Abstract
In pregnant sheep, maternal:fetal exchange occurs across placentomes composed of placental cotyledonary and uterine caruncular tissues. Recently, we reported that fetal weights of obese (OB) ewes [fed a diet of 150% of National Research Council (NRC) recommendations] were ∼30% greater than those of control (C) ewes (fed a diet 100% of NRC recommendations) at midgestation (MG), but fetal weights were similar in late gestation (LG). Transplacental nutrient exchange is dependent on placental blood flow, which itself is dependent on placental vascularity. The current study investigated whether the observed initial faster and subsequent slower fetal growth rate of OB compared with C was associated with changes in cotyledonary vascularity and expression of angiogenic factors (vascular endothelial growth factor, fibroblast growth factor-2, placental growth factor, angiopoietin-1 and -2). Cotyledonary arteriole diameters were markedly greater (P < 0.05) in OB than C ewes at MG, but while arteriole diameter of C ewes increased (P < 0.05) from MG to LG, they remained unchanged in OB ewes. Cotyledonary arterial angiogenic factors mRNA and protein expression were lower (P < 0.05) in OB than C ewes at MG and remained low from MG to LG. In contrast, mRNA levels of angiogenic factors in C ewes declined from high levels at MG to reach those of OB ewes by LG. The increase in cotyledonary arteriole diameter in early to MG may function to accelerate fetal growth rate in OB ewes, while the decreased cotyledonary arterial angiogenic factors from MG-LG may function to protect the fetus from excessive placental vascular development, increased maternal nutrient delivery, and excessive weight gain.
Keywords: pregnancy, angiogenesis, cotyledonary vasculature, sheep
in the united states, approximately 62% of women 20–74 years of age are classified as overweight with a body mass index (BMI) above 25.0, including ∼33%, who are clinically obese (BMI >30.0) (30a). Of interest in relation to problems of pregnancy and fetal development, the prevalence of obesity in women of child-bearing age (20–34 years old) has reached 28.6% in the current U.S. population and continues to increase (30a). Offspring of obese women have an increased risk for developing obesity (51), insulin resistance (29), and other metabolic syndromes (8) than offspring of lean or average weight women. Further, recent evidence suggests that a tendency for fetal obesity and insulin resistance may already be apparent before birth. Catalano et al. (10) compared late-gestation fetuses of obese and lean pregnant women, delivered by cesarean section at 37–40 wk, and reported that fetuses from obese mothers were similar in weight to those of lean mothers, but already exhibited a greater fat mass and percent body fat. Furthermore, these researchers determined that late-gestation fetuses of obese mothers were more insulin resistant than fetuses of lean mothers and reported a significant positive correlation between maternal pregravid BMI and fetal insulin resistance (9).
In sheep pregnancy, 75 to 120 placentomes distributed throughout the uterus constitute the sites of maternal:fetal exchange (44). Each placentome has a maternal caruncular (CAR) and a fetal cotyledonary (COT) component. Both CAR and COT tissues are richly endowed with blood vessels that interdigitate at the fetal:maternal interface to facilitate nutrient and gas exchange. Placentomal vascularity is positively related to blood flow through the fetal:maternal interface and with nutrient and oxygen delivery to the fetus (6, 43). Branching angiogenesis, a process involving the branching of new capillaries from preexisting vessels, is crucial for the proliferation of the COT and CAR capillary beds (3). Angiogenic factors, including vascular endothelial growth factor (VEGF) and its receptors FMS-like tyrosine kinase (FLT)-1 and kinase insert domain receptor (KDR); angiopoietin-1 (ANG-1) and ANG-2, and their receptor Tie-2; basic fibroblast growth factor (FGF- 2); and placenta growth factor (PLGF) are known to play key roles in increasing placental vasculogenesis and angiogenesis (1, 9, 38). Other molecules, such as hypoxia inducible factor (HIF)-1α also impact placental angiogenesis. Under hypoxic condition, HIF-1α interacts with the hypoxia-responsive element in the promoter region of VEGF and the first intron of ANG-2 to promote transcription of these two genes (41, 42).
To date, the mechanisms whereby maternal obesity affects placental vascular development, leading to changes in fetal growth and development, are still unclear. This study investigated the impact of maternal obesity and excess dietary intake before and during pregnancy on fetal growth, COT vascularity, and COT arterial angiogenic factor expression from midgestation to near term.
MATERIALS AND METHODS
Animal care and tissue collection.
This study was conducted at the University of Wyoming, and all procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia cross ewes of similar weight and body condition score (BCS) were studied. These ewes were 3–4 years old and had carried 2 or 3 pregnancies (only produced singles or twins) before assignment to the study and were randomly distributed across dietary treatment groups. A BCS on a scale of 1 (emaciated) to 9 (obese) was assigned by 2 trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5), the ribs and the region around the tail head. BCS is highly related to carcass lipids and has been used to estimate energy reserves available to ewes (40).
Ewes of similar BCS were assigned at random to be fed either a highly palatable diet, at 100% (control, C: n = 20) of National Research Council (NRC) recommendations (12) or 150% (obese, OB: n = 20) of NRC recommendations, as previously reported (16). Experimental diets were delivered on a dry matter basis to meet the total digestible nutrients requirements for maintenance for an early pregnant ewe based on metabolic BW (BW0.75) from 60 days before mating to day 75 or day 135 of gestation. The number of fetuses carried by each ewe was determined by ultrasonography (Asonics Microimager 1000 sector scanning instrument, Ausonics, Sydney, Australia) at day 45 of gestation. All ewes used in this study carried twin fetuses and were necropsied at day 75 (n = 5/dietary group) or day 135 (C, n = 4; OB, n = 6) of gestation. Placentas of both twin fetuses were selected for study. All ewes were weighed weekly, and diets were adjusted for weight gain as previously described (15). BCS was recorded monthly to evaluate changes in body fatness.
At either day 75 or day 135 of gestation, ewes were weighed, sedated with ketamine (22.2 mg/kg body wt), and anesthetized via isoflurane inhalation (1–2%). Ewes were euthanized by exsanguination, while under general anesthesia, and the gravid uterus was recovered. Fetal body weights and crown rump lengths (CRL) were recorded. From each placenta, two Type A placentomes (45) of similar weight and size within 15 cm of the umbilical cord attachment site were selected for immunohistochemical localization of VEGF, PLGF, and FGF-2 and for determination of arteriole diameter and number. Another five Type A placentomes of similar size and weight from each placenta were used for collection of COT arteries. Only the smallest arteries entering the COT tissues (0.5–1.0 mm in diameter) were collected; they were immediately frozen in liquid nitrogen and stored at −80°C until used for real-time PCR and Western blot analysis. All remaining placentomes were then dissected from each uterus and counted, and a total placentome weight was determined.
Assessment of arteriole numbers and diameters.
Two placentomes were collected at random from each placenta at the time of necropsy on day 75 or day 135 of gestation. A cross section (∼0.8 cm in thickness) of each placentome containing CAR and COT tissue was placed in a tissue cassette (Tissue Tek, Miles Labs, Elkhart, TN) and fixed with 4% (wt/vol) paraformaldehyde in a phosphate buffer (0.12 M; pH = 7.4) and paraffin embedded. Three 5-μm cross sections (50–75 μm apart) of each placentome were placed on slides and stained with eosin and hematoxylin and evaluated for COT and CAR arteriolar numbers and diameters via image analysis (Optimus Image Analysis Software, Bothell, WA) by an experienced individual blinded to the experimental groups, and the results were averaged. For each of the 5-μm sections, total arteriole number in fetal COT and maternal CAR areas were determined. Arteriole number from each COT and CAR was then corrected with the total COT or CAR area measured (per unit area as mm2). Arteriole numbers per unit area from all 3 slices of one placentome were then averaged. For each arteriole, its area and perimeter were measured, and the arteriole diameter was calculated accordingly. Diameters of all arterioles on one section were averaged.
Immunohistochemistry.
Processing of placentomal sections for immunostaining was accomplished as described above for vascular density measurement. Immunostaining of specific proteins was accomplished following the general immunohistochemistry protocol with the ABC kit previously described by Zhu et al. (53). Briefly, two 5-μm sections per placentome were utilized for staining of each target protein, with another section from the same placentome serving as a negative control. First, tissue sections were deparaffinized and hydrated by routine methods; then antigen retrieval was accomplished through boiling in citrate buffer (pH = 6.0) for 30 min. Nonspecific antigenic sites were blocked with blocking buffer: normal goat serum (Vector Laboratories, Burlingame, CA) diluted in PBS [1.5% (wt/vol) in PBS buffer (pH = 7.0), with 0.05% (wt/vol) Tween 20 for 1 h at room temperature]. Sections were incubated for 2–3 h at room temperature with anti-VEGF (1:50 dilution), anti PLGF (1:20 dilution), or anti-FGF-2 (1:50 dilution) antibody diluted in blocking buffer. Negative controls were incubated only with blocking buffer for the same period of time. The sections were then incubated at room temperature with a biotinylated second anti-rabbit antibody (1:200 dilution) diluted in blocking buffer for 1 h followed by incubation with a peroxidase-conjugated biotin-avidin complex (Vectastain ABC kit; Vector Laboratories) for 30 min at room temperature. The target proteins were revealed by reacting with 3,3-diaminobenzidine color substrate, according to the manufacturer's instructions (Vector Laboratories), and further counterstained with Harris-modified hematoxylin.
Total RNA extraction and single-strand DNA synthesis.
COT arteries were pulverized in liquid nitrogen. Total RNA was extracted from COT arteries (50–100 mg) using TRIzol reagent (Invitrogen, Carlsbad, CA) treated with DNase I (Qiagen, Valencia, CA), and then purified by RNeasy minicolumn (Qiagen), according to the respective protocols. One microgram of purified RNA of each COT arterial tissue preparation was used to synthesize single-strand DNA using Promega ImProm- II Reverse Transcription System (Promega BioSciences, San Luis Obispo, CA), according to the kit protocol.
Real-time PCR.
Genes studied in the real-time PCR experiments are listed in Table 1. All real-time PCR reactions were conducted using a Bio-Rad IQ5 real-time PCR Reaction System (Bio-Rad Laboratories, Hercules, CA). Reactions for each gene were run in duplicate. A temperature gradient PCR reaction was run for all of the primer sets before conducting real-time PCR to determine the optimal annealing temperature for all primer sets. According to gradient PCR, the optimal annealing temperature of all primer sets overlapped at 60°C. Correspondingly, the following protocol was designed and applied to all real-time PCR reactions: step 1) 1 cycle at 95°C for 3 min; step 2) 40 repeat cycles at 95°C for 10 s, following by annealing at 60°C for 30 s; and step 3) 55.0°C–95.0°C, with melting temperature increasing 0.5°C for each 30 s. Fluorescence was detected at both step 2 and step 3. Real-time PCR analysis was enabled at step 2, and melt curve data collection and analysis were enabled at step 3. An amplification efficiency of 90–105% is considered to be high in an optimized real-time PCR reaction (7). In our real-time PCR experiments, all reactions reached 100% efficiency. Final data were analyzed through the 2−ΔΔCt method (26), where 18s RNA was used as a reference gene to normalize all the selected gene expression data.
Table 1.
Primer sequences used in real-time PCR quantification of selected genes
| Gene | Primer Sequences |
|---|---|
| VEGF | F: 5′-CGA AAG TCT GGA GTG TGT GC-3′ |
| R: 5′-TAT GTG CTG GCT TTG GTG AG-3′ | |
| PLGF | F: 5′-GCC CTT CTT TGT GGA GAT GA-3′ |
| R: 5′-GTA TCA CCG CAC CTT TTT GG-3′ | |
| FGF-2* | F: 5′-CGA CGG CCG AGT GGA C-3′ |
| R: 5′-CTC TCT TCT GCT TGA AGT TGT AGT TTG-3′ | |
| ANG-1* | F: 5′-AAA TGA AAA GCA GAA CTA CAG GTT GTA T-3′ |
| R: 5′-GCA AGA TCA GGC TGC TCT GTT-3′ | |
| ANG-2* | F: 5′-AAA TAG GGA CCA ACC TGC TCA A-3′ |
| R: 5′-TGT TGT CTG ATT TAA TAC TTG GGC TT-3′ | |
| FLT-1* | F: 5′-TGG ATT TCA GGT GAG CTT GGA-3′ |
| R: 5′-TCA CCG TGC AAG ACA GCT TC-3′ | |
| KDR* | F: 5′- CTT CCA GTG GGC TGA TGA CC-3′ |
| R: 5′- GCA ACA AAC GGC TTT TCA TGT-3′ | |
| Tie2* | F: 5′-CCT CGG AGG CAG GAA GAT G-3′ |
| R: 5′-TCA GGC AGG TCA TTC CCG-3′ | |
| HIF-1α* | F: 5′-CGC ATC TTG ATA AGG CTT CTG TT-3′ |
| R: 5′-CAC CAG CAT CCA GAA GTT TCC T-3′ | |
| 18 s RNA* | F: 5′-AGC CTG CGG CTT AAT TTG AC-3′ |
| R: 5′-CAA CTA AGA ACG GCC ATG CA-3′ |
Primers were designed according to the references as listed in materials and methods. F and R, forward and reverse primers, respectively.
Primers.
VEGF and PLGF primers were designed using Primer 3 based on genetic sequence X89506 and AY157708, respectively. FLT-1, KDR, ANG-1, ANG-2, and Tie-2 primers were synthesized according to Redmer et al. (33), while HIF-1α and 18s RNA primers were synthesized, according to Johnson et al. (23) and Lopez-Andreo et al. (27), respectively. All primers were synthesized by Invitrogen (Carlsbad, CA). Their sequences are as listed in Table 1.
Total protein extraction and Western blot analysis.
Liquid-nitrogen pulverized COT arterial samples (80–100 mg) were homogenized in a polytron homogenizer, with ice-cold lysis buffer [50 mM Tris-HCl, 100 mM NaF, 1 mM MgCl2, 2.5 mM EDTA (4 Na), 100 mM NaCl, 2% SDS, 1% NP-40, 0.5 M CaCl2, 2 mM Na3VO4, pH = 7.4]. Homogenates were then sonicated and clarified by centrifugation. After centrifugation, the supernatant was mixed with SDS sample loading buffer and heated at 95°C for 5 min. A standard SDS-PAGE was run to separate proteins followed by the transfer of separated proteins to a nitrocellulose membrane and blocking with primary and secondary antibodies dissolved in 5% (wt/vol) and 2% (wt/vol) skim milk, respectively. VEGF, PLGF, FGF-2, ANG-1, or ANG-2 primary antibodies were used at dilutions of 1:200, 1:40, 1:400, 1:400, and 1:400, respectively, in 5% (wt/vol) skim milk for blocking. All secondary antibodies were used at a dilution of 1:2,000 in 2% (wt/vol) skim milk. The membrane was then visualized using ECL Western blotting detection reagents and exposed to film. The density of bands was quantified by using an Imager Scanner II (Amersham Biosciences, Piscataway, NJ) and ImageQuant TL software (Amersham Biosciences). The target gene band density was normalized according to the density of a reference sample, as well as β-actin content in the same samples.
Antibodies.
Anti-VEGF antibody (A-20, rabbit IgG, sc-152), ANG-1 antibody (C-19, goat IgG, sc-6320), ANG-2 antibody (C-19, goat IgG, sc-7015), and anti-goat IgG antibody (sc-2020) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-PLGF antibody (rabbit IgG, ab9542) was purchased from Abcam (Cambridge, MA). Anti-FGF-2 antibody (rabbit IgG, AB-33-NA) was purchased from R&D Systems (Minneapolis, MN). Anti-β-actin antibody (mouse IgG, A-1978) was purchased from Sigma-Aldrich (St. Louis, MO). Secondary anti-mouse IgG (#7076) and secondary anti-rabbit IgG (#7074) antibodies were both purchased from Cell Signaling Technology (Danvers, MA).
Statistical analysis.
When comparing across dietary treatment and day of gestation, data were analyzed as a complete randomized design using GLM (general linear model of Statistical Analysis System) (SAS 9.1, 2002–2003; SAS Institute, Cary, NC). Mean separation was performed using LSMEANS. Student's t-tests were performed to compare differences of individual measurements between the two dietary groups at either day 75 or day 135 of gestation (Microsoft Excel 2008 for Mac OX). Additional Student's t-tests were performed to determine differences of a measurement within a dietary group at different stages of pregnancy. Data are presented throughout as means ± SE and are considered significantly different when P < 0.05, while P values between 0.05 and 0.10 were considered trends.
RESULTS
Ewes on the OB diet increased their body weight by ∼30% from diet initiation to mating (71.6 ± 3.2 and 92.8 ± 2.9 kg, respectively; P < 0.05) and increased 43% and 52% in body weight from diet initiation to necropsy on day 75 or day 135 of gestation, respectively. In contrast, C ewes, whose body weight was similar to that of OB ewes at diet initiation, exhibited only modest nonsignificant increases in body weight from diet initiation to conception (2.9%), or necropsy on day 75 or day 135 of gestation (5.7% and 7.0%, respectively). Similarly, BCS of OB ewes increased (P < 0.05) from diet initiation to mating (5.0 ± 0.3 and 7.2 ± 0.2, respectively) and had further increased (P < 0.05) to 8.0 ± 0.2 by day 75, and 8.6 ± 0.2 by day 135, while BCS of control ewes remained relatively constant from diet initiation to day 135 of gestation averaging 4.9 ± 0.4.
Fetuses from OB ewes necropsied on day 75 of gestation were ∼30% heavier (P < 0.05) and had greater (P < 0.05) CRL than fetuses gestated by C ewes on day 75 of gestation (Table 2). In contrast, fetal weight and CRL of C and OB ewes on day 135 of gestation were not different from each other. Total placentome number, total placentome weight, and average placentome weight did not differ across the two dietary groups on day 75 of gestation (Table 2). Total placentome number of C ewes on day 135 of gestation tended to be reduced (P = 0.08) compared with day 135 OB ewes or compared with day 75 C and OB ewes. In contrast, total placentome weight and average placentome weight of OB ewes on day 135 were markedly reduced (P < 0.05) compared with that of C ewes on day 135, as well as C and OB ewes on day 75 (Table 2). The fetal-to-placental weight ratio, an index of placental efficiency, was greater in OB than C ewes on both day 75 and day 135 of gestation (P < 0.05).
Table 2.
Fetal and maternal data from control ewes fed 100% of the NRC-recommended diet, and obese ewes fed 150% of the NRC-recommended diet on both day 75 and day 135 of gestation
| Day 75 of Gestation |
Day 135 of Gestation |
|||
|---|---|---|---|---|
| Measurements | C | OB | C | OB |
| Number of twin pregnancies | 5 | 5 | 4 | 6 |
| Fetal weight, g | 185.7 ± 6.9a | 234.4 ± 6.6b | 5024.5 ± 163.4c | 4827.4 ± 169.1c |
| Crown rump length, cm | 17.3 ± 0.5a | 19.6 ± 0.4b | 58.1 ± 0.7c | 57.1 ± 0.8c |
| Number of placentomes | 88.6 ± 4.8a | 93.4 ± 5a | 72.8 ± 3.5b,e | 84.3 ± 4.6a,f |
| Total placentome weight, g | 709.5 ± 38.9a,e | 654.6 ± 37.6a | 623.3 ± 15.8a,f | 486 ± 14.2b |
| Average placentome weight, g | 16.3 ± 0.9a | 14.4 ± 1.1a | 17.2 ± 0.6a | 11.6 ± 0.6b |
| Fetal/placental weight ratio | 0.27 ± 0.02a | 0.37 ± 0.02b | 8.1 ± 0.3c | 10 ± 0.4d |
NRC, National Research Council; C, control; OB, obese.
a,b,c,dValues are means ± SE within a row when superscripted letters differ (P < 0.05). e,fValues are means ± SE within a row when superscripted letters differ (P < 0.10).
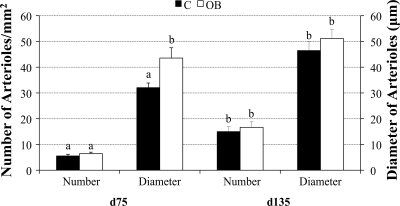
COT arteriole numbers per placentome were similar for C and OB ewes on day 75, but vessel diameters were greater (P < 0.05) in OB than C ewes (Fig. 1). On day 135 of gestation, while COT arteriole numbers per placentome remained similar between C and OB ewes, vessel numbers in both dietary groups were greater (P < 0.05) than those on day 75. In contrast to day 75, there were no significant differences in COT arteriole diameter in C vs. OB ewes on day 135. COT arteriole diameters of C ewes dramatically increased (P < 0.05) from day 75 to day 135, while arteriole diameters of OB ewes remained unchanged over the same interval (Fig. 1). The CAR arteriole numbers followed a pattern similar to that of the COT. The numbers of CAR arterioles were similar in C and OB ewes on days 75 and 135, with vessel numbers in both groups increasing (P < 0.05) over this period and averaging 4.02 ± 0.98 and 7.39 ± 1.13 per mm2, respectively. In contrast to the COT, however, CAR arteriole diameters were similar for C and OB ewes and remained relatively constant from day 75 to day 135, averaging 108 ± 13.70 and 83.73 ± 15.76, respectively.
Fig. 1.
Average numbers of arterioles per mm2 cotyledonary (COT) tissue and average diameters of arterioles of control (C) ewes and obese (OB) ewes at both day 75 (C, n = 8; OB, n = 10) and day 135 (C, n = 4; OB, n = 6) of gestation a,bMeans ± SE within a measurement with different superscripts differ (P < 0.05).
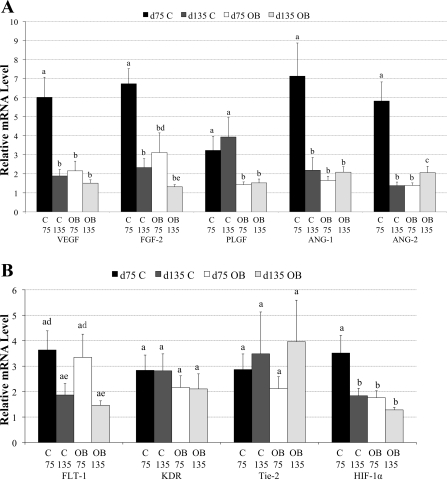
The mRNA level of VEGF, FGF-2, PLGF, ANG-1, and ANG-2 in OB ewe COT arteries were decreased 64.2%, 53.7%, 55.8%, 76.8%, and 76.1%, respectively, compared with mRNA levels expressed in C ewe COT arteries at day 75 of gestation (P < 0.05, Fig. 2A). HIF-1α mRNA expression also exhibited a reduction of 49.9% in OB ewe COT arteries vs. that of C ewes (Fig. 2B) at day 75 of gestation. In contrast, on day 135 of gestation, only PLGF mRNA expression was reduced (P < 0.05), and FGF-2 tended (P = 0.07) to be lower in COT arterial tissue of OB compared with C ewes, while ANG-2 mRNA expression was higher (P < 0.05, Fig. 2B). No differences were detected in FLT-1, KDR, and Tie-2 mRNA expression in COT arterial tissue between the two dietary groups (Fig. 2B) at either midgestation or late gestation.
Fig. 2.
A: real-time PCR quantification of selected angiogenic factors mRNA levels in COT arterial tissue from both C and OB ewes at day 75 of gestation (C, n = 5; OB, n = 5) and day 135 of gestation (C, n = 4, OB, n = 6). B: receptors of selected angiogenic factors and hypoxia inducible factor-1α (HIF-1α) mRNA levels in COT arterial tissue from both C and OB ewes at day 75 of gestation (C, n = 5; OB, n = 5) and day 135 of gestation (C, n = 4, OB, n = 6) a,b,cValues are expressed as means ± SE, where superscripted letters that differ show significant difference (P < 0.05) d,eValues are expressed as means ± SE within a treatment group where superscripted letters that differ show significant difference (P < 0.10). VEGF, vascular endothelial growth factor; FGF-2, fibroblast growth factor; PLGF, placenta growth factor; FLT-1, FMS-like tyrosine kinase-1; KDR, kinase insert domain receptor.
The mRNA expression of all the angiogenic factors in COT arterial tissue, except PLGF, markedly decreased (P < 0.05) from midgestation to late gestation in C ewes (Fig. 2A). FLT-1 also tended to decline (P = 0.07) from day 75 to day 135 in C ewes (Fig. 2B). In contrast, when angiogenic factor mRNA expression was compared for COT arterial tissue of OB ewes from midgestation to late gestation, it was determined that levels of all angiogenic factors measured remained low and relatively constant over this interval (Fig. 2A). Only FLT-1 showed a trend toward a decrease (P = 0.08) in OB ewes from day 75 to day 135 (Fig. 2B).
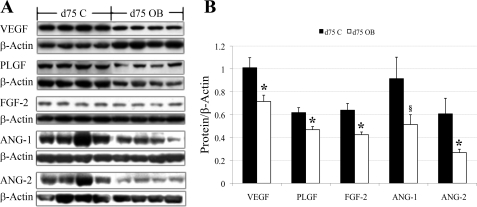
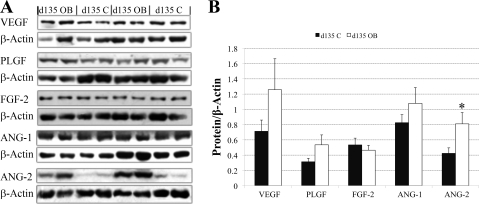
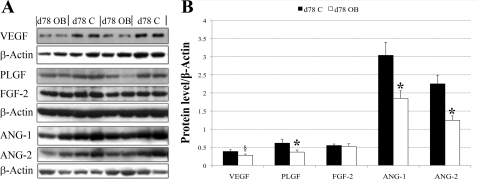
In agreement with the mRNA expression data, Western blot analysis confirmed that protein expression of VEGF, PLGF, FGF-2, and ANG-2 in COT arteries on day 75 in OB ewes were 41%, 32%, 50%, and 56% lower (P < 0.05) than those in COT arteries of C ewes (Fig. 3, A and B), while ANG-1 expression tended to be lower (P = 0.08). Also, in agreement with the real-time RT-PCR data, protein expression of VEGF, PLGF, FGF-2, and ANG-1 of C and OB ewes were similar on day 135 of gestation (Fig. 4, A and B). In contrast, protein expression of ANG-2 was increased in COT arteries on day 135 in OB vs. C ewes (P < 0.05). In Western blot analysis, VEGF and PLGF were detected at ∼28 kDa in our sheep tissue, exactly the same size as the positive control (sc-2210; Santa Cruz Biotechnology, Santa Cruz, CA). The PLGF antibody, according to the product description, does not cross react with VEGF protein. FGF-2 was detected at ∼25 kDa, slightly higher than the protein size described by the company, probably due to posttranscriptional modification, such as glycosylation. ANG-1 and ANG-2 were detected at ∼55 kDa.
Fig. 3.
A: Western blot measurement of selected protein expression in COT arterial tissue of both C (n = 5) and OB (n = 5) ewes at day 75 of gestation. Representative Western blot bands to VEGF, PLGF, FGF-2, ANG-1, and ANG-2, and the reference protein β-actin are shown. B: statistical analysis on Western blot result of the five proteins in A. Values are expressed as means ± SE; *P < 0.05. Trend toward difference, §P = 0.08.
Fig. 4.
Western blot measurement of selected protein expression in COT arterial tissue of both C (n = 4) and OB (n = 6) ewes at day 135 of gestation. A: representative Western-blot bands to PLGF, FGF-2, and ANG-2 and the reference protein β-Actin. B: statistical analysis on Western blot result of the five proteins in A. Values are means ± SE; *P < 0.05.
To relate differences in angiogenic factor expression found in COT arterial tissue on day 75 between C and OB females to the surrounding COT tissues, COT tissue from the placentomes supplied by these arteries were evaluated for protein expression of VEGF, PLGF, FGF-2, ANG-1, and ANG-2. As depicted in Fig. 5, A and B, protein expression of PLGF, ANG-1, and ANG-2 were found to be higher (P < 0.05), and VEGF tended to be higher (P = 0.09) in COT tissue from OB vs. C ewes. Protein expression of FGF-2 was similar in COT tissues of C and OB ewes.
Fig. 5.
Western blot measurement of selected protein expression in COT tissue of both C (n = 8) and OB (n = 8) ewes at day 75 of gestation. A: representative Western blot bands to VEGF, PLGF, FGF-2, ANG-1, and ANG-2 and the reference protein β-Actin. B: statistical analysis on Western blot result of the five proteins in A. Values are expressed as means ± SE; *P < 0.05. Trend toward difference, §P = 0.09.
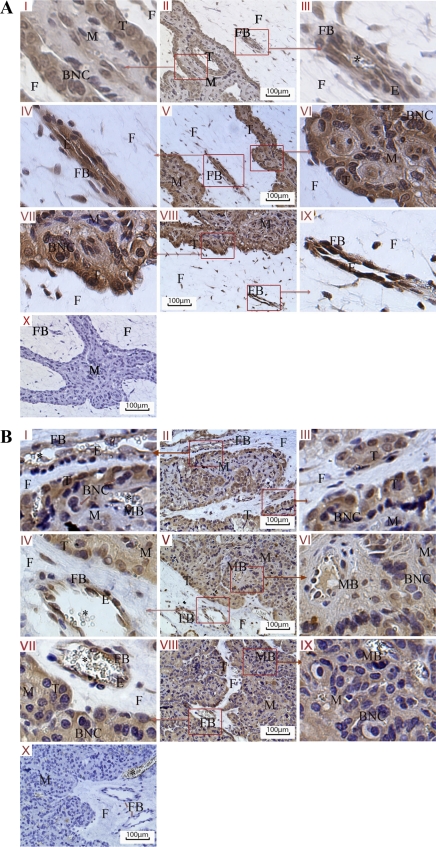
Fig. 6 illustrates endothelial localization of VEGF, PLGF, and FGF-2 in cotyledonary blood vessels of placentomes collected from day 75 (Fig. 6A) and day 135 (Fig. 6B) of gestation, respectively. In nonvascular placentomal tissues, VEGF, PLGF, and FGF-2 were mainly localized in the cytoplasm and nucleus of trophectoderm cells at the fetal-maternal interface. The nuclei of binuclear cells were stained as the trophoblastic cells due to the Harris-modified hematoxylin stain in the maternal caruncular tissue. However, the cytoplasmic area of those binuclear cells failed to be stained with any of the three angiogenic factors, indicating that the three angiogenic factors were not expressed in those binuclear cells.
Fig. 6.
Immunohistochemical staining (brown) of VEGF, PLGF, and FGF-2 in placentomal tissues on day 75 of gestation (A) and day 135 of gestation (B); nuclei are counterstained with with hematoxylin (blue). I–III, VEGF (A and B); IV–VI, PLGF (A and B); VII–IX, FGF-2 (A and B); X, negative control incubated with blocking buffer with no primary antibody (A and B). M, maternal caruncular tissue; F, fetal COT tissue; FB, fetal blood vessel; MB, maternal blood vessel; T, trophoblast cells; BNC, binucleate cells; E, Endothelial layer. An asterisk (*) denotes red blood cells.
DISCUSSION
To the authors' knowledge, this is the first large animal model established to study the impacts of increased maternal calorie intake leading to preconceptional maternal obesity and continued excessive weight gain during pregnancy on fetal and placental growth, and placental angiogenic factor expression and vascularity. The sheep is one of the most commonly and extensively investigated precocial species in biomedical studies on human pregnancy (17, 19, 25, 32). These data demonstrate a markedly (∼ 30%) increased fetal weight in OB vs. C ewes at midgestation, indicating a greater fetal growth trajectory in OB females during the first half of gestation. This increase in fetal growth was associated with a marked increase in COT arteriole diameters compared with those of C ewes. Arterioles are the most highly regulated blood vessels in the body, and contribute the most to overall blood pressure. Arterioles respond to a wide variety of chemical and electrical messages and are constantly changing size to speed up or slow down blood flow through the capillary bed (28). In C ewes, the significant increase in diameter of the arterioles in COT tissue from day 75 to day 135 of gestation would be expected to dramatically increase COT blood flow and hence promote nutrient delivery to the fetus to meet the requirement of rapid fetal growth during the second half of gestation. The failure of COT arterioles to increase in diameter coupled with a reduction of capillary growth and proliferation due to reduced COT angiogenic factor expression in OB ewes may have resulted in the slowed fetal growth observed in this group compared with C ewes. Downregulation of growth factors and/or increases in inflammatory factors in the fetal circulation is known to slow placental and fetal growth and development during gestation (11, 52).
While numbers of COT arterioles were similar in C and OB ewes on day 75, arteriole diameters were ∼35.5% greater in OB ewes. If we apply Poiseuille's Law (see right), which describes the variables controlling flow through the vasculature, the blood flow through COT tissue of OB ewes was 337% greater than in C ewes, if length, pressure, and blood viscosity remain constant.
Such an increase in blood flow would provide a mechanism for the observed fetal overgrowth in OB ewes at day 75 of gestation. Alternatively, the increased blood flow might represent a response to the increased growth rather than a cause. Similar explanations would apply to the interesting continuation of C ewes COT arterioles to increase ∼44.9% in diameter (P < 0.05), while OB ewes COT arterioles didn't show an increase in diameter from day 75 to day 135 of gestation. Again applying Poiseuille's Law, there is a potential 440% increase in blood flow through COT tissue from day 75 to day 135 in C ewes if length, pressure, and blood viscosity remain constant. An increase in blood flow of this magnitude would have a markedly increased nutrient delivery to the fetus, thereby providing for the well-documented acceleration in fetal growth in C ewes that occurs during the second half of gestation (5). This increased blood flow in fetuses from C ewes explains the observation of similar body weights and CRL in fetuses of C and OB ewes regardless of treatment. The failure of CAR arterioles from C and OB ewes to increase in diameter from day 75 to day 135 in this study may result from differences in the pattern of vascular growth in COT and CAR tissues. Reynolds et al. (35) reported that capillary number density (number of capillaries per unit area tissue), a common measure of vascular growth (50) increased exponentially and dramatically (12.3 fold) in the fetal COT but only slightly (1.5 fold) in the maternal caruncle from day 50 to day 140 of sheep pregnancy. Further, the CAR arterioles exhibited markedly greater diameters than COT arterioles on both day 75 and 135 of gestation.
Our observations are in agreement with Catalano et al. (10), who reported that fetuses of obese and lean women exhibit similar weights in late-gestation (37–40 wk). Evidence suggesting an accelerated fetal intra-utero growth rate during first half of gestation followed by a reduced fetal growth rate during the second half of gestation in OB vs. C ewes is suggested by the ratio of brain weight to fetal body weight. At midgestation, fetal brain weight was significantly higher in fetuses of OB ewes than C ewes (7.13 ± 0.19 g and 6.20 ± 0.18 g, respectively, P < 0.05). However, the ratio of brain weight to whole body weight was markably lower in OB fetuses than C fetuses (3.02% ± 0.13% and 3.34% ± 0.07%, respectively, P < 0.05), suggesting a greater increase in fetal body mass than fetal brain weight in OB fetuses. In contrast, in late gestation, fetal brain weight (C fetus, 58.75 ± 1.06 g; OB fetus, 57.80 ± 1.15 g, P > 0.1) and brain/whole fetal body weight (C fetus, 1.17% ± 0.05%; OB fetus, 1.22 ± 0.02%, P < 0.1) were similar for OB and C group, suggesting a reduction in growth rate of OB vs. C fetuses from midgestation to late gestation.
As previously discussed, the COT capillary bed grows primarily by branching angiogenesis throughout the last two-thirds of pregnancy, resulting in a large (>10 fold) increase in capillary area density, accompanied by a decrease in capillary size (44). This marked increase in capillary numbers in the COT tissue results in a simultaneous increase in umbilical blood flow and facilitates transplacental exchange. These observations help to explain why the proportion of nutrients and oxygen transported to the fetus increases by 2- to 4-fold from midgestation to late gestation, a requirement for keeping pace with fetal growth (14, 36). While many factors have been implicated in mediating placental angiogenesis, VEGF, PLGF, FGF-2, ANG-1, and ANG-2, as well as their receptors appear to play a major role (35). Throughout the last two-thirds of gestation in the sheep, COT tissue expression of these angiogenic factors is positively correlated with measures of angiogenesis, including capillary area density, capillary number density, and capillary surface density (35). Further, both VEGF and FGF-2 have been implicated in regulating uterine blood flow through increases in endothelial nitric oxide production, a major local vasodilator (4, 20, 37).
In this study, we report decreases in VEGF, PLGF, FGF-2, ANG-1, and ANG-2 mRNA and protein levels on day 75 of gestation in OB ewe COT arteries. Further, we observed that HIF-1α, a compound known to stimulate angiogenic factor expression (41, 42) also tended to be reduced in COT arteries of OB vs. C ewes on day 75. These data suggest the presence of a fetal/maternal signal-reducing COT arterial angiogenic factor expression and vascular growth in the face of maternal overnutrition. This would be expected to reduce nutrient delivery to the fetal maternal interface, slowing fetal growth rate and preventing fetal overgrowth and dystocia at the time of birth. These data are consistent with those of Redmer et al. (33), who reported a reduction of VEGF, ANG-1 and ANG-2, and FLT-1 mRNA expression in whole placentomes recovered from overnourished adolescent ewes on day 81 of gestation compared with those recovered from adolescent ewes fed only to basic requirements. These data, however, were not confirmed at the protein level in that study (33). Further, these authors (33) reported that uterine arterial blood flow was reduced by 50% on day 90 of gestation in these adolescent overnourished ewes compared with adolescent ewes fed to basic requirements. They concluded that these decreases in angiogenic factor expression may be part of the causal mechanisms since they preceded any reduction in placentomal wet weight, which may constitute an early defect in these adolescent overnourished pregnancies. Because mRNA and protein expression of angiogenic factors were markedly reduced in COT arterial tissues of OB vs. C ewes on day 75, it is possible that capillary growth and blood flow were already slowing in the COT of OB ewes, potentially beginning to impact nutrient diffusion into the fetal compartment. Establishment of the timing and cause of the decrease is an important goal of future studies.
From midgestation to late gestation, a slowing of fetal growth in OB vs. C ewes was observed, such that by day 135, fetuses of C and OB ewes were similar in size. These data are in agreement with Catalano et al. (10), who reported that late-gestation fetuses (37–40 wk) of obese and lean women were similar in size in late-gestation (37–40 wk). These data suggest a slowing of fetal growth in OB vs. C ewes during the second half of gestation. While the numbers of COT arterioles doubled from day 75 to day 135 in both C and OB ewes, arteriolar diameter increased significantly in C ewes, while remaining similar in OB ewes. These data are consistent with the concept that at a time when uteroplacental blood flow increases three-fold in normal pregnancies (30), the placentae of OB ewes are limiting uteroplacental blood flow and fetal nutrient delivery. Reductions in fetal-placental flow in the presence of maternal overnutrition in our study would be consistent with the observations of Wallace et al. (48), who reported that late-gestation umbilical blood flow was lower per kilogram fetus, in overnourished adolescent ewes than adolescent ewes fed to requirements, suggesting a reduced perfusion of the placenta by the fetus. While we used mature ewes in the present study and Wallace et al. (2002) used adolescent ewes, the accelerated increase in the body weight of pregnant ewes on the obesogenic diets in both studies may have resulted in similar changes placental vascularity, but additional studies are required to confirm this hypothesis. Lang et al. (24) reported that a chronic reduction in uterine blood flow from day 113 to day 138 of gestation in the ewe decreased fetal body weight by 32%, which is similar to the relative reduction seen in OB vs. C ewes from midgestation to late gestation in this study. Further, these researchers (24) reported that this chronic reduction in uterine blood flow decreased relative placental weight by 34%, which again is similar to the differences observed in total placentome weight between OB and C ewes in the present study on day 135, which averaged ∼28%. In another recent study, Redmer et al. (34) observed a similar decrease (P < 0.05) in placentome weight at day 130 of gestation in the overnourished pregnant adolescent sheep vs. control-fed pregnant adolescent sheep. It is well accepted that placental angiogenesis is one of the earliest events in placental development and is required to supply nutrients and oxygen for these growing tissues (39). The dramatically increased COT arteriole diameters from midgestation to late gestation reported in this study, coupled with increased capillary diameters and densities (S. P. Ford et al., unpublished observations) in C ewes vs. OB ewes would be expected to further increase blood flow into the COT vascular bed, promoting the growth of the placentome itself. The significant increase in placental efficiency (fetal weight/placental weight ratio) of OB vs. C ewes at midgestation, results from fetal overgrowth, while at late gestation, it results from decreased placentomal weight. These data suggest the presence of a fetal signal reducing COT blood flow and thus placental growth and nutrient delivery to the fetal compartment in the face of elevated maternal feed intake and circulating nutrient concentrations. The term coined by Lang et al. (24) to explain the decrease in placental weight resulting in response to this chronic reduction in uterine blood flow was “blood flow-mediated adaptive regression.” This concept is supported by the changes in COT angiogenic factor expression and vascularity observed in this study. While COT arterial tissue angiogenic factor (VEGF, FGF-2, ANG-1, and ANG-2) expression decreased progressively from day 75 to day 135 in C ewes, these same angiogenic factors remained low and constant from midgestation to late gestation in OB ewes.
A similar early gestational acceleration in fetal growth followed by a reduced growth trajectory from midgestation to late gestation has recently been described by Redmer et al. (34) in an overnourished adolescent ewe model. Compared with control fed adolescent ewes, these researchers observed an 11% increase in fetal weight in overnourished adolescent ewes by day 90 of gestation, followed by a 20% decrease by day 130 of gestation. Similar to the results presented here for the mature multiparous ewes, pregnant overnourished adolescent ewes exhibit a reduction in placental mass by late gestation, but unlike the current study, which overnourished mature ewes, their adolescent overnourished ewes gave birth prematurely to low-birth-weight lambs (47, 48). In contrast, using mature ewes, previous research in our laboratory demonstrated similar birth weight between C and OB ewes, which averaged 5,320 ± 470 and 6,000 ± 321 g, respectively (16). A significant decrease in placentome numbers has also been reported in adolescent overnourished ewes vs. control fed adolescent ewes on day 134 (48) and at birth (46). The authors of this report concluded that this decrease was due to a flow-induced atrophy or regression of a portion of the placentomes during the second half of gestation. No similar decrease in placentome numbers was observed in the present study, where numbers of placentomes of OB ewes tended to be greater than C ewes on day 135. Additionally, previous research in our laboratory demonstrated similar placentome numbers between C and OB ewes at term, which averaged 81 ± 4 and 78 ± 8, respectively (n = 5/group; S. P. Ford et al., unpublished observations). These data suggest that in contrast to overnourished adolescent ewes, mature obese overnourished ewes slowed fetal and placental growth in late gestation without significant reductions in placentome number or birth weight. This difference may result from a greater ability of older mature ewes, as used in this study, than adolescent ewes to meet or exceed their own nutrient requirements, while at the same time partitioning adequate nutrients for fetal growth. Our study and that conducted with overnourished adolescent ewes cannot be directly compared, however, since the ewes in our study were already obese at the time of conception, while those in the adolescent ewe study only became obese several weeks into gestation (49).
The lack of any differences in CAR arteriole numbers and diameters between C and OB ewes at either midgestation or late gestation is consistent with the concept that differences in uterine artery arteriogenesis played a minor role, if any, in the group differences in placental vascularity and fetal growth reported here. This is supported by Gassman et al. (18), who reported that uterine arteriogenesis during a gestation is largely irreversible, and thus after several pregnancies, does not play a major role in pregnancy outcome (18).
Regulation of fetal growth is largely a balance between fetal nutrient demand determined by the individual fetal genetic growth potential and the intrauterine environment, in which the maternal to fetal nutrient supply is a major factor. Factors that determine maternal to fetal nutrient supply include maternal nutrition and metabolism, the transplacental concentration gradient, uteroplacental blood flow, placental size, and placental transport capabilities (31, 54). Zhu et al. (54), using the same experimental paradigm utilized in this study, reported that maternal blood glucose and insulin concentrations were markedly higher in OB ewes and fetuses than C ewes and fetuses at midgestation. Jansson et al. (22) summarized evidence suggesting that gestational diabetes mellitus-induced upregulation of placental nutrient transporter activity, with an associated increase in fetal/neonatal size, may result from elevated insulin concentrations in maternal blood. Insulin has been reported to increase placental glucose transport capacity (13) and System A (neutral amino acid transport) activity (21) in vitro.
Perspectives and Significance
These data demonstrate for the first time in a large animal model that prepregnancy maternal obesity resulting from dietary excess causes an initial accelerated fetal growth rate to midgestation, followed by a markedly reduced fetal growth trajectory to term compared with animals fed to basic requirements. By midgestation, COT arteriole diameters of OB conceptuses were markedly larger than those of C conceptuses, potentially increasing COT blood flow and nutrient delivery from mother to fetus, and increasing fetal weight. The marked decrease in expression of key angiogenic factors observed at midgestation in the COT arteries of OB vs. C conceptuses would be expected to decrease capillary proliferation in the COT, slowing fetal growth rate. The signal whereby placental vascular growth is suppressed in the OB placenta is, at present, unknown, but leads to normal birth weights at term in these OB animals. Results from our ovine model are similar to outcomes of human pregnancies, where infants from obese mothers often exhibited macrosomia in gestation but are born at normal weights. These infants, however, exhibit a marked increase in adiposity at term, which is associated with insulin resistance and obesity in postnatal life. The similarity of pregnancy outcomes in our obese sheep model and the human data from obese pregnancies suggest common mechanisms, which could potentially lead to a better understanding of the specific control mechanisms involved.
GRANTS
This project was supported by the University of Wyoming National Institutes of Health Grant INBRE P20-RR–016474.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Adam Uthlaut for help with animal care, sample collection, and sample processing.
REFERENCES
- 1.Anthony RV, Limesand SW, Jeckel KM. Transcriptional regulation in the placenta during normal and compromised fetal growth. Biochem Soc Trans 29: 42–28, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Aron EA, Anthony RV. Angiogenesis. In: Fetal and Neonatal Physiology ( 3rd ed.), edited by Polin RA, Fox WW, Abman SH. Philidelphia: Saunders WB Company, 2003, p. 79–84 [Google Scholar]
- 4.Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res 82: 1007–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology 69: 55–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassingthwaighte JB, Goresky CA. Modeling in the analysis of solute and water exchange in the microvasculature. In: Handbook of Physiology. The Cardiovascular System. Micrcirculation. Bethesda MD: Am. Physiol. Soc., 1984, set. 2, vol. IV, pt. 1, chapt 13, p. 549–626 [Google Scholar]
- 7.Bio-Rad Laboratories Real-Time PCR Applications Guide. Hercules, CA: Bio-Rad, 2006 [Google Scholar]
- 8.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol Reprod 76: 259–267, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32: 1076–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 16: 206–215, 2009 [DOI] [PubMed] [Google Scholar]
- 12.National Research Council Nutrient Requirements of Sheep. Washington DC: National Academic Press; 1985 [Google Scholar]
- 13.Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod 20: 521–530, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ferrell CL. Placental regulation of fetal growth. In: Animal Growth Regulation, edited by Campion DR, Hausman GJ, Martin RJ. New York: Plenum, 1989, p. 1–19 [Google Scholar]
- 15.Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic β cell but not α cell development in the sheep: prenatal and postnatal consequences. Am J Physiol Regul Integr Comp Physiol 297: R835–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowden AL, Forhead AJ. Effects of pituitary hormone deficiency on growth and glucose metabolism of the sheep fetus. Endocrinology 148: 4812–4820, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gassmann M, Manini A, Stallmach T, Saam B, Kuhn G, Grenacher B, Bogdanova AY, Vogel J. Abortion in mice with excessive erythrocytosis is due to impaired arteriogenesis of the uterine arcade. Biol Reprod 78: 1049–1057, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Harding JE, Evans PC, Gluckman PD. Maternal growth hormone treatment increases placental diffusion capacity but not fetal or placental growth in sheep. Endocrinology 138: 5352–5358, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates eNOS message, protein, and NO production in human endothelial cells. Am J Physiol Heart Circ Physiol 274: H1054–H1058, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 88: 1205–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jansson T, Cetin I, Powell TL, Desoye G, Radaelli T, Ericsson A, Sibley CP. Placental transport and metabolism in fetal overgrowth—a workshop report. Placenta 27 Suppl A: S109–S113, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17β on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine 30: 333–342, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lang U, Baker RS, Khoury J, Clark KE. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol Regul Integr Comp Physiol 279: R53–R59, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Andreo M, Lugo L, Garrido-Pertierra A, Prieto MI, Puyet A. Identification and quantitation of species in complex DNA mixtures by real-time polymerase chain reaction. Anal Biochem 339: 73–82, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Melbin J, Detweiler DK. The cardiovascular system and blood flow. In: Duke's Physiology of Domestic Animals ( 11th ed.), edited by Swenson MJ, Reece WO. New York: Cornell University Press, 1993, p. 64–89 [Google Scholar]
- 29.Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 31: 1872–1876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molina RD, Meschia G, Wilkening RB. Uterine blood flow, oxygen and glucose uptakes at mid-gestation in the sheep. Proc Soc Exp Biol Med 195: 379–385, 1990 [DOI] [PubMed] [Google Scholar]
- 30a.National Center for Health Statistics Health, United States, With Chartbook on Trends in the Health of Americans. Hyattsville, MD: National Center for Health Statistics; 2007, p. 88–292 [Google Scholar]
- 31.Pedersen J. The Pregnant Diabetic and Her Newborn: Problems and Management. Baltimore: Williams & Wilkens, 1967 [Google Scholar]
- 32.Recabarren SE, Padmanabhan V, Codner E, Lobos A, Duran C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 289: E801–E806, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Redmer DA, Aitken RP, Milne JS, Reynolds LP, Wallace JM. Influence of maternal nutrition on messenger RNA expression of placental angiogenic factors and their receptors at midgestation in adolescent sheep. Biol Reprod 72: 1004–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Redmer DA, Luther JS, Milne JS, Aitken RP, Johnson ML, Borowicz PP, Borowicz MA, Reynolds LP, Wallace JM. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction 137: 749–757, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta 26: 689–708, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Reynolds LP, Ferrell CL. Transplacental clearance and blood flows of bovine gravid uterus at several stages of gestation. Am J Physiol Regul Integr Comp Physiol 253: R735–R739, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17β in ovariectomized ewes: expression of angiogenic factors. Biol Reprod 59: 613–650, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod 64: 1033–1040, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci 73: 1839–1851, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci 71: 1112–1116, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Shih SC, Claffey KP. Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. J Biol Chem 274: 1359–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol 217: 809–818, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Smith JJ, Kampine JP. Circulatory Physiology-The Essentials ( 3rd ed.). Baltimore: Williams & Wilkins, 1990, 345 p. [Google Scholar]
- 44.Stegeman JHJ. Placental development in the sheep and its regulation to fetal development. Bijdragen Tot De Dierkunde 44: 4–72, 1974 [Google Scholar]
- 45.Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol 15: 351–356, 1991 [PubMed] [Google Scholar]
- 46.Wallace J, Bourke D, Da Silva P, Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction 122: 347–357, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Wallace JM, Aitken RP, Milne JS, Hay WW., Jr Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol Reprod 71: 1055–1062, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol 282: R1027–R1036, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW., Jr Nutritional modulation of adolescent pregnancy outcome—a review Placenta 27Suppl A: S61–S68, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Weibel ER. Point counting methods. J Microsc 95: 373–378, 1972 [Google Scholar]
- 51.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114: e29–e36, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Zhu MJ. Down-regulation of growth signaling pathways linked to a reduced cotyledonary vascularity in placentomes of over-nourished, obese pregnant ewes. Placenta 30: 405–410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu MJ, Du M, Hess BW, Nathanielsz PW, Ford SP. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome. Placenta 28: 1192–1199, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]