Abstract
Skeletal muscle atrophy and weakness are major contributors to frailty and impact significantly on quality of life of older people. Muscle aging is characterized by a loss of maximum tetanic force (Po) generation, primarily due to muscle atrophy, to which mitochondrial dysfunction is hypothesized to contribute. We hypothesized that lifelong overexpression of the mitochondrial heat shock protein (HSP) HSP10 in muscle of mice would protect against development of these deficits. Po generation by extensor digitorum longus muscles of adult and old wild-type and HSP10-overexpressing mice was determined in situ. Muscles were subjected to damaging lengthening contractions, and force generation was remeasured at 3 h or 28 days to examine susceptibility to, and recovery from, damage, respectively. Muscles of old wild-type mice had a 23% deficit in Po generation and a 10% deficit in muscle cross-sectional area compared with muscles of adult wild-type mice. Overexpression of HSP10 prevented this age-related fall in Po generation and reduction in cross-sectional area observed in muscles of old wild-type mice. Additionally, overexpression of HSP10 protected against contraction-induced damage independent of age but did not improve recovery if damage occurred. Preservation of muscle force generation and CSA by HSP10 overexpression was associated with protection against the age-related accumulation of protein carbonyls. Data demonstrate that development of age-related muscle weakness may not be inevitable and show, for the first time, that lifelong overexpression of an HSP prevents the age-related loss of Po generation. These findings support the hypothesis that mitochondrial dysfunction is involved in the development of age-related muscle deficits.
Keywords: aging, heat shock protein, Cpn10, Hspe1, atrophy
skeletal muscle function declines significantly with age and is characterized by the loss of maximum tetanic force (Po) generation and muscle cross-sectional area (CSA) (5, 21, 27). The development of these functional deficits has been hypothesized to result from the increased susceptibility of muscles to, and incomplete recovery from, contraction-induced damage (14). The mechanism(s) responsible for this is unclear, although a failure in the ability to activate the stress or heat shock response has been implicated. The heat shock protein (HSP) content of skeletal muscle of adult mammals is increased following short- and long-term exercise protocols (23, 37). In contrast, the HSP content and the ability to activate a stress response are modified in skeletal muscle with age; this has also been proposed to play a role in the development of age-related muscle deficits (35).
HSPs are molecular chaperones that are rapidly synthesized in the cell following a variety of cellular stresses, including hyperthermia, ischemia, and increased production of reactive oxygen species (ROS) (6, 16). Under normal circumstances, HSPs are involved in the folding, transportation, and conformational maturation of newly synthesized proteins. Under conditions of stress, HSPs are required for stabilization of denatured or misfolded proteins (24, 30). Some chaperones are localized to subcellular compartments. Mitochondria contain several chaperones, including HSP60 (also called Cpn60), HSP10 (also called Cpn10 and Hspe1), and Grp75 (mortalin) (16). HSP60 and HSP10 together form the HSP60/10 chaperonin complex, which is responsible for the folding of proteins transported into the mitochondrial matrix, refolding, and prevention of aggregation of denatured proteins (12). More recent studies demonstrate chaperone roles for HSP10 that are independent of HSP60, as well as nonchaperone (e.g., signaling) roles for HSP10 (11). HSP10 is encoded by a nuclear gene; the newly translated protein is transported into the mitochondria (18), and HSP10 is mostly localized in the mitochondrial matrix (11). In contrast, increased content of HSP10 has been observed in the cytosol of some cancer cells (9, 10), although the mechanisms by which HSP10 is retained in the cytoplasm in these circumstances are not fully understood (11).
The effect of overexpression of mitochondrial HSPs on the development of age-related functional deficits in skeletal muscle has not been investigated. The mitochondrial theory of aging proposed by Harman (19) was that oxidative damage to mitochondrial DNA, proteins, and lipid membranes accumulates over time due to ROS produced by the electron transport chain. Oxidative damage to mitochondrial proteins has been shown to increase with age in skeletal muscle (2, 15). This damage to key components of the mitochondria is thought to result in aberrant ROS production by the electron transport chain, which in turn results in further damage, eventually resulting in mitochondria with impaired ATP production (3, 25, 41). The aim of the present study was to examine the effect of lifelong overexpression of the mitochondrial HSP, HSP10, in skeletal muscle of mice on age-related loss of muscle force and CSA and the susceptibility of muscles to, and recovery from, contraction-induced damage. We hypothesized that overexpression of HSP10 may preserve skeletal muscle function during aging by preserving mitochondrial function by protecting against the accumulation of oxidized mitochondrial proteins.
EXPERIMENTAL PROCEDURES
Mice.
Experiments were performed in accordance with UK Home Office Guidelines under the UK Animals (Scientific Procedures) Act 1986 and received ethical approval from the University of Liverpool Animal Welfare Committee. A total of 32 mice [8 adult (10- to 12-mo-old) wild-type, 8 adult transgenic, 8 old (26- to 28-mo-old) wild-type, and 8 old transgenic] were used, and power calculations were based on previous studies (34). The transgenic mice were originally developed to overexpress HSP10 and HSP60. Mice were generated using chimeric transgenes consisting of human HSP10 and HSP60 genes inserted into pCAGGS vectors (Fig. 1). The pCAGGS construct placed the HSP10 and HSP60 genes under the control of the human cytomegalovirus (CMV) immediate early (hCMV-IE) enhancer and chicken β-actin promoter and intron 1. The CAGGS-HSP10 fragment was cut from the Bluescript II KS vector using Xho I/Not I digestion to produce a ∼3.3-kb fragment. The CAGGS-HSP60 fragment was cut from the Bluescript II KS vector using Sal I/Not I digestion to produce a 4.8-kb fragment. Both fragments were purified and used for microinjection into mouse oocytes (32). Western blot analysis demonstrated that HSP10 content was significantly elevated but HSP60 was not overexpressed in skeletal muscle of these mice (Fig. 2, A and B). The mice used in this study are referred to as HSP10-overexpressing mice. Transgenic mice were identified from DNA extracted from tail snips (DNeasy DNA extraction kit, Qiagen, Crawley, West Sussex, UK) using PCR with the primers 5′-ATTACGGGGTCATTAGTTCATAGCC-3′ (CMVIE71) and 5′-GTAGGAAAGTCCCATAAGGTCATGT-3′ (CMVIE351) to amplify the hCMV-IE enhancer region of the construct. PCR was performed using 1 μl of isolated DNA, each primer at 20 μM, 12.5 μl of BioMix Red PCR mix (Bioline, London, UK), and DNase/RNase-free water to 25 μl. PCR was carried out as follows: 1 min at 95°C, 30 s at 95°C, 1 min at 62°C, and 2 min at 72°C for 30 cycles. Transgenic mice were identified by the presence of one PCR product on an agarose gel at 300 bp; no PCR product was evident in wild-type mice. For distinction between heterozygous and homozygous mice, the same primers with real-time PCR were used, with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 50 ng of template DNA, and primers at 20 μM. Real-time PCR was performed on a Bio-Rad iCycler with the above-described PCR protocol, and the presence of one product was determined by melt curve analysis. Homozygous and heterozygous mice were identified by the difference of one cycle in the threshold cycle. Heterozygous transgenic mice and wild-type littermates were used in this study. HSP10 overexpression in muscles of all mice used in the study was confirmed postmortem by Western blotting for the HSP10 content of protein isolated from gastrocnemius muscles, as described below.
Fig. 1.
A: map of human cytomegalovirus (hCMV) β-actin/heat shock protein (HSP) 10 transgene. Coding region of the human HSP10 transgene (∼0.5 kb) is under the control of the hCMV immediate early (hCMV-IE) enhancer and chicken β-actin promoter with intron 1 (∼2.3 kb) and followed by the SV40 polyadenylation signal region (∼0.47 kb). Digestion at the Xho I/Not I sites was used to release the CAGGS-HSP10 fragment (∼3.3 kb) from Bluescript II KS (∼2.9 kb) for microinjection into mouse oocytes. B: map of hCMV β-actin/HSP60 transgene. Coding region of the human HSP60 transgene (∼2.0 kb) is under the control of the hCMV-IE enhancer and chicken β-actin promoter with intron 1 (∼2.3 kb) and followed by the SV40 polyadenylation signal region (∼0.47 kb). Digestion at the Sal I/Not I sites was used to release the CAGGS-HSP10 fragment (∼4.8 kb) from Bluescript II KS (∼2.9 kb) for microinjection into mouse oocytes.
Fig. 2.
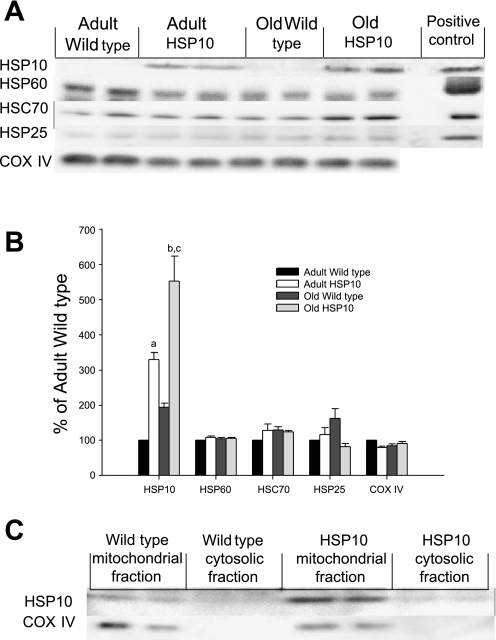
A and B: representative Western blots and densitometry showing HSP10, HSP60, HSC70, HSP25, and cytochrome c oxidase (COX) IV content of gastrocnemius muscles of adult and old wild-type and HSP10-overexpressing mice. aP = 0.014 and bP = 0.001 vs. adult wild-type mice. cP = 0.003 vs. old wild-type mice. HSP70 and Grp75 were undetectable in gastrocnemius muscles (Western blots not shown). C: representative Western blots showing HSP10 and COX IV content of mitochondrial and cytosolic fractions from gastrocnemius muscles of adult and old wild-type and HSP10-overexpressing mice.
Damaging exercise protocol.
Po generated by extensor digitorum longus (EDL) muscles was measured in situ for mice overexpressing HSP10 and wild-type controls, as previously described (34). Mice were anesthetized with ketamine hydrochloride (66 mg/kg body wt) and medatomidine hydrochloride (0.55 mg/kg body wt) by intraperitoneal injection, and anesthesia was maintained with additional ketamine (30 mg/kg body wt) as required. The knee of the right hindlimb was fixed. The distal tendon of the EDL muscle was exposed and attached to the lever arm of a servomotor (Cambridge Technology). The peroneal nerve was exposed, and stainless steel needle electrodes were placed across the nerve. Stimulation voltage and muscle length were adjusted to produce a maximum twitch force. The muscle length that produced the maximum twitch force is also the optimum muscle length (Lo) for the production of Po. For determination of Po, the muscle was electrically stimulated to contract at Lo and optimal stimulation voltage (8–10 V) every 2 min for a total of 500 ms with 0.2-ms pulse width. For each 500-ms stimulus, the frequency of stimulation was increased from 10 to 50 Hz and, subsequently, in 50-Hz increments to a maximum of 400 Hz. Po was identified when the maximum force reached a plateau, despite increasing stimulation frequency.
After identification of Po, mice were subjected to damaging protocols of 225 (for the 28-day study) or 75 (for the 3-h study) lengthening contractions. Muscles were electrically stimulated at 150 Hz for 300 ms and lengthened through a strain of 20% of fiber length (Lf) at a velocity of 1.5 Lf/s at 100 ms. Muscles of adult and old mice are activated to 90% of maximum at this frequency (unpublished observations). Mice were allowed to recover for 3 h for assessment of susceptibility to damage or for 28 days for assessment of recovery from damage (34). Previously, 75 lengthening contractions have been shown to cause submaximal damage in muscles of adult mice and maximal damage in muscles of old mice, where 225 contractions have been shown to cause equal damage in muscles of adult and old mice and, therefore, are used to examine regeneration of muscles following equal amounts of damage (6, 43). Po generation of EDL muscles was determined for eight adult and eight old wild-type mice and eight adult and eight old HSP10-overexpressing mice, all of which contributed to the data in Table 1. Subsequently, EDL muscles of four adult and four old wild-type HSP10-overexpressing mice underwent 75 lengthening contractions and were maintained under anesthesia for 3 h before remeasurement of Po generation to determine the susceptibility of muscle to contraction-induced damage. EDL muscles of the remaining four adult and four old wild-type and HSP10-overexpressing mice underwent 225 lengthening contractions, mice were removed from the platform, the wound was sutured, and the mice were allowed to recover for 28 days before remeasurement of Po under general anesthesia to determine the extent of recovery from contraction-induced damage. After completion of the procedures, mice were killed by cervical dislocation, and muscles were rapidly removed and frozen in liquid nitrogen for later analysis. The length and weight of EDL muscles were measured ex vivo to determine muscle CSA, with the constant 0.44 used to correct fiber length and 1.06 g/ml for the density of muscle (36). EDL muscles were orientated on cork disks, surrounded by optimal cutting temperature mounting compound, and frozen in isopentane precooled in liquid nitrogen. Blocks were stored at −70°C before transverse sections (10 μm) were obtained for histological analysis.
Table 1.
Body mass, muscle mass, and contractile properties of EDL muscles from adult and old wild-type and HSP10-overexpressing mice
| Adult |
Old |
|||
|---|---|---|---|---|
| Wild-Type | HSP10 Transgenic | Wild-Type | HSP10 Transgenic | |
| Body mass, g | 29.7 ± 1.26 | 32.7 ± 1.56 | 29.0 ± 1.45 | 31.2 ± 1.30 |
| Muscle mass, mg | 8.3 ± 0.3 | 9.4 ± 0.4 | 8.1 ± 0.9 | 9.0 ± 0.8 |
| Muscle CSA, mm2 | 1.81 ± 0.04 | 1.84 ± 0.06 | 1.63 ± 0.12* | 1.91 ± 0.15 |
| Fiber area, μm2 | 1,229.8 ± 21.3 | 1,342.0 ± 38.7 | 1,065.2 ± 161.5 | 1,247.6 ± 90.5 |
| Peak twitch force, mN | 99.7 ± 7.3 | 106.6 ± 6.0 | 85.6 ± 10.4 | 101.8 ± 9.1 |
| Time to peak twitch, ms | 11.1 ± 0.7 | 11.3 ± 0.5 | 10.2 ± 0.5 | 10.0 ± 0.7 |
| Twitch half-relaxation time, ms | 13.4 ± 0.8 | 14.0 ± 1.1 | 13.9 ± 1.5 | 11.9 ± 1.3 |
| Po, mN | 335.2 ± 22.3 | 346.4 ± 15.7 | 258.2 ± 27.8† | 322.1 ± 18.3 |
Values are means ± SE. EDL, extensor digitorum longus; HSP10, 10-kDa heat shock protein; CSA, cross-sectional area; Po, maximum tetanic force.
P = 0.045 and
P = 0.02 vs. adult wild-type.
Western blot analysis of HSP content of muscles.
Gastrocnemius muscles were ground under liquid nitrogen, a portion of muscle was homogenized in 1% SDS with protease inhibitors, and the sample was centrifuged at 20,000 g for 10 min at 4°C. The supernatant was analyzed for HSP content by SDS-PAGE and Western blotting, as previously described (31). Protein concentration of supernatants was determined using the bicinchoninic acid assay (Sigma-Aldrich, Dorset, UK). Total protein load was standardized by loading 100 μg of total protein per sample, which was separated on a 12% polyarylamide gel with a 4% stacking gel. Proteins were separated using a 40-mA current, and transfer of proteins onto nitrocellulose membrane was accomplished using a 45-mA current for 1.5 h. The membrane was probed with antibodies for HSP10 (SPA-110, Stressgen, Victoria, AB, Canada), HSP25 (SPA-801, Stressgen), HSP70 (SPA-810, Stressgen), HSC70 (SPA-815, Stressgen), Grp75 (SPS-825, Stressgen), and HSP60 (H3524, Sigma-Aldrich). Membranes were stripped between different primary antibodies, or, where appropriate, multiple gels were analyzed for different proteins. The mouse monoclonal antibody for cytochrome c oxidase (COX) IV (Ab14744, Abcam, Cambridge, UK) was used as a mitochondrial marker. Recombinant HSPs (Stressgen) were analyzed simultaneously as positive controls. Western blots were visualized using an enhanced chemiluminescence detection system (Perbio Science, Northumberland, UK) and analyzed by densitometry using QuantityOne software (Bio-Rad). Graphical data from Western blots are presented as a percentage of the mean adult control value. Statistical analysis was carried out on raw densitometric data.
Isolation of mitochondria from muscle.
To determine whether HSP10 was localized in the mitochondria and/or cytosol of skeletal muscle of wild-type and HSP10-overexpressing mice, a tissue mitochondrial isolation kit (Pierce, Rockford, IL) was used to isolate mitochondria from gastrocnemius muscles of four adult wild-type and four adult HSP10-overexpressing mice. Mice were killed by cervical dislocation, and gastrocnemius muscles were removed, washed twice with 1 ml of PBS, and cut into small pieces. Muscle pieces were then homogenized with a Dounce homogenizer, and mitochondria and cytosolic fractions were isolated by differential centrifugation, as described in the manufacturer's instructions. Mitochondrial pellets were lysed in 1% SDS with a range of protease inhibitors. Protein (30 μg) from cytosolic and mitochondrial fractions was analyzed by SDS-PAGE and Western blotting for HSP10 content, as described above, and COX IV content was analyzed as a mitochondrial marker.
Analysis of skeletal muscle succinate dehydrogenase activity.
Succinate dehydrogenase (SDH) activity was measured in gastrocnemius muscles as previously described (23). A portion of ground gastrocnemius muscle was homogenized (model K43, TRI-R Instruments, Rockville Centre, NY) in 100 mM potassium/sodium phosphate buffer (pH 7.2) containing 2 mM EDTA. Whole homogenate was used to determine SDH activity. Solutions were made in 50 mM potassium phosphate buffer (pH 7.6). Ten microliters of homogenate were added to 210 μl of assay buffer, and 30 μl of 40 mM sodium azide and 20 μl of 0.5 mM 2,6-dichloroindophenol were then added to homogenates. This mixture was vortexed and placed in a 96-well microplate. The reaction was initiated by addition of 30 μl of 0.2 M succinic acid. The decrease in absorbance at 600 nm was measured for 20 min at 37°C using a microplate reader (Powerwave X340, Bio-Tech Instruments), and SDH activity was calculated using the molar extinction coefficient of 2,6-dichloroindophenol (2,100 M−1·cm−1) and corrected for protein concentration, which was measured using the bicinchoninic acid assay.
Histological analysis.
A cryostat (model CM1850, Leica Microsystems) was used to cut transverse sections (10 μm) through the midpoint of the EDL muscle; these sections were transferred onto glass slides and stained with hematoxylin and eosin (Merck, Dorset, UK), as previously described (34). For assessment of fiber area, sections were photographed, and the area of all fibers fully within a 30,000-μm2 box (placed randomly on the muscle section) was measured by a blinded investigator using AxioVision software (Carl Zeiss). Three sections of the undamaged contralateral EDL muscle were analyzed per mouse. Fibers from each EDL muscle were grouped according to size; the percentage of fibers in each size range was then calculated for each EDL muscle and for each experimental group. Mean fiber area per EDL muscle was calculated from all the fibers measured from each EDL muscle, and the mean fiber area for each experimental group was calculated from these data.
Detection of carbonylated proteins in mitochondria.
Carbonylated mitochondrial proteins were detected using the Oxyblot protein detection kit, as described by the manufacturer (Millipore, Billerica, MA) and as previously described (7, 42). Briefly, after isolation of mitochondria as described above, 20 μg of mitochondrial protein were denatured with 12% SDS, then the samples were derivatized with 2,4-dinitrophenylhydrazine, separated on a 12% (wt/vol) polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane. Carbonylated proteins were detected using an antibody against dinitrophenylhyrazone. Total carbonyl content of mitochondrial proteins was analyzed by densitometric analysis of the whole lane for each sample.
Statistical analysis.
Power calculations were undertaken using Minitab version 15 on Po calculations based on previous data (23); alpha was set at 0.05, and beta was set at 0.2. Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS) software version 15. Where multiple comparisons were made, data were analyzed using ANOVA. When a significant F value was observed, least significant difference post hoc analysis was performed to identify where differences occurred. For fiber area data, factorial ANOVA was used. Significance was set at alpha ≤ 0.05. Data are presented as means ± SE.
RESULTS
HSP content of gastrocnemius muscles of wild-type and HSP10-overexpressing mice.
The HSP10 content was approximately threefold higher in the gastrocnemius muscles of adult and old HSP10-overexpressing mice than age-matched wild-type mice (Fig. 2, A and B). HSP70 and Grp75 were undetectable by Western blot analysis in gastrocnemius muscles of wild-type and HSP10-overexpressing mice. No significant differences were observed in the content of HSC70, HSP25, or HSP60 (Fig. 2, A and B) in muscles of transgenic mice compared with wild-type mice. The COX IV content of gastrocnemius muscles of adult and old wild-type and HSP-overexpressing mice was not significantly different (Fig. 2, A and B).
Cellular location of HSP10.
HSP10 was primarily located in the mitochondrial fraction of skeletal muscle in wild-type and HSP10-overexpressing mice (Fig. 2C). HSP10 content was approximately threefold higher in the mitochondrial fraction of skeletal muscle of HSP10-overexpressing than wild-type mice. No HSP10 appeared to be located in the cytosolic fraction of muscle from mice overexpressing HSP10 (Fig. 2C). COX IV was located in the mitochondrial fraction from wild-type and HSP10-overexpressing mice (Fig. 2C).
Effect of HSP10 overexpression on SDH activity.
SDH activity was measured to assess the effect of HSP10 overexpression on the activity of a key mitochondrial enzyme. No significant differences were observed in the SDH activity in gastrocnemius muscles between any of the groups (Fig. 3).
Fig. 3.
Succinate dehydrogenase (SDH) activity in gastrocnemius muscles of adult and old wild-type HSP10-overexpressing mice.
Body mass, muscle mass, and contractile properties of EDL muscles of adult and old wild-type and HSP10-overexpressing mice.
The body mass, muscle mass, and contractile properties of EDL muscles of adult and old wild-type and HSP10-overexpressing mice are shown in Table 1. Body mass was not significantly different between the groups. EDL muscles of adult mice overexpressing HSP10 showed no significant differences in muscle CSA, mean fiber area, or any of the contractile properties measured compared with EDL muscles of adult wild-type mice. Muscle CSA was significantly (P = 0.045) reduced in old wild-type mice compared with adult wild-type mice. This age-related decrease in CSA was not observed in muscles of old mice overexpressing HSP10. This age-related decrease in CSA in muscles of old wild-type mice was accompanied by a significant (P = 0.02) decrease in Po generation compared with muscles of adult wild-type mice. This decrease in Po generation was not observed in muscles of old mice overexpressing HSP10 compared with muscles of adult mice overexpressing HSP10 (Fig. 4A). No significant differences were observed in peak twitch force, time taken to reach peak twitch, and half-relaxation time of EDL muscles between any of the groups.
Fig. 4.
A: maximum tetanic force generated by extensor digitorum longus (EDL) muscles of adult and old wild-type and HSP10-overexpressing mice. aP = 0.02 vs. adult wild-type mice. B: percentage of fibers in each size range calculated from mean area of fibers from undamaged contalateral EDL muscles from adult and old wild-type and HSP-overexpressing mice from all experimental groups and time points (n = 8).
Individual fiber areas for EDL muscles of adult and old mice and wild-type and HSP10-overexpressing mice were grouped by size (Fig. 4B). Factorial ANOVA showed a main effect, with a significant interaction between strain and fiber size (P = 0.039) but no interaction between age and fiber size. Further investigation showed a reduced number of the smallest (<500-μm2) fibers and a greater number of larger (2,001- to 2,500-μm2) fibers in adult and old HSP10-overexpressing mice.
Effect of HSP10 overexpression on susceptibility of EDL muscles to lengthening contraction-induced damage.
To assess the susceptibility of EDL muscles of wild-type and HSP10-overexpressing mice to contraction-induced damage, the Po generated by EDL muscles was measured at 3 h following a protocol of 75 lengthening contractions. Data demonstrated maintenance of a greater Po generation following damage in EDL muscles of adult HSP10-overexpressing than adult wild-type mice (75.8 ± 5.5% of predamage force generation for muscles of adult mice overexpressing HSP10 compared with 50.7 ± 4.2% for muscles of wild-type mice at 3 h following the protocol). In addition, muscles of old mice overexpressing HSP10 also demonstrated reduced susceptibility to damage at 3 h following 75 lengthening contractions, with a Po generation of 58.3 ± 8.5% of the preexercise value compared with 36.7 ± 2.5% for muscles of old wild-type mice (Fig. 5A).
Fig. 5.
Maximum tetanic force generated by EDL muscles of adult and old wild-type and HSP10-overexpressing mice as a percentage of predamage force at 3 h following 75 lengthening contractions (A) and at 28 days following 225 lengthening contractions (B). aP = 0.02 vs. adult wild-type mice. bP = 0.005 vs. old wild-type mice.
Effect of HSP10 overexpression on recovery of EDL muscles from lengthening contraction-induced damage.
To assess the effect of HSP10 overexpression on the recovery from contraction-induced damage, EDL muscles of adult and old mice were subjected to 225 lengthening contractions following the initial Po measurement. Mice were allowed to recover for 28 days before remeasurement of Po. The Po generated by EDL muscles of adult wild-type and adult HSP10-overexpressing mice had recovered to 92.3 ± 10.1 and 90.4 ± 3.6% of the predamage force, respectively, and the forces generated were not significantly different from predamage values. In contrast, muscles of old wild-type and old HSP10-overexpressing mice had only recovered to 66.9 ± 6.2 and 68.1 ± 9.6% of the predamage force, respectively (Fig. 5B).
Effect of HSP10 overexpression on the carbonyl content of mitochondrial proteins.
A significant (P = 0.003) 60% increase in the total content of protein carbonyls in mitochondria from muscles of old wild-type mice was observed compared with muscles of adult wild-type mice (494.1 ± 50.2 vs. 310.0 ± 23.4 arbitrary units; Fig. 6). In contrast, this significant difference was not observed in muscles of old HSP10-overexpressing mice compared with adult HSP10-overexpressing mice (192.1 ± 17.7 and 241.4 ± 38.2 arbitrary units, respectively). This increase in carbonyl content in mitochondria from muscles of old wild-type mice was particularly evident in ∼40-, 45-, and 80-kDa proteins.
Fig. 6.
Representative carbonyl blot showing carbonylated mitochondrial proteins from muscles of adult and old wild-type and HSP10-overexpressing mice.
DISCUSSION
This is the first demonstration that lifelong overexpression of a single HSP preserves muscle CSA and prevents loss of Po generation during aging and potentially has wide-reaching implications for the prevention of age-related muscle weakness and atrophy.
The aims of this study were to investigate the effect of overexpression of a mitochondrial HSP, HSP10, in skeletal muscle of mice on 1) the development of age-related loss of force generation and CSA, 2) the susceptibility of muscles of adult and old mice to contraction-induced damage, 3) the recovery of muscles of adult and old mice from contraction-induced damage, and 4) the accumulation of oxidized proteins in the mitochondria.
Data demonstrated that lifelong overexpression of HSP10 prevented the loss of CSA and Po generation in EDL muscles of old wild-type mice. EDL muscles of old wild-type mice had a mean ∼10% deficit in the CSA and a mean 23% deficit in Po generation, which were statistically significant compared with adult wild-type mice (Table 1, Fig. 4A). This decline in Po generation and CSA is representative of sarcopenia and is similar to that seen in our previous studies (23, 34) and those of others (5, 43). The mean (although nonsignificant) 13% decrease in fiber area in muscles of old wild-type mice (Table 1) accounts for the 10% decrease observed in muscle CSA with age. Further deficit in maximum force is evident with a 23% decline in muscles of old wild-type mice, suggesting the presence of muscle fibers with a reduced ability to contract, possibly consistent with denervation. In contrast, CSA and Po generated by EDL muscles of old mice overexpressing HSP10 were not significantly decreased compared with adult wild-type or HSP10-overexpressing mice. Thus, lifelong overexpression of HSP10 had preserved the CSA and Po generation in EDL muscles of old mice. This study examined a cohort of old mice at an age where >50% of the cohort had died before reaching the age previously used as a determinant of old age (i.e., 26–28 mo) (23, 34); therefore, the effect of overexpression of HSP10 on muscle atrophy and force loss at extreme old age (e.g., 10% survival) could not be determined. McArdle et al. (34) demonstrated that overexpression of HSP70 prevented the loss of specific force generation, but this did not prevent the loss of Po generation in muscles of old HSP70-overexpressing mice compared with old wild-type mice. EDL muscles of adult and old mice overexpressing HSP10 had a greater percentage of large fibers and fewer smaller fibers (Fig. 4B) than adult and old wild-type mice, and this may account for the maintenance of CSA in the muscles of old HSP10-overexpressing mice.
Susceptibility of muscles to contraction-induced damage was determined by measurement of the force generation of EDL muscles of adult and old mice at 3 h following 75 lengthening contractions. In contrast to a more severe contraction protocol, this protocol has been used to demonstrate that muscles of old wild-type mice are more susceptible to contraction-induced damage than muscles of adult wild-type mice (43). Thus, in the present study, Po generated at 3 h following 75 contractions was 50.7 ± 4.2% of predamage force generated in muscles of adult wild-type mice compared with 36.7 ± 2.5% of predamage force generated in muscles of old wild-type mice. Overexpression of HSP10 offered significant protection against lengthening contraction-induced damage. EDL muscles of adult and old mice overexpressing HSP10 maintained a greater maximum force generation following the 75 contractions than did muscles of age-matched wild-type mice (Fig. 5A); therefore, the effect of HSP10 overexpression on protection against damage is independent of age. The mechanisms underlying this dramatic protection against force loss following lengthening contraction-induced damage are unclear. Studies have demonstrated that the damage that occurs within the first few hours following lengthening contractions is primarily mechanical, with damage to sarcomeres, including widening of I bands, Z line streaming, and loss of staining for the structural protein desmin (1, 28, 38). It has been demonstrated that the small cytosolic HSPs, HSP25 and αB-crystallin, translocate to the Z disk following lengthening contractions and may stabilize or repair these structural proteins following contraction-induced damage (26). However, HSP10 was demonstrated to be primarily in the mitochondria (Fig. 2C), and the expression of other cytosolic HSPs remained unchanged in muscles of mice overexpressing HSP10 (Fig. 2, A and B). It is difficult to see a direct mechanism by which HSP10 protected cytoskeletal components from mechanical damage in the first few hours following contraction-induced damage, unless mitochondrial disruption plays a role in this mechanical damage, and this possibility warrants further study. A greater number of larger fibers (Fig. 4B) in EDL muscles of adult and old mice overexpressing HSP10 than adult and old wild-type mice may have offered some protection against contraction-induced damage, although the mechanism by which this may occur is unclear. We are unable to identify whether this is due to neonatal changes in fiber size distribution in muscles of mice overexpressing HSP10 or selective hypertrophy.
McArdle et al. (34) demonstrated that lifelong overexpression of HSP70 facilitated the successful regeneration of muscles of old mice following a severe protocol of lengthening contractions. At 28 days following the lengthening contraction-induced damage, muscles of old mice overexpressing HSP70 had recovered, such that force generation was not significantly different from the predamage force generation. In contrast, old wild-type mice had a 44% force deficit. In the present study, mice overexpressing HSP10 demonstrated reduced susceptibility to contraction-induced damage. Thus, when studying regenerative capacity, it was important to damage muscles of adult and old wild-type and overexpressor mice to the same extent prior to allowing them to recover for 28 days before remeasurement of maximum force generation. A protocol of 225 contractions was used, as Brooks and Faulkner (6) demonstrated that this protocol results in equal force loss in muscle of adult and old mice at 3 h after damage. At 28 days following the 225 lengthening contractions, EDL muscles of adult wild-type and adult mice overexpressing HSP10 had completely recovered maximum force generation (Fig. 5B), such that these were not significantly different from their predamage forces. This is in accordance with a number of studies (6, 23, 34) demonstrating complete recovery of muscle of adult wild-type mice at 28 days following lengthening contraction-induced damage. A main objective of the present study was to examine whether HSP10 overexpression enhanced recovery of maximum force generation in muscles of old mice. At 28 days following the protocol of 225 lengthening contractions, EDL muscles of old wild-type and old HSP10-overexpressing mice had a similar (33.1 and 31.9%, respectively) deficit in Po generation compared with their predamage force generation (Fig. 5B). This deficit in force generation is comparable to data from old wild-type mice in other studies (6, 23) and suggests that, unlike overexpression of the cytosolic HSP70 (34), overexpression of HSP10 did not enhance recovery of muscles of old mice following contraction-induced damage and, therefore, has a different effect on lifelong overexpression of HSP70 in skeletal muscle.
The mechanisms by which overexpression of HSP10 prevented atrophy of the EDL muscle and preserved maximum force generation in muscles of old mice is unclear. McArdle et al. (34) demonstrated protection against development of age-related muscle deficits in transgenic mice, with a 10- to 20-fold increase in the muscle content of HSP70; this protection was not evident when HSP70 was elevated by 2-fold following treadmill training (23), suggesting a threshold level for protection. In contrast, data from the present study demonstrated that a threefold overexpression of HSP10 in muscle of adult and old mice overexpressing HSP10 resulted in protection against loss of Po and CSA and the initial injury following lengthening contractions, and this overexpression did not affect the content of other HSPs (Fig. 2, A and B). It was important to determine the cellular location of the overexpressed HSP10, since other studies demonstrated that, in large bowel and uterine cancer cells, HSP10 is overexpressed, but the majority of the protein remains in the cytosol (9, 11). Isolation of mitochondria from the muscles of adult wild-type and HSP10-overexpressing mice demonstrated that HSP10 was primarily located in the mitochondria (Fig. 2C), suggesting that the preservation of Po generation and prevention of atrophy by overexpression of HSP10 were mediated through functions of HSP10 within the mitochondria. No significant difference was observed in SDH activity or COX IV expression in muscles of mice overexpressing HSP10 (Figs. 2 and 3), suggesting that overexpression of HSP10 had not affected mitochondrial number in these muscles. Previous data demonstrated that overexpression of HSP60 and HSP10 augmented insulin-like growth factor I receptor signaling in cardiomyocytes (40), although the data suggested that the major overriding effect was likely to be provided by HSP60 overexpression in the cytosolic compartment, suggesting that this is unlikely to be the protective mechanism in this study.
In contrast, a previously proposed mechanism for the age-related loss of force and atrophy in skeletal muscle involves the development of mitochondrial dysfunction. Defective mitochondria have been shown to accumulate in skeletal muscle with age (4); however, the mechanisms by which accumulation of defective mitochondria in muscle during aging may result in the development of the age-related deficits is unclear. It has been proposed that defective mitochondria accumulate within a muscle fiber, resulting in abnormal ATP production, and this may eventually result in fiber splitting and atrophy (20). In agreement with the “mitochondrial theory of aging,” several studies have demonstrated an accumulation of mitochondria with mutations in key regions involved in the electron transport chain in skeletal muscle fibers with age (4, 8, 20), and it is proposed that mitochondria with mutations may produce aberrant ROS, resulting in further damage to mitochondrial proteins and membranes (41).
To examine the possibility that HSP10 overexpression provided protection against the detrimental effects of ROS generation, total protein carbonyl content of mitochondrial proteins was analyzed. Data demonstrated a 60% increase in the carbonyl content of mitochondria from muscles of old wild-type mice compared with adult wild-type mice (Fig. 6). In contrast, this significant increase was not observed in muscles of HSP10-overexpressing mice. Furthermore, adult and old HSP10-overexpressing mice demonstrated reduced protein carbonyl content compared with adult wild-type mice (77 and 62% of adult wild-type, respectively). Several studies demonstrated increased content of protein carbonyls in mitochondria of muscles of old rodents (2, 15), and this increase was enhanced by sedentary behavior (15). Our findings suggest that overexpression of HSP10 prevents the age-related accumulation of oxidized mitochondrial proteins observed in muscles of old wild-type mice. In Escherichia coli, it has been demonstrated that HSP10 and HSP60 are involved in the degradation of abnormal proteins alongside the Lon protease (22). In addition, mutations leading to a reduction in HSPs in E. coli and a failed heat shock response demonstrated reduced protein degradation and Lon protease content (17). It is therefore likely that lifelong overexpression of HSP10 in the muscles of old mice has decreased the accumulation of oxidized mitochondrial proteins compared with muscles of wild-type mice through increased degradation, therefore preventing the accumulation of defective mitochondria in the muscles of old HSP10-overexpressing mice.
The mechanism by which defective mitochondria lead to cellular dysfunction and atrophy is the focus of a number of studies. Mitochondria are considered to be key regulatory organelles for apoptosis; therefore, accumulation of dysfunctional mitochondria and, consequently, ATP depletion in skeletal muscle fibers with age may stimulate apoptosis through mitochondrial signaling, resulting in fiber loss and weakness (for review see Refs. 13, 33). Thus, overexpression of HSP10 may protect against age-related skeletal muscle force loss and atrophy through the prevention of apoptosis by preventing accumulation of oxidized mitochondrial proteins and, thus, maintaining mitochondrial function. Studies in cardiac muscle cells demonstrated that overexpression of HSP60 and HSP10 has protective effects by preventing apoptosis (29, 39).
Perspectives and Significance
Skeletal muscle atrophy and weakness are major causes of increased frailty and loss of mobility in the elderly and are, in part, responsible for the increased incidence of falls, which impact on quality of life. We have demonstrated that lifelong overexpression of the mitochondrial chaperone HSP10 in skeletal muscle of mice prevented the age-related loss of force and CSA observed in muscles of old wild-type mice and, furthermore, protected muscles of adult and old mice from damage following contraction-induced injury. These data demonstrate that the development of age-related muscle weakness and atrophy is not inevitable, although extreme ages were not studied, and the protective effect of overexpression of HSP10 in skeletal muscle supports the hypothesis that mitochondrial dysfunction is involved in the development of these deficits. The mechanism underlying these functional improvements appears to be a reduction in the accumulation of oxidatively damaged mitochondrial proteins. Whether this is a result of the independent action of HSP10 or the greater activity of the HSP60/10 chaperonin complex is unclear, and further studies are required to elucidate the underlying mechanisms, as this will further our understanding of the mechanisms underlying the loss of force and atrophy during aging and give rise to a potential therapeutic target in its prevention.
GRANTS
This study was supported by Research into Aging and the Biotechnology and Biological Sciences Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54: 80–93, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett 532: 103–106, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Brierley EJ, Johnson MA, James OF, Turnbull DM. Mitochondrial involvement in the ageing process. Facts and controversies. Mol Cell Biochem 174: 325–328, 1997 [PubMed] [Google Scholar]
- 4.Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM. Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann Neurol 43: 217–223, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J 20: 1549–1551, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 79: 469–480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappello F, Bellafiore M, David S, Anzalone R, Zummo G. Ten kilodalton heat shock protein (HSP10) is overexpressed during carcinogenesis of large bowel and uterine exocervix. Cancer Lett 196: 35–41, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cappello F, Bellafiore M, Palma A, David S, Marciano V, Bartolotta T, Sciume C, Modica G, Farina F, Zummo G, Bucchieri F. 60-kDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem 47: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Czarnecka AM, Campanella C, Zummo G, Cappello F. Heat shock protein 10 and signal transduction: a “capsula eburnea” of carcinogenesis? Cell Stress Chaperones 11: 287–294, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11: 116–128, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5: 179–195, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci 50: 124–129, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo PA, Powers SK, Ferreira RM, Amado F, Appell HJ, Duarte JA. Impact of lifelong sedentary behavior on mitochondrial function of mice skeletal muscle. J Gerontol A Biol Sci Med Sci 64: 927–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gething M. (Editor). Molecular Chaperones and Protein-Folding Catalysts. New York: Oxford University Press, 1997 [Google Scholar]
- 17.Goff SA, Casson LP, Goldberg AL. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci USA 81: 6647–6651, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen JJ, Bross P, Westergaard M, Nielsen MN, Eiberg H, Borglum AD, Mogensen J, Kristiansen K, Bolund L, Gregersen N. Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localised head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet 112: 71–77, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc 20: 145–147, 1972 [DOI] [PubMed] [Google Scholar]
- 20.Herbst A, Pak JW, McKenzie D, Bua E, Bassiouni M, Aiken JM. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: evidence for a causal role in muscle fiber loss. J Gerontol A Biol Sci Med Sci 62: 235–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadhiresan VA, Hassett CA, Faulkner JA. Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493: 543–552, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandror O, Busconi L, Sherman M, Goldberg AL. Rapid degradation of an abnormal protein in Escherichia coli involves the chaperones GroEL and GroES. J Biol Chem 269: 23575–23582, 1994 [PubMed] [Google Scholar]
- 23.Kayani AC, Close GL, Jackson MJ, McArdle A. Prolonged treadmill training increases HSP70 in skeletal muscle but does not affect age-related functional deficits. Am J Physiol Regul Integr Comp Physiol 294: R568–R576, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80: 183–201, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood TB. Molecular gerontology. J Inherit Metab Dis 25: 189–196, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol 286: C713–C722, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80: 278–284, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 103: 1787–1792, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6: D12–D25, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol 548: 837–846, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 95: 1446–1456, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41: 1234–1238, 2006 [DOI] [PubMed] [Google Scholar]
- 34.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18: 355–357, 2004 [DOI] [PubMed] [Google Scholar]
- 35.McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev 1: 79–93, 2002 [DOI] [PubMed] [Google Scholar]
- 36.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol 61: 293–299, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol 101: 176–182, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci 61: 109–122, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol 35: 1135–1143, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Shan YX, Yang TL, Mestril R, Wang PH. Hsp10 and Hsp60 suppress ubiquitination of insulin-like growth factor-1 receptor and augment insulin-like growth factor-1 receptor signaling in cardiac muscle: implications on decreased myocardial protection in diabetic cardiomyopathy. J Biol Chem 278: 45492–45498, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol 36: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Vasilaki A, Simpson D, McArdle F, McLean L, Beynon R, Van Remmen H, Richardson A, McArdle A, Faulkner JA, Jackson M. Formation of 3-nitrotyrosines in carbonic anhydrase III is a sensitive marker of oxidative stress in skeletal muscle. Proteomics Clin Appl 1: 362–372, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol Cell Physiol 258: C429–C435, 1990 [DOI] [PubMed] [Google Scholar]