Abstract
Pathways and densities of descending vasa recta (DVR) and ascending vasa recta (AVR) in the outer zone of the inner medulla (IM) were evaluated to better understand medullary countercurrent exchange. Nearly all urea transporter B (UT-B)-positive DVR, those vessels exhibiting a continuous endothelium, descend with little or no branching exclusively through the intercluster region. All DVR have a terminal fenestrated (PV-1-positive) segment that partially overlaps with the UT-B-positive segment. This fenestrated segment descends a distance equal to ∼15% of the length of the connecting UT-B-positive segment before formation of the first branch. The onset of branching is indicative of vessel entry into the intracluster region. The number density of UT-B-positive DVR at 3,000 μm below the OM-IM boundary is ∼60% lower than the density at 400 μm below the OM-IM boundary, a result of DVR joining to fenestrated interconnecting vessels and an overall decline in UT-B expression. AVR that lie in the intercluster region (designated AVR2) lie distant from CDs and ascend to the OM-IM boundary with little or no branching. AVR2a represent a subcategory of AVR2 that abut DVR. The mean DVR length (combined UT-B- and PV-1-positive segments) nearly equals the mean AVR2a length, implying a degree of overall equivalence in fluid and solute countercurrent exchange may exist. The AVR2/DVR ratio is ∼2:1, and the AVR2a/DVR ratio is ∼1:1; however, the AVR/DVR ratio determined for the full complement of fenestrated vessels is ∼4:1. The excess fenestrated vessels include vessels of the intracluster region (designated AVR1). Countercurrent exchange between vasa recta occurs predominantly in the intercluster region. This architecture supports previous functional estimates of capillary fluid uptake in the renal IM.
Keywords: concentrating mechanism, countercurrent system, computer-assisted reconstruction, NaCl transport, urea transport
during the past two decades, the three-dimensional architecture of the renal medulla has become increasingly pertinent to the development of advanced computational models of medullary function (4, 10, 11, 13, 30, 31). Recent advances have been made in understanding the geometrical relationships of collecting ducts (CDs) and nephrons of the outer medulla (OM) and inner medulla (IM) (25, 32–34). However, much remains to be learned about compartmentation arising from dynamic flow of fluid and solutes between these structures, compartmentation that is essential for understanding the role of the renal medulla in processes of urine concentration, sodium and water balance, and the long-term control of arterial pressure (5, 19). For each of these processes, blood flow through the medullary vasculature is highly integrated with epithelial fluid and solute fluxes, and a more complete understanding of vessel architecture and connectivity between descending vasa recta (DVR), interconnecting capillary networks, and ascending vasa recta (AVR) will more clearly define this compartmentation.
IM vasculature consists of two types: vessels with a continuous endothelium (DVR), expressing the urea transporter B (UT-B), and vessels with a discontinuous (fenestrated) endothelium (AVR), expressing PV-1, a component of the fenestral diaphragm. A type of AVR commonly referred to as “interconnecting” or “communicating” capillaries, exhibits no known structural or functional distinctions but is considered to be spatially resolved from remaining vessels (19). DVR and AVR exhibit little or no branching, and interconnecting capillaries generally exhibit repeated branching and form sparse networks (17, 19, 27). With the tip of the papilla as a reference point, the DVR are all afferent (that is, they carry blood in a descending direction) and AVR and interconnecting capillaries are generally considered efferent. However, functional studies of Zimmerhackl and colleagues (36) suggest that 10–15% of DVR may have terminal fenestrated segments that carry blood in a descending direction.
DVR, grouped in vascular bundles, enter the IM and at various levels give rise to interconnecting capillaries, which form networks that route blood flow along the corticopapillary axis. Some DVR, albeit relatively few, descend to the tip of the papilla before breaking up into capillary networks (17, 27). At various levels, interconnecting capillaries join AVR that are grouped with DVR in vascular bundles. These AVR then ascend along the corticopapillary axis, exiting the IM into the OM.
DVR and some AVR participate in countercurrent solute exchange, a process considered important for preservation of the IM interstitial solute gradient. AVR and interconnecting capillaries theoretically could also engage in countercurrent exchange with descending thin limbs of Henle (DTLs), prebend segments, and CDs. Exchanges in a cocurrent fashion potentially occur between AVR and ascending thin limbs of Henle (ATLs) (23–25). In all cases, the parallel expression of fluid and solute channels and transporters in adjacent vessels and tubules would define, in part, the exchange processes that take place.
One important goal in understanding renal medullary function is to clearly define the limits to which vascular networks, perhaps variably, lead to homogeneous or heterogeneous interstitial compartmental composition. The analysis reported herein provides a framework for quantitative studies that explore how fluid and solute are distributed and exchanged amongst medullary compartments by way of vascular pathways.
METHODS
Animals.
Young male Munich-Wistar rats (average wt, 90 g) were purchased from Harlan (Indianapolis, IN). The animals were euthanized with pentobarbital sodium (0.2 ml/100 g body wt) or with CO2. All experiments were conducted in accordance with The Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996) and approved by the Institutional Animal Care and Use Committee.
Tissue preparation and immunohistochemistry.
Kidneys were prepared for immunohistochemistry as described previously (23, 24). Serial, transverse sections 1 μm thick and no greater than 5 μm apart were prepared for each kidney. DVR were labeled with a polyclonal antibody raised in rabbits against rat UT-B (diluted 1:200, provided by Drs. Jeff Sands and Janet Klein, Emory University). Fenestrated vessels were labeled with a polyclonal antibody raised in chickens against rat PV-1, a plasmalemmal vesicle protein formerly known as gp68 (diluted 1:500, provided by Radu Stan, Dartmouth College). PV-1 is a component of fenestral diaphragms. The structural and functional role of PV-1 is poorly understood. In the rat IM, the AVR and interconnecting capillaries are fenestrated and believed to have diaphragms. CDs were labeled for aquaporin-2 (AQP2; diluted 1:200, raised in goats, Santa Cruz Biotechnology). Secondary antibodies conjugated to fluorescent probes (Invitrogen/Molecular Probes or Jackson ImmunoResearch) were applied as described previously (23, 24). Sections were mounted with Dako fluorescent mounting medium (Carpinteria, CA) and were viewed with epifluorescence microscopy.
Image analysis.
Separate stacks of digitized, serial images were generated by capturing AQP2, UT-B, or PV-1 immunofluorescence from each tissue section. Partial three-dimensional reconstructions were created for some, but not all tissue. Quantitative analyses were carried out on two-dimensional images using PhotoShop (Adobe) and the Image Processing Toolkit (Reindeer Graphics).
Determining CD cluster boundaries with a Euclidean distance map.
Each pixel in the background is assigned a luminosity value equal to its distance from the nearest edge of any CD; in this case, pixels at a greater distance are assigned a darker value (28). This value encodes the straight-line distance to the nearest point on any CD edge. The Euclidean distance map (EDM) is then skeletonized to produce a boundary around each CD. The process of skeletonization sets pixels in white if they have neighbors that are darker. The boundaries are then linked so as to encompass individual CD clusters. Boundaries are represented in images as contour lines that correspond to the linear array of pixels that are most distant from any CD.
The volume shrinkage factor for ethanol dehydration of rat medullary tissue has been reported to be ∼20% (1), and the linear shrinkage factor has been reported to be ∼20–25% (6). Thus the linear distances that we report would underestimate the distances measured for fresh tissue by a maximum of ∼20–25%.
Statistical analyses.
Data combined from three or more samples are reported as means ± SE (n = number of replicates). The statistical significance of differences between the means for each category of multiple data sets was determined with ANOVA and Duncan's post hoc test (P < 0.05).
RESULTS
Clusters of CDs that coalesce as they descend the corticopapillary axis form the organizing motif in the outer zone of the IM (the first 3–3.5 mm of the IM). At the OM-IM boundary, each primary CD cluster consists of ∼6–12 CDs that coalesce into a single CD deep in the outer zone of the IM. The terminal CDs from these primary CD clusters also coalesce to form a single CD >3–3.5 mm below the OM-IM boundary (in the inner zone of the IM). Therefore, about five to six of the primary clusters seen at the OM-IM boundary make up a larger secondary CD cluster that ends at or near the ducts of Bellini. This organization of CDs produces two clearly defined interstitial regions in the transverse dimension, the intercluster and intracluster regions (26). The intercluster region is composed of the interstitial space that separates CDs of adjacent primary clusters. The boundary for each primary CD cluster is defined by the EDM border (see methods). This border lies in the intercluster region. The intracluster region is the region encompassed by CDs of each cluster (8). DTLs, ATLs, and blood vessels are arranged within and outside clusters in an organized radial fashion. This distinctive radial tubulovascular organization may be important in establishing functionally distinct radial compartments; integration of radial and axial compartmentation may be essential to the urine concentrating mechanism (12, 14, 25).
Vascular bundles in intercluster region of the outer zone of the IM.
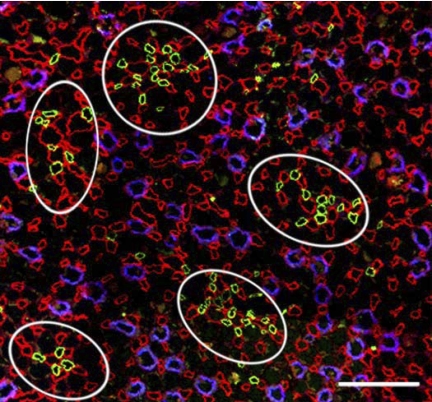
Vascular bundles carrying DVR (UT-B-positive vessels) and associated AVR2 (PV-1-positive vessels) lie within the intercluster region (intercluster AVR are designated AVR2). For the first several hundred micrometers below the OM-IM boundary, these vessels remain relatively tightly packed (Fig. 1). However, even in this region of the IM, these vascular bundles are more loosely organized than those of the OM, with wider interstitial space between vessels (19). As the UT-B-positive DVR descend through the outer IM, the bundles break up and DVR tend to fan out from each other along the EDM borders, remaining compartmentalized within the intercluster region throughout their entire descent of the IM outer zone. This architecture can be seen for DVR and AVR2 in transverse sections at 400 and 1,500 μm below the OM-IM boundary (Fig. 2). DVR and AVR2 in the nine vascular bundles of Fig. 2A are the same as those shown in the bundles of Fig. 2B.
Fig. 1.
Five vascular bundles from a transverse section at 100 μm below the outer medullary (OM)-inner medullary (IM) boundary [collecting ducts (CDs), aquaporin-2 (AQP2)/blue; descending vasa recta (DVR), urea transporter B (UT-B)/green; vessels of the intracluster region (AVR1) and in the intercluster region (AVR2), PV-1/red]. Vascular bundles are located predominantly in the intercluster region, distant from CDs. In the intracluster region, fenestrated vessels abut CDs. Scale bar = 100 μm.
Fig. 2.
Transverse sections that include 2 secondary CD clusters (see results for definition of secondary CD cluster) (CDs, AQP2/blue; DVR, UT-B/green; AVR1 and AVR2, PV-1/red). A: 400 μm below the OM-IM boundary. B: 1,500 μm below the OM-IM boundary. Within bundles, DVR descend from, and AVR2 ascend to, the upper boundary of reconstruction at 400 μm below the OM-IM boundary. Each secondary CD cluster consists of 5 primary clusters; secondary cluster 1, bottom 5 white borders; secondary cluster 2, top 5 white borders (compare with Figs. 1–3 in Ref. 26). Four vascular bundles (VB) are associated with cluster 1, and 5 vascular bundles are associated with cluster 2 (outlined with green). Inset: magnification of vascular bundle 2 from cluster 1 (arrows) (CDs, AQP2/blue; DVR, UT-B/green; AVR2, PV-1/overdrawn with white; AVR1, PV-1/red). Scale bars = 100 μm (figure) and 25 μm (inset).
Fenestrated descending vessels of the outer zone of the IM.
All UT-B-positive DVR lying within the intercluster regions of two secondary CD clusters were reconstructed beginning at a point ∼400 μm below the OM-IM boundary and continuing at least as far as their first branch point. Beyond this point, the degree of branching becomes more frequent, signaling initiation of a capillary network, and presumably ascending flow. Few DVR within these two clusters directly join AVR in a hairpin-type loop configuration, at least through the first 3,200 μm below the OM-IM boundary; instead, they connect to fenestrated vessels that form networks within the intracluster region (8).
Each UTB-positive descending vas rectum overlaps with a PV-1-positive segment at its terminus for virtually all reconstructed DVR. This confirms earlier observations (23). The vascular arrangement is depicted diagrammatically in Fig. 3. Along this overlap, in the axial descent, there is a gradual reduction in UT-B expression and a gradual increase in PV-1 expression. The lengths of the overlapping UT-B- and PV-1-positive segments for DVR in reconstructed secondary CD clusters 1 and 2 averaged 137 ± 23 (n = 42) and 167 ± 48 μm (n = 46; means ± SE), respectively.
Fig. 3.
Diagram of vasa recta architecture in the outer zone of the IM. UT-B-positive (shaded light, nonfenestrated) DVR descends along the corticopapillary axis. UT-B-positive segment overlaps (small bracket) with PV-1 (shaded dark, fenestrated) and is continuous with a descending PV-1-positive, UT-B-negative DVR (large bracket). This PV-1-positive segment is continuous with AVR1, which lie within the CD cluster (intracluster region) (8). AVR1 connect to AVR2 in vascular bundles of the intercluster region. Arrows denote blood flow direction. AVR1, PV-1-positive intracluster AVR and interconnecting capillaries that do abut CDs; AVR2, ascending PV-1-positive intercluster vessels that do not abut CDs, and do or do not abut DVR.
The terminal fenestrated segment (PV1-positive, UT-B-negative) continues to descend a variable distance before forming its first branch, for 75% of all DVR. Fenestrated descending segments (distinguishable only for reconstructed vessels) are designated DVR. For the remaining 25% of DVR, a secondary fenestrated vessel immediately branches off from the DVR at the beginning of the terminal (primary) fenestrated segment. The primary fenestrated segment that continues descending beyond this secondary branch potentially carries blood in either descending or ascending direction. For vessels of clusters 1 and 2, these segments measured 203 ± 48 (n = 32) and 201 ± 40 μm (n = 31; means ± SE), respectively, equal to 14 ± 4 and 18 ± 4% of the length of the connecting UT-B-positive DVR. The length of the terminal fenestrated segment is not significantly correlated with the length of the connecting UT-B-positive descending vas rectum segment (R2 = 0.026 and 0.1113 for clusters 1 and 2).
AVR/DVR number density ratios of the outer zone of the IM.
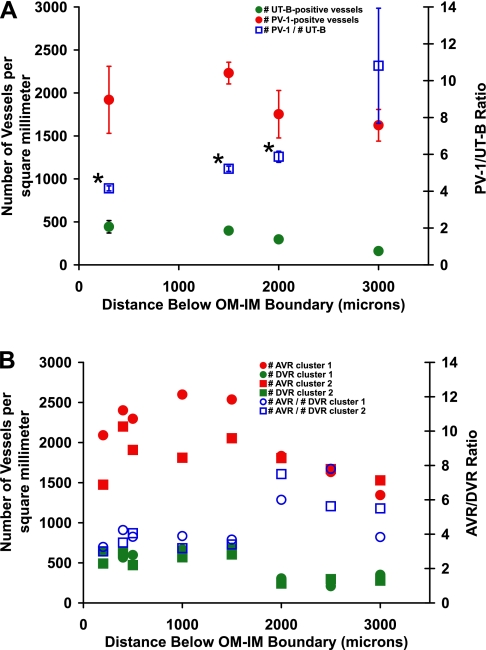
The number densities of all PV-1-positive vessels and all UT-B-positive vessels within random IM transverse sections, such as the section shown in Fig. 1, are summarized in Fig. 4A. Through the first 2,000 μm of the IM, the PV-1/UT-B number density ratio ranges between 4.2 and 5.9. At levels deeper than 2,000 μm, the fractional decrease in number density of UT-B-positive vessels is greater than the fractional decrease in number density of PV-1-positive vessels, resulting in a very high PV-1/UT-B ratio. UTB-positive vessels are nearly undetectable at deeper than 3,000 μm below the OM-IM boundary (24).
Fig. 4.
Number densities and ratios of IM blood vessels. A: number densities and ratios for UT-B-positive and PV-1-positive vessels that lie within random cross-sectional areas of the IM from 3 kidneys. Values are means ± SE. Ratio means marked with an asterisk are not significantly different from each other (ANOVA; P < 0.05). B: number densities and ratios for reconstructed DVR and AVR that lie within the borders of CD clusters 1 and 2. Tracing UT-B-positive DVR and their fenestrated segments becomes increasingly less accurate beyond 2 mm for technical reasons. Incomplete recognition of fenestrated DVR combined with biological variation probably account for higher AVR/DVR ratios at 2,000 μm below the OM-IM boundary. Compare with Fig. 4 in Ref. 8.
As noted, some fraction of PV-1-positive (fenestrated) vessels are actually descending vessels. To assess this fraction, we analyzed vessels in two clusters that have been reconstructed through the first 3,200 μm below the OM-IM boundary. In addition to distinguishing fenestrated descending segments that might otherwise be considered as ascending, these reconstructions make clear distinctions between AVR2 that ascend through the intercluster region, and AVR1, those AVR that ascend through the intracluster region (8). The AVR/DVR ratio of these vessels through the first 1,500 μm below the OM-IM boundary is ∼4:1, rising at deeper levels (Fig. 4B). For the outer zone of the IM, the highest percentage of fenestrated DVR occurs at 3,000 μm below the OM-IM boundary (Table 1).
Table 1.
Number densities and ratios of reconstructed ascending vasa recta (ascending fenestrated vessels) and descending vasa recta (UT-B-positive or PV-1-positive descending vessels) that lie within cross-sectional areas defined by borders of 2 secondary CD clusters
| Distance Below OM-IM Boundary, μm |
||||
|---|---|---|---|---|
| Number of Vessels/mm2 | 400 | 1,500 | 2,000 | 3,000 |
| Cluster 1 | ||||
| AVR2 | 1,098 | 1,143 | 611 | 588 |
| AVR2a | 429 | 536 | 305 | 235 |
| DVR | 566 | 571 | 191 | 118 |
| %DVRfen | — | 15.8 | 37.5 | 66.7 |
| AVR2/DVR ratio | 1.94 | 2.00 | 2.50 | 3.00 |
| AVR2a/DVR ratio | 0.76 | 0.94 | 1.33 | 1.33 |
| Cluster 2 | ||||
| AVR2 | 860 | 707 | 632 | 318 |
| AVR2a | 467 | 390 | 271 | 45 |
| DVR | 627 | 415 | 150 | 91 |
| %DVRfen | 32.0 | 37.5 | 66.7 | |
| AVR2/DVR ratio | 1.37 | 1.71 | 3.33 | 3.50 |
| AVR2a/DVR ratio | 0.75 | 0.94 | 1.50 | 0.50 |
UT-B, urea transporter B; OM, outer medulla; IM, inner medulla; AVR, ascending vasa recta; DVR, descending vasa recta. AVR that do not abut collecting ducts (CDs) and either do or do not abut DVR (AVR2) lie primarily within the intercluster region (Fig. 2A). AVR that do not abut CDs and do abut DVR (AVR2a) lie solely within the intercluster region (Fig. 2A). %DVRfen, percentage of DVR that are fenestrated.
AVR/DVR number density ratios of countercurrent exchanging vessels in intercluster region.
AVR2 of the outer zone of the IM do not abut CDs and can be spatially discriminated from PV-1-positive vessels that lie in the intracluster region and do abut CDs (AVR1) (Figs. 1 and 2). AVR2 likely play a dominant role in the process of countercurrent exchange with DVR due to their proximity to DVR. For random transverse sections from various depths below the OM-IM boundary, we determined the number densities of all fenestrated vessels that do not abut CDs and 1) that do or do not abut UT-B-positive DVR (AVR2) or 2) that do abut UT-B-positive DVR (AVR2a). Note that AVR2a are a subset of AVR2. The distribution of AVR1 is assessed in a separate manuscript (8).
Through the first 3,000 μm of the outer zone of the IM the AVR2a/DVR ratio ranges between 0.71 and 1.17 (Table 2). In fact, each descending vas rectum may abut as many as two to three AVR as a result of compact bundling (Fig. 2A, inset). Through the first 3,000 μm of the outer zone of the IM, the AVR2/DVR ratio ranges between 1.69 and 2.51 (Table 2). Notably, the AVR2a/DVR ratios are significantly lower than the AVR2/DVR ratios along nearly the entire corticopapillary axis of the outer IM (ANOVA; P < 0.05); the AVR2a/DVR ratio is ∼1.0, and the AVR2/DVR ratio is ∼2.0.
Table 2.
Number densities and ratios of UT-B-positive vessels (DVR) and PV-1-positive vessels (AVR) that lie within random cross-sectional areas of IM from 3 kidneys
| Distance Below OM-IM Boundary, μm |
||||
|---|---|---|---|---|
| Number of Vessels/mm2 | 300 | 1,500 | 2,000 | 3,000 |
| AVR2 | 748 ± 105 | 503 ± 16 | 653 ± 172 | 385 ± 10 |
| AVR2a | 535 ± 185 | 325 ± 31 | 327 ± 86 | 108 ± 16 |
| DVR | 443 ± 72 | 397 ± 24 | 297 ± 33 | 160 ± 27 |
| AVR2/DVR ratio | 1.69 ± 0.04 | 1.80 ± 0.01 | 2.21 ± 0.61 | 2.51 ± 0.52 |
| ANOVA | 3,9 | 4,10 | 1,5,7,11 | 2,6,8,12 |
| AVR2a/DVR ratio | 1.17 ± 0.24 | 0.82 ± 0.03 | 1.10 ± 0.27 | 0.71 ± 0.19 |
| ANOVA | 1,2 | 3,4,5,6 | 7,8 | 9,10,11,12 |
Values are means ± SE. AVR that do not abut CDs and either do or do not abut DVR (AVR2) lie primarily within the intercluster region (Fig. 2A). AVR that do not abut CDs and do abut DVR (AVR2a) lie solely within the intercluster region (Fig. 2A). Fenestrated descending vessels cannot be discerned in these images. Ratio means that share a common numeral (ANOVA, P < 0.05) are significantly different from each other.
The AVR2/DVR ratios and AVR2a/DVR ratios that were determined in random transverse sections from multiple kidneys are comparable to values determined for reconstructed vessels in two secondary CD clusters from a single kidney (Table 1).
Relative lengths of DVR and AVR2a within vascular bundles of the outer zone of the IM.
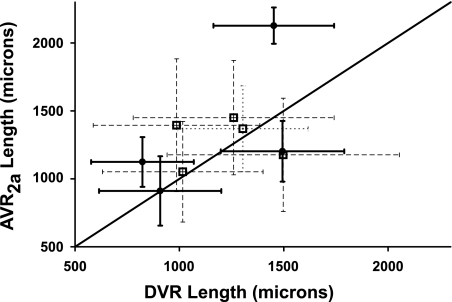
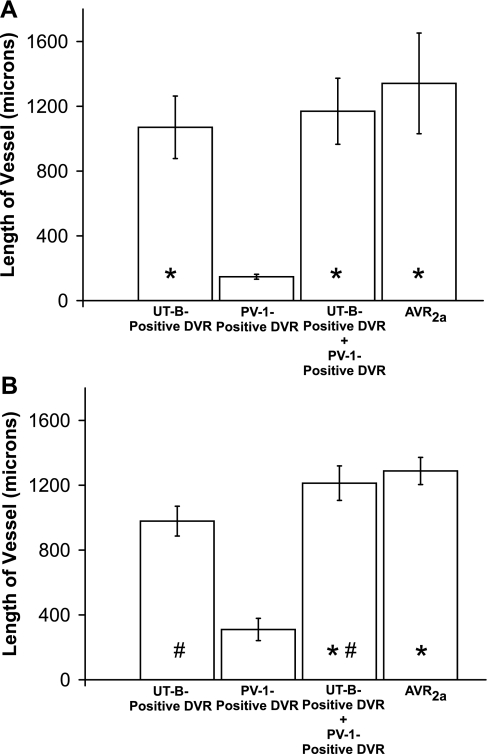
The lengths of descending and ascending vessels that lie adjacent to each other along the corticopapillary axis should provide an index of the relative degree of fluid and solute countercurrent exchange that takes place along the corticopapillary axis. The lengths of DVR (including fenestrated descending segments) and apposing nonbranching AVR2a within nine vascular bundles from two secondary CD clusters were compared to evaluate this degree of coupling between adjacent vessels. These vessel lengths are depicted in Supplemental Figs. 1 and 2 (all supplementary material for this article is available on the AJP-Renal Physiology website). As noted, apposing AVR2a include those AVR that do not abut CDs and do abut DVR in transverse sections, as shown for vascular bundles in Fig. 2A. All vessels that lie within the EDM border plus those lying within ∼1 vessel diameter outside the EDM border were included for each bundle. These criteria are sufficient to identify all DVR and most AVR2a that form individual vascular bundles. The bundles consist of variable numbers of DVR and AVR2a. Vessel lengths within bundles are highly variable (Fig. 5). The lengths of AVR2a in bundles of two secondary CD clusters are longer on average than the lengths of UT-B-positive DVR (reaching statistical significance in one of two secondary CD clusters) and are not significantly different from the lengths of UT-B-positive DVR including their fenestrated descending segments (Fig. 6). The fenestrated descending segments bring the mean DVR lengths nearer to the mean AVR2a lengths. Although vascular pairs repeatedly dissociate and reassociate with different partners along the corticopapillary axis, countercurrent exchange overall appears to occur between a relatively fixed ratio and nearly equal lengths of AVR2a and DVR within the outer zone of the IM.
Fig. 5.
Comparisons between mean lengths of DVR (including PV-1-positive segment) and AVR2a for vessels of 4 vascular bundles from secondary CD cluster 1 (black circles) and for 5 vascular bundles from secondary CD cluster 2 (white squares). Values are means ± SE. The 2 clusters and 9 bundles are outlined in Fig. 2A. DVR and AVR2a lie within the reconstructed interval of 400 and 3,200 μm below the OM-IM boundary. Line of identity is shown.
Fig. 6.
Lengths of vessel segments in vascular bundles of the outer zone of the IM. A: secondary CD cluster 1 (n = 4). B: secondary CD cluster 2 (n = 5). Values are means ± SE. Sample means that share a common symbol are not significantly different from each other (ANOVA; P < 0.05).
The lengths of ascending vessels within bundles give an indication of the approximate depths at which AVR1 networks (8) feed into the countercurrent exchange process. For two secondary CD clusters that were reconstructed between the interval of 400 and 3,200 μm below the OM-IM boundary, nearly every vascular bundle includes at least several AVR2a that are shorter than and several that are longer than the length of the longest DVR (Supplemental Figs. 1 and 2). Thus most DVR are sufficiently long so as to connect to AVR2a that lie within the same bundle. Also, for most bundles there are relatively few DVR that extend to levels deeper than 3,200 μm below the OM-IM boundary.
DISCUSSION
Three-dimensional functional reconstructions of IM vasa recta reveal a number of spatial relationships that may be significant for the process of vascular countercurrent exchange and the urinary concentrating mechanism. Previous studies have shown that CD clusters form the organizing motif in the IM with nephrons and vessels arranged within and around them in an organized fashion (22, 23). This architecture leads to two distinct interstitial compartments in the transverse dimension, the intercluster and intracluster interstitial regions. The vascular elements associated with these two compartments are summarized in Fig. 3. In addition, there appears to be a repeating array of discrete compartments that we refer to as interstitial nodal spaces (INSs) that are formed by two fenestrated capillaries adjacent to a CD, with one or two ATLs or prebend segments lying opposite the CD (23). We and our colleagues have hypothesized that INSs may play a significant role in a solute-separation, solute-mixing mechanism for concentrating urine in the IM (14, 21, 25).
We have shown here that unbranched fenestrated vessels that are positioned within the intercluster region and near UTB-positive DVR within the vascular bundles (AVR2, Tables 1 and 2) constitute a significant fraction of the intercluster vessels at each transverse level examined. Their spatial orientation, such as their position within the intercluster region, their distance from CDs, their proximity to DVR, and their unbranched segmentation uniquely characterize these vessels as AVR2. These characteristics of AVR2 distinguish them from fenestrated vessels that typically lie within 0.5–1 μm of a CD (23) and which undergo branching and fusing to form capillary networks (AVR1). Importantly, AVR1, which lie in the intracluster region, connect to AVR2, which lie in the intercluster region. We have described elsewhere the intracluster architecture of AVR1 in detail for reconstructed IM vascular networks (8). For purposes of categorizing populations of fenestrated vessels that are structurally and possibly functionally distinct, we consider any ascending vas rectum that abuts a CD (AVR1) to lie within the CD cluster. However, some of these vessels may have one surface abutting a CD and an opposite surface facing DVR in the intercluster region; these vessels could be uniquely positioned to engage in countercurrent exchange and also interact with INSs.
IM blood flow is a dynamic process involving multiple regulatory mechanisms constrained within a fixed architecture (8, 18, 19). Blood flow rates and flow patterns likely influence the degree to which fluid and solute derived from different endothelial and epithelial compartments become intermixed (35) and the extent of fluid and solute countercurrent exchange that occurs within each vascular bundle. The marked variation in length between DVR and AVR2a for each vascular bundle (Fig. 5), combined with near equivalent average length overall for DVR and AVR2a within each secondary CD cluster (Fig. 6) imply that a degree of countercurrent exchange uniformity may exist for each bundle. This would be particularly true if DVR-to-AVR2a connections exist as a uniform, cascading arrangement along the corticopapillary axis. However, since for some bundles, a number of AVR2a in the IM outer zone are longer than UT-B-positive DVR (Supplemental Figs. 1 and 2), some fraction of countercurrent exchange appears to occur between AVR2a that arise from the deep IM and DVR that recycle solutes to shallower levels. This would seem at odds with a system whose primary goal is to recycle urea to the deepest IM.
Blood flow through AVR1 will further influence countercurrent systems. In this regard, the arrays of INSs or microdomains that lie alongside CDs in the intracluster region may be sites of high osmolality due to reabsorption of NaCl, urea, and water from ATLs and CDs (14, 25). Abutting capillaries may provide a low-resistance sink for water and solutes. Solutes are either retained within AVR1 of the intracluster region or pass into the intercluster region as AVR1 ascend the corticopapillary axis. The extent to which vessels distribute solutes among these two compartments may further promote or constrain the multiple countercurrent systems that have been hypothesized to exist in the intracluster and intercluster regions (25).
The AVR/DVR number density ratio is important in accounting for mass balance of fluid and solute flows into and out of the IM. All vessels leaving the IM are generally considered to pass into the OM by way of vascular bundles (19), although there is evidence that some take an alternate pathway (8). The AVR2/DVR ratio of ∼2:1, determined for vessels positioned in the intercluster region along the entire corticopapillary axis to a point 3,000 μm below the OM-IM boundary (outer zone of the IM) (Tables 1 and 2) provides a structural basis supporting previous functional studies (2, 16, 36) that have derived a comparable ratio. Notably, Zimmerhackl et al. (36) visualized blood flow patterns with videomicroscopy and combined these data with protein concentration determinations in the Munich-Wistar rat, and estimated the functional AVR/DVR ratio to lie between 2.1:1 and 2.4:1 at ∼2,000 μm above the papilla tip. Böttcher and Steinhausen (2) estimated an AVR/DVR ratio of 2.2:1 for the Munich-Wistar rat based on blood flow patterns in situ for exposed papilla at 500 μm above the papilla tip on the papillary surface. Also, for the hamster, Marsh and Segel (16) reported a mean AVR/DVR ratio of 1.71:1, estimated from blood flow patterns in situ for exposed papilla at ∼3,000 μm below the OM-IM boundary on the papillary surface.
On the other hand, an AVR/DVR ratio of between about 4:1 and 6:1, based on number densities of all fenestrated and UTB-positive vessels along the outer zone of the IM (Fig. 4A), approximates the ratio of 3.96:1 determined at ∼2,000 μm above the papillary tip by Holliger et al. (7) but varies from the ratio of ∼2:1 for the same region in the Sprague-Dawley rat (15). AVR/DVR ratios determined in the latter two studies were based on morphological criteria for the full complement of fenestrated and nonfenestrated vessels. Assuming our study and the study by Holliger et al. (7) include representative areas from the IM, our ratio of 10.8:1 for random cross-sectional areas at 3,000 μm below the OM-IM boundary (Fig. 4A) accounts only for ∼40% of all DVR. Reconstructed vessels confirm that at 3,000 μm below the OM-IM boundary the percentage of DVR that are fenestrated is ∼60–70% (66.7% for both clusters) (Table 1). This confirms and extends our previous report (24) and that of Kim et al. (9), citing evidence of reduced UT-B expression in the terminal papilla. Existence of a significant number of fenestrated DVR supports functional studies that have estimated total plasma outflow from the IM via AVR exceeds inflow by ∼30% (36), the excess outflow representing fluid uptake. A significant degree of uptake would occur in the intracluster region (8).
A higher ratio of fenestrated to UT-B-positive vessels in the inner zone of the IM (the terminal 1.5–2 mm of the IM) (3, 15, 29) suggests that UTB-mediated countercurrent urea exchange between AVR and DVR is a feature primarily of the outer zone of the IM; if a significant degree of solute recycling does occur between descending and ascending vessels in the inner zone of the IM, it likely involves primarily fenestrated vessels. A disproportionately large fraction of fenestrated descending and ascending vessels in the inner zone of the IM could account for the remarkably equivalent Na and urea permeabilities reported by Pallone et al. (20) for DVR and AVR at the papillary tip of the adult female Munich-Wistar rat. Countercurrent exchange between DVR and AVR in the outer zone of the IM may therefore be more selective for urea, and in the inner zone of the IM may more permissively include NaCl and urea, though both solutes are exchanged to some degree throughout the IM (19). This further supports the concept that the inner and outer zones play functionally distinct roles in the urine concentrating mechanism (25, 26), a concept based, in part, on the gradual disappearance of distinct intracluster and intercluster regions in the inner zone.
In summary, we have introduced a set of parameters that more clearly define those fenestrated vessels that play a major role in the process of countercurrent exchange between IM DVR and AVR. This population of AVR lies outside CD clusters and accounts for, at most, ∼50% of all fenestrated vessels in the outer zone of the IM. Additional fenestrated vessels are spatially more closely associated with CDs (AVR1). The existence of fenestrated descending segments at DVR termini tends to equalize the overall lengths of descending and ascending segments participating in countercurrent exchange. The vascular architecture described here supports former functional studies suggesting countercurrent-exchanging AVR outnumber DVR by a factor of about two (36).
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK16294.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Insightful discussions about the dynamic urinary concentrating mechanism with long-time colleagues Harold Layton, Anita Layton, and Bill Dantzler are gratefully acknowledged. We thank Radu Stan of Dartmouth College, and Jeff Sands and Janet Klein of Emory University for providing antibodies. Brandi Hoopes and Collin Laufenberg contributed to image analysis as part of their undergraduate degree requirements at the University of Arizona.
REFERENCES
- 1.Bankir L, Fischer C, Fischer S, Jukkala K, Specht HC, Kriz W. Adaptation of the rat kidney to altered water intake and urine concentration. Pflügers Arch 412: 42–53, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Bottcher W, Steinhausen M. Microcirculation of the renal papilla of rats under control conditions and after temporary ischemia. Kidney Int 10: S74–S80, 1976 [PubMed] [Google Scholar]
- 3.Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat 118: 685–722, 1966 [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla: I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F537–F548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Han JS, Thompson KA, Chou CL, Knepper MA. Experimental tests of three-dimensional model of urinary concentrating mechanism. J Am Soc Nephrol 2(12): 1677–1688, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Holliger C, Lemley K, Schmitt C, Thomas F, Robertson C, Jamison RL. Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res 53: 401–413, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol (doi:10.1152/ajprenal.00072.2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol 282: F530–F540, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Layton AT, Layton HE. A region-based model framework for the rat urine concentrating mechanism. Bull Math Biol 65: 859–901, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Layton AT, Layton HE, Dantzler WH, Pannabecker TL. The mammalian urine concentrating mechanism: hypotheses and uncertainties. Physiology 24: 250–256, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816–F839, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacPhee PJ. Fluid uptake by the renal medullary vasa recta: An estimate based on a quantitative analysis of the distribution of fenestrae in the vasa recta of young Sprague-Dawley rats. Exp Physiol 83: 23–34, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Marsh DJ, Segel LA. Analysis of countercurrent diffusion exchange in blood vessels of the renal medulla. Am J Physiol 221: 817–828, 1971 [DOI] [PubMed] [Google Scholar]
- 17.Moffat DB, Fourman J. The vascular pattern of the rat kidney. J Anat 97: 543–553, 1963 [PMC free article] [PubMed] [Google Scholar]
- 18.Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: 212–222, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pannabecker TL. Loop of Henle interaction with interstitial nodal spaces in the renal inner medulla. Am J Physiol Renal Physiol 295: F1744–F1751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pannabecker TL, Dantzler WH. Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla. Am J Physiol Renal Physiol 293: F696–F704, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannabecker TL, Henderson C, Dantzler WH. Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla. Am J Physiol Renal Physiol 294: F1306–F1314, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Rollhauser H, Kriz W, Heinke W. Das gefass-system der rattenniere. Z Zellforsch 64: 381–403, 1964 [PubMed] [Google Scholar]
- 28.Russ JC. The Image Processing Handbook Boca Raton, FL: CRC, 1999 [Google Scholar]
- 29.Schwartz MM, Karnovsky MJ, Venkatachalam M. Ultrastructural differences between rat inner medullary descending and ascending vasa recta. Lab Invest 35: 161–170, 1976 [PubMed] [Google Scholar]
- 30.Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. I. Modeling results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F368–F383, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. II. Sensitivity results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F384–F394, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77–88, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Zhai XY, Birn H, Jensen KB, Thomsen JS, Andreasen A, Christensen EI. Digital three-dimensional reconstruction and ultrastructure of the mouse proximal tubule. J Am Soc Nephrol 14: 611–619, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: 2937–2944, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Zimmerhackl B, Roberston CR, Jamison RL. Effect of arginine vasopressin on renal medullary blood flow. A videomicroscopic study in the rat. J Clin Invest 76: 770–778, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerhackl B, Robertson CR, Jamison RL. Fluid uptake in the renal papilla by vasa recta estimated by two methods simultaneously. Am J Physiol Renal Fluid Electrolyte Physiol 248: F347–F353, 1985 [DOI] [PubMed] [Google Scholar]