Abstract
High-salt intake can change the effect of adenosine on arterial tone in mice. The aim of this study was to clarify the mechanism by which this occurs. Using aortas from mice fed a 4% NaCl (HS) or 0.45% NaCl (NS) diet for 4–5 wks, concentration-response curves for ACh, 5′-N-ethylcarboxamidoadenosine (NECA; adenosine analog) and 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate [CGS-21680; A2A adenosine receptor (A2A AR) agonist] were obtained with Nω-nitro-l-arginine methyl ester (l-NAME; nitric oxide inhibitor, 10−4 M), methylsulfonyl-propargyloxyphenylhexanamide [MS-PPOH; a CYP (cytochrome P-450) epoxygenase blocker, 10−5 M including CYP2J2], 12-(3-adamantan-1-yl-ureido)dodecanoic acid [AUDA; soluble epoxide hydrolase (sEH) blocker, 10−5 M], dibromo-dodecenyl-methylsulfimide [DDMS; CYP ω-hydroxylase (CYP4A blocker), 10−5 M], glibenclamide (KATP channel blocker; 10−5 M) and 5-hydroxydecanoate (5-HD; mitochondrial-KATP channel blocker, 10−4 M). HS dose response to ACh (10−7 − 10−5 M) was not different from NS (P > 0.05). Relaxation to 10−6 M NECA was greater in the HS group (28.4 ± 3.9%) than in the NS group (4.1 ± 2.3%). Relaxation to 10−6 M CGS-21680 was also greater in HS (27.9 ± 4.5%) than in NS (4.9 ± 2.2%). l-NAME was able to block the dose response of ACh (10−7 − 10−5 M) equally in both HS and NS (P > 0.05), whereas l-NAME did not block CGS-21680-induced response in HS. In HS the CGS-21680 response was greatly reduced by MS-PPOH (to 4.7 ± 2.0%) and 5-HD (to 8.9 ± 2.2%), and also abolished by glibenclamide (−1.0 ± 5.9%). In NS, the CGS-21680 response was increased by AUDA (to 26.3 ± 3.4%) and DDMS (to 27.2 ± 3.0%). Compared with NS, HS vessels showed increased CYP2J2 and A2A AR expression (46 and 74% higher, respectively) but decreased sEH, CYP4A, and A1 AR expression (75, 30, and 55% lower, respectively). These data suggest that in mice fed NS-containing diet, upregulation of arterial A1 receptor causes vasoconstriction via increased sEH and CYP4A proteins. However, in mice fed HS-containing diet, upregulation of A2A receptor protein triggers vascular relaxation through ATP-sensitive (K+) channels via upregulation of CYP2J2 enzyme.
Keywords: KATP channels, relaxation, contraction, adenosine
in our recent studies (33), we found that the nonselective adenosine analog 5′-(N-ethylcarboxamido)adenosine (NECA) and a selective A2A adenosine receptor (AR) agonist [2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680)] dilated aortas from mice fed with a 7% NaCl-containing diet, while they had no significant effect on aortas from mice fed with a 0.45% NaCl-containing diet. Vasodilation is primarily caused by the activation of A2A receptors in the coronary and renal arteries and other vessels (1, 18, 28, 32, 33). Through the activation of A2A receptors, the increase in renal adenosine concentration could possibly contribute to a reduction in macula densa-mediated renin secretion, dilation of pre- and postglomerular vessels, and inhibition of tubular sodium reabsorption (25, 44), leading to enhanced sodium excretion to maintain the constancy of body fluid volume and arterial pressure (2, 44, 45). In contrast, stimulation of A1 receptors produce preglomerular vasoconstriction, activation of tubuloglomerular feedback response, increased cortical and medullary tubular sodium reabsorption, and, consequently, a reduction of sodium excretion (40, 44).
The recent report from us (33) also suggested that the CGS-21680-induced dilation of vessels from 7% NaCl-containing diet-fed mice was inhibited by the cytochrome P450 (CYP450) epoxygenase inhibitor methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH). Other investigators have shown regulatory control of the renal epoxygenase by dietary salt-loading in rats (4). It has also been demonstrated that inhibition of CYP epoxygenase with clotrimazole gives rise to an increase in blood pressure in rats maintained on a high-salt diet, and the salt itself does not increase blood pressure (26). Downregulation of CYP epoxygenase activity and inhibition of epoxyeicosatrienoic acid (EET) formation is associated with salt-sensitive hypertension (17, 26, 55). Conversion of arachidonic acid epoxides to diols by soluble epoxide hydrolase (sEH) diminishes the beneficial cardiovascular properties of these epoxyeicosanoids. Inhibition of sEH results in the accumulation and longer retention of EETs after their formation (9). Expression of sEH in renal cortical tissue and renal microvessels is also increased in rats made hypertensive by the infusion of ANG II (20, 56). Urinary (±)14,15-DiHETrE-d11 (DHET; EET hydrolysis products) are increased in spontaneously hypertensive rats and ANG II-induced hypertensive rats (20, 54).
In our recent studies (33), the CYP ω-hydroxylase (CYP4A)-triggered contraction of vessels from 0.45% NaCl-fed mice was inhibited by the CYP4A inhibitor dibromo-dodecenyl-methylsulfimide (DDMS). CYP4A enzymes hydroxylate arachidonic acid to 20-HETE, which is a potent vasoconstrictor in small arteries and other vessels, including aortas (12, 13, 32, 33). Also, 20-HETE formation was lower in glomeruli as well as in cortex isolated from rat kidneys fed a high-salt compared with a low-salt diet (21).
Opening of cardiac KATP channels shortens the cardiac action potential, thereby reducing intracellular Ca2+ overload and leading to cardiac protection (19, 29–31, 43). In contrast, the activation of the vascular KATP channels leads to membrane hyperpolarization that reduces the calcium influx and regulates vascular tone (53). Vascular KATP channels are known to be more sensitive to sulphonylurea drugs (i.e., glyburide and tolbutamide) as well as to the K+ channel opener diazoxide, implying that the properties one of the subunits of these channels (sulfonylurea receptor) may contribute to the diverse functions of the KATP channel isoforms.
Relatively very little is known regarding the mouse model of salt feeding compared with rats. It is difficult to isolate murine-resistant vessels, and also very challenging to study their vascular responses. However, mice offer significant advantages in that specific gene transgenics and gene knockout technology have been developed and used successfully as a powerful tool. The aorta is less challenging to study and is commonly used to directly characterize adenosine receptors and CYP450 signaling (32, 33). Some reports (6, 42, 48) suggested the use of diet containing 4% NaCl to be considered as a high-salt diet in mice instead of 7% NaCl (33). Therefore, we used 4% NaCl (HS)- and 0.45% NaCl (NS)-containing diets in this study to determine the effects on vascular response in mouse aorta. We hypothesized that HS enhances adenosine A2A receptor-dependent relaxation through CYP epoxygenase (CYP2J2) via KATP channels, while NS diet reduces relaxation through sEH and CYP4A in mice.
MATERIALS AND METHODS
Our animal use experimental protocol was approved and carried out in accordance with the West Virginia University Institutional Animal Care and Use Committee and was in accordance with the principles and guidelines of the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals. Ten-week-old inbred male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were placed on a whole-grain diet containing either NS or HS (TD.88311 and TD.92034 diets, respectively; Teklad, Madison, WI). We have previously shown that these mice remain normotensive when placed on a high-salt diet (33, 37). All mice were studied 4–5 wk after assignment to either the NS or HS group.
Mouse aortic rings.
Mice were killed with pentobarbital sodium (100 mg/kg ip). According to our previously described protocol (32, 33), after thoracotomy the aorta was gently removed, cleaned of fat and connective tissue, and cut transversely into rings of 3–4 mm in length. Extreme care was taken not to damage the endothelium. The rings were mounted vertically between two stainless steel wire hooks. Two rings were suspended in 10-ml organ baths containing modified Krebs-Henseleit buffer (32, 33). At the end of equilibration period, tissues were contracted with KCl (50 mM) to check the viability of the tissue. Aortic rings were then constricted with phenylephrine (PE; 10−7 M), and changes in tension were monitored continuously with a fixed-range precision force transducer (model TSD 125 C; Biopac Systems, Goleta, CA) connected to a differential amplifier (model DA 100B; Biopac Systems). Data were recorded using digital acquisition system MP100 WSW and analyzed using Acknowledge 3.5.7 software (both from Biopac Systems). The vascular endothelium was tested to determine whether it was intact, as previously described by our laboratory (32, 33) through ACh (10−7 M) on precontracted (PE) aortic rings. Preparations were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began. For all tests the contraction and relaxation responses are expressed as %decrease or %increase of PE-induced precontraction.
Response of aortas to NECA/CGS-21680 in mice fed HS and NS.
The responsiveness of the precontracted aortic rings was determined by using a nonselective adenosine analog (NECA) and CGS-21680 (selective A2A AR agonist). Concentration-response curves were obtained by cumulative addition of drugs in one-log increments as described by us (32, 33). One concentration-response curve was constructed for each ring, and all concentration-response determinations were run in parallel on pairs of rings from either HS or NS in the same organ bath.
Effect of nitric oxide inhibitor on response of aortas to ACh in mice fed HS and NS.
A nitric oxide (NO) inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) was added 30 min before contraction of the tissue with PE and was present throughout the ACh dose-response experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with l-NAME.
Effect of NO or cyclooxygenase inhibitor on response of aortas to CGS-21680 in mice fed HS and NS.
l-NAME (100 μM), an NO inhibitor or indomethacin (10 μM), a cyclooxygenase (COX) inhibitor was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with l-NAME or indomethacin.
Effects of CYP epoxygenase, sEH, CYP4A, and KATP channel inhibitors on response of aortas to CGS-21680 in mice fed HS and NS.
Selective CYP epoxygenase inhibitor MS-PPOH (10 μM) or selective sEH inhibitor 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA; 10 μM) or selective CYP4A inhibitor DDMS (10 μM) or KATP channel blocker glibenclamide (10 μM) or selective mitochondrial KATP channel blocker 5-hydroxydecanoate (5-HD; 100 μM) was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with MS-PPOH or AUDA or DDMS or glibenclamide or 5-HD.
Protein extraction, gel electrophoresis, and Western blot analysis.
In brief, aortas from both HS and NS mice were isolated, and each sample was treated with 1 ml of lysis buffer and protein extraction. Gel electrophoresis and Western blot analysis were done according to the protocol previously described by us (32, 33). Following blocking with nonfat dry milk, the nitrocellulose membranes were incubated with polyclonal antibodies cross-reacting with primary antibody for CYP2J2 (from Dr. D. C. Zeldin), sEH (from Dr. A. Marowsky), CYP4A (Affinity Bio-Reagents), A1 AR (Sigma, St. Louis, MO), A2A AR (from Dr. S. J. Mustafa, West Virginia University, Morgantown, WV), and β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used. The secondary antibody was a horseradish peroxidase-conjugated anti-rabbit IgG. The membranes were developed using enhanced chemiluminescence (Amersham BioSciences) and exposed to X-ray film for the appropriate time.
Chemicals, drugs, and antibodies.
PE and ACh were dissolved in distilled water. NECA and CGS-21680 (Sigma) were dissolved in 100% DMSO as 10 mM stock solutions, which were followed by serial dilutions in distilled water. MS-PPOH (from Dr. J. R. Falck), and DDMS (Cayman, Ann Arbor, MI) were dissolved in 100% ethanol, AUDA (from Dr. C. Morisseau) was dissolved in DMSO. CYP2J2 (from Dr. D. C. Zeldin), sEH (from Dr. A. Marowsky), CYP4A antibody (Affinity BioReagents, Golden, CO), A1 AR (Sigma), and A2A AR (Dr. S. J. Mustafa) were obtained and used for Western blot analysis.
Statistical analysis.
Statistical data are reported as means ± SE. One-way ANOVA was used to compare difference among groups, and two-way ANOVA was used for repeated measures, followed by Tukey's post hoc test to compare the vascular responses to antagonist (l-NAME, MS-PPOH, AUDA, DDMS, glibenclamide, and 5-HD). Differences were considered significant if P < 0.05. Furthermore, densitometry of Western blot analysis (CYP2J2, sEH, CYP4A, A2A AR, and A1 AR) was expressed as means ± SE in arbitrary units. All of the statistical analyses were performed using Graph Pad Prism statistical package.
RESULTS
Response of aortas to ACh in mice fed HS and NS.
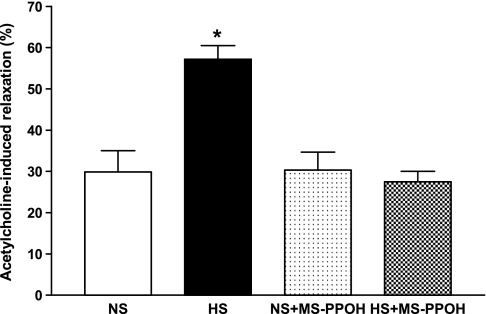
The vascular endothelium was tested to determine whether it was intact. The protocol was used as previously described by our laboratory (32, 33) through ACh (10−7 M) for 5 min (single dose and short-term response) on PE precontracted aortic rings. Relaxation to 10−7 M ACh was significantly greater in PE precontracted HS (57.2 ± 3.3%) compared with NS (29.0 ± 5.1%) aortas (P < 0.05, Fig. 1). MS-PPOH (10 μM), a selective CYP epoxygenase inhibitor, was able to reduce ACh-dependent relaxation in HS aortas significantly (27.5 ± 2.5% vs. 55.2 ± 4.3% for untreated controls, P < 0.05). No significant difference was found between MS-PPOH treated and nontreated NS aortas (P > 0.05, Fig. 1).
Fig. 1.
Acetylcholine (10−7 M)-dependent responses of aortas from mice fed 0.45% NaCl (NS)-containing diet and a 4% NaCl (HS)-containing diet. Values are means ± SE. MS-PPOH, methylsulfonyl-propargyloxyphenylhexanamide.*P < 0.05 compared with NS, NS+MS-PPOH, and HS+MS-PPOH, n = 6.
Response of aortas to NECA and CGS-21680 in mice fed HS and NS.
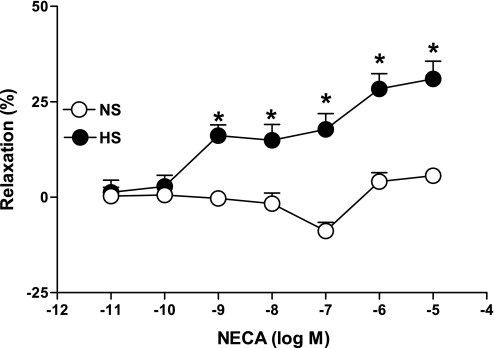
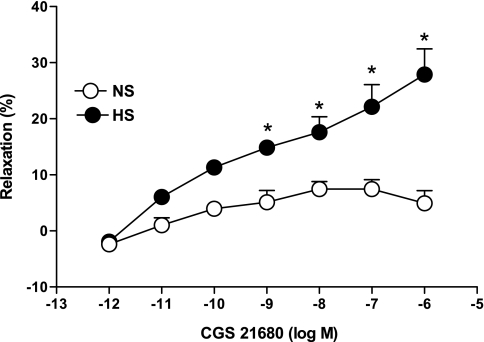
NECA produced a concentration-dependent relaxation in aortas from HS as opposed to contraction in NS (Fig. 2). For example, the response to 10−6 M NECA in HS aortas was 28.37 ± 4.0% relaxation, while in NS aortas 4.0 ± 2.3% contraction was produced. Concentration-response curves in HS vs. NS were significantly different from each other at each concentration of NECA (10−9 − 10−5 M, P < 0.05). CGS-21680 produced a concentration-dependent relaxation (P < 0.05) in aortas from HS and NS mice, but the relaxation response was significantly greater for HS at all concentrations (10−9 − 10−6 M, P < 0.05; Fig. 3). For example, at 10−6 M CGS-21680, the relaxation response was 27.9 ± 4.5% in HS compared with 4.9 ± 2.2% in NS.
Fig. 2.
5′-N-ethylcarboxamidoadenosine (NECA)-induced vascular responses in aortic rings of mice fed NS- and HS-containing diet. Values are means ± SE. *P < 0.05 compared with NS, n = 6.
Fig. 3.
2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate (CGS-21680)-induced vascular responses in aortic rings of mice fed NS- and HS-containing diet. Values are means ± SE. *P < 0.05 compared with NS, n = 6.
Effect of NO inhibitor on dose-response curve of aortas to ACh in mice fed HS and NS.
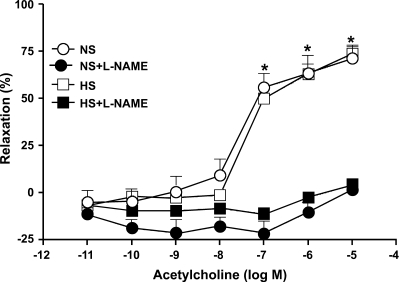
Dose (10−7 − 10−5 M)-dependent ACh-induced relaxation was observed in both HS and NS, but the response was not significantly different (P > 0.05) between aortas from HS and NS mice (Fig. 4). For example, the responses to 10−6 M ACh in HS and NS aortas were 62.92 ± 5.4% and 63.46 ± 9.3% relaxation, respectively, and there was no significant difference observed in concentration-response curves between HS and NS (P > 0.05). An NO inhibitor (l-NAME; 100 μM) altered vascular responses significantly (P < 0.05) in both HS (−2.5 ± 2.3% at 10−6 ACh) and NS (−10.2 ± 5.1% at 10−6 ACh) compared with no l-NAME. But, there was no significant difference observed in concentration-response curves between l-NAME-treated HS and NS mice (P > 0.05, Fig. 4).
Fig. 4.
Effect of Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) on ACh-induced vascular response in aortic rings of mice fed NS- and HS-containing diet. Values are means ± SE. *P < 0.05 between HS and NS vs. NS+l-NAME and HS+l-NAME, n = 6.
Effects of NO, COX, CYP epoxygenase, sEH, CYP4A, and KATP channel inhibitors on response of aortas to CGS-21680 in mice fed HS and NS.
CGS-21680 produced a concentration-dependent relaxation (P < 0.05) in aortas from HS and NS mice, but the relaxation response was significantly greater for HS at all concentrations (10−9 − 10−6 M, P < 0.05). l-NAME (100 μM), an NO inhibitor, did not alter vascular responses in both treated (25.2 ± 2.2% at 10−6 CGS-21680) and control HS (27.9 ± 4.5%, P > 0.05) tissues. There was no significant difference observed in concentration-response curves between treated (l-NAME) and control NS aortas (3.9 ± 2.8 vs. 4.9 ± 2.2% at 10−6 CGS-21680). Similarly, indomethacin (10 μM), a COX inhibitor, also did not alter the concentration-response curve in HS (29.2 ± 3.5% at 10−6 CGS-21680) compared with nontreated HS (27.9 ± 4.5%, P > 0.05). In addition, there was no significant difference observed in concentration-response curves between treated (indomethacin) and control NS aortas (5.3 ± 3.9 vs. 4.9 ± 2.2% at 10−6 CGS-21680).
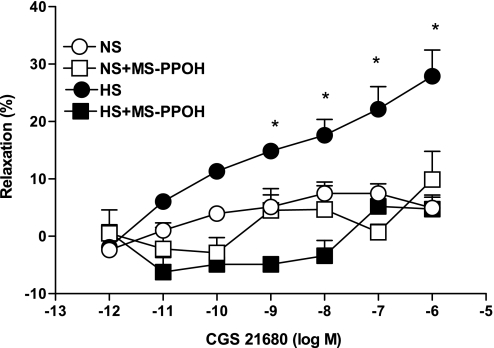
Total abolition of the CGS-21680-induced relaxation response in HS was achieved with MS-PPOH (10 μM), a highly selective CYP epoxygenase inhibitor. Use of MS-PPOH caused a significant reduction in relaxation (to +4.7 ± 2.0%), while the absence of MS-PPOH does not have any effect on relaxation (27.9 ± 4.5%; P < 0.05, at 10−6 M CGS-21680, Fig. 5) in HS. No significant effect of MS-PPOH was observed on CGS-21680-induced responses in NS mouse aortas (P > 0.05, Fig. 5).
Fig. 5.
Effect of MS-PPOH (10 μM) on CGS-21680-induced vascular response in aortic rings of mice fed NS- and HS-containing diet. The control curve is the same as in Fig. 3. Values are means ± SE. *P < 0.05 between HS vs. NS, HS+MS-PPOH, and NS+MS-PPOH, n = 6.
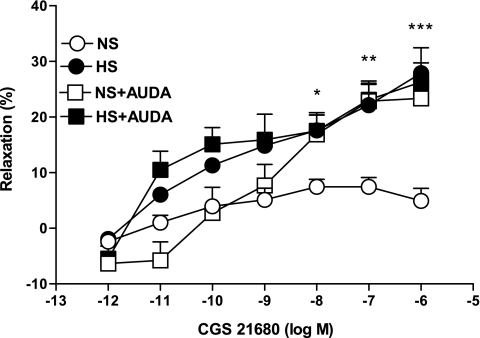
AUDA (10 μM), an sEH inhibitor, produced a significantly higher relaxation (23.3 ± 1.4%, P < 0.05) in the CGS-21680-induced vascular response in NS aortas as opposed to control (4.9 ± 2.2%; Fig. 6). In contrast, AUDA had no significant effect on CGS-21680-mediated relaxation in HS aorta. There were no significant differences in responses to CGS-21680 among NS aortas treated with AUDA and treated and untreated HS mouse aortas (P > 0.05).
Fig. 6.
Effect of 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA; 10 μM) on CGS-21680-induced vascular response in aortic rings of mice fed NS- and HS-containing diet. The control curve is the same as in Fig. 3. Values are means ± SE. *P < 0.05 between HS and NS **P < 0.05 between NS and NS+AUDA, ***P < 0.05 between NS and HS+AUDA, n = 6.
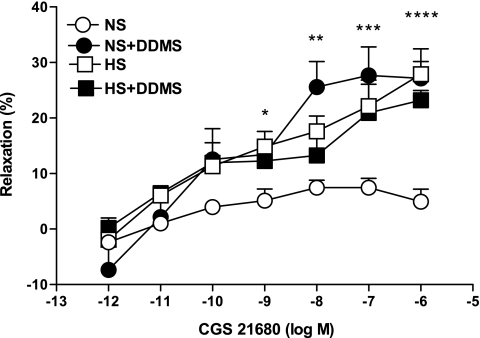
DDMS (10 μM), a CYP4A inhibitor, produced significantly higher relaxation (27.1 ± 3.0%, P < 0.05) in the CGS-21680-induced vascular response in NS aortas as opposed to control (4.9 ± 2.2%; Fig. 7). In contrast, DDMS had no significant effect on CGS-21680-mediated relaxation in HS aorta. There were no significant differences in responses to CGS-21680 among NS aortas treated with DDMS and treated and untreated HS mouse aortas (P > 0.05).
Fig. 7.
Effect of dibromo-dodecenyl-methylsulfimide (DDMS; 10 μM) on CGS-21680-induced vascular response in aortic rings of mice fed NS- and HS-containing diet. The control curve is the same as in Fig. 3. Values are means ± SE. *P < 0.05 between HS and HS+DDMS, **P < 0.05 between HS and NS, ***P < 0.05 between NS and NS+DDMS, ****P < 0.05 between NS and HS+DDMS, n = 6.
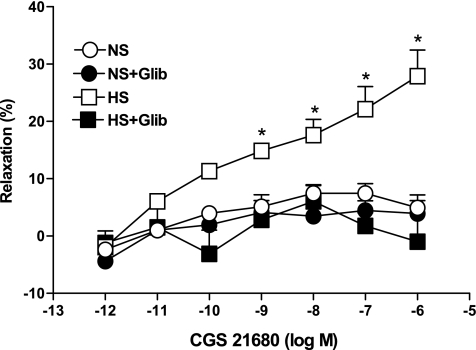
Complete abolition of the CGS-21680-induced relaxation response in HS was achieved with glibenclamide (10 μM), a KATP channel blocker. Use of glibenclamide caused −0.9 ± 5.9% contraction, while in the absence of glibenclamide 27.9 ± 4.5% relaxation was observed (P < 0.05, at 10−6 M CGS-21680, Fig. 8) in HS. There were no significant differences in responses to CGS-21680 among NS aortas treated with glibenclamide and untreated NS mouse aortas (P > 0.05, Fig. 8).
Fig. 8.
Effect of glibenclamide (Glib; 10 μM) on CGS-21680-induced vascular responses in HS compared with nontreated HS mouse aortic rings. The control curve is the same as in Fig. 3. Values are means ± SE, *P < 0.05 between HS vs. HS+Glib, NS+Glib, and NS groups, n = 6.
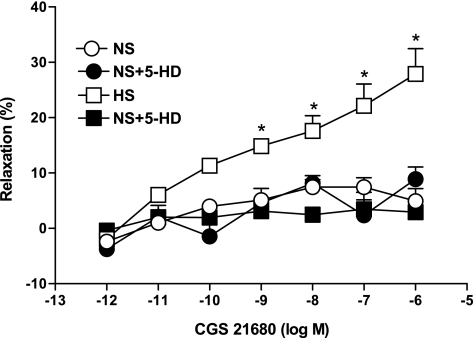
5-HD (100 μM), a specific mitochondrial KATP channel blocker, lowers the relaxation (8.8 ± 2.2%, P < 0.05) in the CGS-21680-induced vascular response in HS aortas as opposed to control (27.9 ± 4.5%; Fig. 9). In contrast, 5-HD had no significant effect on CGS-21680-mediated vascular response in NS aorta. There were no significant differences in responses to CGS-21680 among NS aortas treated with 5-HD and untreated NS mouse aortas (P > 0.05).
Fig. 9.
Effect of 5-hydroxydecanoate (5-HD; 100 μM) on CGS-21680-induced vascular responses in HS compared with nontreated HS mouse aortic rings. The control curve is the same as in Fig. 3. Values are means ± SE, *P < 0.05, between HS vs. HS+5-HD, NS+5-HD, and NS groups, n = 6.
Expression of CYP2J2, sEH, CYP4A, A1 AR, and A2A AR proteins in aortas from mice fed HS and NS.
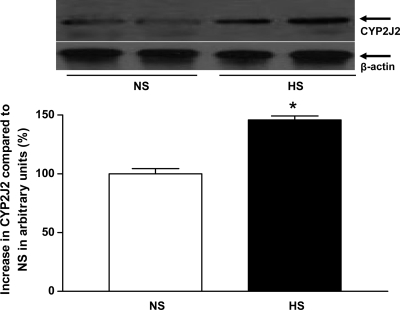
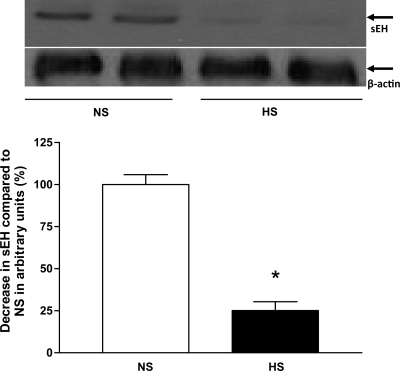
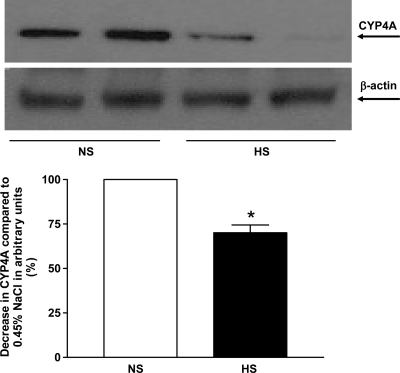
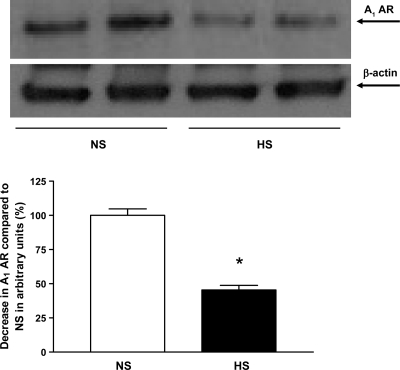
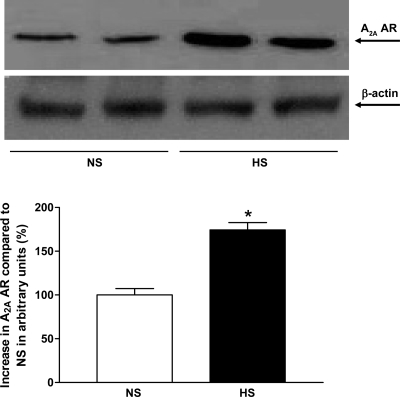
Western blot analysis for CYP2J2 (∼58 kDa) protein showed ∼46% more CYP2J2 protein expressed in HS mouse aortas than in NS mouse aortas (P < 0.05, Fig. 10). Western blot analysis for sEH (∼60 kDa) protein, the amount of sEH protein in HS mouse aortas, was reduced by ∼75% compared with NS mouse aortas (P < 0.05, Fig. 11). The amount of CYP4A (∼50 kDa) protein in HS mouse aortas was reduced by ∼30% compared with NS mouse aortas (P < 0.05, Fig. 12). Western blot analysis for A1 AR (∼37 kDa) protein showed ∼55% less A1 AR protein in HS mouse aortas than in NS mouse aortas (P < 0.05, Fig. 13). The amount of A2A AR (∼45 kDa) protein in HS mouse aortas was increased by 74% compared with NS mouse aortas (P < 0.05, Fig. 14).
Fig. 10.
Representative Western blots and densitometric data for CYP2J2 (∼58 kDa) protein in aortas of mice fed HS- and NS-containing diets. Values are means ± SE,*P < 0.05 compared with NS aortas, n = 4.
Fig. 11.
Representative Western blots and densitometric data for soluble epoxide hydrolase (sEH; ∼60 kDa) protein in aortas of mice fed HS- and NS-containing diets. Values are means ± SE, *P < 0.05 compared with NS aortas, n = 3.
Fig. 12.
Representative Western blots and densitometric data for cytochrome P-450 4A (CYP4A; ∼50 kDa) protein in aortas of mice fed HS- and NS-containing diets. Values are means ± SE, *P < 0.05 compared with NS aortas, n = 3.
Fig. 13.
Representative Western blots and densitometric data for A1 adenosine receptor (A1 AR; ∼37 kDa) protein in aortas of mice fed HS- and NS-containing diets. Values are means ± SE, *P < 0.05 compared with NS aortas, n = 4.
Fig. 14.
Representative Western blots and densitometric data for A2A AR (∼45 kDa) protein in aortas of mice fed HS- and NS-containing diets. Values are means ± SE, *P < 0.05 compared with NS aortas, n = 3.
DISCUSSION
We earlier concluded that either 4% or 7% NaCl enhances the expression of A2A AR and induces vascular relaxation through CYP epoxygenase (33). In the present study, the nonselective adenosine analog NECA and a selective A2A receptor agonist, CGS-21680, dilate in a similar way (i.e., 7% NaCl; Ref. 33) in aortas from mice fed an HS (4% NaCl) diet, while they had no significant effect on aortas from mice fed an NS (0.45% NaCl) diet. Also, the expression of A1 adenosine receptor was lower, and A2A receptor expression was higher in HS compared with NS. Taken together, our earlier report (33) and these present findings suggest that HS intake leads to an increase in the density of aortic A2A AR and a decrease in the density of A1 AR receptors. As a matter of fact, the role of A1 AR and A2A AR receptors in the modulation of vascular response is well established in mouse aortas (32–34, 46). Our present data also demonstrate that ACh induces dose-dependent relaxation (10−7 − 10−5 M) equally in both HS and NS mouse aorta. HS feeding does not make any difference in ACh dose-dependent relaxation (10−7 − 10−5 M, P > 0.05) compared with NS; l-NAME was able to block ACh-induced dose-dependent relaxation (10−7 − 10−5 M, P < 0.05) in both HS and NS equally. These data suggest that HS does not increase or decrease ACh-induced dose-dependent relaxation (10−7 − 10−5 M, P > 0.05) compared with NS-fed mouse aorta. In contrast, HS enhances in CGS-21680 (A2A AR)-induced dose-dependent relaxation compared with NS (33). Therefore, l-NAME and indomethacin were unable to block the CGS-21680-induced relaxation in HS diet-fed mouse aorta, indicating that NO and COX do not contribute to A2A AR-induced relaxation in HS diet-fed mouse aortas as it does in ACh-induced dose-dependent response in both HS and NS the same as with l-NAME. Similar responses have been reported by others (5, 7) in which NO and PGI2 were not involved in adenosine-induced vascular relaxation in rat preglomerular microvessels.
It has also been demonstrated that inhibition of CYP epoxygenase with clotrimazole causes an increase in blood pressure in rats maintained on a high-salt diet, and the salt itself does not have the ability to increase blood pressure (26). But, the downregulation of CYP epoxygenase activity and inhibition of EET formation are associated with salt-sensitive hypertension (17, 26, 55). Furthermore, an exaggerated relaxation response to adenosine has been reported in kidneys from HS-fed rats through EETs (24), which suggests that the failure to upregulate A2A adenosine receptor-EET pathway was the main factor in the development of hypertension in Dahl salt-sensitive rats (23). Our present data and previously published work by us (32, 33) and others (5, 7) indicated that HS feeding for 4–5 wk enhances A2A AR-induced relaxation through CYP epoxygenases, and neither NO nor COX is involved in this process. Therefore, ACh-induced dose-dependent response did not change in the HS group compared with NS group. We have also observed recently that HS feeding in endothelial NO synthase (eNOS)−/− mice enhances A2A AR-induced vascular relaxation with upregulation of CYP epoxygenase compared with NS-fed eNOS−/− mice (MA Nayeem, DC Zeldin, MA Boegehold, and JR Falck, unpublished observation). In contrast, others have reported (37) that an HS diet also increases oxidant activity, decreases NO-availability, and reduces vascular response in rat skeletal muscle arterioles. Also, acute salt increase in diet has adverse effect in humans (47), and salt reduction improves vasodilation in humans (8). These differences in vascular response may be due to use of various vascular beds as well as different species.
Also, in the present study, CYP450 epoxygenase inhibitor, MS-PPOH was able to block the CGS-21680-induced dilation of vessels obtained from HS mice. Our data also suggest that the endothelium-dependent CGS-21680-induced vascular relaxation is mediated by CYP epoxygenase, including CYP2J2 in HS mouse aorta, whereas, NS-reduced dilation was abolished by AUDA (an sEH inhibitor) and changed vascular response into vasodilation. No significant difference was observed between AUDA-treated and -nontreated control (HS) tissues, suggesting that the sEH enzyme is involved in the induction of reduced vascular response in the NS-containing diet group. It has been reported that the conversion of arachidonic acid derived epoxides to diols by sEH, diminishes the beneficial cardiovascular properties of these epoxyeicosanoids (9), and inhibition of sEH causes EETs to accumulate and be retained for longer periods after they are formed (9).
The CYP4A inhibitor, DDMS also changed vascular response and enhanced vasodilation in NS vessels. No significant difference was observed between DDMS-treated and -nontreated control (HS) tissues. These data suggest that the CYP4A enzyme reduced vascular relaxation in NS and the HS downregulates CYP4A protein expression in mice. In contrast, others have reported that a high-salt diet upregulates CYP4A protein in rat mesenteric arteries (49) and reduces vasodilator response through 20-HETE in rat arterioles (12, 14). These differences in vascular response may be due to use of various vascular beds as well as different species. But immunoblot analysis indicated that the expression of CYP4A protein in glomeruli from the kidneys of rats fed a low-salt diet was sixfold higher than in kidneys of rats fed a high-salt diet (21). Current findings from this study also suggest that the activation of A2A receptor increases the activity of CYP2J2 epoxygenase and decreases the activities of sEH and CYP4A in aortas from HS mice. Downregulation of A2A receptors appears to lower CYP2J2 (CYP epoxygenase) and increase sEH and CYP4A protein expression in aortas from NS mice. As reported by others (23), the failure to upregulate A2A adenosine receptor-EET pathway was the main factor in the development of hypertension in Dahl salt-sensitive rats and an exaggerated relaxation response to adenosine have been observed in kidneys from HS-fed rats through CYP epoxgenase-synthesized product EETs (24).
ATP-sensitive (K+) channels blocker, glibenclamide, changed CGS-21680-induced enhanced vasodilation response into reduced vasodilation in HS diet-fed mouse aorta. No significant difference was observed between glibenclamide treated and nontreated control (NS) tissues. These data suggest that ATP-sensitive (K+) channels are involved in vasodilation in HS diet group. The activation of the vascular KATP channels lead to membrane hyperpolarization that reduces the calcium influx, and enhances vascular relaxation (53). Obiefuna and Obiefuna (38) has suggested that ATP-sensitive potassium channels associated with the vascular smooth muscle membrane plays an important role in the modulation of vascular response. The concentration of glibenclamide (10 μM) that was used in this study seemed to be appropriate for its selectivity, because it matches to the concentration used by us and others (29, 30, 35, 39, 41, 50). However, some workers (16, 22, 52) have also used 1 μM glibenclamide to block KATP channels. It may possible that other K+ channels may also be involved (3, 27, 57) in CGS-21680-induced vasodilation in HS-fed mouse aorta. Additionally, we have also observed the involvement of mitochondrial KATP channels in the regulation (opening and closing) of vascular tone in both HS- and NS-containing diet-fed groups. This conclusion was drawn due to the inhibitory action of a specific mitochondrial KATP channels inhibitor (5-HD) and changed vascular response in HS-containing diet-fed mouse aorta.
CYP epoxygenase activities and the formation of endothelium-derived hyperpolarization factor (EDHF) have been explored over the last several years in different blood vessels by others as well as by us in different animal models (10, 15, 32, 33, 36, 51). A substance released from endothelial cells through CYP epoxygenases (CYP2C, CYP2J2) activity is called EDHF, which stimulates hyperpolarization in smooth muscle cells and thus induces vasodilation (11). In the present study, significant expression of CYP2J2 protein in aortas from HS compared with NS was observed. These data suggest that an HS-containing diet enhances CYP epoxygenase activity, not only in mouse kidneys, but also in mouse aortas as confirmed by our research team earlier (33). The present findings are in agreement with those reported earlier in renal microvessels of high-salt-fed rats (24) as well as in mouse aortas (33). Also, the vascular responses seen in our study were similar to those reported earlier by others in rat kidneys, where CYP epoxygenases played an important role in the generation of EDHFs leading to vasorelaxation in preglomerular microvessels from rats fed a high-salt diet (5, 7) and by our research team in mouse aortas (33).
Interestingly, we have also found the involvement of both sEH and CYP4A in eliciting the contraction or less vasodilation response in aortas from mice fed the NS diet in the presence of NECA and CGS-21680. High levels of CYP epoxygenase (CYP2J2) and low levels of both sEH and CYP4A proteins were observed with enhanced relaxation in the presence of NECA and CGS-21680 in aortas from mice fed HS than those from mice fed NS. The upregulation of sEH [in renal cortical tissue and renal microvessels of rats made hypertensive (20, 56)] and urinary DHETs (the EET hydrolysis products) were increased in spontaneously hypertensive rat and ANG II-induced hypertensive rats (20, 54). In this study, we found significant downregulation of sEH, CYP4A, and A1 AR in HS compared with NS mouse aorta. This is further supported by the NECA and CGS-21680-elicited enhanced relaxation in HS mouse aortas compared with NS mouse aortas and greater availability of adenosine A2A receptors and CYP2J2 in HS compared with NS mouse aorta.
Perspectives and Significance
The present findings provide evidence that NECA and CGS-21680-induced relaxation were significantly higher in aortas from HS than NS, as reported earlier by others in renal vessels of salt-loaded rats (4, 5, 7, 24). Also, we reported earlier (33) that this phenomenon of HS-induced vascular response is not limited to kidneys but also extends to larger vessels like the aorta, as seen in this study. The larger vessels (including aorta) may also be prone to the effects of hypertensive or salt-sensitive hypertensive conditions. The present data suggest that there is a relationship among the availability of A2A ARs, functional capacity of endothelium to activate CYP2J2, downregulation of sEH and CYP4A, activated ATP-sensitive (K+) channels in NECA, and CGS-21680-enhanced relaxation in HS mouse aorta. In contrast, the reduced relaxation with NECA and CGS-21680 in NS diet-fed mice provide evidence that there is a possible link among the less available A2A ARs, less functional capacity of endothelium to activate CYP2J2, upregulation of sEH, CYP4A, A1 AR, and deactivation of ATP-sensitive (K+) channels. Therefore, we conclude that upregulation of CYP2J2, A2A AR, and downregulation of sEH, CYP4A, and A1 AR protein expression contribute to NECA and CGS-21680-induced dilation of HS aorta, while the action of CYP4A, sEH, and A1 AR proteins are predominant when NS aortas are treated with NECA and CGS-21680, producing a net vasoconstriction.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-027339 (to S. J. Mustafa) and HL-094447 (to S. J. Mustafa and M. A. Nayeem, coinvestigator), American Heart Association Grant 2250298 (to M. A. Boegehold), National Institute of General Medical Sciences Grant GM-31278 (to J. R. Falck Jr), and the Intramural Research Program of the National Institute of Environmental Health Sciences/National Institutes of Health Grant Z01-ES-025034 (to D. C. Zeldin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. S. J. Mustafa of West Virginia University, Morgantown, WV for his support.
REFERENCES
- 1.Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther 284: 1066–1073, 1998 [PubMed] [Google Scholar]
- 2.Biaggioni I. Contrasting excitatory and inhibitory effects of adenosine in blood pressure regulation. Hypertension 20: 457–465, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem 267: 21720–21726, 1992 [PubMed] [Google Scholar]
- 5.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol 291: F155–F161, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chen D, La Greca L, Head GA, Walther T, Mayorov DN. Blood pressure reactivity to emotional stress is reduced in AT1A-receptor knockout mice on normal, but not high salt intake. Hypertens Res 32: 559–564, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441–448, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 ω-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol 278: H1517–H1526, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC, Lombard JH. Parenchymal tissue cytochrome P450 4A enzymes contribute to oxygen-induced alterations in skeletal muscle arteriolar tone. Microvasc Res 63: 340–343, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol 280: H1066–H1074, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, McGiff JC, Quilley J. Pharmacological evaluation of an epoxide as the putative hyperpolarizing factor mediating the nitric oxide-independent vasodilator effect of bradykinin in the rat heart. J Pharmacol Exp Ther 287: 497–503, 1998 [PubMed] [Google Scholar]
- 16.Haba M, Kinoshita H, Matsuda N, Azma T, Hama-Tomioka K, Hatakeyama N, Yamazaki M, Hatano Y. Beneficial effect of propofol on arterial adenosine triphosphate-sensitive K+ channel function impaired by thromboxane. Anesthesiology 111: 279–286, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71: 1105–1115, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hein TW, Belardinelli L, Kuo L. Adenosine A2A receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther 291: 655–664, 1999 [PubMed] [Google Scholar]
- 19.Hoag JB, Qian YZ, Nayeem MA, D'Angelo M, Kukreja RC. ATP-sensitive potassium channel mediates delayed ischemic protection by heat stress in rabbit heart. Am J Physiol Heart Circ Physiol 273: H2458–H2464, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ito O, Roman RJ. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol Regul Integr Comp Physiol 276: R1749–R1757, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita H, Matsuda N, Kaba H, Hatakeyama N, Azma T, Nakahata K, Kuroda Y, Tange K, Iranami H, Hatano Y. Roles of phosphatidylinositol 3-kinase-Akt and NADPH oxidase in adenosine 5′-triphosphate-sensitive K+ channel function impaired by high glucose in the human artery. Hypertension 52: 507–513, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 295: F1696–F1704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol 289: F386–F392, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Ling BN. Luminal adenosine receptors regulate amiloride-sensitive Na+ channels in A6 distal nephron cells. Am J Physiol Renal Fluid Electrolyte Physiol 270: F798–F805, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ Res 83: 501–507, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Nayeem MA. Sublethal simulated ischemia promotes delayed resistance against ischemia via ATP-sensitive (K+) channels in murine myocytes: role of PKC and iNOS. Antioxid Redox Signal 6: 375–383, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Nayeem MA, Matherne GP, Mustafa SJ. Ischemic and pharmacological preconditioning induces further delayed protection in transgenic mouse cardiac myocytes over-expressing adenosine A1 receptors (A1AR): role of A1AR, iNOS and KATP channels. Naunyn-Schmiedebergs Arch Pharmacol 367: 219–226, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Nayeem MA, Mustafa SJ. Mechanisms of delayed preconditioning with A1 adenosine receptor activation in porcine coronary smooth muscle cells. Pol J Pharmacol 54: 443–453, 2002 [PubMed] [Google Scholar]
- 32.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol 296: R567–R574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Mustafa SJ. Role of CYP2C generated metabolites in adenosine-mediated relaxation using A2A AR−/− mice. FASEB J 22: 755–755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niiya K, Uchida S, Tsuji T, Olsson RA. Glibenclamide reduces the coronary vasoactivity of adenosine receptor agonists. J Pharmacol Exp Ther 271: 14–19, 1994 [PubMed] [Google Scholar]
- 36.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurkiewicz TR, Boegehold MA. High-salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Obiefuna PC, Obiefuna IP. Salt-induced hypertension in rats alters the response of isolated aortic rings to cromakalim. West Indian Med J 50: 17–21, 2001 [PubMed] [Google Scholar]
- 39.Olanrewaju HA, Gafurov BS, Lieberman EM. Involvement of K+ channels in adenosine A2A and A2B receptor-mediated hyperpolarization of porcine coronary artery endothelial cells. J Cardiovasc Pharmacol 40: 43–49, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pawlowska D, Granger JP, Knox FG. Effects of adenosine infusion into renal interstitium on renal hemodynamics. Am J Physiol Renal Fluid Electrolyte Physiol 252: F678–F682, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Petersson J, Zygmunt PM, Hogestatt ED. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br J Pharmacol 120: 1344–1350, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294: H1258–H1265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension 17: 117–130, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Taubes G. The (political) science of salt. Science 281: 898–901, 903–897, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288: H1411–H1416, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Roman RJ, Falck JR, de la Cruz L, Lombard JH. Effects of high-salt diet on CYP450–4A ω-hydroxylase expression and active tone in mesenteric resistance arteries. Am J Physiol Heart Circ Physiol 288: H1557–H1565, 2005 [DOI] [PubMed] [Google Scholar]
- 50.White R, Hiley CR. Effects of K+ channel openers on relaxations to nitric oxide and endothelium-derived hyperpolarizing factor in rat mesenteric artery. Eur J Pharmacol 357: 41–51, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem 272: 12551–12559, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol 288: H358–H364, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C25–C37, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709–714, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15: 1244–1253, 2004 [PubMed] [Google Scholar]
- 57.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Fluid Electrolyte Physiol 270: F822–F832, 1996 [DOI] [PubMed] [Google Scholar]