Abstract
We hypothesized that early life stress enhances endothelin (ET-1)-dependent acute stress responses in adulthood. We utilized a unique rat model, wild-type (WT) and ETB receptor-deficient spotting lethal (sl/sl) rats, as well as pharmacological blockade of ET receptors, in a model of early life stress, maternal separation (MS). MS was performed in male WT and sl/sl rats 3 h/day from day 2 to 14 of life. Acute air jet stress (AJS)-induced responses (elevation in blood pressure, plasma corticosterone, and plasma ET-1) were evaluated in adult MS rats compared with the nonhandled littermate (control) rats. MS significantly augmented the acute AJS-induced blood pressure response (area under the curve) in WT rats compared with control, while the AJS-induced pressor responses were similar in sl/sl MS and control rats. ET receptor blockade significantly blunted the AJS-induced pressor response in WT MS and control rats. Moreover, AJS-induced plasma corticosterone levels in control rats were sensitive to ET receptor blockade, yet, AJS did not alter plasma corticosterone levels in MS rats. MS significantly increased circulating ET-1 levels, and AJS-induced plasma ET-1 levels were similarly increased in control and MS rats. MS induced a significant downregulation in expression of ETA and ETB receptors in aortic tissue compared with control rats. These results indicate that early life stress reduced expression of ETA and ETB receptors, leading to alterations in the ET pathway, and an exaggerated acute stress-mediated pressor response in adulthood.
Keywords: early life stress, maternal separation, air jet stress, blood pressure, endothelin, corticosterone
in humans, there is a large body of epidemiological evidence that early social experiences can promote phenotypic changes that persist in adult life (5, 12, 28). An adverse environment during early life increases the susceptibility to develop heart disease, as well as depression, drug abuse, obesity, and diabetes (21, 24, 32).
Acute behavioral stress responses in adult life are influenced by early life maternal care in both humans (53) and rodents (36). Maternal separation is a widely used animal model of early life stress and chronic behavioral stress (20). Rat pups separated from their mothers daily for a prolonged period (up to 3 h) during the hyporesponsive period display abnormal anxiety-related behavior (41, 43), as well as increased acute behavioral and endocrine stress reactivity as adults (4, 27, 38, 44).
Individuals with increased susceptibility to stressors may also have an exacerbated rise in blood pressure (26). Acute stressors induce many physiological responses, and greater reactivity to stress or delayed poststress recovery predicts future cardiovascular disease risk (7), such as stroke (14), hypertension (37), and preclinical atherosclerosis (23, 52). An acute stress-induced rise in blood pressure results from autonomic and neuroendocrine-mediated changes in cardiac contractility and peripheral vascular resistance (18). Particularly relevant to this study, acute stress is associated with a rise in blood pressure (9, 10), increased plasma endothelin (ET-1) (50, 51), and plasma corticosterone levels (3, 40).
ET-1 is a 21-amino acid peptide characterized and purified from cultured endothelial cells (54). The actions of ET-1 are the result of activation of two receptor subtypes, ETA and ETB. Initial investigations indicated that the ETA receptor is located on vascular smooth muscle and mediates vasoconstriction, while the ETB receptor is predominant on the vascular endothelium (1, 35); both receptor subtypes are localized on sympathetic nerves (35). Studies have demonstrated an interaction between the ET-1 pathway and stress-induced elevations in blood pressure in humans and rodents (9, 50). Reports have linked ET-1 and attenuation of sympathetically mediated responses; specifically, our laboratory has shown that ETA receptor activation attenuates the acute stress-induced pressor response (10). In addition, both ET receptor subtypes have been implicated in triggering the release of corticosterone (2, 46). Some rodent models display acute stress-mediated corticosterone release; thus, alterations in the expression or function of the ET pathway may be involved in the regulation of short- and/or long-term stress responses.
We designed experiments to test the hypothesis that MS enhances ET-1-dependent acute air jet stress (AJS) responses in adult rats. We monitored three responses to AJS (blood pressure, plasma corticosterone, and plasma ET-1) in MS rats and nonhandled littermate rats with both genetic and pharmacological approaches to evaluate the involvement of the ET pathway. We utilized a unique colony of transgenic rats, the ETB receptor-deficient spotting lethal rat that has a loss of function in ETB receptor in nonneuronal cells along with wild-type (WT) genetic control rats (15). We further analyzed the effect of MS on acute stress responses in WT rats treated with a nonselective ETA/B receptor antagonist. Finally, we assessed the impact of MS in WT rats on the expression of ET-1, ETA, and ETB receptors in the vasculature.
METHODS
Animal model.
Wild-type rats (WT; +/+) or rats homozygous (sl/sl) for ETB receptor deficiency were obtained from our local breeding colony. The spotting lethal rat carries a natural 301-bp deletion of the ETB receptor gene that abrogates functional activity of the ETB receptor resulting in the lethal phenotype of intestinal agangliosis (17). The sl/sl rats were rescued by expression of a transgene containing the β-hydroxylase promoter upstream of the ETB receptor gene. This manipulation maintains the loss of a functional ETB receptor in nonneuronal cells; thus, these rats are described as ETB receptor deficient. WT animals also express the transgene (17). Rats were housed in the animal care facility at the Medical College of Georgia, and all protocols were approved by the Institutional Animal Care and Use Committee. Rats had free access to water and normal rat chow. Separate groups of WT rats were treated with the nonselective ETA/B receptor antagonist, A-182086, (30 mg·kg−1·day−1; Abbott Laboratories, Chicago, IL) in ground food for 3 days.
Early life stress (maternal separation) protocol.
Close to delivery, pregnant rats were carefully monitored to determine the exact day of birth (postnatal day 0). Approximately half of the male pups from each litter were removed from the mother's cage, tails snipped, and cauterized with silver nitrate for identification as “maternally separated” (MS). From postnatal days 2 to 14 of life, animals were transferred to a clean cage with bedding and placed into an incubator for 3 h (30°C) at the same time of day. Counterpart littermates normally reared and nonhandled were used as nonseparated or control rats (control). This protocol was previously described in detail by Huot et al. (22). Weaning was performed at postnatal day 28, and ears were punched for identification.
AJS protocol.
We have utilized a model of acute stress that combines restraint with pulsatile, unavoidable bursts of air to the forehead for 3 min in MS and control rats, similar to our previous studies (9, 10). Briefly, all rats were acclimatized to the restrainer tube prior to actual experimentation to minimize trauma associated with entry into the restrainer tubes. Blood pressure, heart rate, and locomotor activity were monitored in animals by telemetry for 15–30 min in their home cages and then placed in tubular Plexiglas restrainers for 15 min while continuing telemetry monitoring. AJS consisted of pulses (2 s in duration delivered every 10 s for 3 min) of compressed air (15 lb/in2) aimed at the forehead from a 1/8“ opening at the front of the tube.
Telemetry blood pressure measurements and AJS analysis.
Telemetry transmitters (Data Sciences International, St, Paul, MN) were implanted according to the manufacturer's specifications. Rats (10 wk old) were anesthetized with ketamine/xylazine (50 mg/kg/10 mg/kg ip). Transmitter catheters were inserted into the abdominal aorta just proximal to the iliac bifurcation. The transmitter body was attached to the abdominal wall along the incision line with 4–0 proline suture, as the incision was closed. Rats were allowed to recover from surgery (8–10 days) and returned to individual housing for data acquisition. Mean arterial pressure (MAP), heart rate (HR), and locomotor activity were continuously recorded throughout the study using the Dataquest ART Acquisition program (Data Sciences). The total (integrated) pressor response during the acute (3 min) stress period was determined by the equation: Σ[(AJS pressure − pre-AJS pressures) × 0.067 min], where pressure refers to the individual values during the delivery of the AJS, the pre-AJS is the average pressure during the 3-min interval before the start of the AJS. Data are expressed as the area under the curve (AUC; mmHg × 3 min), as previously described (8–10).
Chronic venous catheter implantation.
Under ketamine/xylazine (50 mg/kg/10 mg/kg ip) anesthesia, the right jugular vein was isolated and cannulated with a rat thoracic jugular vein catheter (R-JVC; Braintree Scientific; Braintree, MA). Catheter ends were tunneled subcutaneously to exit between the scapulae and filled with heparin (1,000 U/ml). Catheters were flushed daily with heparin.
Blood sampling and plasma analysis.
Each of the 2 days after the implantation of the venous catheter, animals were placed in restrainer tubes for at least 15 min, consistent with the duration used for the acute stress protocol, and catheters were flushed to maintain patency. On days 3 and 4 postimplantation, blood (1 ml) was drawn for analysis of baseline (nonstressed) plasma. On day 5 postsurgery, rats were subjected to AJS, blood (500 μl) was drawn during the 30–60-s interval of AJS. Plasma was aliquoted and frozen at −80°C until analyses were performed. After plasma was removed, red blood cells were resuspended in isotonic solution (0.9% NaCl) containing EDTA 0.075%, BSA 6% (Sigma) was reinfused, and catheters were refilled with heparin. Subsets of rats were subjected to a second acute stress after a 1-wk period. Three days prior to the second stress, rats were treated with the ETA/B receptor antagonist, A-182086, (30 mg·kg−1·day−1; Abbott Laboratories, Chicago, IL) in ground food. Plasma corticosterone and ET-1 were assessed by ELISA, according to manufacturer's specifications (corticosterone EIA kit; Cayman Chemical, Ann Arbor, MI; and ET-1 QuantiGlo; R&D Systems, Minneapolis, MN).
Real-time PCR in aortic tissue.
Relative levels of mRNA expression for prepro-ET-1, ETA, and ETB receptors were determined in aortic tissue. RNA was extracted from tissue using RNeasy mini kit (Qiagen, Valencia, CA). Genomic DNA elimination and reverse transcription were performed using a QuantiTaq kit (Qiagen). RNA quantification and the 260/280 ratio were determined by spectrophotometric analysis (NanoDrop ND-1000; Thermo Scientific, Waldorf, Germany). Forward (sense) and reverse (antisense) primers for GAPDH (Entrez ID: 35728), prepro-ET-1 (Entrez ID: 24323), ETA receptor (Entrez ID: 24326), and ETB receptor (Entrez ID: 50672) (Qiagen) were analyzed using real-time PCR Systems (Step One Software ver. 2.0. Applied Biosystems, Foster City, CA). To calculate the relative expression levels in each sample, the CT (cycle of threshold fluorescence) value for GAPDH was subtracted from the CT value of the gene of interest to give a ΔCT value. The ΔCT value of the calibrator was then subtracted from each individual sample to give a ΔΔCT value. This number was then inserted into the formula 2−ΔΔCT to give the expression level relative to the calibrator.
Statistical analysis.
All data are expressed as means ± SE. AUC and real-time PCR data were analyzed using t-test comparison between the baseline and stress period for individual groups of rats or control and MS groups, respectively. Plasma ET-1 and corticosterone levels in the WT group were compared using a 2-way ANOVA (5.01, GraphPad Software, San Diego, CA) followed by a Tukey post hoc test.
RESULTS
Baseline cardiovascular parameters.
Baseline telemetry data were collected at 12 wk of age (table 1). Body weights were similar between control and MS rats. We observed elevated MAP in the sl/sl rats compared with the WT strain as described previously in this model (17, 25, 48). Furthermore, in agreement with previously reported data with the MS model (50), we did not find changes in 24-h MAP or HR in rats of either strain exposed to MS (Table 1). In addition, locomotor activity was similar between control and MS rats from both strains (Table 1).
Table 1.
Baseline parameters in telemetry-implanted WT and sl/sl rats
| WT |
sl/sl |
|||
|---|---|---|---|---|
| Control (n = 8) | MS (n = 7) | Control (n = 8) | MS (n = 14) | |
| MAP, mmHg | 100 ± 2 | 94 ± 3 | 114 ± 2* | 116 ± 3† |
| Heart rate, beats/min | 356 ± 7 | 365 ± 9 | 389 ± 16 | 368 ± 5 |
| Locomotor activity, AU | 5.1 ± 0.9 | 6.4 ± 0.8 | 6.3 ± 0.9 | 5.8 ± 0.8 |
Data are expressed in 24-h averages. WT, wild type; MS, maternal separation; MAP, mean arterial pressure.
P < 0.05 vs. WT control;
P < 0.05 vs. WT MS.
MS- and AJS-mediated blood pressure responses.
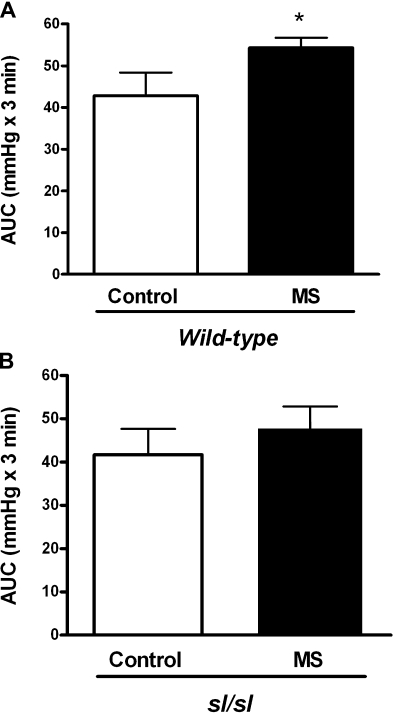
AJS-induced pressor responses were monitored by telemetry in rats similar to our previous studies (10). The average MAP from before and during the AJS protocol in control and MS rats is shown in Fig. 1. MS significantly enhanced the total AJS-induced pressor response (AUC) in WT rats (Fig. 2A). Interestingly, the exaggerated stress-mediated pressor response seen with MS was not observed in sl/sl rats (Fig. 2B).
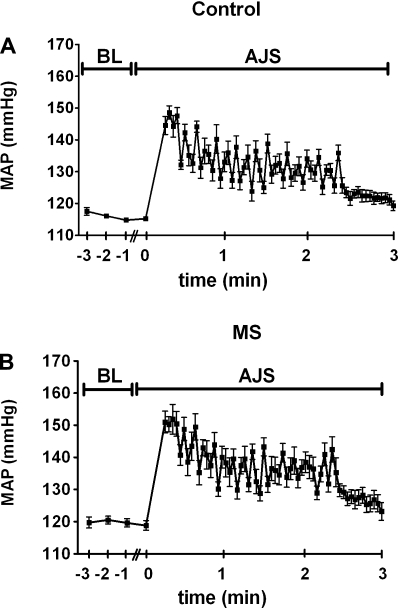
Fig. 1.
Average trace of mean arterial pressure (MAP) of control (A) and maternal separation (MS) (B) rats before and during the acute air jet stress (AJS) protocol. BL denotes baseline or not exposed to AJS; n = 6–8.
Fig. 2.
AJS-induced pressor responses in control and MS from the wild-type (WT; A) and ETB receptor deficient spotting lethal (sl/sl; B) rat strains. Data are expressed as the area under the curve (AUC) during the stress period; n = 8–11. *P < 0.05 vs. control.
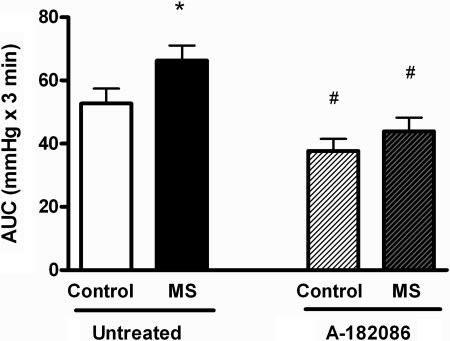
To further probe the dependency of the ET pathway on the exaggerated AJS-induced pressor response in MS rats, we treated adult WT control and MS rats with a nonselective ETA/B receptor antagonist, A-182086. Selective ETB receptor blockade results in ETA-dependent vasoconstriction and increases blood pressure that would confound the interpretation of stress-mediated changes in blood pressure. Therefore, we utilized the dual ETA/B receptor blocker to pharmacologically probe the ET pathway. Blockade of ET receptor activation significantly decreased the AJS-induced pressor response in both control and MS groups to the same degree (Fig. 3). Treatment with A-182086 decreased 24 h MAP in control (untreated: 105 ± 1 vs. A-182086: 101 ± 1 mmHg, P < 0.05) and MS rats (untreated: 104 ± 1 to A-182086: 101 ± 1 mmHg, P < 0.05). Similarly, HR was decreased by treatment with A-182086 (control: 370 ± 4 vs. A-182086: 358 ± 4 beats/min P < 0.05; MS: 363 ± 2 vs. A-182086: 353 ± 1 beats/min, P < 0.05).
Fig. 3.
AJS-induced pressor responses in WT untreated or treated, control and MS rats. WT rats were treated with A-182086, nonselective ETA/B receptor antagonist, for 3 days prior to AJS. Data are expressed as the area under the curve (AUC) during the stress period; n = 9 or 10. *P < 0.05 vs. control; #P < 0.05 vs. corresponding untreated group.
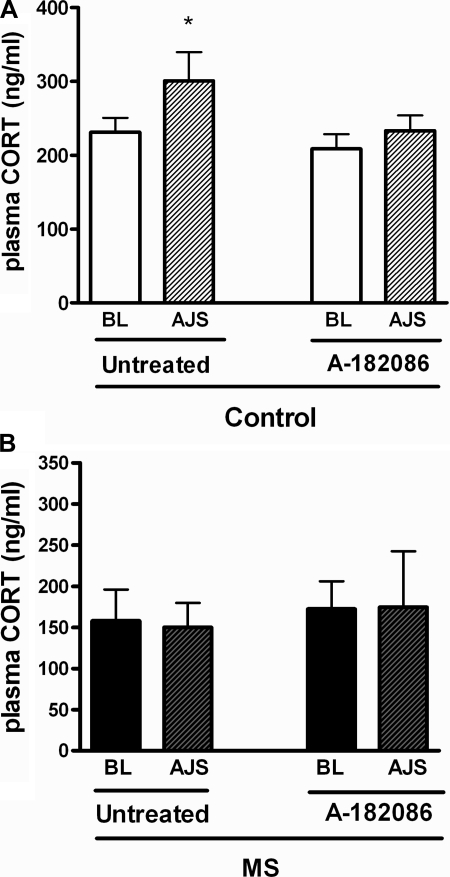
MS- and AJS-mediated responses in plasma corticosterone.
In WT rats, AJS significantly increased plasma corticosterone (Fig. 4A). ETA/B antagonism significantly inhibited this response. In contrast, AJS had no effect on plasma corticosterone in MS rats, irrespective of ET receptor blockade (Fig. 4B).
Fig. 4.
Plasma corticosterone (CORT) levels at BL or during AJS in untreated and treated control (A) or MS (B) rats. WT rats were treated with A-182086, nonselective ETA/B receptor antagonist, for 3 days prior to experimentation; n = 7 or 8, *P < 0.05 vs. baseline.
MS- and AJS-mediated responses in plasma ET-1.
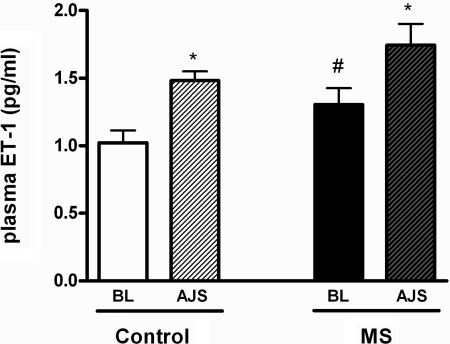
MS significantly increased baseline plasma ET-1 levels (Fig. 5). AJS increased plasma ET-1 levels in both control and MS rats, similarly (Fig. 5).
Fig. 5.
Plasma ET-1 levels at BL or during AJS in WT control or MS rats; n = 7–9. *P < 0.05 vs. baseline. #P < 0.05 vs. corresponding control.
MS-induced changes in vascular expression of preproET-1, ETA, and ETB receptors.
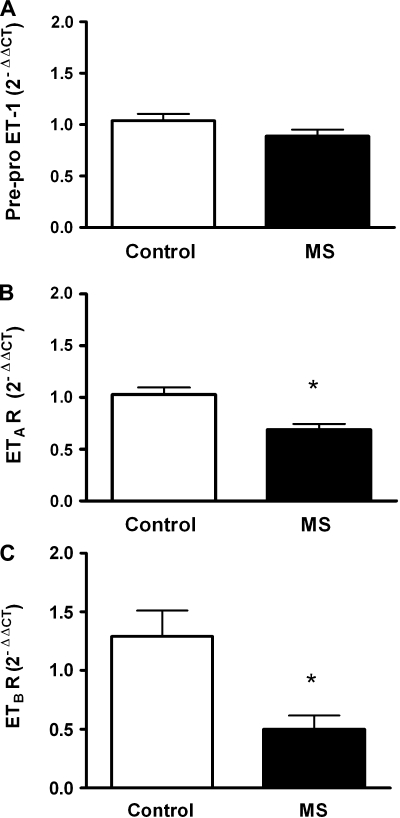
We determined mRNA expression of components in the ET pathway in aortic tissue from WT control and MS rats. The prepro-ET-1 levels were similar in control and MS rats (Fig. 6A). Aortic mRNA expression of ETA (Fig. 6B) and ETB receptors (Fig. 6C) were significantly reduced in MS rats compared with control rats.
Fig. 6.
Expression of prepro-ET-1 (A), ETA receptor (B), and ETB receptor (C) in aortic tissue from control and MS rats; n = 5–11. *P < 0.05 vs. control.
DISCUSSION
The focus of this study was to elucidate whether early life stress induces a hyperreactive, ET-dependent cardiovascular phenotype in adult rats. Our major finding is that MS, a model of early life stress, produced an exaggerated AJS-mediated pressor response in adult WT rats but not in sl/sl rats. The sl/sl rats lack a functioning ETB receptor in nonneuronal cells, indicating that the systemic, peripheral ET pathway enhances the AJS response. Also, AJS elicited an ET-dependent increase in plasma corticosterone only in nonseparated WT rats; no changes in plasma corticosterone were noted in MS rats in the absence or presence of a nonspecific ETA/B receptor antagonist. Dual ETA/B receptor blockade blunted the pressor response to a similar degree in nonseparated and MS rats, further validating the relevance of the ET pathway in the AJS response. MS induced an increase in circulating levels of ET-1 and a downregulation in vascular ETA and ETB receptor expression; however, vascular expression of prepro-ET-1 was similar in MS and nonseparated rats. These results have led us to hypothesize that early life stress induces a chronic downregulation of vascular ET receptor expression that exerts a dysfunctional acute stress-mediated cardiovascular response, specifically an exaggerated pressor response.
In humans, Lindgarde et al. (29) demonstrated that low socioeconomic status, particularly during childhood, increases the risk of cardiovascular disease. In a large epidemiological study of more than 17,000 adults, Dong et al. (12) showed a highly significant correlation of adverse childhood experiences with ischemic heart disease. Also, relevant to our finding, Steptoe's laboratory recently reported on a meta-analysis of existing literature (6) that an enhanced stress (mental) reactivity, specifically, blood pressure and heart rate responses, predicts a greater cardiovascular risk status in males and younger populations (7). In rodents, maternal separation has been used to study the influence of chronic behavioral stress, and we reasoned that this model resembles, in part, an adverse environment during early life that may increase susceptibility to cardiovascular disease. Interestingly, the MS model does not appear to induce significant baseline differences in the cardiovascular phenotype in rats (33, 47). Consistent with our report, several studies in rodents demonstrate that MS does not affect locomotor activity (4, 19, 27, 45). It is known that MS sensitizes rats to acute stressors later in life, in terms of behavioral parameters (27, 30, 36), although acute stress-mediated mechanisms of cardiovascular phenotypic changes have not been explored previously in this model to our knowledge. Indeed, we found that MS induces an exaggerated pressor response to acute stress via an ET-dependent mechanism.
Our results indicate that MS induced an enhanced pressor response to stress that is observed in WT but not in sl/sl rats. The sl/sl rats express a nonfunctioning mutant ETB receptor in nonneuronal tissue, while neuronal tissue expresses the transgene with a functioning ETB receptor (17). The WT rats also express the transgene; thus, the difference between the two strains is the lack of a functioning ETB receptor in nonneuronal tissue, such as the vasculature. We hypothesized that the MS-induced exaggerated pressor response may be due to alterations in the vascular ET pathway. Interestingly, we found MS induced decreased expression of ETA and ETB receptors in vascular tissue.
It is known that ET-1 modulates sympathetically mediated responses (39). Furthermore, we previously reported that endogenous ETA receptor activation attenuates the air jet stress-mediated pressor response (9), suggesting cross-talk between the ETA receptor and the adrenergic pathway. We would speculate that reduced ETA receptor expression might lead to increased activation of the adrenergic pathway. Thus, down-regulation of ETA receptor expression may contribute to the mechanism of the exaggerated pressor response through an increased responsiveness in vascular tissue to adrenergic activation.
Reduced ETB receptor expression in MS rats is most likely related to higher circulating ET-1 levels. The ETB receptor is characterized as a “clearance receptor” to sequester and remove circulating ET-1, presumably to regulate ETA receptor activation (17). Indeed, ETB receptor-deficient rats have higher circulating ET-1, leading to a higher basal level of ETA receptor activation and elevated baseline blood pressure (13). Since ETA receptor activation attenuates stress-mediated pressor responses (9), we speculate that this heightened endogenous ETA receptor activation counterbalances the MS-induced exaggerated pressor response in the sl/sl rat. Further studies are necessary to confirm these hypotheses.
Several reports have shown that plasma ET-1 levels increase in response to physical stressors (e.g., cold pressor and immobilization) and acute mental stressors (e.g., mental arithmetic) (11, 16, 42, 49, 50). Plasma ET-1 levels are influenced by the production of ET-1, as well as by the clearance of circulating ET-1 by ET receptors, especially ETB receptors. The stress-mediated increase in plasma ET-1 levels observed in both the control and MS rats most likely originates from activation of ET-1 production and not reduced clearance of ET-1. We have concluded this since both control and MS rats have similar AJS-induced plasma ET-1 levels, even though the MS rats were shown to have reduced expression of vascular ETB receptors.
MS is associated with activation of the hypothalamic-pituitary-adrenal (HPA) axis, and in some studies, higher corticosterone post-acute stress (30, 41). Our study showed that MS and control rats have similar circulating levels of plasma corticosterone. Our data showed that the control rats responded to the acute stress by increasing plasma corticosterone levels, yet somewhat surprisingly, our data demonstrated that the MS rats lack a stress-induced increase in plasma corticosterone. These data do not support our initial hypothesis that MS elevates the acute stress-mediated corticosterone response. A possible explanation for this disparity may be related to the strain of rat or other environmental factors that impact the sensitivity of the HPA axis. Our findings are, at least in part, consistent with other studies that have reported no significant effects of early life stress linked to the HPA axis (5, 19, 31, 34). Renard et al. (45) reported that normally reared Wistar rats exposed to random acute stress responded with increased plasma corticosterone, and similar to our findings, Wistar rats exposed to MS did not have an increase in plasma corticosterone. The WT and sl/sl rats are reported to be on a Wistar-Kyoto background (17). Further research is needed to examine the level of maternal care and the sensitivity of the HPA axis in the stress-mediated response to resolve the findings in the literature.
It has been shown that ET-1 stimulates plasma corticosterone levels associated with a stress response (35) and that stimulation of the ETB receptor mediates the release of corticosterone from the adrenal cortex (2, 46). Our data show that nonseparated rats respond to acute stress by increasing plasma corticosterone levels in an ET-dependent manner. MS rats did not elicit a similar response, and we would speculate that ET receptor expression or downstream signaling proteins in the adrenal gland may be downregulated or dysfunctional. Future experiments are planned to analyze MS-induced changes in ET receptor expression in these various cell and tissue types.
Perspectives and Significance
In conclusion, early life stress induces a downregulation of ETA and ETB receptors, leading to alterations in the ET pathway, and exaggerated acute stress-mediated pressor responses in adulthood. These data demonstrate that a model of early life stress induces an enhanced cardiovascular reactivity phenotype involving the ET pathway, suggesting that early life stress is a possible new risk factor in the development of cardiovascular disease. We hypothesize that the mechanisms associated with an adverse postnatal environment may lead to prevention strategies for control of cardiovascular risk. Thus, the ET pathway may be a potential therapeutic target for controlling excessive cardiovascular reactivity to acute stress.
GRANTS
Funding was provided by the National Institutes of Health through Grant P01 HL 69999 (J. S. Pollock and D. M. Pollock) and the American Heart Association (Postdoctoral fellowship to A. S. Loria; Scientist Development Grant to G. D'Angelo; Established Investigator Awards to J. S. Pollock and D. M. Pollock).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Hiram Ocasio, Jeff Bobo, Heather Socha, and Amy Dukes for outstanding technical assistance.
Present address for G. D'Angelo: Institutes for Pharmaceutical Discovery, 23 Business Park Dr., Branford, CT 06405.
REFERENCES
- 1.Barton M, d'Uscio LV, Shaw S, Meyer P, Moreau P, Luscher TF. ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension 31: 499–504, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Belloni AS, Rossi GP, Andreis PG, Neri G, Albertin G, Pessina AC, Nussdorfer GG. Endothelin adrenocortical secretagogue effect is mediated by the B receptor in rats. Hypertension 27: 1153–1159, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Bernatova I, Rigatto KV, Key MP, Morris M. Stress-induced pressor and corticosterone responses in oxytocin-deficient mice. Exp Physiol 89: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci 16: 187–197, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry 48: 1164–1174, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull 134: 829–885, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. A meta-analysis of prospective evidence. Hypertension 55: 1026–1032, 2010 [DOI] [PubMed] [Google Scholar]
- 8.D'Angelo G, Pollock DM, Pollock JS. Oxidative stress mediates the pressor response to acute environmental stress in Dahl salt-sensitive rats. FASEB J 20: A357, 2006 [Google Scholar]
- 9.D'Angelo G, Pollock JS, Pollock DM. Endogenous endothelin attenuates the pressor response to acute environmental stress via the ETA receptor. Am J Physiol Heart Circ Physiol 288: H1829–H1835, 2005 [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo G, Pollock JS, Pollock DM. In vivo evidence for endothelin-1-mediated attenuation of alpha1-adrenergic stimulation. Am J Physiol Heart Circ Physiol 290: H1251–H1258, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Danese C, Parlapiano C, Zavattaro E, Di Prima M, Campana E, Rota C, Tonnarini G, Di Siena G, Borgia MC. ET-1 plasma levels during cold stress test in sclerodermic patients. Angiology 48: 965–968, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110: 1761–1766, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Elmarakby AA, Dabbs Loomis E, Pollock JS, Pollock DM. ETA receptor blockade attenuates hypertension and decreases reactive oxygen species in ETB receptor-deficient rats. J Cardiovasc Pharmacol 44Suppl 1: S7–S10, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Everson SA, Lynch JW, Kaplan GA, Lakka TA, Sivenius J, Salonen JT. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke 32: 1263–1270, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun 199: 1461–1465, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Fyhrquist F, Saijonmaa O, Metsarinne K, Tikkanen I, Rosenlof K, Tikkanen T. Raised plasma endothelin-I concentration following cold pressor test. Biochem Biophys Res Commun 169: 217–221, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herd JA. Cardiovascular response to stress. Physiol Rev 71: 305–330, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res 542: 286–292, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev 29: 1335–1346, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Holness MJ, Langdown ML, Sugden MC. Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of Type 2 diabetes mellitus. Biochem J 349: 657–665, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long-Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 158: 366–373, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation 110: 2198–2203, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 203: 311–319, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Konishi F, Okada Y, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y. Role of endothelin ET(B) receptors in the renal hemodynamic and excretory responses to big endothelin-1. Eur J Pharmacol 451: 177–184, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychol Bull 96: 435–464, 1984 [PubMed] [Google Scholar]
- 27.Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res 107: 133–144, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 30: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Lindgarde F, Furu M, Ljung BO. A longitudinal study on the significance of environmental and individual factors associated with the development of essential hypertension. J Epidemiol Community Health 41: 220–226, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol 12: 5–12, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Loizzo A, Loizzo S, Galietta G, Caiola S, Spampinato S, Campana G, Seghieri G, Ghirlanda G, Franconi F. Overweight and metabolic and hormonal parameter disruption are induced in adult male mice by manipulations during lactation period. Pediatr Res 59: 111–115, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci 20: 1017–1024, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Malendowicz LK, Nussdorfer GG, Meneghelli V, Nowak M, Markowska A, Majchrzak M. Effects of endothelin-1 on the rat pituitary-adrenocortical axis under basal and stressful conditions. Endocr Res 23: 349–364, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto Y, Yoshihara T, Yamasaki Y. Maternal deprivation in the early versus late postnatal period differentially affects growth and stress-induced corticosterone responses in adolescent rats. Brain Res 1115: 155–161, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004 [DOI] [PubMed] [Google Scholar]
- 38.McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav 73: 77–85, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Milner P, Loesch A, Burnstock G. Endothelin immunoreactivity and mRNA expression in sensory and sympathetic neurones following selective denervation. Int J Dev Neurosci 18: 727–734, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Mohn CE, Fernandez-Solari J, De Laurentiis A, Prestifilippo JP, de la Cal C, Funk R, Bornstein SR, McCann SM, Rettori V. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc Natl Acad Sci USA 102: 6213–6218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann ID, Wigger A, Kromer S, Frank E, Landgraf R, Bosch OJ. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience 132: 867–877, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Luscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation 93: 866–869, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Okimoto DK, Blaus A, Schmidt MV, Gordon MK, Dent GW, Levine S. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive period. Endocrinology 143: 1717–1725, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18: 195–200, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Renard GM, Rivarola MA, Suarez MM. Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci 25: 373–379, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Rossi GP, Andreis PG, Neri G, Tortorella C, Pelizzo MR, Sacchetto A, Nussdorfer GG. Endothelin-1 stimulates aldosterone synthesis in Conn's adenomas via both A and B receptors coupled with the protein kinase C- and cyclooxygenase-dependent signaling pathways. J Investig Med 48: 343–350, 2000 [PubMed] [Google Scholar]
- 47.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res 183: 25–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high-salt diet. Hypertension 41: 657–662, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Treiber FA, Jackson RW, Davis H, Pollock JS, Kapuku G, Mensah GA, Pollock DM. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension 35: 722–725, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med 65: 46–62, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Treiber FA, Kapuku GK, Davis H, Pollock JS, Pollock DM. Plasma endothelin-1 release during acute stress: role of ethnicity and sex. Psychosom Med 64: 707–713, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and preweanling heart rate in spontaneously hypertensive and Wistar Kyoto rats. Dev Psychobiol 17: 587–600, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Williams RB, Marchuk DA, Siegler IC, Barefoot JC, Helms MJ, Brummett BH, Surwit RS, Lane JD, Kuhn CM, Gadde KM, Ashley-Koch A, Svenson IK, Schanberg SM. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosom Med 70: 32–39, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]