Abstract
The mRen(2).Lewis (mRen2) strain is an ANG II-dependent model of hypertension expressing marked sex differences in blood pressure and tissue injury that also exhibits estrogen and salt sensitivity. Because estrogen and salt influence angiotensinogen (AGT), circulating and renal expression of the protein were assessed in the mRen2 using a sensitive and specific ELISA. Hemizygous female and male mRen2 were placed on normal (1% NaCl, NS)- or high (8% NaCl, HS)-salt diets from 5 to 15 wk of age while a separate NS cohort was ovariectomized (OVX). The OVX mRen2 exhibited higher blood pressure (184 ± 6 vs. 149 ± 5 mmHg, n = 6), a 16-fold increase in urinary AGT (uAGT) (0.2 ± 0.02 vs. 0.01 ± 0.01 μg·kg−1·day−1, P < 0.01), but no change in proteinuria (PROT). Excretion of AGT was correlated with blood pressure and PROT in the female groups. The HS diet led to higher blood pressure (224 ± 8 mmHg), a 180-fold increase in uAGT (1.8 ± 0.2 μg·kg−1·day−1), and increased PROT (98 ± 9 vs. 7 ± 1 mg·kg−1·day−1). Compared with females, NS males expressed higher excretion of uAGT (3.0 ± 0.4 μg·kg−1·day−1) and PROT (32 ± 5 mg·kg−1·day−1); both were increased eightfold with HS (uAGT: 23 ± 3 μg·kg−1·day−1; PROT: 285 ± 28 mg·kg−1·day−1) without a change in blood pressure. Although uAGT was markedly higher in the OVX and HS groups, neither renal cortical AGT mRNA or protein expression was increased. Moreover, AGT release in cortical slices was similar for the NS and HS females. We conclude that the increase in uAGT with estrogen depletion or HS likely may be a biomarker for glomerular damage reflecting filtration of the circulating protein in the mRen2.
Keywords: angiotensin II, normal salt diet, high-salt diet
the congenic mRen(2).Lewis rat is a hypertensive strain that exhibits marked sex differences in blood pressure, cardiac function, and renal injury (7). The basis for male-female differences in the mRen(2).Lewis and other experimental models of hypertension likely reflects, in part, the influence of sex steroids on the renin-angiotensin-aldosterone system (RAAS) (4, 13, 27). ANG II levels are higher in the circulation and the kidney of the male mRen(2).Lewis compared with the female congenics, as well as male and female Lewis rats, the normotensive control strain (24). The female mRen(2).Lewis still maintain higher levels of circulating ANG II than the Lewis rats, but exhibit lower renal ANG-(1–7), suggesting an imbalance in the ANG II to ANG-(1–7) axis within the kidney. Ovariectomy alone increases the degree of hypertension associated with increased circulating and renal ANG II, as well as diastolic dysfunction, cardiac fibrosis, and higher levels of aldosterone (6, 14). Moreover, ovariectomy exacerbates the salt-sensitive increases in blood pressure and proteinuria in the female congenics (8). Estrogen (17β-estradiol) replacement in the ovariectomized (OVX) congenic reverses increases in blood pressure and deleterious alterations in the RAAS (6).
Although the protective effects of estrogen may encompass downregulation of the AT1 receptor and angiotensin-converting enzyme, androgens may exacerbate hypertension and tissue injury through stimulation of the ANG II-AT1 receptor arm of the RAAS (2). Depletion of androgens by orchidectomy or testosterone receptor antagonism reduces blood pressure and renal injury in the spontaneously hypertensive rat (SHR), Dahl salt-sensitive (Dahl S), and mRen(2)27.Sprague Dawley strains (3, 9, 11, 30). Indeed, a key target for the stimulatory actions of androgen is the precursor protein angiotensinogen (9, 11). Yanes et al. (30) recently demonstrated that orchidectomy reduced salt-sensitive hypertension in male Dahl S rats and the salt-dependent increase in renal angiotensinogen. However, experimental and clinical studies suggest that estrogen also stimulates angiotensinogen synthesis, which may mitigate the beneficial actions of the steroid on the cardiovascular system (4, 15). In the current study, we tested the hypothesis as to whether ovariectomy or a high-salt diet alters angiotensinogen expression in the ANG II-dependent hypertensive mRen(2).Lewis. We used a highly sensitive and specific angiotensinogen assay recently developed by Kobori and colleagues (16) to measure both circulating and urinary levels of the protein, as well as assess the release of the protein from the kidney.
MATERIALS AND METHODS
Animals.
Hemizygous male (n = 12) and female (n = 18) mRen(2).Lewis rats at 4 wk of age were obtained from the Hypertension and Vascular Research Center Congenic colony of Wake Forest University. Rats were housed in an American Association of Laboratory Animal Care approved facility in a temperature-controlled room (22 ± 2°C) with a 12:12-h light-dark cycle (lights on 6:00 A.M. to 6:00 P.M.) and allowed free access to food and water. A bilateral ovariectomy (OVX) was performed on a separate cohort of females (n = 6) under 4% isoflurane. At 5 wk of age, male (n = 6) and intact female (n = 6) animals remained on a 1% [normal salt (NS); Purina Mills Richmond, VA] or started a 8% [high salt (HS); Harlan TEKLAB, Madison, WI] sodium diet for an additional 10 wk. Rats were housed in metabolic cages (Harvard Bioscience, South Natick, MA) for 24-h assessment of food and water intake and urine collection. Systolic blood pressure (SBP) was measured in trained rats (mean of 5 determinations/data point) with an automated tail cuff system (Narco Bio-systems, Houston, TX) under mild restraint. At the end of the study, animals were euthanized, and the trunk blood and tissues were collected as previously described (24). These procedures were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee.
Western blot (immunoblot analysis).
Renal cortical and urinary angiotensinogen were assessed by immunoblot with an antibody directed against an epitope on the NH2-terminus region of the protein (residues 44–56) which detects both ANG I-containing and des-ANG I forms of the protein. The antibody was produced in rabbits by coupling the COOH-terminus of Gln-Leu-Glu-Asp-Pro-Ser-Val-Glu-Thr-Leu-Pro-Thr-he-Glu-Val-Pro-[Cys] via an added Cys residue to keyhole limpet hemocyanin. Kidney cortical homogenates were prepared as previously described (24). The soluble fractions (25 μg protein), plasma (0.38 μl), or urine (50 μl neat or concentrated) were then separated by gel electrophoresis and transferred to polyvinylidene difluoride membranes. Densities of the 55- and 60-kDa immunoreactive bands among experimental groups were determined by an IMCID imaging system; the results were expressed as arbitrary units of intensity and normalized to elongation factor-1α (24).
Angiotensinogen ELISA.
Plasma and urinary concentrations of angiotensinogen were measured with a total angiotensinogen assay kit (Immuno Biological Laboratories, Minneapolis, MN). The ELISA uses two specific antibodies for the capture and detection of the protein. The capture antibody is directed against the NH2-terminal region [amino acids (AA): 135–150] of rat angiotensinogen, and the detection antibody recognizes the COOH-terminal sequence (AA: 405–420). The minimum detectable level of the angiotensinogen assay is 10 pg/ml of sample. Both plasma and urinary samples were initially diluted in the ELISA buffer before the assay.
RNA isolation and real-time RT-PCR.
Cortical tissues were analyzed using real-time PCR as previously described (12). All reactions were performed in triplicate, and 18S ribosomal RNA served as an internal control.
Angiotensinogen release.
Kidneys were removed from NS and HS female congenic rats (n = 3/group) at 15 wk of age. Two 2-mm transverse sections (100 mg/section) were cut using a tissue block at the midline of each kidney and immediately transferred to a vial containing 3 ml of oxygenated Krebs buffer maintained at 37°C. A 1.5-ml sample of the kidney perfusate was collected at 30 and 60 min and frozen at −80°C. The samples were thawed, concentrated 10-fold on a 5-kDa cut-off filter (Millipore, Bedford, MA), diluted in the ELISA buffer, and analyzed for angiotensinogen content. The release data were expressed as picograms angiotensinogen per minute of incubation per milligram weight of cortical tissue (pg/min/mg).
Urine analysis.
Urine was collected over a 24-h period in metabolic cages, and urinary concentrations of ANG II and ANG-(1–7) were determined by RIA as previously described (29). An additional urine sample was collected for angiotensinogen, creatinine, and protein over a 24-h period and stored at −20°C. Serum and urinary creatinines were measured by a Quantichrom Creatinine Assay Kit (Bioassay Systems, Hayward, CA). Urinary protein was measured by a Bradford protein assay (Bio-Rad, Hercules, CA).
Statistical analyses.
All measurements are expressed as means ± SE. Comparisons between the NS intact, NS OVX, and HS intact female rats were evaluated by ANOVA and the Tukey's multicomparison test post hoc analysis. All other data were analyzed by the Student's unpaired t-test, and Figs. 1–7 were constructed with GraphPad Prism V plotting and statistical software. A probability value of <0.05 was required for statistical significance.
Fig. 1.
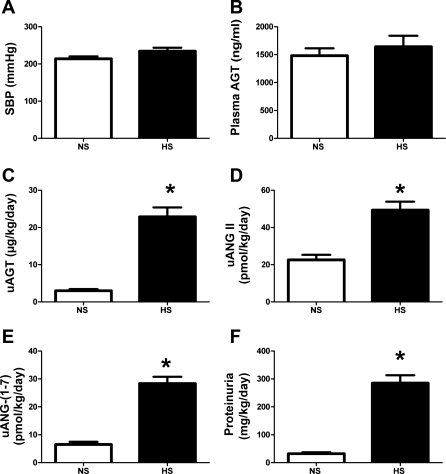
Influence of a high-salt (HS) diet on blood pressure, angiotensinogen levels, and other parameters in 15-wk-old male mRen(2).Lewis. A: systolic blood pressure (SBP) in conscious male mRen(2).Lewis congenic rats receiving either normal salt (NS, 1%) or HS (8%) diet were similar as measured by tail cuff plethysmography. B: plasma angiotensinogen levels (ng/ml) were not changed by the HS diet as determined by ELISA. C: urinary angiotensinogen (uAGT; μg·kg−1·day−1) was significantly increased by the HS diet at 15 wk. D: urinary ANG II (uANG II) peptide levels were increased with the HS diet. E: urinary ANG-(1–7) [uANG-(1–7)] levels increased with HS. F: proteinuria was increased with HS. Values are means ± SE; n = 6 rats/group. *P < 0.05 vs. NS males.
Fig. 2.
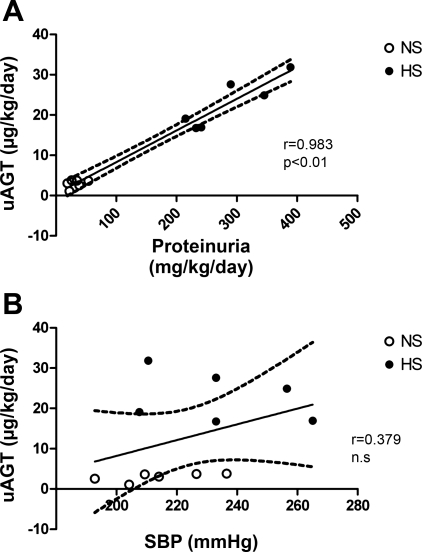
Urinary angiotensinogen associates with proteinuria but not SBP in NS- and HS-fed male mRen(2).Lewis. A: regression analysis of male uAGT and proteinuria. B: regression analysis of male uAGT and SBP. NS, not significant.
Fig. 3.
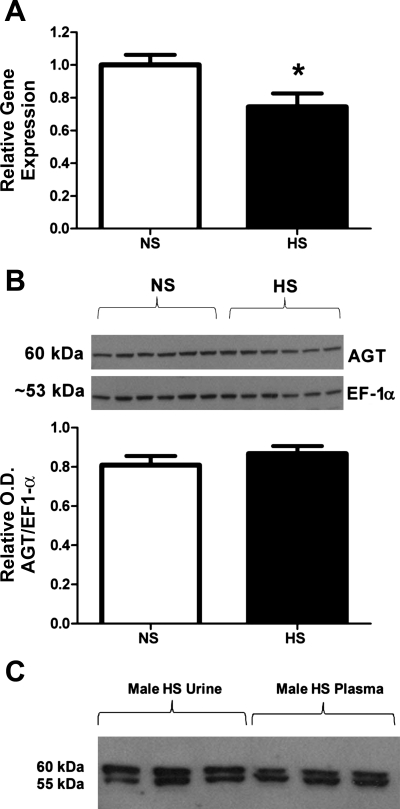
Influence of a HS diet on angiotensinogen expression in the renal cortex of 15-wk-old male mRen(2).Lewis. A: angiotensinogen mRNA levels, measured by real-time RT-PCR and expressed as a ratio of 18S ribosomal subunit, were lower in the HS vs. NS group diets (n = 6; *P < 0.01). B: angiotensinogen protein expression was not changed by the HS diet. Protein density was determined by immunoblot of the soluble fractions from the renal cortex using an NH2-terminally directed angiotensinogen antibody and an antibody to elongation factor-1α (EF-1α) to normalize protein content (n = 6/group). OD, optical density. C: immunoblots of plasma and urinary angiotensinogen in HS male mRen(2).Lewis reveal bands at 60 and 55 kDa in both urine and plasma samples.
Fig. 4.
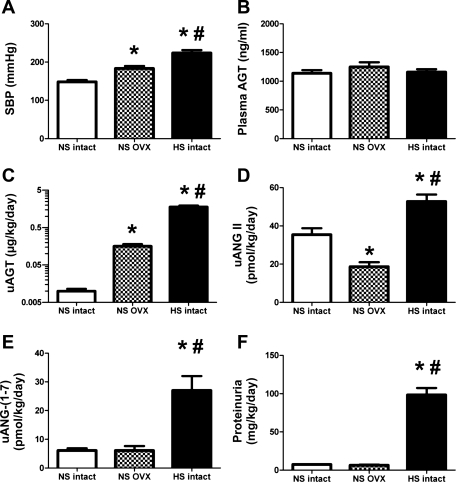
Influence of estrogen depletion or a HS diet on blood pressure, angiotensinogen levels, and other parameters in 15-wk-old female mRen(2).Lewis. A: SBP in conscious female mRen(2).Lewis congenic rats measured by tail cuff plethysmography were higher in the ovariectomized (OVX) and HS groups compared with intact NS mRen(2).Lewis. B: plasma angiotensinogen levels (ng/ml) were similar among the NS, OVX, and HS groups. C: uAGT (μg·kg−1·day−1) was significantly higher in the OVX and HS groups. D: uANG II peptide levels were increased with the HS diet. E: uANG-(1–7) levels increased with HS. F: proteinuria was increased with HS. Values are means ± SE; n = 6/group. P < 0.01 vs. NS intact (*) and vs. NS OVX (#).
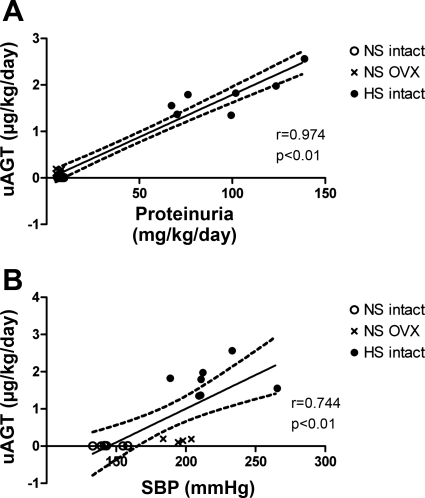
Fig. 5.
Urinary angiotensinogen associates with blood pressure and proteinuria in female mRen(2).Lewis. A: regression analysis of female uAGT and proteinuria. B: regression analysis of female uAGT and SBP.
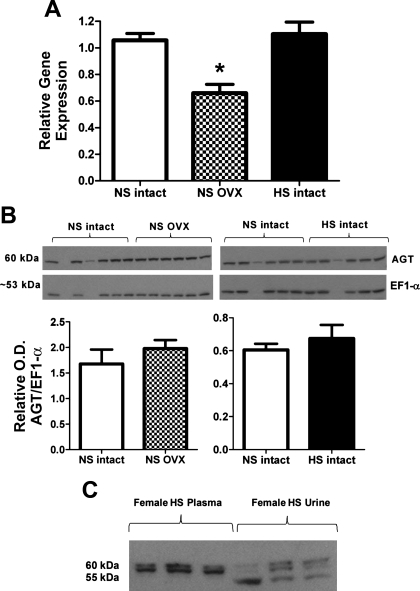
Fig. 6.
Influence of estrogen depletion or a HS diet on angiotensinogen expression in the renal cortex of 15-wk-old female mRen(2).Lewis. A: angiotensinogen mRNA levels measured by real-time RT-PCR and expressed as a ratio of 18S ribosomal subunit were lower in the OVX group (*P < 0.01 vs. NS and HS intact; n = 6/group). B: angiotensinogen protein expression was not changed by OVX or the HS diet. Protein density was determined by immunoblot of the soluble fractions from the renal cortex using an NH2-terminally directed angiotensinogen antibody and an antibody to EF-1α to normalize protein content (n = 5/group). C: immunoblot of plasma and urinary angiotensinogen in HS intact female mRen(2).Lewis rats.
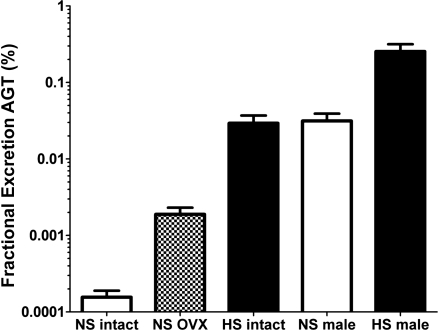
Fig. 7.
Fractional excretion of angiotensinogen in the female and male mRen(2).Lewis. Both OVX and the HS diet increased fractional angiotensinogen excretion although the overall values were <0.05% for females and 0.3% for males.
RESULTS
Male mRen(2).Lewis.
Maintenance of the male mRen(2).Lewis on the HS diet for 10 wk did not significantly influence SBP (214 ± 6 vs. 234 ± 10 mmHg; Fig. 1A) or plasma angiotensinogen levels (1,482 ± 132 vs. 1,642 ± 197 ng/ml; Fig. 1B). However, the urinary excretion of angiotensinogen was significantly higher in the HS males compared with the NS diet males (23 ± 2.5 vs. 3.0 ± 0.4 μg·kg−1·day−1, P < 0.01; Fig. 1C and Table 1). Consistent with the increase in angiotensinogen, the urinary levels of both ANG II (49.3 ± 4.6 vs. 22.6 ± 2.7 pmol·kg−1·day−1, P < 0.01) and ANG-(1–7) (28.4 ± 2.4 vs. 6.5 ± 1.0 pmol·kg−1·day−1; P < 0.01) were significantly higher in the HS-fed males (Fig. 1, D and E). Moreover, the urinary levels of ANG II were higher than ANG-(1–7) for both the NS (4-fold) and HS (2-fold) diets (Fig. 1, D and E). Proteinuria increased approximately ninefold with the HS diet (285 ± 28 vs. 32 ± 5 mg·kg−1·day−1, P < 0.01; Fig. 1F and Table 1). Angiotensinogen excretion was highly associated with proteinuria; however, the increase in urinary angiotensinogen with the HS diet did not correlate with systolic blood pressure (Fig. 2, A and B). Because these data suggest that the HS diet may stimulate renal angiotensinogen, we next assessed the mRNA and protein expression in the renal cortex. In contrast to urinary content, the mRNA levels for cortical angiotensinogen were lower in the HS-fed group compared with those maintained on the NS diet (Fig. 3A). Moreover, protein expression as determined by Western blot did not reveal tissue differences in angiotensinogen between the HS and NS male congenics (Fig. 3B). The immunoblot for cortical angiotensinogen revealed a single band at 60 kDa consistent with the molecular size of the protein reported in the kidney of the Dahl S strain (30). Finally, we assessed the molecular forms of angiotensinogen in the plasma and urine of the HS males by immunoblot. As shown in Fig. 3C, the gel reveals two predominant immunoreactive bands at 60 and 55 kDa for both the plasma and urine samples.
Table 1.
Magnitude (fold) change in response to ovariectomy or high-salt diet in mRen2.Lewis rats
| OVX to NS Females | HS to NS Intact Females | HS to NS Intact Males | |
|---|---|---|---|
| AGT excretion | 16 | 178* | 7.7† |
| Proteinuria | 0.8 | 12* | 8.9* |
| ANG II excretion | 0.5 | 1.4 | 2.8* |
| ANG-(1–7) excretion | 1.0 | 3.6* | 4.4* |
AGT, angiotensinogen; NS, normal salt diet; HS, high-salt diet; OVX, ovariectomy.
P < 0.05, vs. OVX to NS females (*) and vs. HS to NS intact females (†)
Female mRen(2).Lewis.
We then assessed the influence of the HS diet or ovariectomy on expression of angiotensinogen in the female mRen(2).Lewis. In contrast to the male mRen(2).Lewis, HS intake markedly increased blood pressure in the intact females (224 ± 8 vs. 149 ± 5 mmHg, P < 0.01; Fig. 4A). OVX mRen(2).Lewis females maintained on a NS diet also exhibited significantly higher blood pressure compared with intact females (184 ± 6 vs. 149 ± 5 mmHg; Fig. 4A). Plasma angiotensinogen levels were not influenced by diet or ovariectomy (Fig. 4B); however, angiotensinogen excretion increased 180-fold (1.8 ± 0.2 vs. 0.01 ± 0.001 μg·kg−1·day−1, P < 0.01; Fig. 4C and Table 1) with the HS diet and 16-fold (Table 1) following early ovariectomy (0.2 ± 0.02 vs. 0.01 ± 0.001 μg·kg−1·day−1, P < 0.01; Fig. 4C). The magnitude of change in urinary angiotensinogen observed in the HS females was significantly greater than that in HS males. The HS diet also increased the excretion of both ANG II (52.8 ± 3.6 vs. 35.5 ± 3.3 pmol·kg−1·day−1, P < 0.01; Fig. 4D) and ANG-(1–7) (27.0 ± 5.0 vs. 6.1 ± 0.8, pmol·kg−1·day−1, P < 0.01; Fig. 4E). Moreover, the urinary content of ANG II was higher than ANG-(1–7) in the intact mRen(2).Lewis on the NS (6-fold) and HS (2-fold) diets. Despite the increase in urinary angiotensinogen in the NS OVX females, ANG-(1–7) urinary levels were similar to the intact NS group, whereas ANG II levels were significantly less than both NS and HS intact (18.7 ± 2.4 vs. 35.5 ± 3.3 and 52.8 ± 3.6 pmol·kg−1·day−1, P < 0.01; Fig. 4, D and E). Protein excretion was 12-fold (Table 1) higher in the HS females compared with either the NS intact (98.3 ± 9.1 vs. 7.4 ± 0.6 mg·kg−1·day−1, P < 0.01) or the NS OVX (6.5 ± 0.8 mg·kg−1·day−1, P < 0.01; Fig. 4F) group; however, proteinuria was not different between the NS intact and NS OVX females. Similar to the males, the female data exhibited a high correlation between urinary angiotensinogen and protein excretion (r = 0.974, P < 0.01; Fig. 5A). Moreover, blood pressure and angiotensinogen excretion were correlated in the mRen(2).Lewis females (r = 0.744, P < 0.01; Fig. 5B). Because HS and ovariectomy enhanced angiotensinogen excretion to a greater extent than proteinuria, we determined whether angiotensinogen expression was induced in the kidney. The mRNA levels of angiotensinogen in the renal cortex were similar between the NS and HS groups but significantly lower in the NS OVX females (Fig. 6A). Protein expression for cortical angiotensinogen was not changed with the HS diet or following ovariectomy (Fig. 6B). It is possible that the HS diet stimulates the release of angiotensinogen in the tubular fluid, which may account for the greater excretion of the precursor. To address this possibility, we determined the secretion rate of angiotensinogen from cortical slices of intact NS and HS females. Although angiotensinogen was detectable in the perfusate by ELISA, we found no difference in protein release between the NS and HS groups (1.2 ± 0.2 vs. 1.3 ± 0.1 pg/min/mg, n = 3/group). We also assessed the molecular forms of angiotensinogen in the circulation and urine obtained from the NS and HS females by immunoblot. As observed in the males, we found two bands for angiotensinogen in the plasma and urine fractions migrating at 60 and 55 kDa, as well as an additional immunoreactive band at 50 kDa that was present in the female urine samples (Fig. 6C).
Finally, we compared the fractional excretion of angiotensinogen excretion in the mRen(2).Lewis groups based on the creatinine estimation of glomerular filtraion rate (Fig. 7). Fractional angiotensinogen excretion amounted to 0.0002% in the NS female but increased to 0.002% in the OVX group and 0.03% in the HS groups. Fractional excretion in NS males was 0.031% and increased to 0.25% with the HS diet.
DISCUSSION
The congenic mRen(2).Lewis rat is an ANG II-dependent model of hypertension that exhibits sex differences in blood pressure, renal injury, and expression of different components of the RAAS (24). The female congenics also show marked blood pressure sensitivity to ovariectomy and increased salt intake (7). The present study investigated whether alterations in the precursor protein angiotensinogen were associated with salt sensitivity in the male and female mRen(2).Lewis, as well as estrogen sensitivity in the females. We used a highly sensitive double-antibody-based ELISA originally developed by Kobori et al. (16) to detect circulating and urinary levels of angiotensinogen, in addition to Western blot for tissue content and to assess the molecular forms of the protein (24). In this study, both male and female mRen(2).Lewis exhibit increased urinary excretion of angiotensinogen and greater proteinuria in response to the HS diet. Females exhibited a salt-dependent increase in systolic blood pressure. Associated with the salt-induced increase in angiotensinogen excretion, males and females exhibited higher urinary levels of ANG II and ANG-(1–7). Ovariectomy of females on the NS diet also increased urinary levels of angiotensinogen, but did not result in an increase in the excretion of ANG II or ANG-(1–7) nor was proteinuria significantly changed. Despite the increase in urinary angiotensinogen with the HS diet or ovariectomy, we did not find an associated increase in renal tissue angiotensinogen. Indeed, ovariectomy or salt loading in the males resulted in a significant decline in angiotensinogen gene expression in the renal cortex. Moreover, we found no difference in the rate of angiotensinogen release ex vivo from cortical tissue slices of NS and HS females. In addition to the similar molecular forms of angiotensinogen in the plasma and urine, our data suggest that increased angiotensinogen excretion in response to salt or ovariectomy likely reflects the enhanced filtration of circulating angiotensinogen rather than an increase in intrarenal synthesis and release in the mRen(2).Lewis hypertensive strain.
The kidney plays a key role in the control of blood pressure, and various cellular elements of the kidney are targets of the circulating RAAS. However, accumulating evidence suggests that the intrarenal RAAS also contributes to the renal regulation of blood pressure (17, 23). Overexpression of angiotensinogen within the proximal tubules increases blood pressure, oxidative stress, and proteinuria but does not lead to higher levels of circulating ANG II (10, 18, 25). Moreover, increased angiotensinogen expression exacerbates the extent of tubular damage and cellular atrophy in streptozotocin-induced diabetes (19, 20). Indeed, several groups demonstrate that testosterone-dependent stimulation of intrarenal angiotensinogen may contribute to sex differences in blood pressure and renal injury (3, 9, 30). Yanes et al. (30) reported that castration reduced the salt-induced increase in blood pressure, proteinuria, and renal angiotensinogen expression in male Dahl S rats. Moreover, angiotensinogen gene expression was significantly higher in the renal cortex of male Dahl S rats compared with females regardless of the salt diet (30). We also found that urinary levels of angiotensinogen were significantly higher in the male vs. the female mRen(2).Lewis rats maintained on either the NS or HS diets. The HS diet increased angiotensinogen excretion approximately eightfold, but we did not find a commensurate increase in gene or protein expression within the renal cortex. Moreover, Western blots revealed similar molecular mass bands at 60 and 55 kDa for angiotensinogen in the plasma and urine of the HS males, whereas a single band at 60 kDa was evident in cortical tissue. Although the molecular size of glycosylated angiotensinogen (55–60 kDa) does not favor glomerular filtration of the protein in the normal kidney (21), urinary levels of angiotensinogen in the male mRen(2).Lewis may reflect the filtration of the circulating protein arising from glomerular damage (24). Urinary levels of ANG II and ANG-(1–7) also increased with salt, and the higher ANG II may contribute to the greater extent of renal damage or proteinuria. The presence of angiotensin peptides in the urine and tubular fluid has been shown by our group and others (5, 12, 22); however, it is not known in the present study whether the increase in urinary angiotensins arises from the processing of the filtered angiotensinogen, the direct release from renal tissue, or filtration of the intact peptides themselves.
In contrast to the male mRen(2).Lewis, the females exhibited a significant increase (∼50 mmHg) in blood pressure with the HS diet consistent with our previous study (8, 14). Associated with the salt-sensitive rise in pressure, urinary angiotensinogen levels markedly increased (>100-fold). Proteinuria and urinary angiotensins also increased with the HS diet, but to a lesser extent than angiotensinogen. Compared with the HS males, the HS females exhibited a significantly greater increase in urinary angiotensinogen (7.7- vs. 180-fold) but not proteinuria (8.9- vs. 12-fold) and an attenuated response in urinary ANG II (2.8 vs. 1.4; Table 1). However, angiotensinogen gene expression and cortical tissue content were not different between the NS and HS females. Because proximal tubules constitutively secrete angiotensinogen, a significant accumulation in tissue content may be difficult to detect. In this case, we found no difference in the release of angiotensinogen from cortical slices of the NS and HS groups. These data do not readily support an increase in secretion of angiotensinogen as the predominant mechanism for the salt-induced excretion of the protein but that filtration contributes to urinary levels. Immunoblot analysis revealed two primary bands (60 and 55 kDa) in both plasma and urine fractions but a single 60-kDa band in cortical tissue consistent with the males. We noted an additional band of 50 kDa in the female urine samples, which may reflect a different degree of glycosylation; the native protein exhibits a molecular mass of 48 kDa. As to the male-female difference in the blood pressure to the HS diet, the mechanism underlying this response is not currently known. The males on a NS diet exhibit significantly higher urinary levels of protein and angiotensinogen than the female congenics, as well as higher circulating and renal levels of ANG II (24). We speculate that the male kidneys may have sustained a greater degree of renal damage than the females, which may impair their ability to regulate sodium and water. Thus the increase in blood pressure required to excrete the higher sodium load in the females may not be operative in the males. Alternatively, the HS diet may induce some degree of heart failure, which would reduce their ability to maintain a sustained increase in pressure. Chronic salt loading in young male SHR results in cardiac dysfunction associated with lower blood pressure (1). We observed no change in body weight nor increased mortality in the HS males (data not shown); however, an assessment of cardiac function is required to establish whether the HS diet induces heart failure in the male mRen(2).Lewis.
Finally, we determined the renal expression and excretion of angiotensinogen in the OVX female mRen(2).Lewis maintained on the NS diet. Estrogen stimulates angiotensinogen expression, and higher levels of the protein may potentially offset the beneficial effects of estrogen via increased levels of ANG II. Early ovariectomy in the female congenics augmented the hypertension and induced a 16-fold increase in urinary levels of angiotensinogen, significantly higher than the HS males but less than the HS females (Table 1). In contrast to the effects of HS diet, ovariectomy did not increase the extent of proteinuria consistent with our earlier study (8). The fact that urinary protein did not increase following ovariectomy would argue for a renal source of the angiotensinogen. However, ovariectomy did not increase angiotensinogen gene expression or cortical tissue content of the protein. Indeed, gene expression was significantly lower in the OVX females. Thus, it is possible that the increased urinary levels of angiotensinogen arises from subtle damage to the glomerulus and the subsequent filtration of circulating angiotensinogen. The significant increase in urinary angiotensinogen but not protein in the OVX mRen(2).Lewis may reflect the high sensitivity (picogram range) of the angiotensinogen ELISA compared with the microgram quantities required for protein detection. We previously reported that ovariectomy of the mRen(2).Lewis did not increase albuminuria or proteinuria despite the sustained increase in blood pressure and activation of the RAAS (8). The albumin ELISA used in the previous study exhibits a detection limit in the nanogram range (20 ng/ml; SPI-BIO), which is more sensitive than the protein assay, but an order of magnitude less than the angiotensinogen assay (10 pg/ml). Given the low concentrations of angiotensinogen in the urine from the NS females regardless of estrogen status, we could not determine whether the molecular forms of the protein were similar for the serum and urine. Moreover, we have not assessed whether release of angiotensinogen is altered in the OVX mRen(2).Lewis; however, the fact that gene expression was reduced and the cortical protein unchanged suggests that it is unlikely that a sustained increase in angiotensin secretion accounts for the 16-fold increase in the urinary levels of the protein.
In summary, the findings in the present study reveal marked differences in the salt-sensitive responses in blood pressure and renal injury between the male and female mRen(2).Lewis hypertensive stain. The male mRen(2).Lewis exhibited significantly higher urinary angiotensinogen and protein than either the intact or OVX females, which may in part reflect greater renal damage in the males. The HS diet or ovariectomy increased angiotensinogen excretion in the absence of an increase in gene or protein expression within the kidney cortex. Moreover, the higher urinary levels of angiotensinogen in the OVX group were not associated with proteinuria. In this regard, Saito et al. (26) recently reported that increased excretion of angiotensinogen precedes the development of albuminuria in type 1 diabetic patients. Urushihara et al. (28) also reported a strong positive correlation for angiotensinogen excretion and proteinuria in patients with chronic glomerulonephritis. Although it remains to be determined whether angiotensinogen excretion precedes increased proteinuria in the mRen(2).Lewis, the assessment of urinary angiotensinogen should be explored as a potential biomarker for the development of renal injury.
GRANTS
These studies were supported by National Institutes of Health Grants HL-56973, HL-51952, and T32-RR-07009.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Brian Westwood for technical assistance.
Portions of this study were presented at the 63rd Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research-2009, Chicago, IL.
REFERENCES
- 1.Ahn J, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ Physiol 287: H767–H772, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bachmann J, Ganten U, Stock G, Ganten D. Sexual dimorphism of cardiovascular function: The role of androgens. In: Sex Steroids and the Cardiovascular System, edited by Ramwell P, Rubanyi G, Schillinger E. Berlin, Germany: Springer-Verlag, 1992 [Google Scholar]
- 3.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, Bader M. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse Ren-2 gene. J Am Soc Nephrol 13: 2681–2687, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Brosnihan KB, Senanayake PS, Li P, Ferrario CM. Bi-directional actions of estrogen on the renin-angiotensin system. Braz J Med Biol Res 32: 373–381, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2.Lewis rat. Hypertension 42: 781–786, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2. Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med, 5Suppl A: S65–S75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol 291: R1557–R1563, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics 1: 3–9, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Groban l Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, Chappell MC. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci 63: 3–11, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Herrington DM, Pusser BE, Riley WA, Thuren TY, Brosnihan KB, Brinton EA, MacLean DB. Cardiovascular effects of droloxifene, a new selective estrogen receptor modulator, in healthy postmenopausal women. Arterio Thromb Vasc Biol 20: 1606–1612, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol 294: F1257–F1263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol 286: F965–F971, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 19: 269–280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 75: 156–166, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol 10, Suppl 11: S189–S195, 1999 [PubMed] [Google Scholar]
- 23.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2).Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295: H10–H20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int 69: 1016–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci 338: 478–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal renin-angiotensin system activity. Am J Nephrol 31: 318–325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada K, Iyer SN, Chappell MC, Brosnihan KB, Fukuhara M, Ferrario CM. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept 80: 57–66, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]