Abstract
The renal thiamin reabsorption process plays an important role in regulating thiamin body homeostasis and involves both thiamin transporters-1 and -2 (THTR1 and THTR2). Chronic alcohol use is associated with thiamin deficiency. Although a variety of factors contribute to the development of this deficiency, effects of chronic alcohol use on renal thiamin transport have not been thoroughly examined. We addressed this issue by examining the effect of chronic alcohol feeding of rats with liquid diet on physiological and molecular parameters of renal thiamin transport. Chronic alcohol feeding caused a significant inhibition in carrier-mediated thiamin transport across the renal brush-border membrane and was evident as early as 2 wk after initiation of alcohol feeding. Similarly, thiamin transport across the renal basolateral membrane was significantly inhibited by chronic alcohol feeding. The inhibition in renal thiamin transport was associated with a marked decrease in the level of expression of THTR1 and -2 proteins, mRNAs, and heterogeneous nuclear RNAs. Chronic alcohol feeding also caused a significant reduction in the level of expression of thiamin pyrophosphokinase but not that of the mitochondrial thiamin pyrophosphate transporter. These studies show that chronic alcohol feeding inhibits the entry and exit of thiamin in the polarized renal epithelial cells and that the effect is, at least in part, mediated at the transcriptional level. These findings also suggest that chronic alcohol feeding interferes with the normal homeostasis of thiamin in renal epithelial cells.
Keywords: transporter, vitamin B1, kidney, thiamin transporter-1, thiamin transporter-2
thiamin, a water-soluble vitamin, is essential for cellular function, growth, and development. The vitamin is enzymatically converted into its active form, thiamin pyrophosphate (TPP), in the cytoplasm via the action of thiamin pyrophosphokinase (TPKase), a rate-limiting enzyme that plays an important role in regulating cellular thiamin homeostasis. While all mammalian nucleated cells possess the ability to convert thiamin to TPP, the liver and kidneys are especially active in this regard (10, 22, 36). TPP is then either used in the cytoplasm or is imported in the mitochondria via a carrier-mediated process that involves the mitochondrial TPP transporter (encoded by the SLC25A19 gene; see Ref. 19), for utilization in carbohydrate metabolism and energy production. The importance of thiamin for human health and well-being is evident by the range of clinical abnormalities that result from its deficiency, which include cardiovascular and neurological disorders (4, 31, 35). Beriberi and the Wernicke-Korsakoff syndrome are well-known thiamin deficiency diseases (24, 35).
All mammals cannot synthesize thiamin; thus, they must obtain the vitamin from exogenous sources via intestinal absorption. Circulating thiamin is filtered in the renal glomeruli and then salvaged via reabsorption by renal epithelial cells to prevent its loss in the urine. Thus the kidneys play an important role in maintaining and regulating normal body homeostasis of thiamin. The mechanism(s) involved in the renal thiamin reuptake process has been examined using a variety of human and animal kidney preparations (2, 9, 34). It is known now that the renal thiamin uptake process is carrier-mediated and involves both thiamin transporters-1 and -2 (THTR1 and THTR2, respectively) (2). It is also known that these two thiamin transporters are expressed differentially at the cell membrane of the polarized renal epithelial cells, with expression of THTR2 being restricted to the apical brush-border membrane (BBM) domain only, while that of the THTR1 is expressed at both the BBM and basolateral membrane (BLM) domains of the renal reabsorptive epithelial cells (6). Furthermore, studies from our laboratory have shown that the renal thiamin uptake process is adaptively upregulated in thiamin deficiency via transcriptional regulatory mechanism(s) involving both the hTHTR1 and -2 (2).
Thiamin deficiency is common in chronic alcoholism and may lead to Wernicke-Korsakoff syndrome, a neuropsychiatric condition characterized by ophthalmoplegia, ataxia, and memory loss (17, 24, 30, 32, 33, 35). Although many factors contribute to the development of thiamin deficiency in chronic alcoholism, an inhibition in the renal thiamin uptake process may also be one of the factors. A study in rats has shown that chronic alcohol feeding leads to an increase in urinary loss of thiamin (1). Little, however, is known about the effect of chronic alcohol feeding on physiological and molecular parameters of the thiamin transport process across the polarized renal epithelial cells, i.e., transport across the BBM and the BLM domains. Little is also known about the effect of chronic alcohol use on level of expression of TPKase and the mitochondrial TPP transporter, two important players that are involved in regulating thiamin homeostasis in renal epithelial cells. We addressed these issues using rats that were fed on alcohol liquid diet (ethanol provided 36% of total calories) for different periods and compared the findings from those obtained with control rats that were pair-fed the same liquid diet but without alcohol (maltose-dextrin isocalorically replaced ethanol in the diet). The results showed that chronic alcohol feeding causes a significant inhibition in thiamin transport across the renal BBM and BLM domains of renal epithelial cells. This inhibition was associated with a marked decrease in levels of expression of THTR1 and -2 at the protein and mRNA levels; it was also associated with a marked decrease in the level of expression of heterogeneous nuclear RNAs (hnRNA) of THTR1 and -2, suggesting possible effects at the transcriptional level. Moreover, chronic alcohol use was found to cause a significant reduction in the level of expression of TPKase but not that of the mitochondrial TPP transporter.
MATERIALS AND METHODS
Materials.
[3H]thiamin (specific activity 20 Ci/mmol; radiochemical purity >99%) was obtained from American Radiolabel (St. Louis, MO). Nitrocellulose filters (0.45-μm pore size) were purchased from Fisher Scientific. Unlabeled thiamin and other chemicals, including molecular biology reagents, were obtained from commercial vendors and were of analytical grade.
Alcohol feeding of rats.
Male Wister rats (Charles River, Wilmington, MA) weighing ∼120 g (∼14 wk old) were housed at the Animal Core of the National Institute on Alcohol Abuse and Alcoholism-Funded Southern California Research Center for Alcoholic Liver and Pancreatic Diseases and Cirrhosis at the University of Southern California, Los Angeles, CA. Animal use committees of both the University of Southern California and the Long Beach Veterans Affairs Medical Center approved the experimental protocols. Rats were fed the Lieber-DeCarli alcohol liquid diet (ethanol provided 36% of total ingested calories; 5 g ethanol/dl diet) (18) for 2, 4, or 6 wk. Control rats were pair-fed with the same liquid diet but without alcohol (maltose-dextrin isocalorically replaced ethanol). Rats were killed at the time of study, and their kidneys were removed and processed immediately for isolation of renal brush-border membrane vesicles (BBMV) or basolateral membrane vesicles (BLMV). For molecular biological studies (mRNA and hnRNA), part of the fresh kidney tissue from the alcohol-fed rats and their pair-fed controls were removed and stored at −80°C in Trizol (Invitrogen, Carlsbad, CA) for later use.
Preparation of rat renal BBMV and BLMV and transport studies.
Purified rat renal BBMV and BLMV were freshly prepared utilizing the divalent (Mg2+) cation chelation method and the Percoll-gradient differential centrifugation method, respectively, as previously described by us and others (5, 11, 12, 27, 28, 37). The final BBMV and BLMV preparations were preloaded with a buffer of 280 mM mannitol and 20 mM Tris·HCl, pH 5.5; incubation was performed in a buffer of 100 mM NaCl, 80 mM mannitol, and 20 mM HEPES, pH 7.4, in the presence of 0.25 μM [3H]thiamin. Transport studies were performed using freshly isolated membrane vesicles at 10 s (initial rate; see Ref. 34) at 37°C using a rapid-filtration method as previously described (13).
Real-time PCR analysis.
Total RNA (5 μg) was isolated from the kidneys of alcohol-fed rats and their pair-fed controls and was primed with oligo(dT) primers to synthesize first-strand cDNA (Superscript First Strand Synthesis RT-PCR kit; Invitrogen). To amplify the coding region of rat THTR-1, THTR-2, and β-actin, we used gene-specific primers for rat THTR1, THTR2, and β-actin as described in Table 1. Real-time PCR was performed as described previously (23), and data were normalized to β-actin and then quantified using a relative relationship method supplied by the iCycler manufacturer (Bio-Rad) as described before (20, 23).
Table 1.
Combination of primers used to amplify open reading frame of the respective genes by real-time and semiquantitative PCR
| Gene name | Forward and Reverse Primers (5′–3′) |
|---|---|
| Real-time PCR | |
| rTHTR1 | GCTGTCATCTACAATGGCGG; GATGTACACTGCAGCAGCAATC |
| rTHTR2 | CGTGATACTCTGCTTGTTCGG; GGTAAGAGTACGTCCAAACAGG |
| rβ-Actin | GTCAGGTCATCACTATCGGC; CATGGATGCCACAGGATTCC |
| hnRNA primers | |
| rTHTR1 | CACTTTGTACCTGTGTGTG; GAATGACAGGCTTGTAACG |
| rTHTR2 | CTACCGTAACAGGACACAG; TGTGAAAATGGAGGCTCAC |
| rTPKase | GCCTTGTGGTTCTTAATCAG; GAGAACCCAGGCTATGATC |
| rβ-Actin | CTGCTCTTTCCCAGATGAG; CTCATAGATGGGCACAGTG |
| Semiquantitative RT-PCR primers for thiamin metabolizing enzyme and slc25a19 | |
| rTPKase | GACCAAGACCACACTGAC; GTTGGAGGAGGTAGATGAG |
| slc25a19 | GGCCATACGCACCATG; GGGTCTTGCTGATGACTC |
| rβ-Actin | GTCAGGTCATCACTATCGGC; CATGGATGCCACAGGATTCC |
r, Rat; THTR, thiamin transporter; TPKase, thiamin pyrophosphokinase; hnRNA, heterogeneous nuclear RNA.
Western blot analysis.
Western blot analysis was performed on purified BBM and BLM as well as kidney cortex homogenate prepared from the kidneys of alcohol-fed rats and their pair-fed controls as described earlier (21, 29). BBM, BLM, and homogenate (to detect TPKase protein expression) preparations (30–60 μg) were resolved on premade 4–12% Bis-Tris mini-gel (Invitrogen) as described before (29). After electrophoresis, proteins were electroblotted on polyvinylidene difluoride membrane (Bio-Rad) and then blocked with a PBS-Tween 20 solution containing 5% dried milk (Bio-Rad) for 1 h at room temperature. The membranes were then incubated either with rat THTR1 or -2 specific polyclonal antibodies that were raised in rabbits against the KKCRKQEDPNSSPQ and EPYLQEPRDVSTKE peptides, which correspond to amino acids 481–494 and 468–481 of the rat THTR1 and -2, respectively, using a commercial vendor (Thermo Fisher Scientific, Huntsville, AL). Specificity of the rat THTR1 and -2 polyclonal antibodies was determined by treating these antibodies with the synthetic antigenic peptides for 1 h at 37°C and then at 4°C overnight before use (21, 29). For rat TPKase detection, the membrane was incubated with TPKase polyclonal antibodies raised in rabbits (Protein Tech Group, Chicago, IL). Immunodetection of the specific bands was performed by incubating the membrane with secondary antibodies [goat anti-rabbit conjugated to horseradish peroxidase (HRP); Santa Cruz Biotechnology, Santa Cruz, CA] and with enhanced chemiluminescent (ECL) substrate (Amersham, Arlington Heights, IL) as described before (21, 29). The appropriate membranes were stripped using reblotting stripping solution (Chemicon, Temecula, CA) and incubated with β-actin antibodies raised in goat (Santa Cruz) and then incubated with bovine anti-goat conjugated HRP secondary antibodies (Santa Cruz). The immunoreactive bands were developed using ECL substrate as described above and then quantitated (as unitless measurements) using the UNSCAN-IT gel automated digitizing system, version 6.1 (Silk Scientific).
hnRNA analysis.
To examine the effect of chronic alcohol feeding on the level of expression of hnRNA of THTR1, THTR2, and TPKase, total RNA [treated with DNase I (1 μg RNA/unit; Invitrogen) to avoid genomic DNA presence during the amplification process] was isolated from the kidneys of alcohol-fed rats and their pair-fed controls as described previously (3, 8, 14). DNase I-treated RNA was then reverse transcribed with the random hexomer as described above (Invitrogen). To ensure the amplification of hnRNA, the forward and reverse primers were designed in exon and intron, respectively. Semiquantitative RT-PCR was performed using the rat THTR1, THTR2, TPKase, and β-actin gene-specific primers as described in Table 1. The semiquantitative PCR conditions consisted of a 3-min 95°C melt followed by 33 cycles of 95°C melt for 30 s, 58°C annealing for 30 s, 72°C extension for 1 min, and final extension at 72°C for 8 min. The amplified products were run in 1% agarose gel, then image was captured using Gel-doc (Bio-Rad), and specific bands were quantified (as unitless measurement) using UNSCAN-IT software 6.1 (Silk Scientific).
Semi-quantitative RT-PCR.
First-strand cDNA synthesis was performed as described above, and semiquantitative RT-PCR was performed using gene-specific primers (Table 1) designed from the open reading frame (ORF) of rat TPKase, mitochondrial thiamin pyrophosphate transporter (slc25a19), and β-actin. Semiquantitative RT-PCR conditions were followed as described above with minor modifications. The amplified products were analyzed on 2% agarose gel, then the image was captured, and bands were quantified as described above.
Statistical analysis.
Uptake data presented in this paper are the results of three separate experiments and are expressed as means ± SE in ficomoles per milligram protein per 10 s. Differences between the means of alcohol-fed and their pair-fed control rats were tested for a significance level at P < 0.05 using the Student's t-test analysis. Uptake of 0.25 μM [3H]thiamin by the carrier-mediated process was determined by subtracting uptake in the presence of a high pharmacological concentration of unlabeled thiamin (1 mM) from uptake in its absence. Western blot, real-time PCR, hnRNA analysis, and semiquantitative RT-PCR were performed on at least three separate occasions.
RESULTS
Thiamin uptake by freshly isolated rat renal BBMV and BLMV.
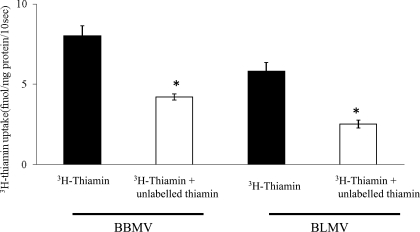
The mechanism of thiamin transport across the apical membrane of renal epithelial cells has been investigated at the functional (2, 6, 9) and molecular (2, 6) levels. The mechanism of exit of thiamin out of the renal epithelial cells, i.e., transport across the BLM, however, is less studied, although findings in our laboratory have shown expression of the THTR1 at this membrane domain (6). We confirmed the existence of a carrier-mediated uptake process for thiamin in rat renal BBMV by demonstrating a significant (P < 0.01) inhibition in the uptake of 0.25 μM [3H]thiamin by 1 mM unlabeled thiamin (Fig. 1). We also functionally showed, for the first time, the existence of a carrier-mediated process for thiamin in renal BLMV by demonstrating a significant (P < 0.01) inhibition in [3H]thiamin (0.25 μM) by unlabeled thiamin (1 mM) (Fig. 1).
Fig. 1.
Uptake of [3H]thiamin by rat renal brush-border membrane vesicles (BBMV) and basolateral membrane vesicles (BLMV). Initial rate of uptake (10 s) of [3H]thiamin (0.25 μM) was examined at 37°C in the presence and absence of 1 mM unlabeled thiamin. Data are means ± SE of 4 determinations from different rats. *P < 0.01.
Effect of chronic alcohol feeding on carrier-mediated thiamin uptake by rat renal BBMV and BLMV.
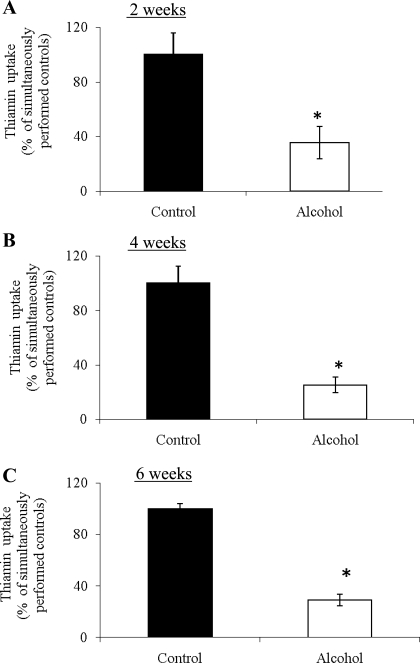
In these investigations, we examined the effect of chronic alcohol feeding of rats for 2, 4, and 6 wk on carrier-mediated thiamin uptake across the renal BBM. We used purified BBMV isolated from the renal cortex of alcohol-fed rats and their pair-fed controls. Our results showed significant (P < 0.01) inhibition in thiamin (0.25 μM) uptake by renal BBMV of alcohol-fed rats compared with their pair-fed controls (Fig. 2). This inhibition in thiamin uptake was observed as early as 2 wk after the start of alcohol feeding. We used a 4-wk alcohol feeding regimen as the standard feeding period in all of our subsequent investigations.
Fig. 2.
Effect of chronic alcohol feeding for different periods on [3H]thiamin uptake by rat renal BBMV. A, B, and C: carrier-mediated uptake of [3H]thiamin (0.25 μM) by renal BBMV of rats fed alcohol-liquid diet for 2, 4, and 6 wk, respectively, was examined and compared with uptake by BBMV of pair-fed controls. Data are means ± SE of 3–4 separate uptake determinations from multiple sets of rats. *P < 0.01.
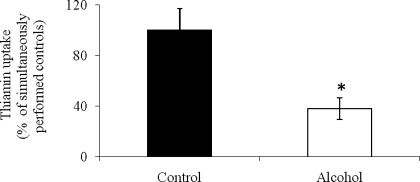
We also examined the effect of chronic alcohol feeding (for 4 wk) on carrier-mediated thiamin (0.25 μM) uptake by purified renal BLMV. The results showed a significant (P < 0.01) inhibition in thiamin uptake by renal BLMV from alcohol-fed rats compared with those isolated from their pair-fed controls (Fig. 3).
Fig. 3.
Effect of chronic alcohol feeding on [3H]thiamin uptake by rat renal BLMV. Carrier-mediated uptake of [3H]thiamin (0.25 μM) by renal BLMV of rats fed alcohol liquid diet for 4 wk and their pair-fed controls was examined as described in materials and methods. Data are means ± SE of 3–4 separate uptake determinations from multiple sets of rats. *P < 0.01.
Effect of chronic alcohol feeding on level of expression of THTR1 and -2 proteins in rat renal BBM and BLM.
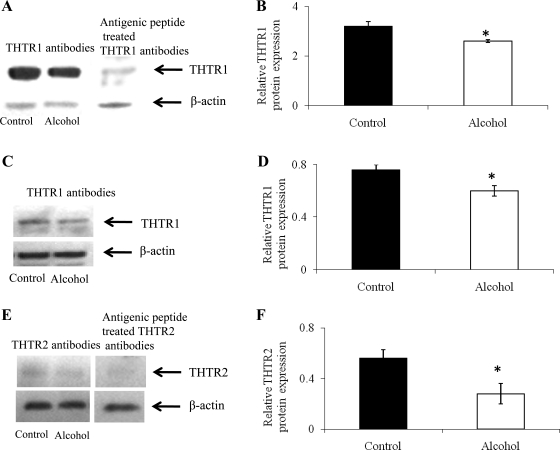
In this study, we used the Western blotting technique to investigate the effect of chronic ethanol feeding on the level of expression of the rat THTR1 and -2 proteins at the renal BBM and BLM domains. Rat THTR1 and -2 polyclonal antibodies were used in this study as detailed in materials and methods. The results showed a significant (P < 0.05) reduction in the level of expression of THTR1 and -2 proteins in BBM preparations isolated from alcohol-fed rats compared with those isolated from their pair-fed controls (Fig. 4, A and B, for THTR1 and Fig. 4, E and F, for THTR2). Similarly, a significant (P < 0.05) reduction was observed in the level of expression of THTR1 protein in renal BLM preparations isolated from alcohol-fed rats compared with those isolated from their pair-fed controls (Fig. 4, C and D) [as seen before (6), no expression for THTR2 protein was detected in renal BLM preparations; data not shown]. Specificity of the rat anti-THTR1 and -2 polyclonal antibodies used in the above experiments was confirmed by demonstrating blocking of the specific bands upon pretreatment of the antibodies with their respective antigenic peptides (Fig. 4, A and E, right).
Fig. 4.
Western blot analysis of renal brush-border membrane (BBM) and basolateral membrane (BLM) proteins from alcohol-fed rats and their pair-fed controls. A: renal BBM (30 μg) proteins were resolved on premade 4–12% Bis-Tris mini-gel as described in materials and methods. Blots were incubated with either rabbit polyclonal anti-rat (r) thiamin transporter (THTR1) antibodies (left) or anti-rTHTR1 antibodies pretreated with the antigenic peptide (right). B, D, and F: densitometry values for the respective blots. C: renal BLM (60 μg) proteins were detected as described above in A. E: renal BBM (60 μg) proteins were resolved as described, and blots were incubated with either rabbit polyclonal anti-rTHTR2 antibodies (left) or anti-rTHTR2 antibodies pretreated with the antigenic peptide (right). Bottom: respective blots were striped and reprobed with rat β-actin antibodies to normalize equal loading in each well. Immunoreactive band was detected using enhanced chemiluminescent (ECL) substrate as described in materials and methods. Each data point represents the mean ± SE of at least 3 separate experiments involving at least 3 sets of rats. *P < 0.05.
Effect of chronic alcohol feeding on level of expression of THTR1 and -2 mRNA in rat kidneys.
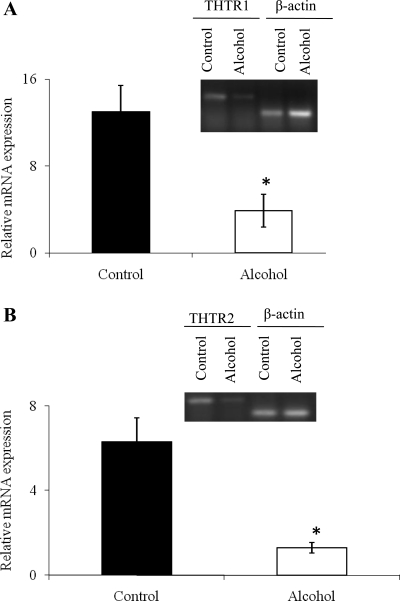
The effect of chronic alcohol feeding on the steady-state mRNA level of expression of THTR1 and -2 in rat kidney cortex was examined. A real-time PCR using gene-specific primers (Table 1) designed from the ORF of rat THTR1 and -2 was performed on mRNA isolated from alcohol-fed rats and their pair-fed controls (materials and methods). Data were normalized relative to the rat housekeeping gene β-actin. The results showed a significant (P < 0.01) decrease in the level of expression of THTR1 and -2 mRNA in the kidneys of alcohol-fed rats compared with pair-fed controls (Fig. 5, A and B). These findings raised the possibility that chronic alcohol feeding may affect the transcription rate of the Slc19a2 and Slc19a3 genes (which encode rat THTR1 and -2, respectively). This issue was addressed in the next section.
Fig. 5.
Real-time PCR analysis of renal mRNA of alcohol-fed rats and their pair-fed controls. Levels of mRNA of rTHTR1 (A) and rTHTR2 (B) in the kidney cortex of alcohol-fed rats and their pair-fed controls were quantified by real-time PCR using gene-specific primers for THTR1, THTR2, and β-actin as described in materials and methods. Inset: a representative real-time PCR product run on 2% agarose gel. Each data point represents the mean ± SE of at least 3 separate experiments involving at least 3 sets of rats. *P < 0.01.
Effect of chronic alcohol feeding on level of expression of THTR1 and -2 hnRNA levels.
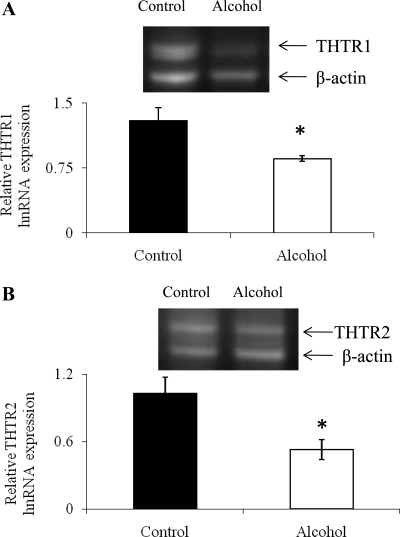
The steady-state level of an hnRNA of a given gene is widely believed to reflect the transcription rate of that gene and is often used as a surrogate for the nuclear run-on assay (3, 7, 8, 14, 26). In this study, we examined the effect of chronic alcohol use on the level of expression for THTR1 (Slc19a2) and THTR2 (Slc19a3) hnRNA in rat kidneys. hnRNA was isolated from kidney cortex of alcohol-fed rats and their pair-fed controls, and the level of expression of THTR1 and -2 was determined as described in materials and methods. The results showed a significant (P < 0.05 for both) decrease in the level of expression of hnRNA of THTR1 and -2 in the kidneys of alcohol-fed rats compared with their pair-fed controls (Fig. 6, A and B). On the other hand, a negative control run without reverse transcriptase enzyme and isolated RNA treated with DNase I and subjected to PCR amplification with THTR1, THTR2, and β-actin primers showed no band in these PCR reactions (data not shown).
Fig. 6.
Effect of chronic alcohol feeding on heterogeneous nuclear RNA (hnRNA) expression of THTR1 (Slc19a2) and THTR2 (Slc19a3). A and B: semiquantitative RT-PCR products for Slc19a2 and Slc19a3, respectively, in the kidneys of alcohol-fed and their pair-fed controls were performed using gene-specific primers for Slc19a2, Slc19a3, and β-actin as described in materials and methods. Data were normalized to rat β-actin. Data represent means ± SE of 3 separate experiments involving 3 sets of rats. *P < 0.05.
Effect of chronic alcohol feeding on level of expression of TPKase and the mitochondrial TPP transporter in rat renal epithelial cells.
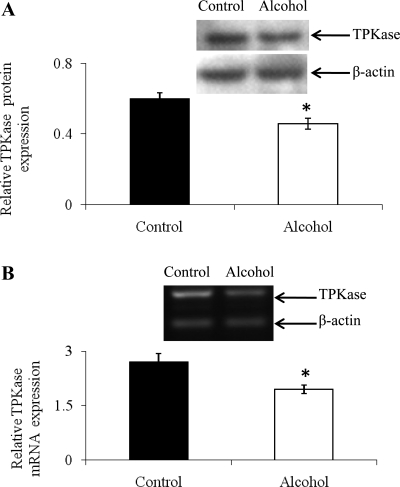
In this study, we examined the effect of chronic alcohol feeding on the level of expression of TPKase [a rate-limiting enzyme in thiamin metabolism (16)] and of the mitochondrial TPP transporter (which is responsible for mitochondrial internalization of the coenzyme) to gain an insight into the effect of chronic alcohol feeding on thiamin homeostasis in renal epithelial cells. To investigate the effect of chronic alcohol feeding on the level of expression of TPKase protein, we performed Western blotting on homogenate prepared from kidney cortex of alcohol-fed rats and their pair-fed controls. The results showed (Fig. 7A) a significant (P < 0.05) inhibition in the level of expression of TPKase protein in the former compared with the latter rat group. Furthermore, semiquantitative RT-PCR using gene-specific primers (Table 1) to amplify the ORF of the TPKase was performed on RNA isolated from alcohol-fed rats and their pair-fed controls (see materials and methods). The amplified products were run on 2% agarose gel, images were captured, and bands were quantified as described above. The results showed a significant (P < 0.05) decrease in the level of expression of TPKase mRNA in the kidneys of alcohol-fed rats compared with their pair-fed controls (Fig. 7B). We also examined the effect of chronic alcohol feeding on the level of expression of hnRNA of Tpk1 (which encodes rat TPKase) in rat kidneys but observed no significant changes in the level of expression in alcohol-fed rats compared with their pair-fed controls (1.49 ± 0.08 and 1.46 ± 0.08 for alcohol and pair-fed control rats, respectively, in arbitrary units).
Fig. 7.
Effect of chronic alcohol feeding on protein and mRNA expression of thiamin pyrophosphokinase (TPKase) in kidney cortex of alcohol-fed rats and their pair-fed controls. A: kidney cortex homogenate (60 μg) was resolved on premade 4–12% Bis-Tris mini-gel as described in materials and methods. Blots were incubated with rabbit polyclonal anti-TPKase antibodies as described in materials and methods. B: level of mRNA expression of rat TPKase in the kidney cortex of alcohol-fed and their pair-fed control rats were quantified by semiquantitative RT-PCR using gene-specific primers for rat TPKase and β-actin as described in materials and methods. The PCR products were run on 2% agarose gel, and images were captured using Gel-doc software. Each data point represents the mean ± SE of 3 separate experiments from at least 3 sets of rats. *P < 0.05.
In contrast to the effect of chronic alcohol feeding on the level of expression of TPKase, chronic alcohol feeding did not affect the level of expression of the mitochondrial TPP transporter mRNA (determined by semiquantitative RT-PCR and expressed in arbitrary units 1.20 ± 0.15 and 1.24 ± 0.13 for alcohol and pair-fed control rats, respectively).
DISCUSSION
Our aim in this study was to investigate the effect(s) of chronic alcohol feeding on physiological and molecular parameters of thiamin transport in and out of the polarized renal epithelial cells. We were also interested in examining the effect of chronic alcohol feeding on specific aspects of intracellular thiamin handling that are important for renal thiamin nutrition, namely the level of expression of TPKase and mitochondrial TPP transporter, which play important roles in regulating cellular thiamin metabolism and compartmentalization. Our interest in addressing these issues stems from the fact that, while chronic alcohol feeding affects normal thiamin body homeostasis and physiology, its effect on renal transport is less well studied. Also, chronic alcohol feeding appears to affect thiamin metabolism in a tissue-dependent manner (25). We used a well-established pair-feeding regimen of rats with alcohol (and control) liquid diets. Our results showed thiamin transport across the renal BBM (studied using purified renal BBMV preparations) to be significantly reduced in alcohol-fed rats compared with pair-fed controls. This inhibition was evident as early as 2 wk after the initiation of alcohol feeding. Similarly, transport of thiamin across renal BLM (studied using purified renal BLMV) was significantly reduced in alcohol-fed rats compared with their pair-fed controls. The inhibition in thiamin transport across renal BBM and BLM domains was associated with a significant inhibition in the level of expression of both THTR1 and -2 proteins in rat renal epithelial cells as indicated by the data of the Western blot analysis. As shown before (6), THTR1 protein was found to be expressed at both the BBM and BLM of the rat renal epithelial cells while that of THTR-2 was expressed only at the BBM.
The above-described changes in the level of expression of THTR1 and -2 proteins was associated with a parallel reduction in the level of expression of mRNA of both transporters. This finding is of interest when compared with our recent findings in the intestine (unpublished observations) where chronic alcohol feeding was found to inhibit thiamin uptake via inhibiting the level of expression of THTR1 without affecting the level of expression of THTR2. The observed inhibition in the level of expression of THTR1 and -2 mRNA in the kidneys of alcohol-fed rats suggests that the inhibition may be mediated, at least in part, via transcriptional mechanism(s). This possibility was tested by determining the level of expression of hnRNA for THTR1 and -2 in kidney cortex of alcohol-fed rats and their pair-fed controls. Changes in hnRNA expression have been used as an indicator for the changes in transcription rate for the involved genes (3, 7, 8, 14, 26). Our results showed a significant reduction in the level of THTR1 and -2 hnRNA in alcohol-fed rats compared with pair-fed controls. These findings raise the possibility that the inhibitory effect of chronic alcohol feeding on thiamin uptake may be, at least in part, mediated via a transcriptionally mediated mechanism(s). Further studies are needed to confirm this suggestion and also to examine if other mechanisms (e.g., changes in RNA stability) are involved in causing the decrease in the level of expression of THTR1 and -2 mRNA.
Our studies also showed chronic alcohol feeding to cause a reduction in the level of expression of TPKase in renal epithelial cells, a finding that is in line with previous functional data showing a decrease in the activity of this enzyme in kidneys of alcohol-fed rats compared with controls (16). The effect, however, does not appear to be transcriptionally mediated, since no change in the level of expression of TPKase in the two rat groups was observed. This suggests that other mechanism(s) (i.e., changes in RNA and/or protein stability) may be involved. Further studies are required to address these issues. In contrast to the effect of chronic alcohol feeding in the level of expression for THTR1, THTR2, and TPKase, alcohol feeding did not affect the level of expression of the mitochondrial TPP transporter. This finding suggests that not all genes are similarly affected by chronic alcohol feeding, a conclusion that is also supported by a recent observation that chronic alcohol feeding differentially influences the pattern of gene expression (15).
In summary, results of these studies show chronic alcohol feeding leads to an inhibition in thiamin transport across rat renal BBM and BLM and that the effect is exerted, at least in part, at the transcriptional level. In addition, chronic alcohol feeding appears to negatively affect renal thiamin metabolism.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK-71538 to V. S. Subramanian, P50AA-11999 to H. Tsukamoto, and DK-56061 and AA-18071 to H. M. Said).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abe T, Itokawa Y. Effect of ethanol administration on thiamine metabolism and transketolase activity in rats. Int J Vitam Nutr Res 47: 307–314, 1977 [PubMed] [Google Scholar]
- 2.Ashokkumar B, Vaziri ND, Said HM. Thiamin uptake by the human-derived renal epithelial (HEK-293) cells: cellular and molecular mechanisms. Am J Physiol Renal Physiol 291: F796–F805, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berdanier CD. Advanced Nutrition Micronutrients New York, NY: CRC, 1998 [Google Scholar]
- 5.Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochim Biophys Acta 647: 169–176, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Boulware MJ, Subramanian VS, Said HM, Marchant JS. Polarized expression of members of the solute carrier SLC19A gene family of water-soluble multivitamin transporters: implications for physiological function. Biochem J 376: 43–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttice G, Kurkinen M. A polyomavirus enhancer A-binding protein-3 site and Ets-2 protein have a major role in the 12-O-tetradecanoylphorbol-13-acetate response of the human stromelysin gene. J Biol Chem 268: 7196–7204, 1993 [PubMed] [Google Scholar]
- 8.Elferink CJ, Reiners JJ., Jr Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Gastaldi G, Cova E, Verri A, Laforenza U, Faelli A, Rindi G. Transport of thiamin in rat renal brush border membrane vesicles. Kidney Int 57: 2043–2054, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Goodhart RS, Sinclair HM. The estimation of cocarboxylase [vitamin B(1) diphosphate ester] in blood. Biochem J 33: 1099–1108, 1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid A, Kaur J. Chronic alcoholism alters the transport characteristics of folate in rat renal brush border membrane. Alcohol 38: 59–66, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hamid A, Kaur J. Decreased expression of transporters reduces folate uptake across renal absorptive surfaces in experimental alcoholism. J Membr Biol 220: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248: 25–32, 1973 [PubMed] [Google Scholar]
- 14.Kohler CU, Roos PH. Focus on the intermediate state: immature mRNA of cytochromes P450–methods and insights. Anal Bioanal Chem 392: 1109–1122, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Laforenza U, Patrini C, Gastaldi G, Rindi G. Effects of acute and chronic ethanol administration on thiamine metabolizing enzymes in some brain areas and in other organs of the rat. Alcohol Alcohol 25: 591–603, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Leevy CM, Baker H. Vitamins and alcoholism. Am J Clin Nutr 21: 1325–1328, 1968 [DOI] [PubMed] [Google Scholar]
- 18.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 19.Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, Chen A, Castegna A, Verhoeven N, Mathews CK, Palmieri F, Biesecker LG. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci USA 103: 15927–15932, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Nabokina SM, Subramanian VS, Said HM. Comparative analysis of ontogenic changes in renal and intestinal biotin transport in the rat. Am J Physiol Renal Physiol 284: F737–F742, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ochoa S. Enzymic synthesis of cocarboxylase in animal tissues. Biochem J 33: 1262–1270, 1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rindi G, Patrini C. Thiamin. Encylc Dietary Suppl 1: 677–686, 2005 [Google Scholar]
- 25.Rindi G, Reggiani C, Patrini C, Laforenza U. Effect of ethanol administration on the in vivo kinetics of thiamine phosphorylation and dephosphorylation in different organs. I. Chronic effects alcohol. Alcohol 26: 285–301, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Ryu DY, Levi PE, Fernandez-Salguero P, Gonzalez FJ, Hodgson E. Piperonyl butoxide and acenaphthylene induce cytochrome P450 1A2 and 1B1 mRNA in aromatic hydrocarbon-responsive receptor knock-out mouse liver. Mol Pharmacol 50: 443–446, 1996 [PubMed] [Google Scholar]
- 27.Said HM, Redha R. A carrier-mediated transport for folate in basolateral membrane vesicles of rat small intestine. Biochem J 247: 141–146, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scalera V, Storelli C, Storelli-Joss C, Haase W, Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J 186: 177–181, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallaksen CM, Bell H, Bohmer T. Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Wernicke encephalopathy. Alcohol Clin Exp Res 17: 712–716, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Shils ME V, Olsen JA, Shike M. Tanphaichirt In: Modern Nutrition in Health and Disease New York, NY: Lea and Febiger, 1994, p. 359–375 [Google Scholar]
- 32.Thomson AD, Baker H, Leevy CM. Thiamin absorption in alcoholism. Am J Clin Nutr 21: 537–538, 1968 [Google Scholar]
- 33.Tomasulo PA, Kater RM, Iber FL. Impairment of thiamine absorption in alcoholism. Am J Clin Nutr 21: 1341–1344, 1968 [DOI] [PubMed] [Google Scholar]
- 34.Verri A, Laforenza U, Gastaldi G, Tosco M, Rindi G. Molecular characteristics of small intestinal and renal brush border thiamin transporters in rats. Biochim Biophys Acta 1558: 187–197, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Victor M, Adams RD, Collins GH. The Wernick-Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition Philadelphia, PA: Davis, 1989 [Google Scholar]
- 36.Westenbrink HGK, Goudsmit J. Investigations on the aneurin and cocarboxylase content of animal tissue, estimated by the thiochrome method. Enzymol 5: 307, 1938 [Google Scholar]
- 37.Yanagawa N, Jo OD, Said HM. Riboflavin transport by rabbit renal brush border membrane vesicles. Biochim Biophys Acta 1330: 172–178, 1997 [DOI] [PubMed] [Google Scholar]