Abstract
Recent studies revealed a striking morphological change of mitochondria during apoptosis. Mitochondria become fragmented and notably, the fragmentation contributes to mitochondrial outer membrane permeabilization and consequent release of apoptotic factors. In renal tubular cells, mitochondrial fragmentation involves the activation of Drp1, a key mitochondrial fission protein. However, it is unclear how Drp1 is regulated during tubular cell apoptosis. In this study, we examined Drp1 regulation during tubular cell apoptosis following ATP depletion. Rat kidney proximal tubular cells (RPTC) were subjected to azide treatment or severe hypoxia in glucose-free medium to induce ATP depletion. During ATP depletion, Drp1 was shown to be dephosphorylated at serine-637. Drp1 dephosphorylation could be suppressed by cyclosporine A and FK506, two calcineurin inhibitors. Importantly, cyclosporine A and FK506 could also prevent mitochondrial fragmentation, Bax accumulation, cytochrome c release, and apoptosis following ATP depletion in RPTC. The results suggest that calcineurin-mediated serine-637 dephosphorylation is involved in Drp1 activation during ATP depletion in renal tubular cells. Upon activation, Drp1 contributes to mitochondrial fragmentation and outer membrane permeabilization, resulting in the release of apoptogenic factors and apoptosis.
Keywords: calcineurin, mitochondrial dynamics, cyclosporine A, FK506, Bax, cytochrome c
the intrinsic or mitochondrial pathway of apoptosis contributes to tubular cell injury and death during renal ischemia-reperfusion, a major cause of acute kidney injury and renal failure (6, 14, 23, 26–29, 34, 38, 41). This apoptosis pathway is characterized by the accumulation of Bax in mitochondria, permeabilization of the mitochondrial outer membrane, and consequent release of apoptogenic factors from the intermembrane space (19). One of the released factors is cytochrome c (cyt.c), which binds to Apaf-1 in cytosol to recruit and activate caspase-9, leading to the activation of the caspase cascade and apoptosis (37). Obviously, a key control point in this pathway is the permeability of the mitochondrial outer membrane. It is recognized that Bcl-2 proteins play critical roles in the control of the outer membrane integrity (1, 11). In addition, permeabilization of the outer membrane may depend on the condition of the mitochondria. In this regard, recent studies suggested that the shape or morphology of mitochondria may also be a determinant of mitochondrial outer membrane permeabilization during apoptosis (2, 31, 32).
Mitochondria are a class of dynamic organelles, constantly undergoing fission and fusion (8). The shape of mitochondria in a cell is thus controlled by fission and fusion events. Under normal conditions, fusion prevails and as a result, mitochondria assume a long or filamentous morphology. However, when a cell is stressed, the dynamic balance is shifted to fission, leading to mitochondrial fragmentation. Interestingly, emerging evidence suggests that mitochondrial fragmentation is involved in mitochondrial outer membrane permeabilization and subsequent release of apoptogenic factors during apoptosis (2, 8, 31, 32). In renal cell and tissues, our recent work indicates that inhibition of mitochondrial fragmentation can suppress tubular cell apoptosis and kidney injury (4), supporting a pathological role for this seemingly morphological change. The mechanism leading to mitochondrial fragmentation during tubular cell apoptosis is largely unknown. However, Drp1, a key fission protein, is activated early on apoptotic stimulation and translocates to mitochondria. Moreover, inhibition of Drp1 via a dominant negative mutant or siRNA can prevent mitochondrial fragmentation. In vivo, a pharmacological inhibitor of Drp1 can ameliorate mitochondrial fragmentation during renal ischemia-reperfusion and cisplatin nephrotoxicity (4, 5). Together, these observations suggest a role for Drp1 in mitochondrial fragmentation and subsequent injury during renal injury. Despite these findings, the mechanism that regulates Drp1 during tubular cell apoptosis is unclear.
Recent studies suggested several interesting mechanisms of Drp1 regulation, including phosphorylation, dephosphorylation, and sumoylation (7, 9, 10, 33, 40). In the present study, we show that Drp1 is dephosphoylated at serine-637 during ATP depletion in renal tubular cells. Drp1 dephosphorylation may be mediated by calcineurin and contribute to mitochondrial fragmentation, outer membrane permeabilization, and ensuing apoptosis.
MATERIALS AND METHODS
Cells and reagents.
The rat kidney proximal tubular cell line (RPTC) was originally obtained from Dr. U. Hopfer (Case Western Reserve Univ., Cleveland, OH) and cultured as described previously (2). Antibodies were from the following sources: monoclonal mouse anti-Bax from MeoMarkers (Fremont, CA), mouse monoclonal anti-cytochrome c and anti-Drp1 from BD Pharmingen (San Diego, CA), mouse monoclonal anti-β-actin from Sigma (St. Louis, MO), rabbit polyclonal antibody specific to phosphorylated Drp1 at serine-637 from Dr. C. Blackstone (National Institute of Neurological Disorders and Stroke, National Institutes of Health), and all secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Digitonin was purchased from ICN Biomedicals (Aurora, OH). Cyclosporin A (CsA) and FK506 were purchased from Calbiochem (San Diego, CA) and AG Scientific (San Diego, CA), respectively. Other reagents and chemicals including azide were purchased from Sigma.
Analysis of mitochondrial morphology.
Mitochondrial morphology was examined as described in our previous work (4, 5). Briefly, RPTC cells were plated at ∼60% confluence for transfection with pDsRed2-Mito (BD Clontech) using Lipofectamine 2000 (Invitrogen). Transfection of pDsRed2-Mito led to the expression of MitoRed, a red fluorescent protein, in mitochondria to label the organelles. The transfected cells were then evaluated for mitochondrial morphology by fluorescence microscopy. Filamentous mitochondria showed a long thread-like tubular structure, whereas fragmented mitochondria were punctate and sometimes rounded. In a given cell, its mitochondria were often either filamentous or fragmented with very rare cases of mix morphologies.
ATP depletion.
ATP depletion was induced in RPTC cells by azide treatment or severe hypoxia. For azide treatment, the cells were incubated with 10 mM azide in a glucose-free Krebs-Ringer bicarbonate solution as previously described (4, 17, 36). For hypoxia, the cells were incubated in a glucose-free Krebs-Ringer bicarbonate solution in a severe hypoxia chamber with >0.2% oxygen (16, 17, 29). In both models, ATP depletion induces key mitochondrial events of apoptosis including Bax accumulation and cyt.c release. However, the development of apoptotic morphology occurs only after the ATP-depleted cells are returned to normal culture medium for recovery (4, 16, 17, 29, 36). To test the effects of CsA and FK506, the compounds were dissolved in DMSO to prepare stock solutions before adding to the cell incubation buffer. The same amount of DMSO was added to the control groups to reveal the specific effects of the inhibitors.
Measurement of cell ATP.
Cell ATP was determined using an ATP Bioluminescent Assay kit (Sigma). Briefly, cells were extracted with perchloric acid. After neutralization of the extract, the sample was mixed at a 1:1 ratio with the Bioluminescence reagent and read for luminescence immediately on a Turner Designs luminometer (Sunnyvale, CA). For each measurement, a standard curve was constructed using serial dilutions of ATP stock solution and the luminescence reading of the sample was converted to nanomolar amounts of ATP accordingly. Parallel dishes of cells were used to determine protein. The cell ATP level was expressed as nanomoles of ATP per milligram of cell protein.
Examination of apoptosis.
Apoptosis was analyzed by morphological methods. Briefly, cells were stained with Hoechst 33342 and then examined by phase contrast and fluorescence microscopy. Apoptotic cells were identified by characteristic morphology including cellular condensation, formation of apoptotic bodies, and condensation and fragmentation of the nucleus. About 200 cells were examined in each 35-mm dish to determine the percentage of apoptotic cells.
Measurement of caspase activity.
Caspase activity was measured by an enzymatic assay as previously described (4, 16, 17, 29, 36). Briefly, cell lysate was collected with Triton X-100 and added to an enzymatic reaction containing DEVD.AFC (carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin; Enzyme Systems Products, Dublin, CA), a fluorogenic substrate of caspase-3, -6, and -7. After 1 h of reaction, fluorescence was measured at excitation 360 nm/emission 530 nm. For each measurement, free AFC (7-amino-4-trifluoromethyl coumarin) was used to construct a standard curve and the fluorescence reading of the samples was converted to nanomolar liberated AFC using the standard curve.
Cellular fractionation.
To investigate the subcellular redistributions of Bax and cyt.c during apoptosis, cells were fractionated by using digitonin at low concentrations (4, 17, 29, 36). Briefly, cells were permeabilized with 0.05% (wt/vol) digitonin in an isotonic sucrose buffer for 2–4 min at room temperature. The released cytosol was collected. Digitonin-insoluble part was further extracted with 2% SDS to collect the membrane-bound organellar fraction enriched with mitochondria.
Immunofluorescence of cyt.c.
Cells were grown on collagen-coated glass coverslips for immunofluorescence as described in our previous studies (4, 17, 29, 36). Briefly, cells were fixed with a modified Zamboni's fixative containing picric acid and 4% paraformaldehyde. The fixed cells were incubated with a blocking buffer containing 2% normal goat serum and then exposed to the primary antibody (mouse monoclonal anti-cyt.c). Finally, the cells were incubated with Cy3-labled goat anti-mouse secondary antibody. To reveal nuclear morphology, the cells were also incubated with Hoechst33342. The signals were examined by fluorescence microscopy.
Immunoblot analysis.
A standard protocol of immunoblot analysis was followed. Briefly, the protein concentration of cell lysate was determined by using the BCA reagent (Pierce, Rockford, IL). Equal amounts of proteins were loaded for reducing SDS-gel electrophoresis and electroblotted onto PVDF membranes. After being blocked, the blots were incubated with a specific primary antibody and then exposed to the horseradish peroxidase-conjugated secondary antibody. Finally, antigens on the blots were revealed using the enhanced chemiluminescence kit from Pierce.
Statistics.
Quantitative data were analyzed by Student's t-test and expressed as means ± SD. Statistical differences between the means were determined using ANOVA followed by Tukey's posttest. P < 0.05 was considered to reflect significant differences. Qualitative data including cell images and immunoblots were representative of at least three independent experiments.
RESULTS
Dephosphorylation of Drp1 at serine-637 during ATP depletion in RPTC cells.
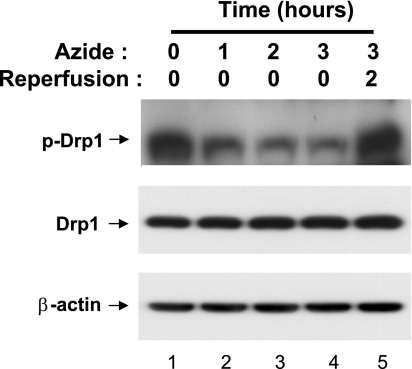
Drp1 is a key regulator of mitochondrial fission. Our recent work suggested that Drp1 is activated early during ATP depletion in renal tubular cells and contributes to mitochondrial fragmentation under the pathological condition (4). To understand the mechanism of Drp1 activation, our study was focused on the changes of its phosphorylation status. Especially, we analyzed Drp1 phosphorylation at serine-637, because phosphorylation of this site has been implicated in the regulation of Drp1 activation on apoptosis induction (7). RPTC cells were subjected to ATP depletion by azide treatment in glucose-free buffer for 1 to 3 h, without or with 2 h of recovery in full culture medium. Whole cell lysate was collected for immunoblot analysis. As shown in Fig. 1, Drp1 was highly phosphorylated at serine-637 in control cells (lane 1). In response to ATP depletion, Drp1 (Ser637) phosphorylation decreased rapidly (lanes 2–4). Drp1 became rephosphorylated after the cells were returned to full culture medium for recovery (lane 5). The expression level of total Drp1 did not show obvious changes during ATP depletion or subsequent recovery (Fig. 1).
Fig. 1.
Dephosphorylation of Drp1 at serine-637 during ATP depletion. Rat kidney proximal tubular cell line (RPTC) cells were subjected to 0–3 h of ATP depletion with 10 mM azide in glucose-free buffer. One group of the cells was returned to full culture medium for 2 h of recovery. Whole cell lysates were collected for immunoblot analysis of phosphorylated (serine-637) Drp1, total Drp1, and β-actin. The blots are representative of 3 experiments.
Inhibition of Drp1 dephosphorylation during ATP depletion by CsA and FK506.
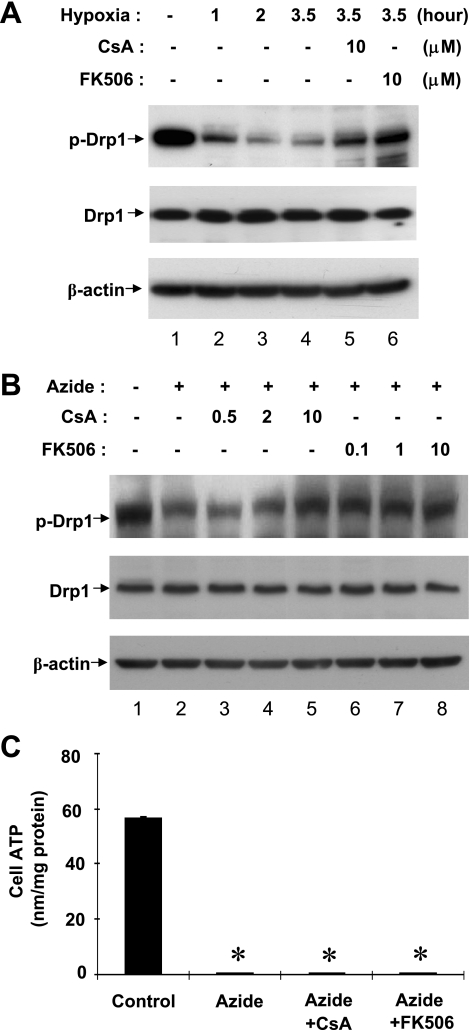
We further examined Drp1 during ATP depletion induced by severe hypoxia or anoxia. As shown in Fig. 2A, severe hypoxia induced a rapid dephosphorylation of Drp1, starting from the first hour and reaching the maximal in 2 h. Recent studies suggested the involvement of calcineurin in Drp1 dephosphorylation (7). Thus, to understand the mechanism of Drp1 dephosphorylation during ATP depletion in RPTC cells, we examined the effects of CsA and FK506, two structurally dissimilar inhibitors of calcineurin. As shown in Fig. 2A, both CsA and FK506 prevented Drp1 dephosphorylation during hypoxia treatment (lanes 5, 6). Consistently, Drp1 dephosphorylation during azide treatment could be inhibited by CsA and FK506 in a dose-dependent manner (lanes 3–8 vs. lane 2). CsA showed significant inhibitory effects at 10 μM, whereas FK506 was effective at 0.1 to 10 μM. ATP depletion induced by azide was not affected by either CsA or FK506 (Fig. 2C). Together, the results suggest that calcineurin may mediate Drp1 dephosphorylation during ATP depletion of RPTC cells.
Fig. 2.
Inhibition of Drp1 dephosphorylation during ATP depletion by cyclosporin A (CsA) and FK506. RPTC cells were pretreated for 30 min without or with indicated concentrations (in μM) of CsA or FK506. The cells were then subjected to ATP depletion by severe hypoxia (A) or 10 mM azide treatment (B) in a glucose-free buffer with or without CsA or FK506. Whole cell lysates were collected for immunoblot analysis of phosphorylated (serine-637) Drp1, total Drp1, and β-actin. The blots are representative of 3–4 experiments. C: RPTC cells were treated with azide for 3 h with or without 10 μM CsA or FK506 to collect lysate to measure ATP and protein. Cell ATP was expressed as nmol per mg protein. Data are expressed as means ± SD, n = 4; *P < 0.01 vs. control.
Suppression of ATP depletion-induced mitochondrial fragmentation by CsA and FK506.
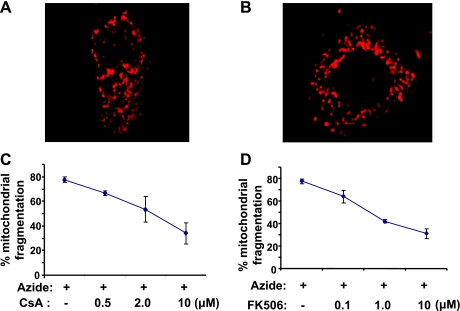
Functionally, Drp1 is a key regulator of mitochondrial fission in pathophysiological conditions. During ATP depletion in renal tubular cells, Drp1 is activated and contributes to mitochondrial fragmentation (4). To determine whether Drp1 dephosphorylation at serine-637 is involved in the regulation of mitochondrial morphology, we examined the effects of CsA and FK506 on ATP depletion-induced mitochondrial fragmentation in RPTC cells. As shown in Fig. 3A, mitochondria in control cells had a long, filamentous shape and formed a network in the cytoplasm. Upon azide-induced ATP depletion, the mitochondrial network broke down into small punctate organelles (Fig. 3B). Notably, both CsA and FK506 could prevent mitochondrial fragmentation in a dose-dependent manner (Fig. 3, C and D). The results suggest that calcineurin-mediated dephosphorylation may play an important role in the function of Drp1 for mitochondrial fission or fragmentation during tubular cell apoptosis.
Fig. 3.
Inhibition of mitochondrial fragmentation during ATP depletion by CsA and FK506. RPTC cells were transfected with MitoRed to fluorescently label mitochondria. The cells were then pretreated for 30 min with or without CsA or FK506. Finally, the cells were subjected to 3 h of ATP depletion with 10 mM azide in glucose-free buffer in the absence or presence of CsA or FK506. A: an untreated control RPTC cell showing long mitochondria with a rod- or worm-like appearance. B: an azide-treated cell showing fragmented or punctate mitochondria. C, D: effects of CsA and FK506 on mitochondrial fragmentation. The percentage of cells with fragmented mitochondria was determined by cell counting. Data are means ± SD, n = 3 (≥100 cells were counted in 3 independent experiments).
Partial inhibitory effects of CsA and FK506 on bax translocation during ATP depletion.
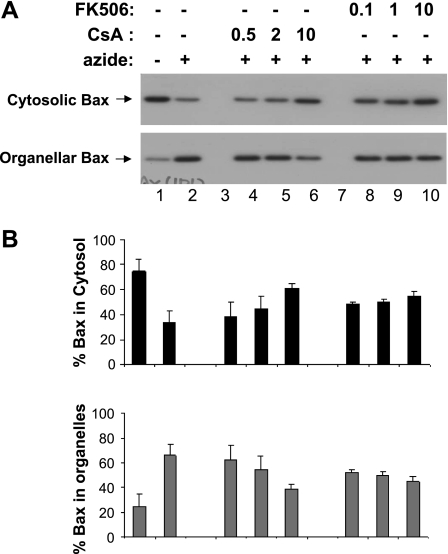
ATP depletion activates the intrinsic pathway of apoptosis in renal tubular cells (3, 36, 39), which is characterized by the accumulation of Bax in mitochondria, permeabilization of mitochondrial outer membrane, and release of apoptogenic factors. To determine whether Drp1 dephosphorylation affects these critical events, we first analyzed Bax accumulation in mitochondria. To this end, cells were fractionated into the cytosolic fraction and the membrane-bound organellar fraction for immunblot analysis of Bax. As shown in Fig. 4, Bax was detected mainly in the cytosolic fraction in control cells (lane 1) and after ATP depletion, it translocated to the mitochondria-containing organellar fraction (lane 2). Bax translocation during ATP depletion was not suppressed by 0.5–2 μM CsA (lanes 4, 5), but was noticeably blocked by 10 μM CsA (lane 6). For FK506, inhibitory effects on Bax translocation were detected at 0.1, 1, and 10 μM (lanes 8–10). By densitometric analysis, we quantified the effects of CsA and FK506 by analyzing Bax immunoblots from separate experiments. The results showed that 10 μM CsA suppressed Bax accumulation in mitochondria from 79 to 34%, while 10 μM FK506 suppressed it to 40%.
Fig. 4.
CsA and FK506 partially decrease Bax translocation during ATP depletion. After pretreatment with CsA or FK506 for 30 min, RPTC cells were subjected to 3 h of ATP depletion with 10 mM azide in the presence or absence of CsA or FK506. The cells were then fractionated into cytosolic fraction and membrane-bound organellar fraction for immunoblot analysis of Bax. A: immunoblot analysis of cytosolic and organellar Bax. B: results of densitometric analysis of Bax blots. Note: the experimental condition represented by each bar in B was the same as the aligned lane in A. Data are means ± SD, n = 4.
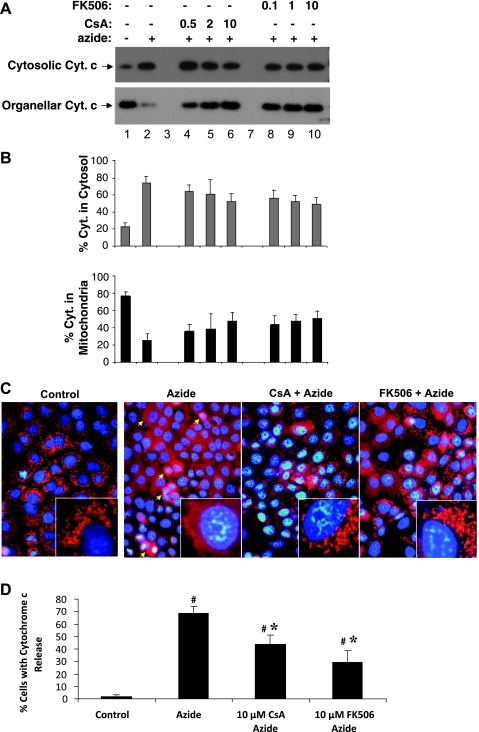
Effect of CsA and FK506 on cyt.c release during ATP depletion.
We further determined the effects of CsA and FK506 on ATP depletion-induced cyt.c release. To this end, we first analyzed cyt.c release by cellular fractionation followed by immunoblotting. Representative immunoblots of cyt.c are shown in Fig. 5A. Clearly, cyt.c was released from mitochondria into cytosol during ATP depletion (lane 2 vs. 1). CsA and FK506 did not seem to prevent the release of cyt.c into the cytosol, although more cyt.c was preserved in mitochondria (Fig. 5A, lanes 4–10). Consistently, densitometric analysis of blots from separate experiments only showed marginal inhibitory effects of CsA and FK506 on cyt.c release during ATP depletion (Fig. 5B). The lack of significant effects of CsA and FK506 on cyt.c release was unexpected, because both inhibitors suppressed ATP depletion-induced Bax accumulation in mitochondria (Fig. 4). To further clarify this issue, we conducted immunofluorescence analysis to further determine the effects of CsA and FK506 on cyt.c release in situ within the cells. In control cells, cyt.c was visualized in perinuclear organelles or mitochondria. After 3 h of ATP depletion, a large population of cells showed diffuse cyt.c in their cytosol (arrowed for representative cells), indicating the release of cyt.c from mitochondria into cytosol. Importantly, the cyt.c release was inhibited by CsA and FK506. Cell counting showed that ATP depletion induced cyt.c release in 67% cells, which was suppressed to 43 and 30% by 10 μM CsA and 10 μM FK506, respectively (Fig. 5D).
Fig. 5.
Effects of CsA and FK506 on cytochrome c (cyt.c) release during ATP depletion. RPTC cells pretreated with CsA and FK506 were subjected to 3 h of ATP depletion in the presence or absence of CsA or FK506. A: immunoblot analysis of cyt.c release. The cells were fractionated into cytosolic and membrane-bound organellar fraction for immunoblot analysis of cyt.c. B: results of densitometric analysis of cyt.c blots. Note: the experimental condition represented by each bar in B was the same as the aligned lane in A. Data are means ± SD, n = 4. C: immunofluorescence of cyt.c. Cells were fixed for immunofluorescence staining of cyt.c. The cells were also stained with Hoechest 33342 to reveal nuclei. Shown are merged images of cyt.c and nuclear staining. Insets: cells at higher magnification. D: quantification of the cells in immunofluorescence analysis that released cyt.c. The cells with cytosolic staining of cyt.c were counted to determine the percentage of cells with cyt.c release. Data are means ± SD (n = 3). #Significantly different from control. *Significantly different from azide-only treated group.
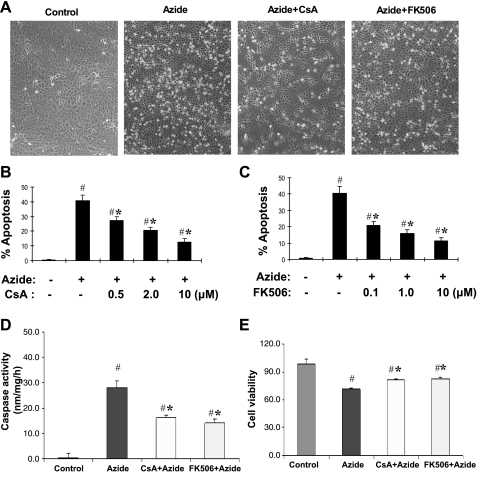
Effects of CsA and FK506 on apoptosis following ATP depletion in RPTC cells.
In RPTC cells, ATP depletion induces the mitochondrial events of apoptosis. However, due to the lack of ATP, the cells cannot complete the apoptotic program to develop apoptotic morphology. When the ATP-depleted cells are returned back to full culture medium, the cells may produce ATP via glycolysis and develop typical apoptotic morphology (4, 17, 36). Thus, to observe the effects of CsA and FK506 on apoptosis, RPTC cells were returned to culture medium for 2 h of recovery. As shown in Fig. 6A, many of the ATP-depleted cells developed apoptotic morphology during the recovery period. These cells became condensed and rounded-up and, in many cases, formed apoptotic blebs or bodies. Both CsA and FK506 had noticeable inhibitory effects on RPTC apoptosis following ATP depletion. To quantify the effects, the cells with apoptotic morphology were counted. As shown in Fig. 6, B and C, 3 h of ATP depletion induced ∼40% apoptosis in RPTC cells, which was suppressed by CsA and FK506 in a dose-dependent manner. At 10 μM, CsA and FK506 suppressed apoptosis to 10∼15%. The morphological evaluation was supported by the measurement of caspase activity (Fig. 6D). To determine whether CsA and FK506 can benefit long-term cell survival, we monitored cell viability at 24-h postazide treatment by MTT assay. As shown in Fig. 6E, there was a decrease of cell viability after azide treatment, which was partially prevented by both CsA and FK506.
Fig. 6.
Effects of CsA and FK506 on RPTC apoptosis following ATP depletion. RPTC cells were pretreated with or without 10 μM CsA or FK506 for 30 min. The cells were then subjected to 3 h of ATP depletion with 10 mM azide in glucose-free buffer in the presence or absence of CsA or FK506, followed by 2 h of recovery in full culture medium. A: cell morphology recorded by phase contrast microscopy. B, C: quantification of the effects of CsA (B) and FK506 (C) on RPTC apoptosis. Cells with typical apoptotic morphology were counted to determine the percentage of apoptosis. D: caspase activity. Cell lysate was collected to determine caspase activity. E: long-term cell survival. After azide treatment, the cells were recovered for 24 h in full culture medium to determine cell viability by MTT assay. Data are expressed as means ± SD (n = 3). #Significantly different from control group without azide treatment. *Significantly different from azide-only treated group.
DISCUSSION
Recent studies suggested an important role for the regulation of mitochondrial dynamics in mitochondrial injury during apoptosis (2, 8, 31, 32). Using both in vitro cell culture and in vivo animal models, our recent work demonstrated mitochondrial fragmentation and its involvement in tubular cell apoptosis, tissue damage, and renal failure in experimental models of acute kidney injury (4). It remains largely unclear as to how the mitochondrial dynamics are altered to result in fragmentation of the organelles. Nonetheless, it showed that Drp1 is rapidly activated and contributes to subsequent mitochondrial fragmentation under the pathological conditions. Mitochondrial fragmentation can be attenuated by the expression of a dominant-negative mutant of Drp1 or Drp1 knockdown siRNA in cultured tubular cells. In mice, mdivi-1, a pharmacological inhibitor of Drp1, can partially suppress mitochondrial fragmentation, tubular cell apoptosis, and renal injury during ischemia-reperfusion and cisplatin nephrotoxicity (4). Despite these observations, very little is known about Drp1 regulation during apoptosis of renal tubular cells. The results of the present study show that Drp1 is rapidly dephosphorylated at serine-637 on ATP depletion in RPTC cells. Drp1 dephosphorylation in these cells may involve calcineurin, as suggested by pharmacological evidence. Inhibition of calcineurin not only prevents Drp1 dephosphorylation but can also suppress mitochondrial fragmentation, Bax activation, cyt.c release, and apoptosis to various degrees. Together, these results suggest that Drp1 dephosphorylation at serine-637 may play an important role in Drp1 activation and subsequent alterations of mitochondrial dynamics during apoptosis of renal tubular cells.
Two pharmacological inhibitors of calcineurin, CsA and FK506, were used in this study. These two inhibitors are structurally dissimilar and functionally they target different receptor proteins with CsA binding to cyclophilin and FK506 to FKBP12. However, both CsA/cyclophilin and FK506/FKBP12 complexes can bind to calcineurin, leading to its inhibition (21). It is important to point out that CsA is also a well-documented inhibitor of mitochondrial permeability transition (MPT), porous defects in the inner membrane of mitochondria that lead to the loss of mitochondrial membrane potential (20, 25). In renal tubular cells, cytoprotective effects have been recently documented for CsA during oxidant-induced cell death (13). As MPT contributes to apoptosis in certain experimental models (25), some of the effects of CsA observed in our present study may result from the suppression of MPT. Although this possibility has yet to be clearly clarified, the fact that FK506 also inhibited Drp1 dephosphorylation and mitochondrial fragmentation in RPTC cells supports the involvement of calcineurin in these regulations.
Calcineurin (also called protein phosphatase 2B) is a calmodulin-dependent, Ca2+-activated phosphatase that mediates the dephosphorylation of a variety of proteins at serine/threonine sites (20). In renal tubular cells, ATP depletion by hypoxia or chemical inhibition of mitochondria leads to increases in intracellular free Ca2+ (15, 24, 42), which may activate calcineurin. In the regulation of apoptosis, calcineurin has been shown to dephosphorylate Bad, a proapoptotic Bcl-2 family protein (35). The dephosphorylation leads to the translocation of Bad from cytosol to mitochondria, where it may interact with and neutralize the anti-apoptotic proteins such as Bcl-2 and Bcl-XL to facilitate apoptosis (35). The results from the current study suggest that calcineurin may promote apoptosis through yet another mechanism, i.e., Drp1 dephosphorylation and mitochondrial fragmentation. These two mechanisms are not mutually exclusive and may actually work together to promote mitochondrial outer membrane permeabilization and the release of apoptogenic factors. Further investigations should examine the regulation of calcineurin in renal tubular cells and delineate the mechanisms underlying calcineurin-mediated mitochondrial damage and apoptosis.
A technical issue noted in our study is the analysis of cyt.c release following cellular fractionation. In situ immunofluorescence showed clearly that both CsA and FK506 attenuated cyt.c release during ATP depletion (Fig. 5, C and D), whereas cellular fractionation using low concentrations of digitonin followed by immunoblot analysis did not show significant effects of the inhibitors (Fig. 5, A and B). The discrepancy of the results was apparently caused by the technical issues of the cellular fractionation method. During cellular fractionation, the cells are broken by physical procedures or chemical methods, leading to the exposure of mitochondria to an unnatural or nonphysiological environment. If the mitochondria have been stressed or weakened, they may be further damaged during cellular fractionation and become leaking. In our study, although the CsA- and FK506-treated cells preserved cyt.c in mitochondria during ATP depletion as shown by immunofluorescence, their mitochondria had been weakened by ATP depletion and as a result, could be damaged to leak cyt.c during cellular fractionation. In contrast, in the immunofluorescence experiment, cells were fixed to preserve cyt.c subcellular localization for analysis.
In the present study, Drp1 dephosphorylation showed a general correlation with mitochondrial fragmentation, Bax accumulation, cyt.c release, and apoptosis following ATP depletion. However, the correlation is not complete. For example, CsA at 0.5 and 2 μM only had marginal effects on Drp1 dephosphorylation and Bax accumulation (Figs. 2 and 4), but it could significantly suppress apoptosis at these concentrations (Fig. 6B). Although the exact cause of the inconsistency remains to be determined, we speculate that, in addition to the intrinsic/mitochondrial pathway, tubular cell apoptosis may also involve other mechanisms that are sensitive to CsA inhibition. One such mechanism may be MPT, which can be effectively inhibited by CsA. We also noticed that low concentrations (0.1–1 μM) of FK506 could almost completely block Drp1 dephosphorylation (Fig. 2), but its effects on mitochondrial fragmentation and Bax accumulation were not as impressive (Figs. 3 and 4). The results suggest that mitochondrial events of apoptosis are not determined only by Drp1. This observation is consistent with the current understanding of the regulation of mitochondrial dynamics, which involves both fission and fusion and several critical regulatory proteins such as Fis1, Drp1, and mitofusins. Although 0.1–1 μM FK506 did not block mitochondrial fragmentation or Bax activation, it significantly prevented apoptosis (Fig. 6C). This result suggests that there are apoptotic events downstream of mitochondrial changes that are sensitive to FK506 inhibition. Apparently, further studies in these areas may gain new insights into the full picture of tubular cell apoptosis.
In addition to their effects on mitochondrial injury and apoptosis, CsA and FK506 also showed beneficial effects in long-term cell survival following ATP depletion injury (Fig. 6E). However, the prosurvival effects shown at late time points appeared much less profound than the acute cytoprotective effects of CsA and FK506 on mitochondria and apoptosis. One plausible explanation is that, besides its role in Drp1 dephosphorylation and mitochondrial injury, calcineurin may also contribute to cell recovery following acute injury and as a result, its inhibition may slow down the recovery or regeneration of RPTC cells. Should this be true, the effects of the calcineurin inhibitors are expected to be “Janus-faced”: protecting against acute injury but suppressing subsequent recovery. The sum result could then be a marginal improvement of long-term cell viability or number. Apparently, further investigation is needed to address this possibility.
In vivo, both CsA and FK506 are immunosuppressive and become nephrotoxic during prolonged use. The effects of these compounds on renal ischemia-reperfusion injury have also been studied, but the results are not consistent in the literature (12, 18, 22, 28, 30, 43, 44). Compared with cultured tubular cells, the in vivo situation of renal ischemia-reperfusion injury is much more complicated and involves changes of renal hemodynamics, inflammation, tubular injury, and recovery, and many other relevant factors or events. In terms of tubular injury and death, cells die in vivo in several forms including both apoptosis and necrosis. While our current results show some protective effects of CsA and FK506 against apoptosis in cultured tubular cells, whether and how these inhibitors may affect other renal injury events remain unclear. Also, these compounds may affect not only initial injury but also renal repair or regeneration. Whether calcineurin inhibitors can protect against or aggravate ischemic renal injury probably depends on the dosage of the inhibitors tested, the time and duration of the drug use, and the characteristics of the injury model such as injury severity.
In conclusion, this study demonstrated Drp1 dephosphorylation during ATP depletion in renal tubular cells. Drp1 dephosphorylation may involve calcineurin and appears to be a key regulatory event of mitochondrial fragmentation and outer membrane permeabilization during tubular cell apoptosis.
GRANTS
The study was supported in part by grants from the National Institutes of Health and Department of Veterans Affairs (VA). Z. Dong is a VA Research Career Scientist.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. C. Blackstone at the National Institutes of Health (National Institute of Neurological Disorders and Stroke) for the antibody against phospho-(serine-637)-Drp1.
REFERENCES
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle 6: 3043–3047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brooks C, Ketsawatsomkron P, Sui Y, Wang J, Wang CY, Yu FS, Dong Z. Acidic pH inhibits ATP depletion-induced tubular cell apoptosis by blocking caspase-9 activation in apoptosome. Am J Physiol Renal Physiol 289: F410–F419, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Brooks C, Wei Q, Cho S, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemia reperfusion injury. Transplantation 76: 50–54, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105: 15803–15808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 116: 205–219, 2004 [DOI] [PubMed] [Google Scholar]
- 12.De Greef KE, Ysebaert DK, Vercauteren SR, Chapelle T, Roeyen G, Bosmans JL, Verpooten GA, De Broe ME. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Transplant Proc 34: 791–794, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297: F749–F759, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Saikumar P, Griess GA, Weinberg JM, Venkatachalam MA. Intracellular Ca2+ thresholds that determine survival or death of energy-deprived cells. Am J Pathol 152: 231–240, 1998 [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J. Upregulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanisms. J Biol Chem 276: 18702–18709, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am J Pathol 163: 663–671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncalves GM, Cenedeze MA, Feitoza CQ, de Paula CB, Marques GD, Pinheiro HS, de Paula Antunes Teixeira V, Antonia dos Reis M, Pacheco-Silva A, Camara NO. The role of immunosuppressive drugs in aggravating renal ischemia and reperfusion injury. Transplant Proc 39: 417–420, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol 47: 117–141, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Inman SR, Davis NA, Olson KM, Lukaszek VA. Simvastatin attenuates renal ischemia/reperfusion injury in rats administered cyclosporine A. Am J Med Sci 326: 117–121, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int 66: 500–506, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kribben A, Wieder ED, Wetzels JF, Yu L, Gengaro PE, Burke TJ, Schrier RW. Evidence for role of cytosolic free calcium in hypoxia-induced proximal tubule injury. J Clin Invest 93: 1922–1929, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pabla N, Wei Q, Dong Z. Apoptosis in acute kidney injury. In: Essentials of Apoptosis: A Guide for Basic and Clinical Research, 2nd editon Clifton, NJ: Humana, 2009, p. 565–579 [Google Scholar]
- 27.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17: 3401–3415, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207: 339–347, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, Fujita T. Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 15: 2320–2333, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339–343, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline upregulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev 15: 2922–2933, 2001 [PubMed] [Google Scholar]
- 38.Wang Y, Knowlton AA, Christensen TG, Shih T, Borkan SC. Prior heat stress inhibits apoptosis in adenosine triphosphate-depleted renal tubular cells. Kidney Int 55: 2224–2235, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol 20: 1919–1928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei Q, Dong Z. Regulation and pathological role of bid in ischemic acute kidney injury. Ren Fail 29: 935–940, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest 87: 581–590, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang CW, Ahn HJ, Han HJ, Kim WY, Li C, Shin MJ, Kim SK, Park JH, Kim YS, Moon IS, Bang BK. Pharmacological preconditioning with low-dose cyclosporine or FK506 reduces subsequent ischemia/reperfusion injury in rat kidney. Transplantation 72: 1753–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Ysebaert DK, De Greef KE, Vercauteren SR, Verhulst A, Kockx M, Verpooten GA, De Broe ME. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int 64: 864–873, 2003 [DOI] [PubMed] [Google Scholar]