Abstract
Prostaglandins, generated within the fetal brain, are integral components of the mechanism controlling the fetal hypothalamus-pituitary-adrenal (HPA) axis. Previous studies in this laboratory demonstrated that prostaglandin G/H synthase isozyme 2 (PGHS-2) inhibition reduces the fetal HPA axis response to cerebral hypoperfusion, blocks the preparturient rise in fetal plasma ACTH concentration, and delays parturition. We also discovered that blockade of N-methyl-d-aspartate (NMDA) receptors reduces the fetal ACTH response to cerebral hypoperfusion. The present study was designed to test the hypothesis that PGHS-2 action and the downstream effect of HPA axis stimulation are stimulated by NMDA-mediated glutamatergic neurotransmission. Chronically catheterized late-gestation fetal sheep (n = 8) were injected with NMDA (1 mg iv). All responded with increases in fetal plasma ACTH and cortisol concentrations. Pretreatment with resveratrol (100 mg iv, n = 5), a specific inhibitor of PGHS-1, did not alter the magnitude of the HPA axis response to NMDA. Pretreatment with nimesulide (10 mg iv, n = 6), a specific inhibitor of PGHS-2, significantly reduced the HPA axis response to NMDA. To further explore this interaction, we injected NMDA in six chronically catheterized fetal sheep that were chronically infused with nimesulide (n = 6) at a rate of 1 mg/day into the lateral cerebral ventricle for 5–7 days. In this group, there was no significant ACTH response to NMDA. Finally, we tested whether the HPA axis response to prostaglandin E2 (PGE2) is mediated by NMDA receptors. Seven chronically catheterized late-gestation fetal sheep were injected with 100 ng of PGE2, which significantly increased fetal plasma ACTH and cortisol concentrations. Pretreatment with ketamine (10 mg iv), an NMDA antagonist, did not alter the ACTH or cortisol response to PGE2. We conclude that generation of prostanoids via the action of PGHS-2 in the fetal brain augments the fetal HPA axis response to NMDA-mediated glutamatergic stimulation.
Keywords: adrenocorticotropic hormone, blood pressure, cortisol
fetal sheep maintain cardiovascular homeostasis in response to hypoxia (1, 9), hypovolemia (23, 24), and hypotension (30, 34) via autonomic and neuroendocrine responses to arterial chemoreceptor and baroreceptor activity (19, 25). Previous studies in this and other laboratories demonstrated that the reflex responses to hypotension are mediated in part by peripheral (carotid sinus) and central (35, 37) receptors. At the same time, it is known that as the fetus develops in late gestation, there is an increase in reflex responsiveness that might be an important component of readiness for extrauterine life (20, 26, 31). In addition to developmental changes in the integrity of the reflex pathways and the activity of the reflexes, there are endogenous modulators of the reflex. We reported previously that indomethacin [a nonselective prostaglandin G/H synthase (PGHS) inhibitor] attenuates the HPA axis response to brachiocephalic occlusion (BCO), a manipulation that results in cerebral hypoperfusion and stimulates autonomic and neuroendocrine responses (30). We subsequently demonstrated that this influence of prostaglandins is dependent on PGHS-2 activity within the fetal brain (i.e., not dependent on prostaglandins in the general circulation) (7, 22, 36).
While fetal central nervous system (CNS)-generated prostaglandins actively modulate the fetal cardiovascular reflexes, the hypothalamus-pituitary-adrenal (HPA) axis response to cerebral hypoperfusion is almost completely dependent on glutamatergic neurotransmission within the fetal brain (18). Previous studies showed that blockade of N-methyl-d-aspartate (NMDA) receptors with ketamine blunts the fetal reflex bradycardic response to hypoxia (2). We subsequently demonstrated that NMDA blockade dramatically reduces the HPA axis response to BCO (18). While we have good evidence that although NMDA-mediated neurotransmission and PGHS-2-mediated prostaglandin biosynthesis are involved in the HPA axis response to cerebral hypotension in fetal sheep, we have not demonstrated that these processes are linked. In the present study, we hypothesized that the HPA axis response to NMDA stimulation is modulated by PGHS-2-mediated prostaglandin generation within the fetal brain. The present experiments were designed to test this hypothesis.
MATERIALS AND METHODS
These experiments were approved by the University of Florida Institutional Animal Care and Use Committee and were performed in accordance with the “Guiding Principles for Use of Animals” of the American Physiological Society. In studies 1, 2, and 3, we studied 12, 5, and 8 pregnant ewes, respectively, with singleton or twin fetuses.
Surgical preparation.
Aseptic surgery was performed ≥5 days prior to the experiment in each animal. In studies 1 and 2, fetal surgery was performed as previously described (30). Polyvinyl chloride catheters were introduced into the tibial artery and saphenous vein in each fetal hindlimb, and another catheter was inserted within the amniotic fluid and secured to the fetal hindlimb. In study 3, fetal surgery additionally included placement of an osmotic minipump (model 2ML2, Alza, Cupertino, CA) for infusion of nimesulide into the lateral cerebral ventricle, as previously described (7). Postoperative care was performed as previously described (7). Each animal was monitored twice per day, with measurement of rectal temperature, and treated with ampicillin (750 mg sc or im; Polyflex, Ft. Dodge Laboratories, Ft. Dodge, IA) for 5 days after the surgery. All catheters were flushed and reheparinized at least once every 5 days.
Experimental protocols.
In study 1, fetuses were studied in one of three groups. In the control group [n = 8, 134 ± 6 (SD) days gestational age], NMDA injection was not preceded by a drug injection. In the second (resveratrol) group (n = 5, 134 ± 6 days gestational age), resveratrol (100 mg iv; Cayman Chemical, Ann Arbor, MI) was administered 30 min before NMDA injection. In the third (nimesulide) group (n = 6, 134 ± 4 days gestational age), nimesulide (10 mg iv; Cayman Chemical) was administered 30 min before NMDA injection. Blood samples (5 ml each) were drawn 30 min before and 0, 10, 20, and 30 min after injection of NMDA (10 mg iv; Acros Organics, Fisher Scientific) and put into chilled Vacutainer tubes containing K+-EDTA. In addition to blood samples drawn for hormone assay, samples (1 ml) for measurement of blood gases were drawn in chilled heparinized syringes. Blood gas samples were immediately analyzed (ABL77 analyzer, Radiometer Copenhagen, Denmark). Plasma for hormone assay was separated from erythrocytes by centrifugation, divided into aliquots, and stored at −20°C. Plasma was assayed for ACTH and cortisol as previously described (36). Some of the plasma samples were assayed for proopiomelanocortin; however, this is an incomplete set, because the assay kits were no longer available. For this reason, the proopiomelanocortin results are not reported.
In study 2, two groups were studied. Blood samples (5 ml) were drawn at 0, 5, 10, 20, and 30 min. In the control group (128 ± 3 days gestational age), PGE2 (100 ng; Cayman Chemical) was administered immediately after the 0-min sample was drawn. In the experimental group (127 ± 3 days gestational age), ketamine was administered 15 min prior to administration of PGE2. Blood and amniotic fluid pressures were measured and blood samples for hormone and blood gas analysis were processed as described for study 1.
In study 3, fetuses were infused intracerebroventricularly with nimesulide (n = 6, 127 ± 2 days gestational age). A smaller number of fetuses were treated with a similar infusion of vehicle (50% deionized water-50% dimethylsulfoxide; Fisher Scientific). The effect of chronic blockade of PGHS-2 was tested on the response to NMDA. In the treated fetuses, nimesulide was infused into the lateral cerebral ventricle (1 mg/day intracerebroventricularly) using an osmotic minipump (model 2ML2, Alza) starting at the time of surgery. Blood samples were drawn at 0, 5, 10, 20, and 30 min after injection of 10 mg of NMDA, as described for study 1. Blood and amniotic fluid pressures were measured and blood samples for hormone and blood gas analysis were processed as described for study 1.
Throughout the experiment, arterial and amniotic pressures were measured using disposable pressure transducers (Transpac IV, Hospira, Lake Forest, IL), an analog-to-digital converter (National Instruments, Austin, TX), and custom-written software (LabView, National Instruments). Fetal intravascular pressures were corrected by subtraction of the amniotic fluid pressure. Fetal heart rate (HR) was calculated from the phasic arterial pressure signal.
Statistical analysis.
Data were analyzed using one- and two-way ANOVA corrected for repeated measures (33). Pairwise comparisons were performed using simple effects contrasts (5). The criterion for assessment of statistical significance was P < 0.05. The computation of the ANOVAs was performed using SPSS version 15 (SPSS, Chicago, IL). In study 1, measured variables in the nimesulide and resveratrol groups were compared separately with the values of those variables in the control group by two-way ANOVA, as designed a priori.
RESULTS
Study 1.
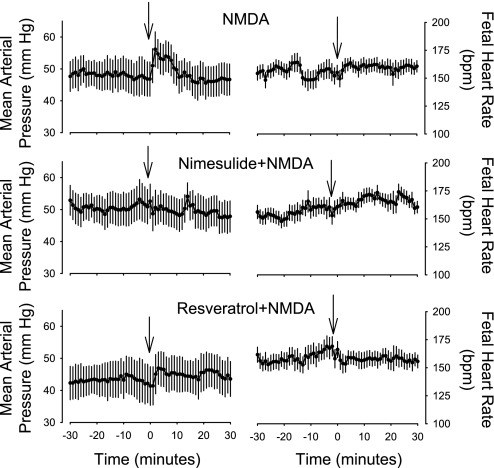
NMDA injection stimulated increases in fetal mean arterial blood pressure that were blocked by injection of nimesulide 30 min before NMDA injection (Fig. 1; P < 0.05 for time × group interaction in ANOVA comparing nimesulide with control group). The increase in blood pressure after NMDA injection was not blocked by resveratrol [Fig. 1; P = not significant (NS) for time × group interaction in ANOVA comparing resveratrol with control group]. NMDA did not significantly alter the fetal HR in any of the groups (P = NS by ANOVA).
Fig. 1.
Fetal mean arterial blood pressure and heart rate (beats/min) as measured in study 1. Fetal mean arterial blood pressure was increased significantly by N-methyl-d-aspartate (NMDA) and resveratrol + NMDA; these responses were not significantly different from each other. Nimesulide significantly blocked the blood pressure response to NMDA. Arrows show time of NMDA injection. Values are means ± SE.
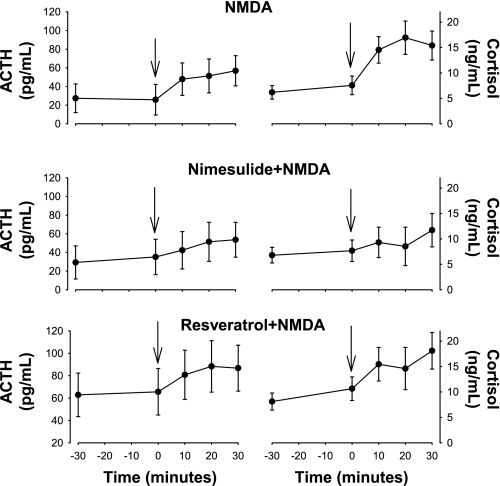
NMDA stimulated increases in fetal plasma concentrations of ACTH and cortisol plasma concentrations in all three groups (Fig. 2). However, comparison of the control group with the nimesulide group by two-way ANOVA revealed a statistically significant interaction between group and time for ACTH and cortisol (P < 0.05 for both variables), demonstrating that acute nimesulide treatment reduced the HPA axis response to NMDA injection. Comparison of the control and resveratrol groups by two-way ANOVA for repeated measures revealed no statistically significant effect of resveratrol on the ACTH or cortisol responses to NMDA (P = NS by ANOVA).
Fig. 2.
Plasma concentrations of ACTH and cortisol as measured in study 1. NMDA stimulated significant increases in ACTH and cortisol in all 3 groups; however, these responses were significantly smaller in the nimesulide + NMDA group. Arrows show time of NMDA injection. Values are means ± SE.
Arterial blood gases in the fetuses of study 1 were similar to those that have been interpreted as representative of healthy fetuses (Table 1). None of the manipulations significantly altered fetal blood gases (Table 1; significance testing using ANOVA).
Table 1.
Arterial Po2, Pco2, and pH in fetuses included in study 1
| Control-NMDA (n = 8) | Nimesulide-NMDA (n = 5) | Resveratrol-NMDA (n = 6) | |
|---|---|---|---|
| Arterial Po2, mmHg | |||
| −30 min | 20.9 ± 1.1 | 18.8 ± 1.4 | 22.2 ± 1.3 |
| 0 min | 21.1 ± 1.1 | 18.6 ± 1.4 | 20.3 ± 1.3 |
| 10 min | 21.4 ± 1.3 | 18.2 ± 1.6 | 23.2 ± 1.5 |
| 20 min | 22.1 ± 1.6 | 18.6 ± 2.0 | 21.7 ± 1.8 |
| 30 min | 20.5 ± 1.1 | 17.8 ± 1.4 | 22.0 ± 1.3 |
| Arterial Pco2, mmHg | |||
| −30 min | 56.1 ± 1.0 | 57.8 ± 1.2 | 53.5 ± 1.1 |
| 0 min | 57.6 ± 1.1 | 56.4 ± 1.4 | 51.5 ± 1.2 |
| 10 min | 57.1 ± 1.4 | 56.8 ± 1.8 | 51.2 ± 1.7 |
| 20 min | 56.9 ± 1.4 | 58.2 ± 1.8 | 51.2 ± 1.6 |
| 30 min | 56.0 ± 1.3 | 56.2 ± 1.6 | 50.2 ± 1.5 |
| Arterial pH | |||
| −30 min | 7.35 ± .01 | 7.32 ± .01 | 7.35 ± .01 |
| 0 min | 7.34 ± .01 | 7.33 ± .01 | 7.34 ± .01 |
| 10 min | 7.34 ± .01 | 7.32 ± .01 | 7.34 ± .01 |
| 20 min | 7.33 ± .01 | 7.31 ± .01 | 7.33 ± .01 |
| 30 min | 7.34 ± .01 | 7.32 ± .01 | 7.34 ± .01 |
Values (means ± SE) are consistent with healthy chronically instrumented fetal sheep. Time is relative to injection of N-methyl-d-aspartate (NMDA). Vehicle, nimesulide, or resveratrol was injected 30 min prior to NMDA injection. There were no statistically significant differences among groups, and no significant changes were caused by NMDA injection.
Study 2.
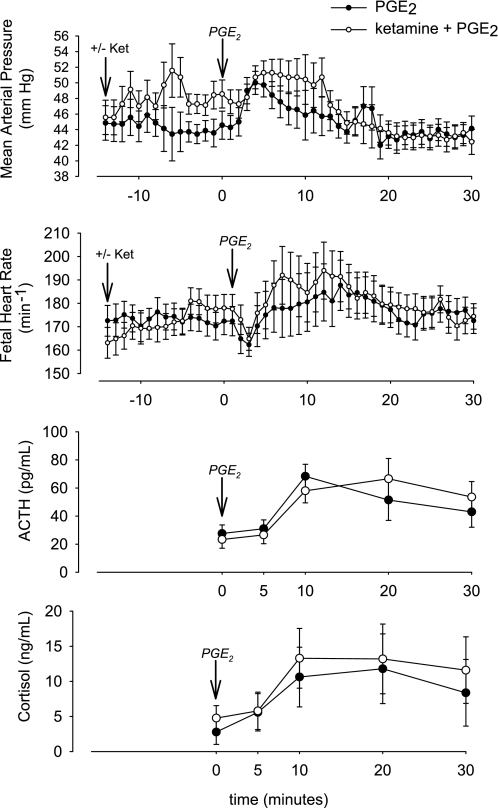
Consistent with results of previous studies in adult patients (14, 32), ketamine increased fetal blood pressure and HR (P < 0.001 for changes in blood pressure between −10 and 0 min as analyzed by 1-way ANOVA for repeated measures). PGE2 stimulated increases in blood pressure and HR that were superimposed on the baseline values at the time of injection (Fig. 3). Overall, the changes in blood pressure in the two groups were significantly different (P < 0.05 for group × time interaction), although the differences were accounted for by the increase in blood pressure acutely after administration of ketamine (Fig. 3). In contrast to the changes in blood pressure, the changes in fetal HR in response to PGE2 with and without ketamine pretreatment were not different in the two groups (P = NS by ANOVA).
Fig. 3.
Fetal mean arterial blood pressure, heart rate, and plasma concentrations of ACTH and cortisol in study 2. Vertical arrows show time of ketamine and PGE2 injections. Mean arterial pressure was significantly increased by ketamine and PGE2, both together and individually. Heart rate was significantly altered by PGE2, but not ketamine. ACTH and cortisol responses to PGE2 were not altered by ketamine. Values are means ± SE.
PGE2 injection significantly increased fetal ACTH and cortisol plasma concentrations (Fig. 3). The increases in fetal plasma ACTH and cortisol were not significantly altered by the ketamine pretreatment, suggesting that the HPA axis response to PGE2 is not dependent on NMDA-mediated glutamatergic neurotransmission. Fetal blood gases were not significantly altered by PGE2 with or without pretreatment with ketamine (P = NS for all 3 variables by ANOVA; Table 2).
Table 2.
Fetal blood gas and pH values in fetuses included in study 2
| Arterial Po2, mmHg |
Arterial Pco2, mmHg |
Arterial pH |
||||
|---|---|---|---|---|---|---|
| −Ket | +Ket | −Ket | +Ket | −Ket | +Ket | |
| 0 min | 17.4 ± 1.1 | 18.3 ± 1.1 | 56.4 ± 1.4 | 58.7 ± 1.4 | 7.34 ± 0.01 | 7.34 ± 0.01 |
| 5 min | 18.1 ± 1.1 | 17.6 ± 1.1 | 56.9 ± 1.6 | 58.3 ± 1.6 | 7.35 ± 0.01 | 7.35 ± 0.01 |
| 10 min | 16.3 ± 1.0 | 15.6 ± 1.0 | 58.1 ± 1.1 | 60.6 ± 1.0 | 7.32 ± 0.01 | 7.33 ± 0.01 |
| 20 min | 17.6 ± 1.1 | 16.3 ± 1.1 | 56.4 ± 1.0 | 58.4 ± 1.0 | 7.34 ± 0.01 | 7.34 ± 0.01 |
| 30 min | 16.4 ± 1.0 | 15.1 ± 1.0 | 56.9 ± 1.2 | 58.9 ± 1.2 | 7.34 ± 0.01 | 7.34 ± 0.01 |
Values are means ± SE. Time is relative to injection of NMDA. Vehicle, nimesulide, or resveratrol was injected 30 min before NMDA injection. Ket, ketamine. There were no statistically significant differences between groups or in response to PGE2 injection.
Study 3.
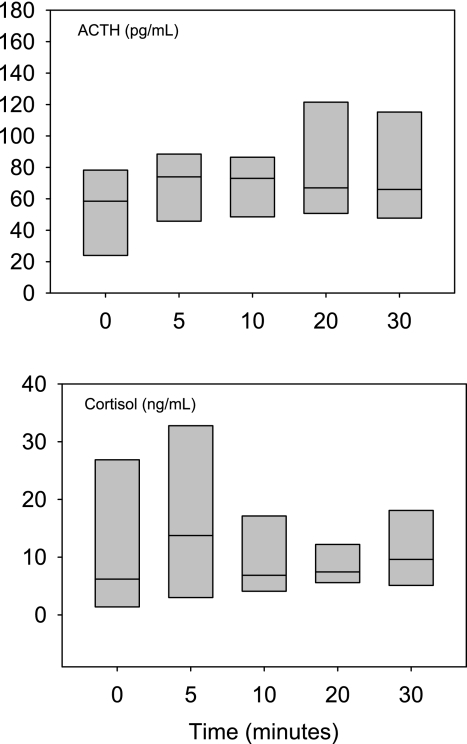
NMDA was injected in fetal sheep that were treated chronically with an intracerebroventricular infusion of nimesulide during the postoperative recovery period (∼5 days). In these fetuses, NMDA did not reproducibly increase fetal ACTH or cortisol concentrations. In the group of six fetuses, plasma ACTH increased after NMDA injection in only three fetuses, and plasma ACTH decreased or remained relatively unchanged in three fetuses. Plasma ACTH and cortisol responses in this group of fetuses are illustrated in Fig. 4. Analysis by parametric (ANOVA) and nonparametric (Friedman's test) statistics showed no statistically significant changes in ACTH or cortisol in this group of fetuses. In a smaller group of three fetuses, we infused vehicle alone into the lateral cerebral ventricle, and we found that all fetuses responded to NMDA with increases in ACTH and cortisol and that the responses to NMDA were indistinguishable from those of fetuses not subjected to chronic intracerebroventricular infusion of vehicle (data not shown). When the chronically nimesulide-treated fetuses were compared with all fetuses that were not treated with nimesulide, two-way ANOVA revealed that nimesulide significantly reduced the magnitude of the ACTH and cortisol responses to the NMDA.
Fig. 4.
Fetal plasma ACTH and cortisol responses to NMDA injection (at 0 min) in fetuses subjected to chronic intracerebroventricular infusion of nimesulide (1 mg/day) in study 3. Data are represented as boxes, which define 90th percentiles. ACTH and cortisol responses to NMDA were attenuated by intracerebroventricular injection of nimesulide compared with responses in unpretreated fetuses (Fig. 2). Horizontal lines within boxes represent medians.
Fetuses chronically treated with nimesulide responded to the NMDA injection with statistically significant increases in mean arterial pressure (P < 0.01 by ANOVA), but no changes in fetal HR (data not shown). Again, the cardiovascular response to NMDA is not different from fetuses not treated with nimesulide or resveratrol in study 1. Baseline values of mean arterial pressure and HR were 45.5 ± 1.5 (SE) mmHg and 184 ± 14 (SE) min−1, respectively. There were no statistically significant changes in fetal blood gas and pH values in response to NMDA in the fetuses chronically treated with nimesulide. Baseline values of arterial Po2, Pco2, and pH were 18.7 ± 2.8 (SE) mmHg, 56.0 ± 2.9 (SE) mmHg, and 7.34 ± 0.01 (SE), respectively.
DISCUSSION
The results of the present study support the concept that there is an association between NMDA-mediated glutamatergic neurotransmission and prostaglandin generation and action in the neuroendocrine control of the HPA axis in fetal sheep. The results of this study suggest that, within one or more regions important for neuroendocrine and cardiovascular control, NMDA receptor-mediated glutamatergic neurotransmission stimulates PGHS-2-mediated prostanoid generation, which, in turn, augments the activity of this pathway. The response of the fetal HPA axis to NMDA is at least partially blocked by nimesulide, a specific PGHS-2 inhibitor.
The role of CNS PGHS-2 in the control of the fetal HPA axis has been demonstrated in acute stress responsiveness (36) and in the mechanisms controlling the initiation of parturition in the sheep fetus (7). Interestingly, this enzyme appears to be a fundamental component of the fetal HPA axis. The present study suggests that the role of PGHS-2 is at least in part dependent on glutamatergic stimulation of the NMDA receptor. What is unclear is where this interaction occurs. PGHS-2 abundance is highest in the cerebral cortex and hippocampus, with lower expression in the hypothalamus and brain stem (8). This pattern of expression in the fetal sheep is similar to that in the adult rat (8, 13). The infusion of nimesulide reduces prostaglandin generation throughout the brain (7), and general levels of expression of PGHS-2 do not provide useful clues as to which regions are important with regard to NMDA-PGHS-2 interactions in control of the fetal HPA axis. Nevertheless, there is significant overlap in the expression of NMDA receptors and PGHS-2 (3, 17, 29). We have found, for example, colocalization of NMDA receptor and PGHS-2 in medullary brain stem (Powers and Wood, unpublished observations).
An interesting outcome of our experiments is that chronic treatment with nimesulide appeared to be more effective in inhibiting the fetal ACTH and cortisol response to NMDA than was acute treatment. This is similar to our previous report regarding the inhibition of fetal ACTH responses to BCO. We reported that intracerebroventricular injection of nimesulide 30 min before BCO did not significantly reduce the ACTH response to BCO (21). However, infusion of nimesulide into the lateral cerebral ventricle for 5 days significantly reduced the fetal ACTH response to BCO (36). This suggests that the effect of PGHS-2 is to augment reflex responsiveness by promoting relatively long-term changes in reflex pathway responsiveness to stimulation. On the other hand, it is possible that the two methods of delivery of nimesulide produced different target tissue concentrations within the fetal brain. Our experimental design could not rule this out as a possibility.
Another interesting aspect of the chronic nimesulide experiments (study 3) is that we seemed to have three “responders” and three “nonresponders” to NMDA. Post hoc analysis of the data revealed that the nonresponders tended to have higher plasma concentrations of cortisol and ACTH before NMDA injection and that the plasma concentrations of ACTH and cortisol were correlated to each other only in the group in which both changed in response to NMDA. Nevertheless, even in the three responders, the HPA axis response to NMDA appeared to have smaller ACTH and cortisol responses to NMDA than the fetuses treated with intracerebroventricular infusion of vehicle. The explanation for this variability in the animals injected intracerebroventricularly with nimesulide is unknown but might be related to effects on fetal autonomic function, which, in turn, affect fetal blood pressure, cardiovascular function, and, likely, hormone secretion and clearance (36).
We propose that, with respect to the control of the fetal HPA axis response to cerebral hypoperfusion, the following model explains the interaction of glutamate and PGHS-2. Within the CNS pathways governing the HPA axis response to BCO, the major controller of the response is a glutamatergic pathway with neurotransmission mediated by NMDA receptors. Stimulation of the relevant NMDA receptors leads to increased prostanoid biosynthesis via the action of PGHS-2. The generated prostanoid augments the effect of the glutamatergic input to the HPA axis, but it is not required for stimulation of the axis. We know this because blockade of NMDA receptors completely blocks the HPA axis response to BCO, but blockade of PGHS-2 reduces, but does not completely block, the ACTH response to BCO (18, 36). Also, in the present study, blockade of PGHS-2 does not consistently result in a complete blockade of the fetal ACTH response to NMDA injection. Not all the cortisol response to BCO is dependent on the stimulation of ACTH secretion. The adrenal cortical response appears to be influenced by factors in addition to ACTH, as blockade of NMDA receptors does not block the cortisol response to BCO (although it completely blocks the ACTH response) (18). This adrenal effect is probably not an NMDA-mediated effect, since blockade of PGHS-2 in the present study completely blocked the increase in cortisol that was observed in response to NMDA injection in control animals.
While the neuroanatomic details of the NMDA-PGHS-2 interaction are not known, there is precedent in the literature for the existence of such an interaction. In adult animals, the influence of glutamate on HPA axis activity, especially with regard to mediation by the NMDA receptor (12), is well known. For example, blockade of the NMDA receptor with ketamine blocks the HPA axis response to chronic stress in rats (6). At the same time, activation of NMDA-dependent glutamatergic neurotransmission upregulates PGHS-2 expression. For example, nociceptive stimuli increase PGHS-2 expression in the dorsal horn of the spinal cord, a response that is blocked by pretreatment with MK-801, a specific antagonist of the NMDA receptor (15). Electrical stimulation increases hippocampal PGHS-2 expression in adult rats; again, the increase in PGHS-2 expression is blocked by MK-801 (38). We have found similar results in our laboratory: blockade of the NMDA receptor with ketamine reduces the expression of PGHS-2 in the fetal brain and blocks the increase in PGHS-2 expression that normally is stimulated by BCO (Powers and Wood, unpublished observations).
The association of PGE2 and ACTH secretion has long been recognized in the fetal sheep (10). While circulating concentrations of PGE2 are higher in fetal plasma than in the adult animal, it is the prostaglandin produced within the fetal brain that is critical for influencing fetal ACTH secretion (4, 28, 30, 36). The role of prostaglandin appears to be to augment the activity of the pathways that, in turn, stimulate activity of the HPA axis. Blockade of PGHS-2 chronically in the fetal brain reduces fetal HPA axis activity and delays parturition but does not completely block fetal HPA axis activity (7, 36). The net effect of fetal brain PGE2 generation appears to be as a mechanism for increasing the gain of the ACTH response to activation of the pathways subserving the response to stress. Interestingly, in response to severe pathophysiological insult, PGHS-2 and PGE2 are implicated in neuronal cell death after glutamatergic stimulation (11, 16, 27). The interaction between glutamatergic neurotransmission and prostaglandin generation, an interaction that can lead to neuronal death, is also a component of the physiological HPA axis response to stress.
Perspectives and Significance
The results of this study support the conclusion that PGHS-2 partially mediates the HPA axis response to NMDA-mediated glutamatergic neurotransmission, although the neuroanatomic site of this interaction is not known. The presence of PGHS-2 in the brains of normoxic and otherwise healthy fetal sheep suggests that this mechanism is a part of normal physiology, and not solely a component of compensatory responses to pathophysiological insult. Nevertheless, the fetus can be said to reside at altitude, precariously living in a low-oxygen environment, while continuing to grow and develop the neuroanatomic pathways that provide the substrate for postnatal survival and behavior. PGHS-2 expression in the fetal brain is responsive to hypoxia and, also, to glutamatergic neurotransmission. Brain prostaglandins, involved in fever generation and inflammatory processes, are damaging to neurons in high concentrations (16). It is possible that the fetal brain expresses these components of the neuroinflammatory response as a part of its normal physiology. If so, anything that tips the balance toward increased PGHS-2 expression increases fetal HPA axis activity, triggering a neuroendocrine stress response and hastening parturition.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-33053 (to C. E. Wood).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Maureen Keller-Wood for advice and collaboration. We acknowledge the outstanding technical expertise of Lisa Fang, Whitney Hartz, and Dr. Yun-Ju He.
REFERENCES
- 1.Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology 94: 588–591, 1974 [DOI] [PubMed] [Google Scholar]
- 2.Boekkooi PF, Baan J, Teitel DF, Rudolph AM. Effect of drugs on chemoreceptor responsiveness in fetal sheep. Pediatr Res 38: 938–943, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Breder CD, DeWitt DL, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355: 296–315, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cudd TA, Wood CE. Prostaglandin E2 releases ovine fetal ACTH from a site not perfused by the carotid vasculature. Am J Physiol Regul Integr Comp Physiol 263: R136–R140, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Field A. Discovering Statistics Using SPSS. London: Sage, 2005 [Google Scholar]
- 6.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 33: 450–455, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Gersting J, Schaub CE, Keller-Wood M, Wood CE. Inhibition of brain prostaglandin endoperoxide synthase-2 prevents the preparturient increase in fetal adrenocorticotropin secretion in the sheep fetus. Endocrinology 149: 4128–4136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gersting JA, Schaub CE, Wood CE. Development of prostaglandin endoperoxide synthase expression in the ovine fetal central nervous system and pituitary. Gene Expr Patterns 9: 603–611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giussani DA, McGarrigle HH, Spencer JA, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. J Physiol 477: 81–87, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingworth SA, Deayton JM, Young IR, Thorburn GD. Prostaglandin E2 administered to fetal sheep increases the plasma concentration of adrenocorticotropin (ACTH) and the proportion of ACTH in low molecular weight forms. Endocrinology 136: 1233–1240, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-d-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA 98: 1294–1299, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jezova D. Control of ACTH secretion by excitatory amino acids: functional significance and clinical implications. Endocrine 28: 287–294, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA 93: 2317–2321, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanning CF, Harmel MH. Ketamine anesthesia. Annu Rev Med 26: 137–141, 1975 [DOI] [PubMed] [Google Scholar]
- 15.Li SQ, Xing YL, Chen WN, Yue SL, Li L, Li WB. Activation of NMDA receptor is associated with up-regulation of COX-2 expression in the spinal dorsal horn during nociceptive inputs in rats. Neurochem Res 34: 1451–1463, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Mori A, Ishii T, Kuroki T, Shigeta N, Sakamoto K, Nakahara T, Ishii K. The prostanoid EP2 receptor agonist ONO-AE1-259-01 protects against glutamate-induced neurotoxicity in rat retina. Eur J Pharmacol 616: 64–67, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Owen D, Setiawan E, Li A, McCabe L, Matthews SG. Regulation of N-methyl-d-aspartate receptor subunit expression in the fetal guinea pig brain. Biol Reprod 71: 676–683, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Powers MJ, Wood CE. Ketamine inhibits fetal ACTH responses to cerebral hypoperfusion. Am J Physiol Regul Integr Comp Physiol 292: R1542–R1549, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raff H, Shinsako J, Dallman MF. Renin and ACTH responses to hypercapnia and hypoxia after chronic carotid chemodenervation. Am J Physiol Regul Integr Comp Physiol 247: R412–R417, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Raff H, Wood CE. Effect of age and blood pressure on the heart rate, vasopressin, and renin response to hypoxia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 263: R880–R884, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Reimsnider S, Wood CE. Differential modulation of ovine fetal ACTH secretion by PGHS-1 and PGHS-2. Neuroendocrinology 83: 4–11, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Reimsnider SK, Wood CE. Does reduction of circulating prostaglandin E2 reduce fetal hypothalamic-pituitary-adrenal axis activity? J Soc Gynecol Investig 12: e13–e19, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Robillard JE, Weitzman RE, Fisher DA, Smith FG. The dynamics of vasopressin release and blood volume regulation during fetal hemorrhage in the lamb fetus. Pediatr Res 13: 606–610, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Rose JC, Morris M, Meis PJ. Hemorrhage in newborn lambs: effects on arterial blood pressure, ACTH, cortisol, and vasopressin. Am J Physiol Endocrinol Metab 240: E585–E590, 1981 [DOI] [PubMed] [Google Scholar]
- 25.Share L. Role of peripheral receptors in the increased release of vasopressin in response to hemorrhage. Endocrinology 81: 1140–1146, 1967 [DOI] [PubMed] [Google Scholar]
- 26.Shinebourne EA, Vapaavuori EK, Williams RL, Heymann MA, Rudolph AM. Development of baroreflex activity in unanesthetized fetal and neonatal lambs. Circ Res 31: 710–718, 1972 [DOI] [PubMed] [Google Scholar]
- 27.Strauss KI, Marini AM. Cyclooxygenase-2 inhibition protects cultured cerebellar granule neurons from glutamate-mediated cell death. J Neurotrauma 19: 627–638, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorburn GD, Hollingworth SA, Hooper SB. The trigger for parturition in sheep: fetal hypothalamus or placenta? J Dev Physiol 15: 71–79, 1991 [PubMed] [Google Scholar]
- 29.Tong H, Gridley KE, Wood CE. Induction of immunoreactive prostaglandin H synthases 1 and 2 and fos in response to cerebral hypoperfusion in late-gestation fetal sheep. J Soc Gynecol Investig 9: 342–350, 2002 [PubMed] [Google Scholar]
- 30.Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 275: R735–R741, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Vapaavouri EK, Shinebourne EA, Williams RL, Heymann MA, Rudolph AM. Development of cardiovascular responses to autonomic blockade in intact fetal and neonatal lambs. Biol Neonate 22: 177–188, 1973 [DOI] [PubMed] [Google Scholar]
- 32.Virtue RW, Alanis JM, Mori M, Lafargue RT, Vogel JH, Metcalf DR. An anesthetic agent: 2-orthochlorophenyl,2-methylaminocyclohexanone HCl (CI-581). Anesthesiology 28: 823–833, 1967 [DOI] [PubMed] [Google Scholar]
- 33.Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill, 1971 [Google Scholar]
- 34.Wood CE. Sinoaortic denervation attenuates the reflex responses to hypotension in fetal sheep. Am J Physiol Regul Integr Comp Physiol 256: R1103–R1110, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Wood CE. Baroreflex and chemoreflex control of fetal hormone secretion. Reprod Fertil Dev 7: 479–489, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Wood CE, Powers MJ, Keller-Wood M. Blockade of PGHS-2 inhibits the hypothalamus-pituitary-adrenal axis response to cerebral hypoperfusion in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 296: R1813–R1819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood CE, Tong H. Central nervous system regulation of reflex responses to hypotension during fetal life. Am J Physiol Regul Integr Comp Physiol 277: R1541–R1552, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11: 371–386, 1993 [DOI] [PubMed] [Google Scholar]