Abstract
Members of the voltage-gated K+ (KV) channel family are suggested to control the resting membrane potential and the repolarization phase of the action potential in urinary bladder smooth muscle (UBSM). Recent studies report that stromatoxin-1, a peptide isolated from tarantulas, selectively inhibits KV2.1, KV2.2, KV4.2, and KV2.1/9.3 channels. The objective of this study was to investigate whether KV channels sensitive to stromatoxin-1 participate in the regulation of rat UBSM contractility and to identify their molecular fingerprints. Stromatoxin-1 (100 nM) increased the spontaneous phasic contraction amplitude, muscle force, and tone in isolated UBSM strips. However, stromatoxin-1 (100 nM) had no effect on the UBSM contractions induced by depolarizing agents such as KCl (20 mM) or carbachol (1 μM). This indicates that, under conditions of sustained membrane depolarization, the KV channels sensitive to stromatoxin-1 have no further contribution to the membrane excitability and contractility. Stromatoxin-1 (100 nM) increased the amplitude of the electrical field stimulation-induced contractions, suggesting also a role for these channels in neurogenic contractions. RT-PCR experiments on freshly isolated UBSM cells showed mRNA expression of KV2.1, KV2.2, and KV9.3, but not KV4.2 channel subunits. Protein expression of KV2.1 and KV2.2 channels was detected using Western blot and was further confirmed by immunocytochemical detection in freshly isolated UBSM cells. These novel findings indicate that KV2.1 and KV2.2, but not KV4.2, channel subunits are expressed in rat UBSM and play a key role in opposing both myogenic and neurogenic UBSM contractions.
Keywords: voltage-gated K+ channel, detrusor muscle, reverse transcriptase-polymerase chain reaction, Western blot, immunocytochemistry
during the bladder-filling phase, urinary bladder smooth muscle (UBSM) relaxes to accommodate increasing volumes of urine. During voiding, the UBSM contracts in a phasic manner to expel the urine. A key regulator of UBSM contractility is the cell membrane potential (3, 6, 7, 13, 16, 24). Spontaneous action potentials determine the phasic nature of the contractions in UBSM (6, 14, 16, 23, 24). Ca2+ entry through L-type voltage-gated Ca2+ channels is responsible for the upstroke of the action potential and leads to an increase in the intracellular Ca2+ concentration, which initiates the UBSM phasic contractions (14). The repolarization phase of the UBSM action potential is mediated by the activity of the large-conductance Ca2+-activated K+ channels (6, 14, 16, 23, 24) and perhaps the voltage-gated K+ (KV) channels (13, 28). After the repolarization phase, the action potential in UBSM displays a prolonged afterhyperpolarization, which is mediated by apamin-sensitive small-conductance Ca2+-activated K+ channels (15) and perhaps KV channels (12, 13). The UBSM resting membrane potential is controlled by the large-conductance Ca2+-activated K+, ATP-sensitive K+, and probably KV channels (12–14, 24, 28). In essence, various K+ channels, including KV channels, play differential roles in regulating both UBSM resting membrane potential and action potentials and, therefore, the related phasic contractions.
Normally, the KV1–4, KV7, and KV10–12 subunits form homotetramers. Interestingly, KV5, KV6, KV8, and KV9 channels do not make their own homotetramers, but rather form heterotetramers with the KV2 subunits (22). For example, association of KV2.1 and KV9.3 subunits can produce oxygen-sensitive K+ channels (17, 20, 21). The KV channels have diverse biophysical and pharmacological properties in different smooth muscles, playing a prominent role in shaping the electrical and contractile behavior (1, 27). The tissue specificity, diverse properties, and various functions of the KV channels make them selective drug targets for diseases such as overactive bladder (4, 22).
In mouse UBSM, KV2, KV5, and KV6 channel subunits are probable candidates for mediating the repolarization phase of the action potential and in maintaining the resting membrane potential (28). In contrast, recent studies on mouse UBSM argue that KV2.1 does not play a role in shaping the action potential (13). The KV1 channels are shown to be expressed and functional in human UBSM (8). In vivo studies suggest a role for KV7 (KCNQ) channels in rat bladder function (26). However, knowledge about the role of the KV channels in UBSM contractility is limited and controversial (8, 11, 13, 28).
In the past, the lack of specific KV channel inhibitors has hampered the study of these channels. The emergence of new, highly specific KV channel inhibitors have enabled researchers to selectively isolate KV channel subtypes and determine their physiological role (22). Stromatoxin-1, a 34-amino acid peptide isolated from African tarantulas, was shown to selectively inhibit KV2.1, KV2.2, and KV4.2 homotetramers, and KV2.1/9.3 heterotetramers with high affinity (9, 10). This recent discovery enables us to employ stromatoxin-1 as a tool to dissect the role of these KV channels in UBSM contractility.
The goal of the present study was to examine the functional role of the KV channels sensitive to stromatoxin-1 in regulating rat UBSM contractility. Using molecular biology approaches, we further identified the expression of the individual KV channels sensitive to stromatoxin-1. We detected mRNA expression of KV2.1, KV2.2, and KV9.3 subunits in freshly isolated UBSM cells and confirmed KV2.1 and KV2.2 protein expression by Western blot and immunocytochemical methods. We revealed that KV2.1 and KV2.2 channels play a key role in opposing rat UBSM myogenic and nerve-evoked contractions.
MATERIALS AND METHODS
Animal care, tissue, and cell preparation.
All animal studies were carried out in accordance with the Animal Use Protocol no. 1482 of the University of South Carolina Institutional Animal Care and Use Committee, which approved this study. In this study, we used 33 adult Sprague-Dawley rats (29 males and 4 females), with an average weight of 325 ± 46 g. Rats were euthanized with CO2 followed by thoracotomy. The urinary bladders were removed and placed in ice-cold Ca2+-free dissection solution composed of 80 mM monosodium glutamate, 55 mM NaCl, 6 mM KCl, 2 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.3. The bladders were cut open and rinsed free of urine, and the entire mucosal layer of the bladder, including the urothelium, was removed. Bladders were then dissected free of fat and connective tissue. The trigone, urethra, and ureter sphincters were also removed, and only detrusor muscle (referred to as “UBSM” throughout) was used in the study.
For reverse transcriptase (RT)-PCR experiments, UBSM whole tissue and freshly isolated single cells were immediately used for RNA extraction. UBSM single cells for RT-PCR and immunocytochemistry were freshly isolated and collected as previously described (7, 16). For RT-PCR and Western blot experiments, the rat brains were also removed and placed in ice-cold Ca2+-free dissection solution (see above).
Isometric UBSM tension recordings.
Isometric UBSM tension recordings were conducted, as previously described (16). Briefly, small UBSM strips (1- to 2-mm wide and 5- to 7-mm long) were cut from the bladder and transferred to a small vial containing Ca2+-free dissection solution (see above). The strips were mounted between a stationary mount and a force-displacement transducer in thermostatically controlled (at 37°C) 10 ml tissue baths. The baths were filled with Ca2+-containing physiological saline solution, which had the following composition: 119 mM NaCl, 4.7 mM KCl, 24 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, and 11 mM glucose, and aerated with 95% O2-5% CO2 (pH 7.4). The UBSM strips were initially stretched to 1 g of tension and then were washed with this solution every 15 min during an equilibration period of 45–60 min. Two groups of treatments were set up for strips exhibiting spontaneous phasic contractions after they stabilized sufficiently. In one group, 100 nM stromatoxin-1 (Sigma-Aldrich) was introduced to the bath, and at least 30–40 min were recorded with the KV channel inhibitor present. In another group, UBSM phasic contractions were induced with depolarizing agents, 20 mM KCl or 1 μM carbachol, and, after the phasic contractions stabilized, 100 nM stromatoxin-1 were introduced into the bath. In a third group, we elucidated the role of stromatoxin-1-sensitive KV channels in response to nerve stimulation. Neurogenic contractions were induced by electrical field stimulation (EFS) using a pair of platinum electrodes, which were mounted in the tissue bath in parallel to the UBSM strip. The neurogenic EFS-induced contractions were characterized in response to increasing EFS frequencies (5–50 Hz) in the presence or absence of 100 nM stromatoxin-1. The EFS pulses were generated using a PHM-152I stimulator (Med Associates), and the EFS pulse parameters were as follows: pulse amplitude was 20 V, pulse width was 0.75 ms, stimulus duration was 3 s, and polarity was reversed for alternating pulses. UBSM contractions were recorded using MyoMed software (Med Associates). Then MiniAnalysis (Synaptosoft) software was used for analyzing data from UBSM contractions. GraphPad Prism 4.03 software (GraphPad Software) was used for further statistical analysis, and CorelDraw Graphic Suite X3 software (Corel) was used for presenting data. To compare the phasic contractions parameters, data were normalized to the spontaneous contractions (taken to be 100%) and expressed as percentages. For the EFS-induced contractions, the maximal contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%, and the data were normalized. Net muscle force (muscle force integral) was determined by integrating the area under the curve of the phasic contractions component. UBSM tone was determined by measuring the phasic contraction baseline curve. Phasic contraction duration was determined by the half-width (width of contraction at 50% of the amplitude). To evaluate the stromatoxin-1 effect on the phasic contractions, a 5-min period before stromatoxin-1 application was analyzed for the controls, and another 5-min period was analyzed following 30 min of 100 nM stromatoxin-1 application. Results are summarized as means ± SE for the n number of UBSM strips isolated from different rats (N = number of rats). Data were compared using paired Student's t-test. A P value <0.05 was considered statistically significant.
RNA extraction, RT-PCR, and sequencing.
Total RNA was isolated from rat brain, intact UBSM tissue, and freshly dispersed UBSM single cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The extracted RNA was reverse transcribed into cDNA using M-MLV RT (Promega) and oligo(dT) primers. Specific primers for KV2.1, KV2.2, KV4.2, and KV9.3 were designed based on the cDNA complete sequences of rat genes in Genbank. Specific primer pair sequences are listed in Table 1. The cDNA production was PCR amplified using GoTaq Green Master Mix (Promega) and specific primers for KV channel subunits. The PCR annealing temperature for each primer pair was optimized using a mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). Thirty cycles were used for detection from tissue samples, and 35 cycles were used for isolated UBSM single cells. Negative control experiments for PCR were performed in the absence of the RT (−RT). PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich) and sequenced at the University of South Carolina Environmental Genomics Facility for sequence confirmation.
Table 1.
RT-PCR primers for KV channels sensitive to stromatoxin-1
| Sense | Antisense | Production, bp | Accession No. | |
|---|---|---|---|---|
| Kv2.1 | GGAGAAAGCCATCAAGCG | CCAGAGGACTGTGGGAGAAT | 551 | NM_013186 |
| Kv2.2 | ACCCTCAGGCGGAGTTAC | GACCACCTCTTCCATTTCTATC | 762 | NM_054000 |
| Kv4.2 | CTCTGAGCGGAGTCTTGG | GCAGGTGGTGGTGTTGTG | 273 | NM_031730 |
| Kv9.3 | ACACCCACCCAGTCACCT | AGCCGTAGCAATAAATCCTT | 645 | AF029056 |
KV, voltage-gated K+.
Western blot.
Brain and UBSM proteins were extracted as previously described (7). Western blot was performed as follows: the protein was mixed with 5× Laemmli buffer (1:4) and denaturized for 5 min at 95°C. Subsequently, equal amounts of brain and UBSM proteins (∼50 μg) were loaded into adjacent lanes, subjected to 4–20% precast SDS-PAGE for 2.5 h at 20 mA, and transferred onto a polyvinylidene difluoride membrane at 40 mA for 2 h using semidry blot. The membrane was then blocked with 5% nonfat dry milk/Tris-buffered saline-Tween (TBST) buffer for 1 h at room temperature, and the blots were incubated with the affinity-purified rabbit polyclonal primary antibodies: anti-KV2.1 (DRK1 1:400), anti-KV2.2 (CDRK 1:2,000), anti-KV4.2 (Kcnd2 1:200) (Alomone Laboratories, Jerusalem, Israel) and anti-KV9.3 [KV9.3 (K-12) 1:200] (Santa Cruz Biotechnology) or anti-KV9.3 (KCNS3 1:200) (Abcam) overnight at 4°C. The membrane was washed with TBST four times and incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase diluted to 1:3,000 (KV2.1, KV2.2, and KV4.2) and donkey anti-goat or goat anti-chicken IgG conjugated with horseradish peroxidase diluted to 1:3,000 (KV9.3) in the blocking buffer for 1 h at room temperature. Four more washes with TBST were followed. Bound antibodies were detected by ECL substrate kit (Amersham, Piscataway, NJ), according to the manufacturer's instructions. Staining specificity was verified by preincubation of the antibodies with a competing peptide.
Immunocytochemistry.
For immunocytochemical studies, freshly isolated UBSM cells were dropped on a glass coverslip for settling for 1.5 h at room temperature before further processing, as described below. UBSM cells were fixed with prewarmed 4% paraformaldehyde and sat for exactly 10 min. Cells were then washed twice in PBS, blocked, and permeabilized in PBS containing 10% normal donkey serum and 0.1% Triton X-100 for 30 min. Once again, cells were washed in PBS and incubated with rabbit polyclonal primary antibodies: anti-KV2.1, anti-KV2.2, and anti-KV4.2 (DRK1, CDRK, and Kcnd2, 1:100, Alomone Laboratories) at 37°C for 1 h. After that, cells were washed four times in PBS and labeled with secondary antibodies [Cy3-conjugated anti-rabbit IgG, at 1:200, PBS/3% normal donkey serum/0.01% Triton X-100 (Jackson ImmunoResearch, West Grove, PA)] for 1 h in the dark. After labeling, UBSM cells were washed with PBS and incubated with Alexa Fluor 488 phalloidin dye (diluted in PBS, 1:50) for 2 h in the dark. Cells were then washed two more times and incubated with 4′,6-diamidino-2-phenylindole for 15 min and washed again and then mounted onto slides with DABCO. Control treatments were carried out as follows: 1) omission of the primary antibody for confirming the specificity of the secondary antibody; 2) absorption of the primary antibody by a competing peptide for confirming the specificity of the primary antibody. Images at ×63 were acquired with a LSM 510 META confocal microscope (Carl Zeiss, Göttingen, Germany). The slides for each group were imaged with the same laser power, gain settings, and pinhole for the controls and antibody treatment.
RESULTS
Isometric UBSM tension recording.
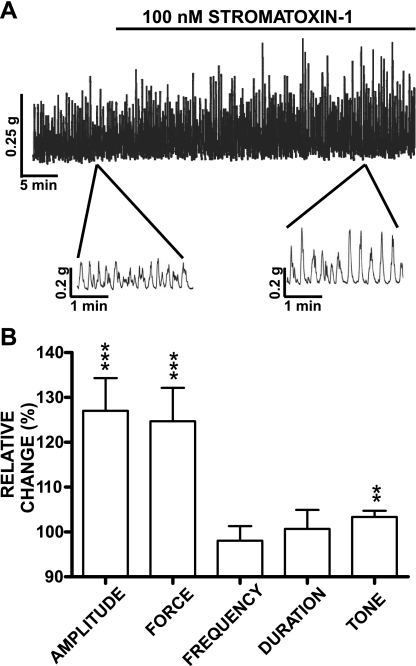
Here, we sought to investigate whether a pharmacological inhibition of stromatoxin-1-sensitive KV channels with stromatoxin-1 leads to potentiation of the physiologically relevant spontaneous (myogenic) phasic and tonic contractions that normally increased during micturition to facilitate urine voiding. In addition to the bladder myogenic activity, the UBSM contractions are also modulated by neurotransmitters released from autonomic nerves located in the bladder wall. Since stromatoxin-1-sensitive KV channels are also located in the nerves (5, 22), blocking them with stromatoxin-1 may cause neuronal cell membrane depolarization, and therefore release of excitatory neurotransmitters such as acetylcholine and ATP. To minimize effects caused by neurotransmitter release, all experiments, with the exception of the EFS-induced contractions, were performed in the presence of 1 μM tetrodotoxin (Sigma-Aldrich), a neuronal Na+ channel blocker. UBSM strips were preincubated for at least 10 min with 1 μM tetrodotoxin before stromatoxin-1 application. Isometric UBSM tension recordings of isolated UBSM strips showed that blocking the KV channels with stromatoxin-1 (100 nM) increased spontaneous phasic contraction amplitude, muscle force integral, and tone without any effect on contraction frequency or duration (n = 20, N = 12; Fig. 1). Following stromatoxin-1 application, the maximal effects on the spontaneous phasic contraction amplitude, muscle force integral, and tone (expressed in percent ± SE) were 127.0 ± 7.3 (P < 0.005), 124.7 ± 7.5 (P < 0.005), and 103.4 ± 1.4% (P < 0.01), respectively (Fig. 1B). The effect of stromatoxin-1 on the spontaneous contractility was not reversible after 10 washes of the preparations with fresh physiological saline solution (n = 8; N = 4). The stromatoxin-1 (100 nM)-induced contractions, along with the spontaneous contractions of UBSM strips, were completely inhibited by 1 μM nifedipine, an L-type voltage-gated Ca2+ channels blocker (n = 6, N = 4). To test the effect of stromatoxin-1 under conditions of sustained membrane depolarization, we used 20 mM KCl or 1 μM carbachol to depolarize the cells and induce high-amplitude phasic contractions. Stromatoxin-1 (100 nM) had no effect on any of the parameters of the UBSM contractions induced by 20 mM KCl or 1 μM carbachol (Table 2). Collectively, these experiments indicate that blocking the KV channels with stromatoxin-1 increases spontaneous UBSM myogenic activity due to membrane potential depolarization and thus activation of Ca2+ entry via L-type voltage-gated Ca2+ channels.
Fig. 1.
Stromatoxin-1 (100 nM) increases spontaneous phasic contraction amplitude, muscle force integral, and tone in isolated rat urinary bladder smooth muscle (UBSM) strips. A: original recordings illustrating the spontaneous phasic contractions followed by application of 100 nM stromatoxin-1. A portion of the recorded trace is shown on an expanded time scale before and after stromatoxin-1 application. B: summary data showing stromatoxin-1 (100 nM) effect on spontaneous phasic contraction amplitude, muscle force integral, contraction frequency, duration (half-width), and tone in isolated UBSM strips. Spontaneous contractions were taken to be 100%. Values are means ± SE; n = 20, N = 12. **P < 0.01; ***P < 0.005.
Table 2.
Stromatoxin-1 (100 nM) does not significantly change any of the UBSM contraction parameters under conditions of UBSM membrane depolarization induced by 20 mM KCl or 1 μM carbachol
| N | n | Amplitude | Force | Frequency | Duration (half-width) | Tone | P Value | |
|---|---|---|---|---|---|---|---|---|
| 20 mM K+-induced contractions | 7 | 9 | 101.23 ± 8.15 | 101.28 ± 8.74 | 101.84 ± 2.46 | 96.83 ± 4.68 | 96.74 ± 2.20 | NS |
| 1 μM carbachol-induced contractions | 7 | 8 | 112.00 ± 7.41 | 113.50 ± 7.02 | 106.19 ± 7.29 | 103.82 ± 1.57 | 91.67 ± 5.67 | NS |
Values are means ± SE in %; N, no. of rats; n, no. of urinary bladder smooth muscle (UBSM) strips. NS, nonsignificant (lack of statistical significance).
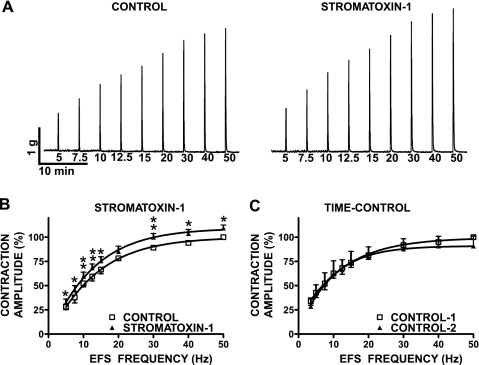
In the next experimental series, we sought to investigate whether UBSM KV channels sensitive to stromatoxin-1 modulate the neurogenic (EFS-induced) contractions of rat urinary bladder. This experimental series was conducted in the absence of tetrodotoxin. In fact, the tetrodotoxin (1 μM) sensitivity of the contractions was used as indicator for the neurogenic origin of the EFS-induced UBSM contractions, because 1 μM tetrodotoxin completely eliminates the EFS-induced contractions (n = 5, N = 4). EFS frequency-response curves were created by measuring the amplitude of the EFS-induced UBSM contraction at stimulation frequencies of 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz (Fig. 2). After an initial EFS frequency-response curve was generated under control conditions, UBSM strips were preincubated for 30 min with 100 nM stromatoxin-1, and then a second EFS frequency-response curve was generated using the same EFS parameters as indicated above. We found that EFS frequency-responses of UBSM strips were significantly increased in the presence of stromatoxin-1 at all stimulation frequencies, except 20 Hz (n = 6, N = 5; P < 0.05; Fig. 2). The amplitudes of the EFS-induced contractions (expressed in percent ± SE) in the presence of 100 nM stromatoxin-1 were as follows: 32.4 ± 3.7 at 5 Hz, 45.4 ± 4.7 at 7.5 Hz, 59.4 ± 4.5 at 10 Hz, 68.3 ± 4.1 at 12.5 Hz, 75.5 ± 4.4 at 15 Hz, 85.2 ± 5.6 at 20 Hz, 100.0 ± 3.9 at 30 Hz, 103.6 ± 4.3 at 40 Hz, and 109.4 ± 3.2 at 50 Hz, respectively. To confirm the stability of the preparations, we also performed time controls, including two runs of EFS-induced contractions without applying stromatoxin-1 (n = 4, N = 4). The second time control (in the absence of stromatoxin-1) followed the pattern of the first one with a slight decrease in contraction amplitude at higher stimulation frequencies (50 Hz), which is an opposite effect of the observed increase in EFS-induced contractions by stromatoxin-1. This experimental series suggests that stromatoxin-1-sensitive KV channels also regulate the neurogenic UBSM contractions. However, the stromatoxin-1-sensitive KV channels play a more important role in contributing to the myogenic UBSM activity compared with the neurogenic contractions (compare Figs. 1 and 2). Using molecular biology techniques such as RT-PCR, Western blot, and immunocytochemistry, we next sought to investigate the molecular identity of the stromatoxin-1-sensitive KV channels that determine the myogenic and neurogenic contractions of the rat urinary bladder.
Fig. 2.
Stromatoxin-1 (100 nM) increases the amplitude of the electrical field stimulation (EFS)-induced contractions in isolated rat UBSM strips. A: original traces of EFS-induced (stimulation frequency 5–50 Hz) contraction in the absence (control) and 30 min after application of 100 nM stromatoxin-1. B: summary data illustrating the changes in the amplitude of the EFS-induced (stimulation frequency 5–50 Hz) contractions following 30-min application of 100 nM stromatoxin-1. The maximal contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%. Values are means ± SE: n = 6, N = 5. *P < 0.05, **P < 0.01. C: summary data illustrating two consecutive time controls without any significant changes in the amplitude of the EFS-induced contractions in the absence of stromatoxin-1. The maximal contraction amplitude at EFS frequency of 50 Hz under control-1 was taken to be 100%. Values are means ± SE; n = 4, N = 4. P > 0.05.
RT-PCR.
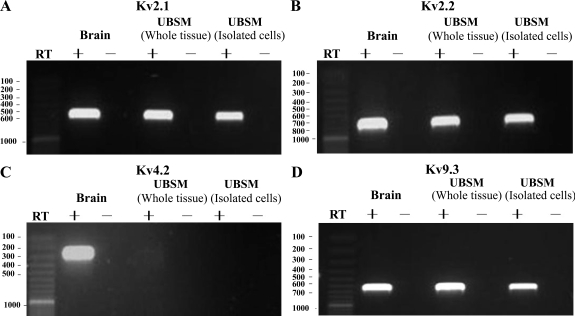
RT-PCR experiments were conducted to detect the expression of mRNA messages for all four stromatoxin-1-sensitive channels, KV2.1, KV2.2, KV4.2, and KV9.3, both in whole UBSM tissues and in freshly dispersed single UBSM cells. Subunit-specific primers were designed to determine the expression pattern of mRNA for the KV2.1, KV2.2, KV4.2, and KV9.3 channels. The expression of mRNA message for all four channels KV2.1, KV2.2, KV4.2, and KV9.3 was detected in rat brain, which was used as a positive control. However, the whole UBSM tissue was determined to express mRNA messages for only KV2.1, KV2.2, and KV9.3, but not for KV4.2 channel (Fig. 3). KV4.2 channel mRNA message was not detected in whole UBSM tissue, even after the initial PCR sample was subjected to a second round of amplification. Negative control experiments performed in the absence of the RT (−RT) demonstrated an absence of genomic DNA contamination (Fig. 3).
Fig. 3.
Detection of mRNA message for voltage-gated K+ (KV) 2.1, KV2.2, KV4.2, and KV9.3 channel subunits in UBSM tissue, as well as freshly dispersed single UBSM cells. A: KV2.1, 551 bp. B: KV2.2, 762 bp. C: KV4.2, 273 bp. D: KV9.3, 645 bp. No products were observed in the negative controls [−reverse transcriptase (RT)] in which RT was left out of the reaction. Rat brain tissue was used as a positive control. Results were verified in at least 15 different preparations, obtained from 5 rats.
The presence of other cell types within the UBSM layer, such as neurons, fibroblasts, and vascular myocytes, may lead to a detection of KV channel subunits expressed in cell types other than UBSM cells. To eliminate contamination from other cell types, we applied single-cell RT-PCR experiments on freshly isolated UBSM cells (7). Again, the single-cell RT-PCR confirmed the expression of mRNA messages for KV2.1, KV2.2, and KV9.3, but not KV4.2 channel (Fig. 3). A lack of genomic DNA contamination in mRNA isolated from single UBSM cells was also confirmed by using the negative control reactions lacking the RT (−RT). RT-PCR-purified products from whole UBSM tissue, freshly dispersed UBSM cells, and rat brain were sequenced to confirm their identity. The purified products were obtained from at least three separate samples. To detect if any proteins for KV2.1, KV2.2, KV4.2, and KV9.3 channels are expressed in UBSM, we next applied Western blot analysis and immunocytochemistry.
Western blot.
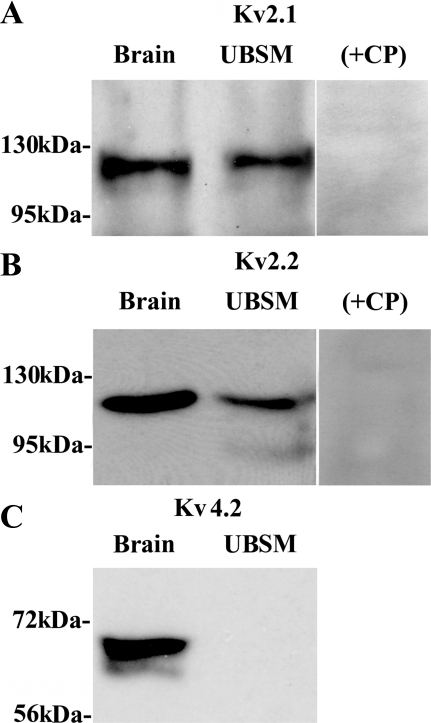
By applying the commercial KV subunit-specific antibodies, the protein expression of KV2.1 and KV2.2 in whole UBSM tissue was confirmed using Western blot (Fig. 4). The expected molecular masses for KV2.1 and KV2.2 proteins were ∼130 and ∼125 kDa, respectively, consistent with the experimentally determined molecular masses (Fig. 4). Preabsorption of the primary antibody with its antigenic competing peptide indicated the specificity of the antibodies for their intended epitope. Consistent with our RT-PCR data, no KV4.2 protein was detected in whole UBSM tissue (Fig. 4). In contrast, an apparent immunoreactive band was expressed in rat brain, which was used as a positive control. The specific antibodies to KV9.3 channel did not show any signal, in both rat brain and whole UBSM (data not shown). This may be due to ineffectiveness of the anti-KV9.3 antibodies used, because KV9.3 channel protein was not detected (using same anti-KV9.3 antibodies) in rat brain controls either.
Fig. 4.
Western blot detection of KV2.1, KV2.2, and KV4.2 channel protein expression in whole UBSM tissues. A: KV2.1. B: KV2.2. C: KV4.2. The immunoreactive band in UBSM tissue was eliminated by a competing peptide (+CP). Rat brain tissue was used as a positive control. The results were verified in 3 separate Western blot reactions using proteins isolated from 3 rats.
Immunocytochemistry.
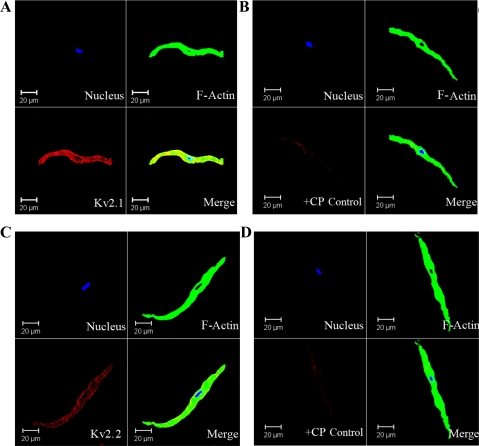
To further verify the presence of KV2.1 and KV2.2 channels in rat UBSM, we applied immunocytochemical labeling of freshly isolated UBSM cells using confocal microscopy. Freshly isolated UBSM cells had bright, distinct edges under confocal phase-contrast optics. Anti-KV2.1 and anti-KV2.2 antibodies specifically labeled the freshly isolated cells, indicating cell membrane localization of the KV2.1 and KV2.2 channels (Fig. 5). However, immunofluorescence staining was not detected for KV4.2 channel in isolated UBSM cells (data not illustrated), which was consistent with our RT-PCR and Western blot experiments. The aforementioned results were carefully controlled for specificity using the omission of the primary antibody and absorption of the primary antibody by a competing peptide (Fig. 5).
Fig. 5.
Immunocytochemical detection of KV2.1 and KV2.2 channels in freshly dispersed single UBSM cells using channel-specific antibodies. Red staining (bottom left panels) indicates detection of KV2.1 channel (A); after absorption of the primary antibody (anti-KV2.1) with a competing peptide (+CP) (B); KV2.2 channel (C); and after absorption of the primary antibody (anti-KV2.2) with a competing peptide (+CP) (D). Cell's nuclei are shown in blue (top left panels); F-actin is shown in green (top right panels). The merged images are also shown (bottom right panels) and illustrate the overlap of others. Images at ×63 were obtained via confocal microscopy. The results were verified in 12 cells freshly isolated from 3 rats.
DISCUSSION
The present study reveals that the KV2 channel subfamily with both of its members, KV2.1 and KV2.2, plays a fundamental role in the control of rat UBSM function. Several lines of evidence suggest that stromatoxin-1-sensitive KV2.1 and KV2.2 channels are the functional and molecular basis of a key regulatory mechanism in rat UBSM myogenic and neurogenic contractions.
First, stromatoxin-1, a specific inhibitor for KV2.1, KV2.2, KV4.2 homomultimeric channels and KV2.1/9.3 heteromultimeric channel, significantly increased spontaneous phasic contraction amplitude, muscle force integral, and tone in isolated UBSM strips. However, stromatoxin-1 had no effect on the UBSM contractions induced by 20 mM K+ or 1 μM carbachol, indicating that, under conditions of sustained membrane depolarization, the KV channels sensitive to stromatoxin-1 have no further contribution to the membrane excitability and contractility. Under normal physiological conditions, the KV channels overcome a physiologically relevant depolarization that appears during an action potential. In our experimental series, we have created artificial conditions of sustained depolarization, such as maintaining high concentrations of K+ or carbachol in the bath solution. Under such conditions, the cell membrane remains constantly depolarized. The high external K+ decreases the driving force for K+ and changes the K+ reversal potential. In addition, the KV channels rapidly inactivate under conditions of depolarization, which further reduces their activity. Our data support such a role for KV channels in rat UBSM. Carbachol, a chemically stable analog of acetylcholine, resistant to the activity of acetylcholinesterase, maintains constant depolarization, unlike the transient depolarization induced by physiological release of the neurotransmitter acetylcholine. Our findings are also supported by recent studies suggesting that the KV channels are potential candidates in modulating the repolarization and afterhyperpolarization phases of UBSM action potentials and the resting membrane potential (8, 12, 28). However, in a recent study by Hayase et al. (13), they reported no effect of stromatoxin-1 on the action potential or resting membrane potential in mouse UBSM using intracellular recordings. Species differences are the most likely reason for such discrepancy in their results and those obtained in our hands. Our observation is also in agreement with Thorneloe and Nelson (28), who suggest that, in mouse UBSM cells, Kv2.1 subunits do not form homotetramers and that this is consistent with a lack of stromatoxin-1 sensitivity. Finally, it is of concern that the Hayase et al. (13) conclusion is based on data obtained only from three preparations that were pretreated with charybdotoxin.
The significant increase in the amplitude of the EFS-induced contractions over a wide range of stimulation frequencies (5–50 Hz) under conditions of blocked KV channels with stromatoxin-1 indicates an additional role for these channels in the nerve-evoked contractions. By decreasing membrane excitability, these channels oppose the activation of UBSM contractions in response to excitatory cholinergic and purinergic nerves. The conclusions drawn from our study are based on the KV2.1-, KV2.2-, KV2.1/9.3-, and KV4.2-specific inhibition by stromatoxin-1, which is confirmed to be the inhibitor with the highest selectivity of KV2.1 channel and the first known specific inhibitor for the KV2.2 channel (9, 10, 25). Stromatoxin-1 is a member of a structural family with a short peptide reticulated by disulfide bridges, and the functional mechanism of this toxin is to inhibit KV channels by specific modification of their gating kinetics (9, 10, 25). A pharmacological dissection of KV2 channels with stromatoxin-1 has also been performed on neurons (5, 25) and cerebral arteries (2). These previous studies have confirmed the high usefulness of stromatoxin-1 for the study of neurological disorders and arterial dysfunction associated with a functional role of KV2 channels.
Second, our combined molecular biology approach indicates the presence of KV2.1 and KV2.2 homomultimeric channels and perhaps also KV2.1/9.3 heteromultimeric channel in rat UBSM. For the purpose of this study, we have applied an alternative technique involving procedures for enzymatic single-cell isolation from fresh rat UBSM tissue in combination with RT-PCR. This approach permits the study of KV channel expression in single-rat UBSM cells, while eliminating contaminations from other surrounding non-UBSM cells. RT-PCR experiments revealed mRNA expression of the KV2.1, KV2.2, and KV9.3 subunits in both rat UBSM whole tissue and freshly isolated UBSM cells. However, the expression of only KV2.1 and KV2.2 proteins was confirmed by Western blot and immunocytochemistry in UBSM whole tissue and freshly isolated cells, respectively. When using whole UBSM tissue for immunohistochemistry, some uncertainty may arise over whether signals come from UBSM cells or from other cell types located within the smooth muscle layer. To address this, we used a combined procedure for single-cell isolation from fresh rat UBSM with immunocytochemistry. With this alternative methodology, we detected KV channel subunit expression initially identified by single-cell RT-PCR on freshly isolated rat UBSM cells.
So far, the KV2.1, but not the KV2.2, channel has been recognized as a predominant KV channel in rat urinary bladder (11, 19). Our study confirms the presence of the KV2.2 channel in rat UBSM cells. Although we also detected mRNA expression of KV9.3 in UBSM whole tissue and isolated cells, we could not confirm the presence of KV9.3 channel protein, which interacts with KV2 channels to form the KV2.1/9.3 heteromultimeric complex. The detection of UBSM KV9.3 subunit protein was not possible at this time because of the lack of an effective specific antibody for this KV channel subunit. Our studies clearly indicate a lack of KV4.2 channel expression in UBSM whole tissue and isolated single cells. Substantial evidence suggests that the KV4 channel family, particularly KV4.2 and KV4.3, may be the major component of an A-type current (rapidly inactivating KV currents) detected predominantly in phasic smooth muscles (1). Only the KV4.3L transcript was identified in rat UBSM, suggesting that maybe the KV4.3L, but not KV4.2, contributes to regulation of rat UBSM contractility (18).
Perspectives and Significance
Two important conclusions may be drawn from our study: 1) stromatoxin-1-sensitive KV2.1 and KV2.2, but not KV4.2, channels are expressed and are functional in rat UBSM; and 2) KV2 channels oppose both myogenic and neurogenic contractions of rat UBSM with primary effect on the myogenic component. Collectively, our findings provide compelling evidence to support that KV2 channels are physiologically relevant regulators of urinary bladder function. Furthermore, a better understanding of KV2 channels is crucial to developing new therapeutic strategies for treatment of overactive bladder. In fact, the KV2 channels may represent an opportunity for novel pharmacological manipulation of the bladder, because drug-induced opening of the KV2 channels should decrease bladder contractions, in contrast to the effect of stromatoxin-1.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK070909 and DK084284 to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Serge Afeli for help with some of the UBSM contraction experiments and Drs. Kiril L. Hristov and Jennifer G. Schnellmann for the critical evaluation of the manuscript.
REFERENCES
- 1.Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol 284: C583–C595, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 291: C348–C356, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol 296: C1271–C1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta 4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AM, Batchelor TJ, Eardley I, Beech DJ. Potassium channel KV alpha 1 subunit expression and function in human detrusor muscle. J Urol 167: 1881–1886, 2002 [PubMed] [Google Scholar]
- 9.Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol 62: 48–57, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon 43: 555–574, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gan XG, An RH, Bai YF, Zong DB. Expressions of voltage-gated K+ channel 2.1 and 22 in rat bladder with detrusor hyperreflexia. Chin Med J (Engl) 121: 1574–1577, 2008 [PubMed] [Google Scholar]
- 12.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayase M, Hashitani H, Kohri K, Suzuki H. Role of K(+) channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181: 2355–2365, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K(+) channels expressed in the pulmonary vasculature. Circ Res 85: 489–497, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett 420: 47–53, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Ohya S, Tanaka M, Watanabe M, Maizumi Y. Diverse expression of delayed rectifier K+ channel subtype transcripts in several types of smooth muscles of the rat. J Smooth Muscle Res 36: 101–115, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J 16: 6615–6625, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, an ATP-dependent delayed-rectifier K+ channel in pulmonary artery myocytes. Ann N Y Acad Sci 868: 438–441, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Petkov GV. Ion channels. In: Pharmacology: Principles and Practice, edited by Hacker M., Messer W., Bachmann K. Boston, MA: Elsevier/Academic, 2009, chapt. 16, p. 385–425 [Google Scholar]
- 23.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Shiau YS, Huang PT, Liou HH, Liaw YC, Shiau YY, Lou KL. Structural basis of binding and inhibition of novel tarantula toxins in mammalian voltage-dependent potassium channels. Chem Res Toxicol 16: 1217–1225, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Streng T, Christoph T, Andersson KE. Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol 172: 2054–2058, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 83: 215–242, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]