Abstract
Intrinsic stem cells (SC) participate in tissue remodeling and regeneration in various diseases and following toxic insults. Failure of tissue regeneration is in part attributed to lack of SC protection from toxic stress of noxious stimuli, thus prompting intense research efforts to develop strategies for SC protection and functional preservation for in vivo delivery. One strategy is creation of artificial SC niches in an attempt to mimic the requirements of endogenous SC niches by generating scaffolds with properties of extracellular matrix. Here, we investigated the use of hyaluronic acid (HA) hydrogels as an artificial SC niche and examined regenerative capabilities of encapsulated embryonic endothelial progenitor cells (eEPC) in three different in vivo models. Hydrogel-encapsulated eEPC demonstrated improved resistance to toxic insult (adriamycin) in vitro, thus prompting in vivo studies. Implantation of HA hydrogels containing eEPC to mice with adriamycin nephropathy or renal ischemia resulted in eEPC mobilization to injured kidneys (and to a lesser extent to the spleen) and improvement of renal function, which was equal or superior to adoptively transferred EPC by intravenous infusion. In mice with hindlimb ischemia, EPC encapsulated in HA hydrogels dramatically accelerated the recovery of collateral circulation with the efficacy superior to intravenous infusion of EPC. In conclusion, HA hydrogels protect eEPC against adriamycin cytotoxicity and implantation of eEPC encapsulated in HA hydrogels supports renal regeneration in ischemic and cytotoxic (adriamycin) nephropathy and neovascularization of ischemic hindlimb, thus establishing their functional competence and superior capabilities to deliver stem cells stored in and released from this bioartificial niche.
Keywords: neovascularization, adriamycin nephropathy, ischemic injury
intrinsic adult stem cells participate in tissue remodeling and regeneration in various chronic diseases and following noxious cardio- and/or nephrotoxic insults (30). Failure of tissue regeneration is in part attributed to the fact that stem cells are not entirely protected from cytotoxic or genotoxic stress of noxious stimuli. One of the striking examples of stem cell vulnerability is represented by the toxicity profile of a commonly used chemotherapeutic agent doxorubicin (adriamycin). This agent leads to severe cardiomyopathy and nephropathy due to induction of oxidative stress, and stem cells are equally affected (4, 7, 15). Similar vulnerability of stem cells has been reported in other chronic diseases, such as atherosclerosis, essential hypertension, preeclampsia, hyperglycemia, smoking, and type I and type II diabetes, to name a few (8, 10, 18, 20, 21, 24, 25, 31, 34, 35). Inexorable progression of these diseases may be in part explained by the reduced ability of stem cells to participate in tissue regeneration. The efficacy of stem cell transplantation in some disease states is the ultimate proof of the concept that stem cell incompetence developing in the course of chronic diseases and after exposure to noxious stimuli results in failed regeneration and progressive tissue degeneration (32).
Intense research efforts have been mounted to develop strategies for stem cell protection and functional preservation. One direction is based on creation of artificial stem cell niches. The concept of stem cell niche is 30 yr old (33) yet adult stem cell niches remain elusive. The best-studied niches are represented by those harboring germline stem cells, bone marrow hematopoietic stem cells, and stem cells of intestinal crypts, to name a few (27, 39). Consensus has been reached that the stem cell niche provides an umbrella microenvironment supporting cell attachment and quiescence by sheltering stem cells from proliferation and differentiation signals, enhances cell survival, regulates stem cell division and renewal, and coordinates the population of resident stem cells to meet the actual requirements of an organ (26). Creation of artificial stem cell niches represents an attempt to mimic some of these requirements by providing cells with a low-oxygen environment within avascular scaffolds, but ensuring their ability to preserve the phenotype, quiescence, recruitability, and protection from the noxious stimuli.
Endothelial progenitor cells (EPC) have been shown to participate in regenerative processes. Transplantation of EPC augments neovascularization of ischemic/infarcted myocardium, ischemic limbs, or brain (4, 42). EPC may play a critical role in the maintenance of integrity of vascular endothelium and in its repair after injury or inflammation (25). EPC are subjected to various stressors, similar to all somatic cells that could impair their competence (31). Hyperglycemia has been reported to reduce survival and impair function of circulating EPC (21). There is emerging evidence that senescence may serve as an important mechanism mediating EPC dysfunction. Decreased numbers and increased proportion of senescent EPC have been reported in patients with preeclampsia or hypertension (21). Angiotensin II can induce EPC senescence through the induction of oxidative stress and influence telomerase activity (34). Oxidized low-density lipoprotein induces EPC senescence and dysfunction (20). In addition, EPC dysfunction has been documented in type I and II diabetes, coronary artery disease, atherosclerosis, vasculitis with kidney involvement, and end-stage renal disease (5, 6, 19, 37, 38). Therefore, the goals of studies presented herein were to expand the knowledge on EPC behavior in hyaluronic acid (HA) hydrogels, examine the resistance of thus stored stem cells to noxious effects of adriamycin, the efficacy of EPC mobilization, and the competence of encapsulated endothelial progenitors to contribute to the postischemic organ repair and neovascularization.
MATERIALS AND METHODS
Cell culture.
A previously established line of mouse embryonic EPC (eEPC) was used for experiments (17). The eEPC were cultured in petri dishes coated with 0.1% gelatin (Sigma), in a medium composed of DMEM high glucose (Invitrogen), 20% FBS, 1% penicillin streptomycin, and 1% nonessential amino acids (17). Cells were labeled using Vybrant CFDA SE Cell Tracker Kit according to the manufacturer's instructions (Invitrogen). Tests were not performed to rule out the presence of mycoplasmal contamination before experiments here.
Animal models.
Balb/c mice received a tail vein injection of 10.2 mg/kg of adriamycin to induce nephropathy and were studied 3 wk later (15, 40). A separate group of FVB/NJ mice (Jackson Laboratories, Bar Harbor, ME) was subjected to acute renal ischemia by clamping the left (or both) renal artery with microserrefines for 30 min, followed by reperfusion until death at 36 h, as detailed previously (29). In a separate series of experiments, Sca-1/GFP+ transgenic mice (16) were used to determine whether stromal cell-derived growth factor-1 (SDF-1)-containing HA hydrogels implanted subcapsularly (via syringe injection) in ischemic kidneys could attract Sca-1/GFP+ cells from the ischemic parenchyma into the implanted gel. As controls, HA hydrogels without SDF-1 were also implanted subcapsularly. For Sca-1/GFP+ experiments, mice were subject to 30-min renal ischemia as previously described, and mice were killed 5 days later, and hydrogels were examined by fluorescence microscopy to determine whether Sca-1/GFP+ cells migrated into implanted hydrogels. In the third arm of the study, femoral artery ligation was performed on FVB/NJ mice and animals were studied at weekly intervals. In each group, animals were sham-treated and received intravenous injection of 5 × 105 EPC or an implantation of HA hydrogels with the equivalent number of EPC embedded. The animal study protocol was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Gel formation and degradation.
The hydrogels were prepared using a thiol-modified HA and a thiol-modified denatured collagen (Gelin-S). Both components were reconstituted in 1 ml of degassed/deionized water following the manufacturer's procedure (Glycosan Biosystems). ExtraLink, a thiol-reactive polyethylene glycol diacrylate cross-linker, was reconstituted following Glycosan's recommended procedure to achieve the intended gel stiffness. By varying Extralinker concentrations from 1 to 10%, we found that optimal concentration for cell viability was a ratio of 4:1, HyStem+Gelin-S:ExtraLink. Additional components, such as pronectin (Sigma) at a concentration of 50 μg/ml, SDF-1 at a concentration of 100 ng/ml (3), and eEPC, were incorporated into the gels before adding the ExtraLink, as detailed in results. Final gels were plated in either individual glass-bottom petri dishes or glass-bottom 24-well plates or were implanted into mice. For cell recovery, gels were digested using 300 U/ml collagenase and 100 U/ml hyaluronidase (Sigma).
For in vivo studies, HA hydrogels were prepared using 4% ExtraLink, 50 μg/ml pronectin, and 5 × 105 eEPC. EPC were fluorescently prelabeled for visualization and tracking. For implantation, 10 μl of hydrogel were aseptically injected into the mouse ear. As an additional control, 10 μl of hydrogel with encapsulated eEPC were implanted directly underneath the capsule of the kidney in the renal ischemia model; however, gel was not subsequently digested with collagenase/hyaluronidase enzymes.
Hydrogel ear injections.
Mice were anesthetized with 60 mg/kg ketamine and 6.6 mg/kg xylazine before injection. The ears were treated with depilatory solution (Nair), restrained to ensure a flat surface, and 10 μl of hydrogel loaded with fluorescently labeled (Cell Tracker, Invitrogen) eEPC (5 × 105 cells total) were injected into one or both ears. The ears were imaged over the next 4 days using intravital fluorescence microscopy. At a designated time point, the gels in the ears were injected with collagenase and hyaluronidase, as detailed above, to permit mobilization of engrafted eEPC. Cell density was estimated by averaging the number of cells per 10× field over the area covered by the hydrogel.
Live/dead assay.
Adriamycin (Sigma) was added to eEPC encapsulated in HA hydrogels with 4% ExtraLink and 50 μg/ml pronectin. Concentrations of 1, 10, 30, 50, and 100 μmol/l adriamycin were added to each well 24 h after cell plating and gel formation. Results were quantified via cell counts, cell size measurements, and a live/dead assay (Invitrogen). Cell counts and measurements were conducted on day 0, day 1 before and after administration of adriamycin, and on day 2. The live/dead assay was conducted on day 2. The LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen) was used to determine cell viaiblity of gel-encapsulated compared with surface-plated eEPC before and after administration of adriamycin. In our experiments, concentrations of 0.75 μmol/l calcein-AM and 0.25 μmol/l ethidium-1 provided optimal staining for eEPC.
Intravital monitoring of hydrogels implanted into the ear was performed as follows. Animals were anesthetized with a ketamine/xylazine solution as above before injection and placed on a thermal plate (Tokai Hit) on the stage of a microscope. The ear was immobilized and intravital fluorescence microscopy of the implanted hydrogel with fluorescently labeled eEPC was performed using a Nikon Y-FL epifluorescence intravital microscope (Nikon, Melville, NY) equipped with an intensified CoolSNAP HQ tube camera (Photometrics, Tucson, AZ), a filter wheel, and a beam splitter (Photometrics). Using objectives with magnification of ×10–20-40, eEPC were monitored within the hydrogels. Fluorescence was recorded and fluorescence intensity was quantified within hydrogels using MetaVue software (Universal Imaging, Downingtown, PA).
Laser Doppler flowmetry and imaging and detection of renal function.
Blood flow on ligated and contralateral hindlimbs was monitored weekly using laser Doppler imaging scanner and flow probes (Periflux System 5000, Kings Park, NY). To assess renal function in mice with acute ischemia and adriamycin nephropathy, serum creatinine concentration was measured using a creatinine assay kit (Abcam, Cambridge, MA) and urine protein/creatinine ratio was quantified using a Bradford Assay (Bio-Rad, Hercules, CA).
Flow cytometry analysis.
eEPC were used for analysis after propagation on the plastic surface, on the surface of the hydrogel, and encapsulated in it for 5 days. Cells were stained with FITC-conjugated rat anti-mouse antibodies for CD31 (BD Pharmingen) and CD34 (BD Pharmingen), PE-conjugated rat anti-mouse antibodies for Flk-1 (BD Pharmingen) and Sca-1 (BD Pharmingen), FITC-conjugated goat anti-mouse antibody for lectin (BD Pharmingen), and goat anti-rat antibody for von Willibrand factor (BD Pharmingen) with the secondary antibody for von Willibrand factor being FITC-conjugated rabbit anti-goat from Jackson ImmunoResearch (West Grove, PA). Primary antibodies were diluted 1:250 to 1:500, whereas secondary antibodies were diluted 1:2,000. For von Willibrand factor staining, cells were permeabolized with 0.5% Triton X-100. After being stained, cells were fixed with 4% paraformaldehyde and subjected to FACS analysis. Data were acquired using a FACScan cytometer equipped with a 488-nm argon laser and a 620-nm red diode laser and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). The setup of FACScan was performed using unstained and secondary antibody-stained cells.
Statistical analysis.
Data are means ± SE. For multiple comparisons between groups, a one-way ANOVA with Tukey's posttest was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Differences were considered significant at P < 0.05.
RESULTS
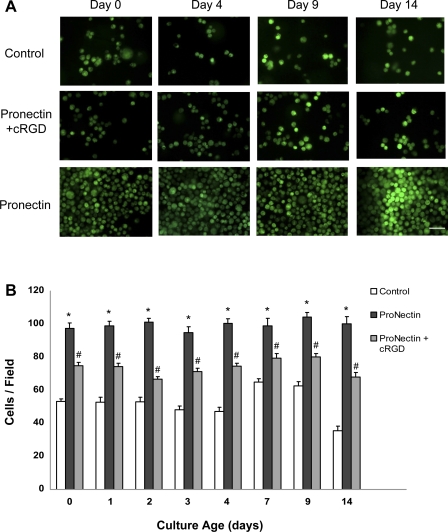
We first optimized conditions for eEPC cultured in 4% HA hydrogels. Since it has been demonstrated that poly-HA is a poor adhesive partner for EPC, we supplemented HA hydrogels with pronectin, a polymeric RGD peptide (9, 23). The use of pronectin in hydrogels showed improved preservation of eEPC in hydrogels, an effect that could be reversed by competition with cyclic RGD peptide (Fig. 1). Interestingly, without pronectin 55% of eEPC that were loaded in hydrogels were retained within the gel, while the addition of pronectin to hydrogels resulted in >95% retention of loaded eEPC in hydrogel (this includes hydrogels that were implanted into animals).
Fig. 1.
Improved engraftment, viability, and maintenance of embryonic endothelial progenitor cells (eEPC) in pronectin-coated hydrogels. A: images of eEPC encapsulated in hydrogels with and without pronectin and cRGD at ×40 magnification. Cells are stained with calcein to confirm viability. Note the improved density on hydrogels containing pronectin. Scale bar is 50 μm. B: quantification eEPC cultured in hydrogels containing pronectin demonstrated optimum cell viability, engraftment, and cell retention through 14 days. *P < 0.05 vs. pronectin + cRGD and control; #P < 0.05 vs. control; n = 4.
To determine whether hydrogel encapsulation altered the phenotype of eEPC or induced differentiation, encapsulated eEPC were analyzed for the expression of cell surface markers, including Flk-1, CD31, CD34, Sca-1, and Ulex Europeus lectin using FACS. In addition, the presence of von Willebrand factor was examined (typically present in eEPC). Comparison of eEPC before and 5 days after encapsulation in HA hydrogels showed equally robust staining with lectin and antibodies to von Willebrand factor, but remained low for Flk-1, CD31, CD34, and Sca-1, suggesting that the cells retained their nondifferentiated phenotype.
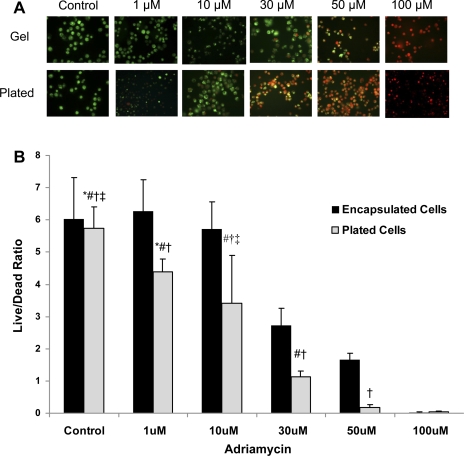
We next examined resistance of eEPC encapsulated in 4% HA hydrogels supplemented with pronectin to adriamycin. The molecular pharmacology profile of anthracycline antibiotic Adriamycin (Doxorubicin) includes cytotoxic and genotoxic effects that lead to the development of cardiomyopathy and nephropathy (4, 7, 15). We recently demonstrated that adriamycin is highly cytotoxic for EPC and that adoptive transfer of intact EPC has beneficial effects on the course of adriamycin-induced nephropathy (40). In addition, it has been demonstrated that stem cells maintained in three-dimensional (3D) matrices exhibit better survival, preservation of stemness, and proliferative capacity when cultured under hypoxic stress conditions (14). Therefore, we hypothesized that HA hydrogel encapsulation may confer protection of progenitor cells against cytotoxic and genotoxic adriamycin insult. In these studies, we compared the viability of eEPC encapsulated in 3D HA hydrogels and eEPC cultured on 2D hydrogel surface in response to adriamycin (Fig. 2). Results show superior tolerance of cytotoxic stress by eEPC encapsulated in 3D HA hydrogel. This indicates that hydrogel encapsulation provides protection to progenitor cells against cytotoxins, a finding that could have ramifications for antineoplastic therapies.
Fig. 2.
eEPC encapsulated in hydrogels show improved viability in response to cytotoxicity of adriamycin. A: images at ×40 magnification of eEPC cultured in either hydrogels (gels) or without hydrogel on normal cell culture plates (plated). After treatment in medium with adriamycin at concentrations of 1, 10, 30, 50, and 100 μmol/l for 24 h, eEPC were subject to a LIVE/DEAD assay in which live cells were stained green with calcein while dead cells were stained red with ethidium homodimer. Scale bar is 50 μm. B: quantification of cell viability of eEPC cultured in hydrogels vs. eEPC cultured without hydrogels on normal culture plates. With increasing adriamycin concentration, cell viability decreased particularly in eEPC cultured on plates unprotected by hydrogel from the cytotoxic effects of adriamycin. *P < 0.05 vs. 30 μmol/l plated cells; ‡P < 0.05 vs. 30 μmol/l encapsulated cells; #P < 0.05 vs. 50 μmol/l plated and encapsulated cells; †P < 0.05 vs. 100 μmol/l plated and encapsulated cells; n = 4.
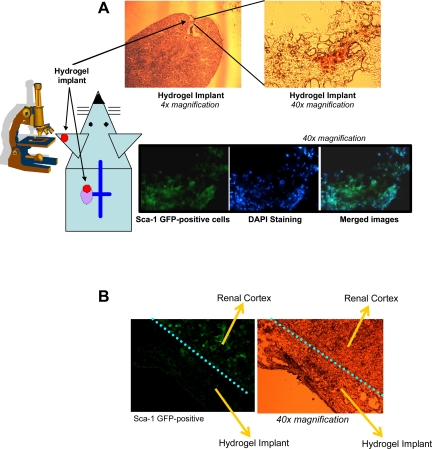
These in vitro findings prompted us to implement hydrogels as artificial stem cell carriers in an in vivo setting. Using these technical approaches, we first studied HA hydrogel engraftment by circulating stem cells (Fig. 3). HA hydrogels containing the stem cell chemoattractant SDF-1, but without encapsulated stem cells, were transplanted in Sca-1/GFP+ transgenic mice. HA hydrogels were implanted under the renal capsule of mouse kidneys that had been subjected to a 30-min ischemia followed by reperfusion. After 5 days, ischemic kidneys were removed and cryosectioned. In Sca-1/GFP+ mice, implanted hydrogels containing SDF-1, but not implanted hydrogels without SDF-1, were engrafted by GFP+ cells after induction of ischemic stress, as shown in Fig. 3. Engraftment of implanted hydrogels demonstrated the potential to serve as suitable microenvironment allowing for cell attraction and attachment, further highlighting the usefulness of the HA hydrogel as a means of a deliverable artificial stem cell niche.
Fig. 3.
Schematic representation of the experimental setup, in this particular case utilizing Sca-1/GFP+ transgenic mice and demonstrating engraftment of the kidney-implanted hydrogel by endogenous Sca-1/GFP+ cells native to the recipient kidney. A: hyaluronic acid (HA) hydrogels containing stromal cell-derived growth factor-1 (SDF-1) demonstrated Sca-1/GFP+ cell engraftment 5 days after renal ischemic insult. Top images illustrate bright-field images obtained after fixation and cryosectioning while bottom images illustrate the engraftment of the implanted hydrogels with fluorescent Sca-1/GFP+ cells. B: HA hydrogel renal implants that did not contain SDF-1 did not show engraftment of Sca-1/GFP+ cells 5 days after renal ischemia.
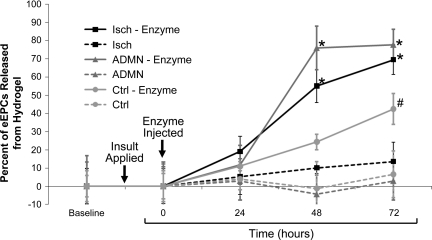
In the next series of experiments, we implanted HA hydrogel into a mouse ear, the site chosen because of the accessibility of fluorescently tagged eEPC encapsulated in hydrogels to intravital microscopy. Renal insult applied was either adriamycin nephropathy or acute renal injury (induced by 30-min renal artery occlusion). Enzymatic digestion of HA hydrogels was employed to facilitate release of encapsulated eEPC. Time lapse monitoring of fluorescently tagged encapsulated eEPC, as summarized in Fig. 4, showed that the number of fluorescently tagged eEPC decreased in ear-implanted hydrogels (only subjected to enzymatic digestion, but not without it) in both disease models studied. This confirms in vivo release of eEPC from ear-implanted hydrogels in control and, to a greater extent, in disease models.
Fig. 4.
Hydrogels loaded with fluorescently tagged eEPC injected into mouse ears show the progression of cell mobilization over time. Graphical image shows the percent increase in release of fluorescently tagged eEPC from hydrogels injected into the ears of 3 groups of mice, adriamycin-treated (ADMN), unilateral renal ischemia (Isch), and control (Ctrl). Digestive enzyme injection (hyaluronidase and collagenase) into HA hydrogels decreased the number of visible cells in the ear, while eEPC not subject to dissolution by enzyme were prone to remain in hydrogels. *P < 0.05 vs. 0 and 24 h; #P < 0.005 vs. 0 h; n = 4.
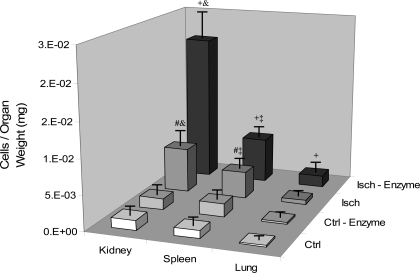
The next question that was addressed is: once eEPC are mobilized from hydrogels, are they competent to engraft injured organs? Analysis of internal organs revealed the appearance of mobilized fluorescently tagged eEPC primarily in the kidneys, lungs, and spleen (Fig. 5) of mice subjected to unilateral renal ischemia. In proportion to organ weight, ischemic kidneys demonstrated the greatest engraftment of eEPC mobilized from ear-implanted hydrogels during ischemic insult. Control organs demonstrated relatively little engraftment of mobilized eEPC, even after hydrogel digestion.
Fig. 5.
Location and quantification of the appearance of eEPC after delivery of fluorescently tagged eEPC delivered by either ear injection of hydrogel-encapsulated cells or intravenous injection with an equal number of cells. Subgroups of mice, Ctrl and Isch, were treated with enzyme (hyaluronidase and collagenase) to dissolve hydrogels thus releasing and mobilizing eEPC. Organs were sectioned and analyzed for engraftment of fluorescently labeled eEPC. Data are depicted as cells (eEPC) per mg of organ weight. Ischemic kidneys demonstrated the greatest engraftment of tagged eEPC. +P < 0.05 vs. Isch, Ctrl-Enzyme, Ctrl; #P < 0.05 vs. Ctrl-Enzyme, Ctrl; &P < 0.05 vs. spleen, lung; ‡P < 0.05 vs. lung; n = 6.
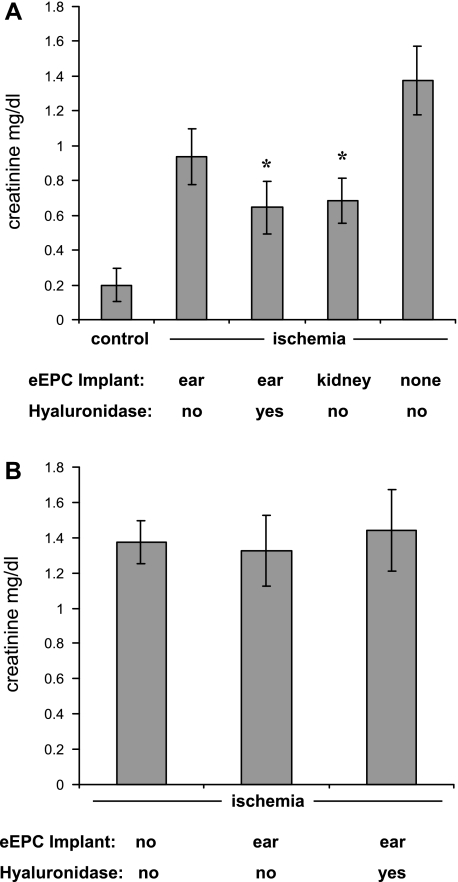
Since previous studies demonstrated that EPC transplantation improves the outcome of renal ischemia (29), we next sought to determine the competence of eEPC encapsulated in HA hydrogel implants in improving renal function after the injury. We analyzed the degree of renal dysfunction, as judged by the serum creatinine levels, in mice 36 h after induction of bilateral renal ischemia (Fig. 6A). Mice with implants subjected to eEPC release by enzymatic digestion demonstrated a significant degree of renoprotection. Curiously, this protective effect facilitated by enzymatic digestion of hydrogels was comparable to a nonenzymatic mobilization of eEPC encapsulated into HA hydrogels implanted subcapsularly. Furthermore, the renoprotective effect was absent when hydrogels were implanted without eEPC (Fig. 6B). These data argue in favor of the therapeutic efficacy of eEPC encapsulated in hydrogels.
Fig. 6.
Improvement of renal function 36 h after bilateral ischemia by eEPC encapsulated into HA hydrogels and implanted in the mouse ear (and treated with hyaluronidase) or implanted subcapsularly in the kidney. A: hydrogel implants containing encapsulated eEPC improved serum creatinine levels 36 h after bilateral ischemia. Hydrogel ear implants that were digested with hyaluronidase enzyme mixture (causing mobilization of eEPC) resulted in significantly improved serum creatinine levels and were statistically equivalent to serum creatinine levels that were observed when hydrogels with encapsulated eEPC were implanted directly under the renal capsule. B: when hydrogels were implanted in mouse ears without encapsulated eEPC (either with or without the addition of digestive enzymes), serum creatinine levels were not changed 36 h after bilateral ischemia. *P < 0.05 vs. ischemia alone; n = 5.
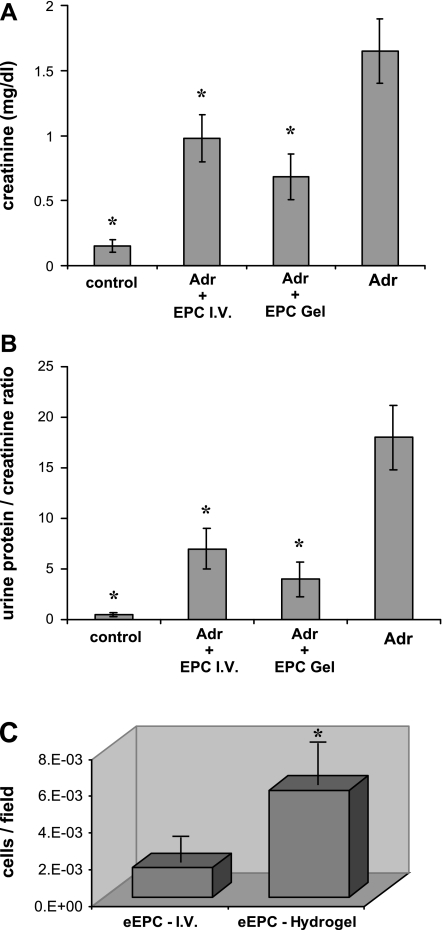
In a model of adriamycin-associated nephropathy (40), Balb/c mice were implanted with 5 × 105 EPC encapsulated in HA hydrogels and received intravenous (10.2 mg/kg body wt) injection of adriamycin. Four days later, HA hydrogels were digested and renal function was examined 3 wk postadriamycin. As controls, mice received intravenous infusion of an equivalent number of eEPC at the same time interval (4 days after adriamycin injection) that mice in the gel group received digestive enzyme to dissolve HA hydrogels. In both cases, eEPC reduced proteinuria and the severity of renal dysfunction (Fig. 7, A and B) and adoptive transfer appeared to be equipotent. Examination of kidneys from adriamycin-treated mice with implanted HA hydrogels revealed increased engraftment of CellTracker-tagged eEPC compared with adriamycin mice that received intravenous infusion of an equivalent number of eEPC (Fig. 7C).
Fig. 7.
Preservation of renal function by adoptive intravenous transfer of eEPC and encapsulated in HA hydrogels. Mice initially received HA hydrogel ear implants containing encapsulated eEPC and then were subsequently treated with adriamycin (Adr) to induce nephropathy, followed by injection of digestive enzymes to dissolve hydrogels and mobilize eEPC. As a control, eEPC were also injected intravenously. Serum creatinine and proteinuria were evaluated 3 wk after adriamycin treatment. eEPC delivered by hydrogels resulted in greatest reduction of serum creatinine (A) and urine albumin:creatinine ratio (B). C: subgroups of adriamycin-treated mice, given either hydrogel-encapsulated cells or intravenous (I.V.) injection of cells, were treated with enzyme (hyaluronidase and collagenase) to dissolve hydrogels thus releasing and mobilizing eEPC to kidneys. eEPC delivered by hydrogel demonstrated the greatest degree of engraftment into kidneys suffering from adriamycin nephropathy. Data are depicted as cells (eEPC) per mg of organ weight. *P < 0.05 vs. adriamycin (n = 6).
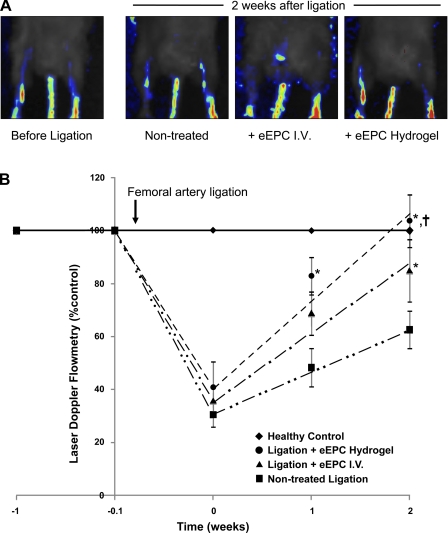
Next, we sought to investigate the competence of EPC encapsulated in HA hydrogels to stimulate neovascularization of the ischemic hindlimb after femoral artery ligation. eEPC (5 × 105) were implanted in the ear and hydrogels were enzymatically digested with collagenase and hyaluronidase 24 h after ligation. In controls, mice were either sham-treated or received intravenous infusion of the equivalent number of EPC. Laser Doppler flowmetry/imaging showed that the recovery of circulation occurred at the highest rate when mice received gel-encapsulated EPC (Fig. 8), with intravenous EPC infusion accelerating recovery of the blood flow at a slower rate.
Fig. 8.
Rate of blood flow recovery in control and EPC-treated mice (adoptive intravenous transfer and HA hydrogel encapsulated) after ligation of the femoral artery. A: representative laser Doppler images of hindlimbs before the ligation and 2 wk after the surgery in nontreated, intravenously treated, and HA hydrogel-delivered eEPC. Pseuodocolor scale from blue to red reflects changes of blood flow from minimum to maximum, respectively. B: summary of laser Doppler flowmetry weekly measurements in control and eEPC-treated mice (adoptive intravenous transfer and HA hydrogel encapsulated) after ligation of the femoral artery. Mice receiving HA hydrogel-delivered eEPC demonstrated the greatest rate of recovery. *P < 0.05 vs. nontreated control; †P < 0.05 between treated groups (n = 6 per group). Equations for best-fit lines are as follows: y = 16.041x + 31.099 (for nontreated ligation control), y = 24.618x + 38.487 (for ligation + eEPC IV), and y = 31.392x + 44.337 (for ligation + eEPC HA hydrogel).
DISCUSSION
Data presented herein provide evidence in support of the model system for bioartificial stem cell niche; HA hydrogel coated with pronectin shows the ability to better protect cells against cytotoxicity of adriamycin, to be populated by circulating Sca-1/GFP+ cells, and the ability of encapsulated eEPC to be recruited from the artificial niches (aided by the scaffold-dissolving enzymes). In three different models of vascular/parenchymal injury, EPC encapsulated in HA hydrogels exerted regenerative properties that were equal or superior to adoptive transfer of cells by intravenous infusion. These findings provide a definitive proof of principle favoring artificial scaffolds as a carrier for delivery of endothelial progenitors. Although HA hydrogels have been extensively used in the past, our data confer novel cytoprotective properties on this material.
The cornerstone idea behind artificial stem cell niches investigated thus far is in generation of scaffolds with properties of extracellular matrix (22). In this regard, chemically engineered synthetic hydrogels (as opposed to natural substances like fibrin or collagen) have been extensively studied based on their mechanical and structural properties emulating extracellular matrix. Among synthetic hydrogels, poly(ethylene oxide) diacrylate, alginate, polycaprolactone, oligo(poly-ethylene glycol) fumarate, poly(N-isopropylacrylamide), poly(hydroxyethyl methacrylate), and others, the use of HA-based hydrogels has received significant attention. It is a nonsulfated linear polysaccharide of (1-beta-4) d-glucuronic acid and (1-beta-3) N-acetyl-d-glucoasamine, which are major constituents of extracellular matrix during early embryogenesis, as well as in adult connective, epithelial, and neuronal tissue (36). HA hydrogels are capable of storing, propagating, and differentiating stem cells (11). Cross-linking HA hydrogels allows to manipulate their mechanical properties such as rigidity and elasticity (12), whereas application of hyaluronidase permits rapid dissolution of hydrogels. These cross-linked 3D scaffolds can be coated with various adhesion molecules and have been used to preserve stem cells for 3 wk (1).
The size of pores, which is a function of the degree of cross-linking, affects stem cell behavior: pores of 30–60 μm allow for a more rapid cell growth than pores of 60–130 μm (28). In our investigations, the optimal results were obtained using 4% crosslinker. The ability to incorporate stem cells, adhesion molecules, and factors responsible for the maintenance of quiescence or proliferation of these cells provides this bioartificial stem cell carrier/niche with substantial flexibility to enable regulation of stem cell fate.
Stem cell delivery for cell therapy is the subject of much investigation. Systemic administration of stem cells is associated with a low level of engraftment efficacy, usually below 10%, due to anoikis and insufficient targeting to and viability at the site of engraftment (41). An alternative strategy of injecting stem cells to the site of injury has been advocated; however, this approach may turn to be suboptimal as it is predicated on preexistence of a known injured tissue and, depending on the tissue, requiring at times nonroutine invasive procedures. In contrast, in vivo storage of stem cells in bioartificial niches could offer substantial benefits. Specifically, improved viability of encapsulated stem cells confronted with cytotoxic agents may represent a prospect for their preservation, whereas on-demand mobilization of stem cells using locally applied enzymatic digestion of the scaffold should ensure their timely liberation. The fact that thus mobilized cells remain functionally competent represents the ultimate proof of the validity of the bioartificial niche approach. Recent demonstration of the efficacy of mesenchymal stem cells encapsulated in alginate-containing scaffolds in providing hypoimmunogenic platform for long-term therapy further advances this argument (13).
In summary, the results provide evidence in support of the model system for artificial stem cell carrier niches; HA hydrogels coated with pronectin demonstrated protection from cytotoxic and genotoxic insults and showed the ability of these scaffolds to be populated by circulating stem cells (Sca-1/GFP+ in the presence of SDF-1), the ability of encapsulated eEPC to be recruited from the artificial niches (aided by the scaffold-dissolving enzymes), mobilization from the bioartificial niche to injured sites, the improvement of renal function in ischemic and nephrotoxic models of kidney injury, as well as in revascularization of an ischemic limb by the EPC encapsulated in an HA hydrogel artificial niche.
GRANTS
These studies were supported in part by National Institutes of Health Grants HL-083958 (A. K. Hatzopoulos), DK-052783, DK-45462, and DK-054602 (M. S. Goligorsky) and the Westchester Artificial Kidney Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The writers thank G. Prestwich at Glycosan for valuable insight regarding the use of hydrogels.
REFERENCES
- 1.Abraham S, Eroshenko N, Rao R. Role of bioinspired polymers in determination of pluripotent stem cell fate. Regen Med 4: 561–578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bladergroen B, Siebum B, Siebers-Vermeulen K, Van Kuppevelt T, Poot A, Feijen J, Figdor C, Torensma R. In vivo recruitment of hematopoietic cells using stromal cell-derived factor-1 alpha-loaded heparinized three-dimensional collagen scaffolds. Tissue Eng Part A 15: 1591–1599, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Burke J, Laucius J, Brodovsky H, Soriano R. Doxorubicin hydrochloride-associated renal failure. Arch Intern Med 137: 385–388, 1977 [PubMed] [Google Scholar]
- 5.Chan CT, Li SH, Verma S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol 289: F679–F684, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 24: 1246–1252, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Couture L, Nash J, Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol Rev 58: 244–258, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 45: 1449–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Franke K, Pompe T, Bornhauser M, Werner C. Engineered matrix coatings to modulate the adhesion of CD133+ human hematopoietic progenitor cells. Biomaterials 28: 836–843, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gennaro G, Menard C, Michaud SE, Deblois D, Rivard A. Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation 110: 3367–3371, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gerecht S, Burdick J, Ferreira L, Townsend A, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA 104: 11298–11303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren X, Rafailovich M, Clark R. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28: 671–679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J 24: 22–31, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Grayson W, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 207: 331–339, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Guest I, Uetrecht J. Drugs toxic to the bone marrow that target the stromal cells. Immunopharmacology 46: 103–112, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Hanson P, Mathews V, Marrus SH, Graubert TA. Enhanced green fluorescent protein targeted to the Sca-1 (Ly-6A) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp Hematol 31: 159–167, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hatzopoulos A, Folkman J, Vasile E, Eiselen G, Rosenberg R. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 125: 1457–1468, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Holmen C, Elsheikh E, Stenvinkel P, Qureshi AR, Pettersson E, Jalkanen S, Sumitran-Holgersson S. Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. J Am Soc Nephrol 16: 3110–3120, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens 23: 1831–1837, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Krankel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, Schuler G, Hambrecht R. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol 25: 698–703, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Leach K. Multifunctional cell-instructive materials for tissue regeneration. Regen Med 1: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Chung E, Rodriguez R, Firpo M, Healy K. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A79: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541–1544, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Moore K, Lemischka I. Stem cells and their niches. Science 311: 1880–1885, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Morrison S, Spradling A. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang A, Ng R, Yang S. Long-term culturing of undifferentiated embryonic stem cells in conditioned media and 3-dimensional fibrous matrices without extracellular matrix coating. Stem Cell 25: 447–454, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Patschan D, Krupincza K, Patschan S, Hamby C, Zhang Z, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after renal ischemia and ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Povsic T, Goldschmidt-Clermont P. Endothelial progenitor cells: markers of vascular reparative capacity. Ther Adv Cardiovasc Dis 2: 199–213, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 108: 457–463, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 99: 42–52, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Schofield R. The relationship between the spleen colony-forming cell and the hematopoietic stem cell. Blood Cells 4: 7–25, 1978 [PubMed] [Google Scholar]
- 34.Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, Yaegashi N, Okamura K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab 90: 5329–5332, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Toole B. Hyaluronan in morphogenesis. J Intern Med 242: 35–40, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development 134: 2001–2006, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Yasuda K, Park HC, Ratliff B, Addabbo F, Hatzopoulos AK, Chander P, Goligorsky MS. Adriamycin nephropathy–a failure of endothelial progenitor cell-induced repair. Am J Pathol 176: 1685–1695, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young P, Vaughan D, Hatzopoulos A. Biological properties of endothelial progenitor cells and their potential for cell therapy. Progr Cardiovasc Dis 49: 421–429, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res 90: 284–288, 2002 [DOI] [PubMed] [Google Scholar]