM. Thus the large increases in AP consistently observed in our experiments following Bic administration into the rostral VLM arose from the disinhibition of vasoconstrictor sympathetic premotor neurons located there and, conversely, the marked fall in AP seen with Musc injections into the rostral VLM can be attributed to the silencing of local vasoconstrictor sympathetic premotor neurons, causing a widespread vasodilation and fall in peripheral resistance. The BAT sympathoinhibitory effects elicited from activation of VLM neurons do not, however, arise from baroreceptor reflex activation during the simultaneously evoked cardiovascular changes since 1) BAT SNA is unaffected by the baroreceptor reflex activation during large pressor responses, such as those evoked by systemic PE administration [Fig. 1A, (35)]; 2) NMDA injections into the caudal VLM, which produced depressor responses, were equally effective in eliciting BAT sympathoinhibition as identical NMDA injections into the rostral VLM, which were accompanied by pressor responses; and 3) when viewed on an expanded time scale, it is clear that the inhibitions of BAT SNA elicited by NMDA and Bic injections into VLM were nearly complete before the onset of simultaneously evoked changes in AP. In addition, the increases in BAT SNA and BAT temperature elicited by inhibition of neurons in the VLM and in the NTS are unlikely to have been due to a fall in core temperature that might have been expected to accompany the inhibition of cutaneous vasoconstrictor sympathetic premotor neurons in the rostral VLM since 1) core temperature was prevented from falling below 36.5°C by the use of servo-controlled external heating (see methods); 2) in experiments in which inhibition of neurons in the rostral VLM preceded inhibition of those in the NTS, there was no increase in BAT SNA until after the injections of Musc into the NTS; and 3) there was often a slight increase in core temperature following combined inhibition of neurons in the rostral VLM and the NTS, likely due to the strong stimulation of BAT thermogenesis. Overall, these results suggest that the BAT sympathoinhibitory mechanisms in the VLM and in the NTS are independent of the local populations of VLM and NTS neurons involved in cardiovascular regulation.
Our results provide only modest information on the pathway through which neurons in the VLM inhibit BAT sympathetic outflow. That disinhibition of VLM neurons inhibits the activation of BAT SNA following transections of the neuraxis at the pontomedullary junction provides strong evidence supporting the proposal that the BAT sympathoinhibition elicited from neurons in the VLM occurs at a site in the neural pathway driving BAT thermogenesis (36) that is caudal to the pontomedullary transections. The BAT sympathetic premotor neurons in the rRPa are a potential medullary locus of the BAT sympathoinhibition evoked from the VLM, since they are potently regulated by GABAergic inhibitory inputs (37). The sources of the GABAergic inputs to rRPa remain to be identified. The effectiveness of Bic in the VLM in inhibiting the potent activation of BAT SNA following Bic in rRPa suggests, however, that, if there is a direct inhibitory input to rRPa from VLM, it is unlikely to be GABAergic. In this regard, it is noteworthy that glycine injections into rRPa were effective in completely reversing the increases in BAT SNA arising from Bic injections into rRPa. That pontomedullary transections are effective in increasing BAT SNA in a manner dependent on the activity of putative BAT sympathetic premotor neurons in rRPa indicates the existence of a medullary source of excitatory input to BAT sympathetic premotor neurons. Although their location remains unknown, such excitatory inputs to BAT sympathetic premotor neurons could also represent a target for VLM inhibitory inputs to reduce BAT SNA.
Regarding spinal sites for the inhibitory action of VLM neurons to reduce BAT SNA, GABAergic and glycinergic neurons with spinal autonomic projections have been identified throughout the rostral ventral medulla (54–56): those in the VLM could directly inhibit excitatory neurotransmission through BAT spinal sympathetic circuits, or those more medially located in the raphe could be driven by VLM neurons to indirectly effect a similar spinal inhibition of BAT sympathetic outflow. Alternatively, spinal GABAergic interneurons in the central region of the spinal cord (16) could be excited by descending inputs from the VLM to provide a substrate for spinal inhibition of BAT sympathetic preganglionic neurons. A spinal locus for the inhibition of BAT sympathetic outflow by VLM neurons would be consistent with all of the data presented here, particularly the capacity of VLM neurons to inhibit the potent BAT sympathetic discharge arising from blockade of GABAA receptors in rRPa. Interestingly, if the VLM-mediated inhibition of BAT SNA is mediated at a spinal site, the finding that simultaneous inhibition of neurons in VLM and in NTS can elicit increases in BAT SNA could suggest that BAT premotor neurons maintain some level of tonic activity (i.e., to excite spinal circuits for BAT SNA) under thermal conditions when BAT SNA is not apparent.
The physiological role of the BAT sympathoinhibitory mechanism in the VLM in body temperature regulation and metabolic homeostasis, as well as the sources of the excitatory and inhibitory inputs to the VLM that normally regulate the level of its BAT inhibitory influence remain to be identified. The finding that Bic into VLM was effective in reversing the activation of BAT SNA induced by pontomedullary transection would be consistent with medullary and/or spinal sources of both a tonic inhibition to the BAT sympathoinhibitory neurons in the VLM that would be relieved by Bic injection and an excitatory input that would account for their neuronal activity following disinhibition with Bic injections. The caveat to these conclusions is that the pontomedullary transections, although extensive, were not complete, but rather they were made through areas surrounding the midline that contain the fibers whose section elicits a rise in BAT SNA. Thus it is possible that inhibitory and/or excitatory inputs from structures rostral to BAT sympathoinhibitory neurons in the VLM may have remained intact in those lateral portions of the brainstem.
Nanoinjection of Bic into the intermediate NTS was as effective as nanoinjection of Bic into the VLM in inhibiting the increases in BAT SNA and BAT thermogenesis evoked by PGE2 into MPO or by nanoinjection of Bic into either DMH or rRPa. Thus BAT sympathoinhibitory neurons in the intermediate NTS are under a tonically active, GABAergic inhibition, but, because inhibition of neurons in the intermediate NTS could contribute, along with simultaneous inhibition of VLM neuronal activity, to an increase in BAT SNA and BAT thermogenesis, we conclude, as with the BAT sympathoinhibitory neurons in the VLM, that those in the intermediate NTS have a level of activity under the conditions of our experiments that allows them to exert a modest inhibitory regulation of BAT sympathetic outflow and BAT energy expenditure.
The mechanism through which disinhibition of neurons in intermediate NTS produces an inhibition of BAT SNA remains unclear. As discussed above for the BAT sympathoinhibitory neurons in the VLM, the spinal cord is a potential site at which NTS neurons could act to inhibit BAT SNA, since NTS neurons project to the medial and intermediolateral regions of the thoracic spinal cord (2, 28, 38). Since the effect on BAT SNA of activating intermediate NTS neurons was not tested following pontomedullary transection, no conclusions can be drawn regarding the role of supramedullary pathways in the BAT inhibition evoked from the intermediate NTS.
Of interest is the recent description of the potent inhibition of BAT SNA that arises from activation of the peripheral chemoreceptor reflex pathway (34) and that is mimicked by nanoinjection of Bic into the comm-NTS, the principal site of second-order sensory neurons for the peripheral chemoreceptor reflex (50), located immediately caudal and medial to the intermediate NTS sites at which Bic was nanoinjected in the present study. Indeed, as in the present study involving the intermediate NTS, nanoinjection of Bic into the comm-NTS elicits a complete inhibition of the increases in BAT SNA evoked by cooling, by injection of Bic into the rRPa and by injection of PGE2 into the MPO (34). Since carotid body afferents project not only into the comm-NTS, but also into the medial and lateral NTS (17), and glutamate receptor antagonism in the lateral NTS blocks the bradycardia and attenuates the pressor response due to chemoreceptor reflex activation (21), we cannot rule out the possibility that neurons in the intermediate NTS, as with those in the comm-NTS, are involved in the arterial chemoreceptor reflex pathway, leading to inhibition of BAT SNA in the face of a reduced oxygen availability. An incidental observation made in the course of the present studies argues for a BAT sympathoinhibitory effect of neurons in the intermediate NTS that is distinct from that of the arterial chemoreceptor reflex pathway in the comm-NTS: following bilateral injections of Musc into the VLM, injection of Musc into the comm-NTS did not elicit an increase in BAT SNA; however, subsequent bilateral injections of Musc into the intermediate NTS resulted in a prompt and large activation of BAT SNA (personal observation).
Two observations in the present study rule out the possibility that the BAT sympathoinhibitions separately evoked from the VLM and the NTS occur due to a serial connection between neurons in these two areas. First, a large increase in BAT SNA was evoked following inhibition of neuronal activity in both the VLM and the intermediate NTS, but not by inhibition of either site separately. A serial connection would imply a dependence of the response evoked from the “distal” site on the activity of neurons in the site “proximal” to the BAT sympathetic outflow and suggest that inhibition of the site “proximal” to BAT sympathetic outflow would be sufficient to activate BAT SNA. Second, the ability to generate increases in BAT SNA was independent of the order of injection of Musc into VLM and intermediate NTS.
In conclusion, these studies reveal two novel sources, in the VLM and in the intermediate NTS, of the inhibitory control of BAT sympathetic outflow. These results contribute to our understanding of the neural circuits subserving the homeostatic regulation of body temperature, energy stores, and metabolism through adjustments in the thermogenesis and energy expenditure of BAT. This information may be useful in the identification of novel therapeutic approaches to regulating BAT energy consumption in a variety of disease states characterized by disruption of body temperature regulation or metabolic homeostasis.
GRANTS
This research was supported by National Institutes of Health grants DK57838 (S. F. Morrison), NS40987 (S. F. Morrison), and DK082558 (C. J. Madden).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull 51: 107–110, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Amendt K, Czachurski J, Dembowsky K, Seller H. Bulbospinal projections to the intermediolateral cell column; a neuroanatomical study. J Auton Nerv Syst 1: 103–117, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, Festuccia WT, Hirabara SM, Ropelle E, Curi R, Carvalheira JB, Vercesi AE, Velloso LA. Hypothalamic actions of tumor necrosis factor α provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology 151: 683–694, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DL, Guyenet PG. Cardiovascular neurons of brain stem with projections to spinal cord. Am J Physiol Regul Integr Comp Physiol 247: R1009–R1016, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cao WH, Morrison SF. Brown adipose tissue thermogenesis contributes to fentanyl-evoked hyperthermia. Am J Physiol Regul Integr Comp Physiol 288: R723–R732, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Catelli JM, Giakas WJ, Sved AF. GABAergic mechanisms in nucleus tractus solitarius alter blood pressure and vasopressin release. Brain Res 403: 279–289, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Cravo SL, Morrison SF, Reis DJ. Differentiation of two cardiovascular regions within the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 261: R985–R994, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci 25: 1063–1070, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol 280: H2891–H2901, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fyda DM, Cooper KE, Veale WL. Nucleus tractus solitarii lesions alter the metabolic and hyperthermic response to central prostaglandin E1 in the rat. J Physiol 442: 337–349, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon FJ. Effect of nucleus tractus solitarius lesions on fever produced by interleukin-1 beta. Auton Neurosci 85: 102–110, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Haibara AS, Bonagamba LG, Machado BH. Sympathoexcitatory neurotransmission of the chemoreflex in the NTS of awake rats. Am J Physiol Regul Integr Comp Physiol 276: R69–R80, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res 1117: 118–124, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol 287: R824–R832, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Jeske I, Morrison SF, Cravo SL, Reis DJ. Identification of baroreceptor reflex interneurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 264: R169–R178, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Loewy AD, Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol 181: 421–449, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology 54: 487–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madden CJ, Morrison SF. Endogenous activation of spinal 5-hyroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 298: R776–R783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122: 5–15, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 286: R320–R325, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue (BAT) in rats. J Physiol 566: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R962–R973, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 93: 773–797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R290–R297, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Mtui EP, Anwar M, Gomez R, Reis DJ, Ruggiero DA. Projections from the nucleus tractus solitarii to the spinal cord. J Comp Neurol 337: 231–252, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 22: 4600–4610, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci 22: 3137–3146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience 161: 614–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold-defense. Am J Physiol Regul Integr Comp Physiol 291: R589–R595, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier, 2007 [Google Scholar]
- 46.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979 [DOI] [PubMed] [Google Scholar]
- 48.Rothwell NJ, Stock MJ, Thexton AJ. Decerebration activates thermogenesis in the rat. J Physiol 342: 15–22, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol 287: R472–R478, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Sapru HN. Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996 [PubMed] [Google Scholar]
- 51.Shibata M, Benzi RH, Seydoux J, Girardier L. Hyperthermia induced by pre-pontine knife-cut: evidence for a tonic inhibition of non-shivering thermogenesis in anaesthetized rat. Brain Res 436: 273–282, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Shibata M, Iriki M, Arita J, Kiyohara T, Nakashima T, Miyata S, Matsukawa T. Procaine microinjection into the lower midbrain increases brown fat and body temperatures in anesthetized rats. Brain Res 716: 171–179, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150: 1705–1711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol 407: 367–380, 1999 [PubMed] [Google Scholar]
- 55.Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol 479: 257–270, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol 492: 477–494, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol Regul Integr Comp Physiol 249: R672–R680, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Sved AF, Ito S, Madden CJ. Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull 51: 129–133, 2000 [DOI] [PubMed] [Google Scholar]
- 59.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett 340: 1–4, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res 928: 113–125, 2002 [DOI] [PubMed] [Google Scholar]