Abstract
Ammonia metabolism is a primary component of acid-base homeostasis but is incompletely developed at time of birth. Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg) are recently recognized ammonia transporter family members expressed in the mammalian kidney. This study's purpose was to establish the expression and localization of Rhbg and Rhcg during kidney development. We examined kidneys from fetal days 16 (E16), 18 (E18), and 20 (E20), and from the first 21 days of postnatal development. Rhbg was expressed initially at E18, with expression only in the connecting tubule (CNT); at E20, Rhbg was expressed in both the CNT and the medullary collecting duct (MCD). In contrast, Rhcg was first expressed at E16 with basal expression in the ureteric bud; at E18, it was expressed in a subset of CNT cells with an apical pattern, followed by apical and basolateral expression in the MCD at E20. In the cortex, Rhbg and Rhcg expression increased in the CNT before expression in the cortical collecting duct during fetal development. In the MCD, both Rhbg and Rhcg expression was initially in cells in the papillary tip, with gradual removal from the tip during the late fetal period and transition during the early neonatal period to an adult pattern with predominant expression in the outer MCD and only rare expression in cells in the initial inner MCD. Double-labeling with intercalated cell-specific markers identified that Rhbg and Rhcg were expressed initially in CNT cells, CNT A-type intercalated cells and non-A, non-B intercalated cells, and in MCD A-type intercalated cells. We conclude that expression of Rhbg and Rhcg parallels intercalated cell development and that immature Rhbg and Rhcg expression at birth contributes to incomplete ammonia excretion capacity.
Keywords: collecting duct, connecting tubule, embryology
renal net acid excretion is critical for acid-base homeostasis, and thereby for normal health. The primary component of net acid excretion is renal ammonia metabolism, which comprises 60–70% of net acid excretion under basal conditions and ∼90% of the increase in net acid excretion in response to chronic metabolic acidosis (11, 55, 57). Recent studies have identified a new family of proteins, the Rh glycoproteins, that mediate important roles in mammalian ammonia transport. Rh A glycoprotein (Rhag) was the first protein to be identified as a member of this family (39); it is expressed exclusively in erythrocytes and erythroid-precursor cells and is not expressed in the kidney (35, 55). Subsequent studies identified Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg) as members of this family (36, 37), and these proteins are expressed in essentially all tissues that transport ammonia (56, 57). In particular, Rhbg is expressed in kidneys, liver, skin, lung, stomach, and gastrointestinal tract (14, 19, 37, 45, 54, 58). Rh C glycoprotein (Rhcg) is expressed in the kidneys, central nervous system, testes, liver, skeletal muscle, lung, stomach, and entire gastrointestinal tract (7, 14, 19, 36, 54, 58).
Increasing evidence suggests that Rhbg and Rhcg mediate important roles in renal ammonia metabolism. They are expressed in the distal convoluted tubule (DCT), connecting tubule (CNT), initial collecting tubule, cortical collecting duct (CCD), outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD) (7, 36, 45, 54), sites where 70–80% of urinary ammonia is secreted (11, 47). Rhbg exhibits basolateral expression (7, 45, 54), whereas Rhcg exhibits both apical and basolateral expression in the mouse, rat, and human kidney (12, 25, 48, 49). Multiple studies show that Rhbg and Rhcg are ammonia-specific transporters (reviewed in Ref. 57), and transport studies show that carrier-mediated transport with functional characteristics identical to those of Rhbg and Rhcg is the predominant mechanism of collecting duct apical and basolateral membrane ammonia transport (17, 18). Finally, both global and collecting duct-specific Rhcg deletion inhibit renal ammonia excretion (2, 30). Thus Rhbg and Rhcg are likely to play important roles in renal ammonia metabolism.
The renal capacity to excrete ammonia is incompletely developed in the developing and newborn mammalian kidney. For example, in the human fetus, studied between 20 and 38 wk of gestational age, urinary ammonia levels average <1 mmol/l (43), which contrast to adult levels of 20–40 mmol/l. Human infants studied shortly after birth have an intact ability to acidify urine, but impaired ability to excrete ammonia (40–43), and premature infants have impaired renal ammonia excretion compared with full-term infants (10). These findings, particularly the intact ability to acidify urine but impaired ammonia excretion, suggest there may be defects in ammonia transport.
Accordingly, the purpose of the current study was to examine the developmental expression of Rhbg and Rhcg in the embryonic and postnatal kidney to determine whether immature Rhbg and Rhcg expression parallels immature ammonia metabolism. We used kidneys from fetuses of timed-pregnant rats and from specific days in the immediate postnatal period. We examined the expression of Rhbg and Rhcg using immunohistochemistry and used colocalization with specific markers of renal epithelial cell types to define the specific sites of expression of Rhbg and Rhcg during renal development.
METHODS
Antibodies.
The antibodies used were antisera and affinity-purified anti-peptide antibodies specific for Rhbg and Rhcg raised in rabbits and characterized previously (12, 19, 48, 49, 54, 58). Antibodies to the B1/B2 subunit of H+-ATPase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA; SC20943). Antibodies to AE1 were obtained from Alpha Diagnostic (San Antonio, TX), and antibodies to pendrin were graciously provided by Dr. Søren Nielsen (University of Aarhus, Aarhus, Denmark).
Animals and tissue preservation.
Animal experiments in this study were performed with the full approval of the Animal Care and Use Committee of the Catholic University of Korea and Ewha Womans University (EMRI 07–0072, 07–0075). Kidneys were obtained from 16 (E16)-, 18 (E18)-, and 20-day-old (E20) fetuses and from 1 (P1)-, 3 (P3)-, 7 (P7)-, 14 (P14)-, and 21-day-old (P21) pups. Kidneys from adult male rats (n = 3) served as a positive reference for the immunohistochemical studies. At least five animals were used in each group. Animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). The kidneys were preserved by in vivo perfusion through the heart or abdominal aorta. The animals were initially perfused briefly with PBS (290 mosmol/kgH2O, pH 7.4) to rinse away the blood. For immunocytochemical studies, the kidneys were then perfused with a periodate-lysine-2% paraformaldehyde (PLP) solution for 5 min. After perfusion, the kidneys were removed and cut into 1- to 2-mm-thick slices that were fixed additionally by immersion in the PLP solution overnight at 4°C. Sections of tissue were cut transversely through the entire kidney on a vibratome at a thickness of 50 μm and processed for immunocytochemical studies using a horseradish peroxidase preembedding technique. Slices of tissue were also embedded in polyester wax.
Preembedding immunohistochemistry.
Fifty-micrometer vibratome sections were processed for immunocytochemistry using an indirect preembedding immunoperoxidase method, as previously described (13, 15). All sections were washed with 50 mM NH4Cl in PBS three times for 15 min. Before incubation with the primary antibody, the sections were pretreated with a graded series of ethanol followed by incubation for 3 h with PBS containing 1% BSA, 0.05% saponin, and 0.2% gelatin (solution A). The tissue sections were then incubated overnight at 4°C with primary antibodies diluted in 1% BSA-PBS (solution B). Control incubations were performed in solution B without the primary antibody. After several washes with solution A, the sections were incubated for 2 h in a peroxidase-conjugated donkey anti-rabbit IgG Fab fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:100 in solution B. The tissues were rinsed first in solution A and subsequently in 0.05 M Tris buffer (pH 7.6). For the detection of horseradish peroxidase, sections were incubated in 0.1% 3,3′-diaminobenzidine in 0.05 M Tris buffer for 5 min, after which H2O2 was added to a final concentration of 0.01% and the incubation was continued for 10 min. The sections were then dehydrated in a graded series of ethanol, embedded in poly/Bed-812 resin (Polysciences, Warrington, CA), and examined by light microscopy.
Double immunohistochemistry.
Two-micrometer wax sections were dewaxed with ethanol, and after rinsing in tap water, incubated with 3% H2O2 for 30 min to eliminate endogenous peroxidase activity. The sections were treated with blocking serum for 30 min and incubated overnight at 4°C in primary antibodies (Rhbg, 1:2,000 or AE1, 1:200). After being washed in PBS, the sections were incubated for 2 h with the peroxidase-conjugated secondary antibodies, washed, and exposed to a mixture of 0.05% 3,3′-diaminobenzidine and 0.01% H2O2 for 5 min at room temperature. After being rinsed with Tris·HCl buffer, the above procedure was then repeated with the substitution of a second primary antibodies (H+-ATPase, 1:200; pendrin, 1:200; or, Rhcg, 1:500) and the substitution of Vector SG for 3,3′-diaminobenzidine. The sections were dehydrated with graded ethanol and xylene, mounted in Permount, and examined by light microscope.
Immunoblot analysis.
For immunoblotting, the kidneys were isolated from neonatal rats at P1, P3, and P7. Kidneys from male adult rats were used as a control. The kidney tissue was stored frozen at −70°C until used. Kidneys were homogenized in 10 volumes of protein extraction solution (Intron, Seoul, Korea), followed by centrifugation (12,000 g, 25 min, 4°C). The supernatant was isolated, and protein concentration was determined using the BCA protein assay kit (Pierce). Thirty micrograms of protein were separated by 12% SDS-PAGE and transferred onto a polyvinylidene membrane (Amersham Biosciences). The membrane was blocked with 5% skim milk for 1 h and incubated with rabbit anti-Rhbg polyclonal antibody and rabbit anti-Rhcg polyclonal antibody (1:500 and 1:1,000 dilution, respectively) overnight at 4°C. Antibody binding was detected using horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000 dilution, Santa Cruz Biotechnology) and visualized using Western blotting luminol reagent (Santa Cruz Biotechnology) and LAS-3000 (Fuji, Kanagawa, Japan).
RESULTS
Expression of Rhbg and Rhcg in adult rat kidney.
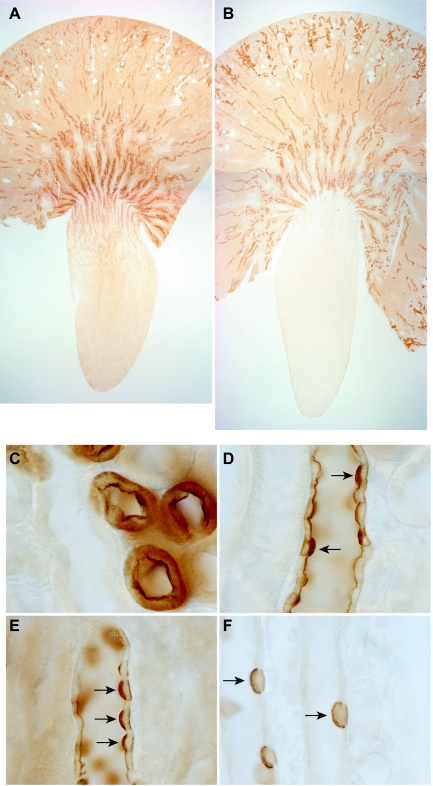
We first examined the immunohistochemical localization of Rhbg and Rhcg in the adult rats used for these studies. Rhbg was predominantly expressed in the inner stripe of the outer medulla, with intermediate expression in the outer stripe of the outer medulla and the cortex, lesser expression in the base of the inner medulla, and only rare positive cells in the distal part of the inner medulla (Fig. 1A). High-power microscopic examination demonstrated a specific basolateral expression (data not shown) reported previously by us and by others (45, 48, 54). The majority of Rhbg expression in the adult kidney is present in the inner stripe of the outer medulla and the cortex, with less in the outer stripe of the outer medulla and expression only in a minority of cells, the intercalated cells, in the IMCD.
Fig. 1.
Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg) expression in adult rat kidney. A: low-power micrograph of Rhbg immunolabel expression in adult rat kidney. The heaviest expression is in inner stripe of outer medulla, with intermediate expression in the cortex and in intercalated cells in the inner medullary collecting duct (IMCD). B: low-power micrograph of Rhcg immunolabel expression in adult kidney. The regional pattern of Rhcg expression is similar to that observed for Rhbg in A. C: intense Rhcg immunolabel expression in high-power micrograph of the connecting tubule (CNT). D: heterogeneous Rhcg expression in the cortical collecting duct (CCD). Intense Rhcg expression is present in A-type intercalated cells (arrows), with less intense expression in principal cells. E: high-power micrograph of outer medullary collecting duct (OMCD) that demonstrates intense Rhcg expression in A-type intercalated cells (arrows) and low-level expression in principal cells. F: high-power micrograph from the IMCD that demonstrates Rhcg expression only in A-type intercalated cells in the IMCD.
Rhcg was distributed in the adult rat kidney in a pattern similar to that for Rhbg, with the exception that both apical and basolateral immunolabel was present (Fig. 1B). High-power microscopy confirmed that Rhcg exhibited apical and basolateral expression in the CNT, CCD, OMCD, and IMCD (Figs. 1, C–F, respectively). These observations of both apical and basolateral Rhcg expression are similar to that reported previously using both light microscopy and immunogold electron microscopy in the rat, human, and mouse kidney (12, 16, 25, 26, 33, 48, 49). Similar to Rhbg, the majority of Rhcg expression in the adult kidney is in the inner stripe of the outer medulla and the cortex, with less in the outer stripe of the outer medulla and expression only in a minority of cells, the intercalated cells, in the IMCD.
Colocalization of Rhcg with intercalated cell-specific cell markers.
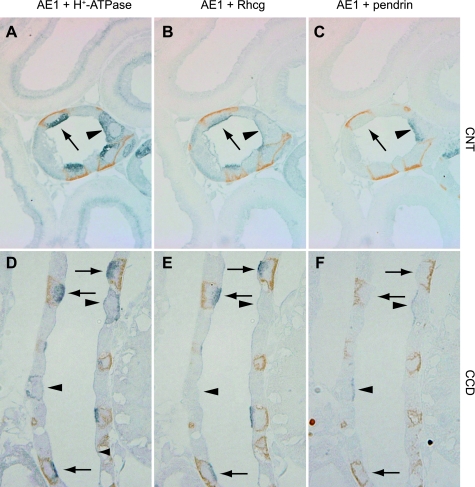
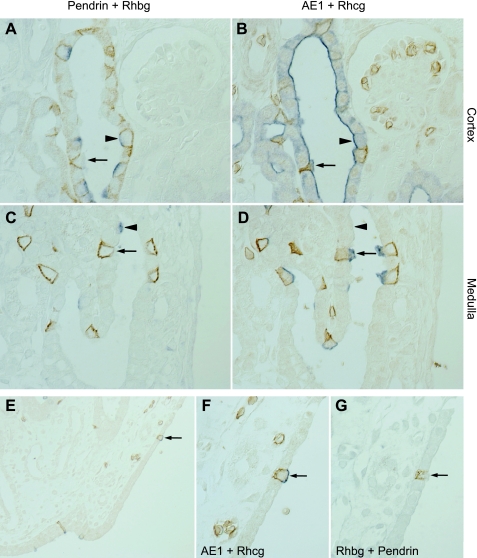
At least four distinct cell types, A-type intercalated cells, B-type intercalated cells, non-A, non-B intercalated cells, and principal cells, are present in the collecting duct. We reported previously that A-type intercalated cells and non-A, non-B intercalated cells express high levels of Rhbg, whereas B-type intercalated cells either do not express Rhbg or express levels below detectable limits by immunohistochemistry (54). To determine whether a similar pattern existed for Rhcg, we performed double-immunolabeling in serial sections using antibodies against Rhcg, pendrin, AE1, and H+-ATPase (Fig. 2). Our findings demonstrate that pendrin-expressing cells that express strong Rhcg immunolabel express, as identified in serial sections, apical H+-ATPase but not basolateral AE1. Thus pendrin-positive, Rhcg-positive cells are non-A, non-B intercalated cells. Pendrin-positive cells that either express very low levels or not-detectable Rhcg express basolateral H+-ATPase and do not express detectable AE1. Thus B-type intercalated cells do not express significant levels of Rhcg. Pendrin-negative cells expressing high levels of Rhcg also expressed apical H+-ATPase and basolateral AE1. This indicates that A-type intercalated cells express high levels of Rhcg.
Fig. 2.
Colocalization of Rhcg with intercalated cell-specific markers. A–C: serial sections of adult rat CNT colabeled for AE1 (brown) and H+-ATPase (blue) in A, AE1 (brown) and Rhcg (blue) in B, and AE1 (brown) and pendrin (blue) in C. A-type intercalated cells, identified by apical H+-ATPase, basolateral AE1, and the absence of pendrin immunolabel, express intense Rhcg immunolabel (arrow). Non-A, non-B intercalated cells, identified by apical H+-ATPase, absence of basolateral AE1, and the presence of apical pendrin (arrowhead), express Rhcg immunolabel. D–F: similar studies in the CCD. Again, A-type intercalated cells identified by apical H+-ATPase, basolateral AE1, and the absence of apical pendrin express Rhcg immunolabel (arrows). In contrast to the non-A, non-B intercalated cell, B-type intercalated cells, identified by basolateral H+-ATPase and apical pendrin immunolabel (arrowheads), do not express detectable Rhcg immunolabel.
Rhbg expression during fetal period.
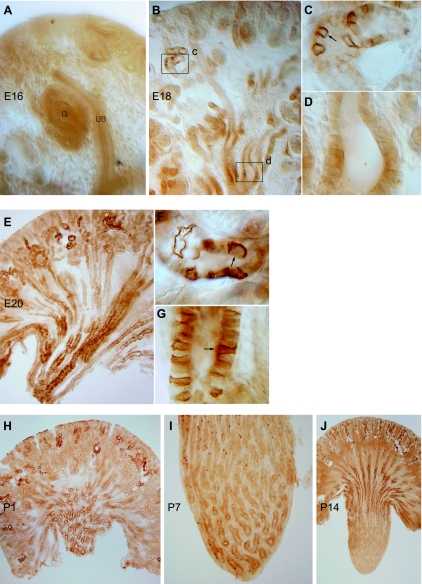
Having defined the characteristics of Rhbg and Rhcg expression in the different intercalated cell types, we began examining the developmental expression of Rhbg in the rat kidney. The first time point examined was E16, at which time there is interaction between the ureteric bud and the condensing mesenchymal tissue. No Rhbg immunolabel was detectable at this time point (Fig. 3A). At E18, there was the onset of detectable Rhbg expression in the cortex, where a subset of cells in convoluted tubules exhibited Rhbg immunolabel (Fig. 3, B–D). Rhbg immunoreactivity was not detectable in collecting ducts in either the cortex or the medulla.
Fig. 3.
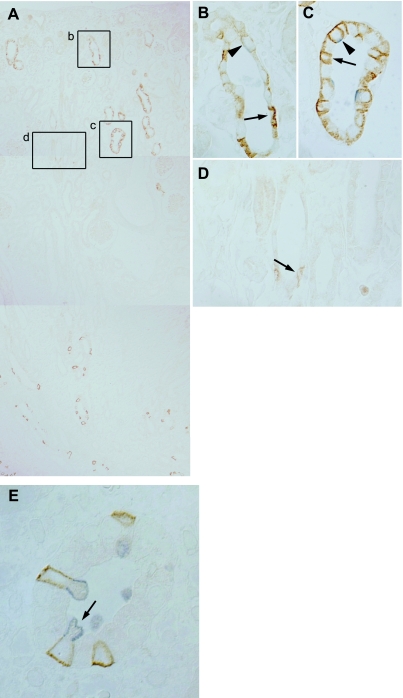
Expression of Rhbg in the developing kidney. A: at embryonic day 16 (E16), detectable Rhbg immunolabel was not expressed in developing glomeruli (G), ureteric bud (UB), or interstitium. B–D: Rhbg expression at E18. B: low-power micrograph of Rhbg immunolabel in the E18 rat kidney. Expression is present in a subset of convoluted tubule cells in the cortex, primarily in the corticomedullary junction region. C: high-power micrograph of cortex showing basolateral Rhbg expression in a subset of connecting segment cells. D: high-power micrograph of medullary collecting duct (MCD) demonstrating that specific Rhbg immunolabel is not present at E18. E–G: Rhbg in the E20 kidney. Rhbg expression in the cortex is predominantly in the inner cortical region in CNT segments and is increased relative to E18. Medullary Rhbg expression is now present and is greatest in the distal medulla. F: high-power micrograph demonstrating basolateral Rhbg immunolabel in a subset of connecting segment cells (arrow). G: simultaneous intense basolateral Rhbg immunolabel in a subset of MCD cells. The distinct basolateral Rhbg expression present in many, but not all, MCD cells is consistent with differential expression in intercalated cells and principal cells. H: low-power micrograph at postnatal day 1 (P1). Rhbg is expressed at increasing levels in connecting segments in the cortex and in collecting duct segments in the medulla. CCD segments do not express significant Rhbg immunolabel. In the medulla, Rhbg expression is greatest in the distal MCD, with relatively less expression in the MCD adjacent to the corticomedullary junction. I: high-power micrograph of the medulla of the P7 kidney. There is the beginning of the disappearance of Rhbg immunolabel from the distal MCD and increased expression in a subset of cells in the MCD closer to the corticomedullary junction. J: Rhbg expression in the P14 kidney. There is a further increase in Rhbg immunolabel in the cortex, almost complete absence of Rhbg-expressing cells in the distal MCD, and increased expression in more proximal regions of the MCD.
E20 demonstrated a substantial increase in Rhbg immunoreactivity. In the cortex, heterogeneous Rhbg expression in connecting segments persisted, although expression was increased compared with E18 (Fig. 3, E–G). Proximal tubule segments, identified by connection to nascent glomeruli, did not express Rhbg. In the medulla, there was a dramatic onset of Rhbg expression in the medullary collecting duct (MCD), but Rhbg expression in MCD was not uniform. In particular, Rhbg immunoreactivity was more intense in the more distal portions of the MCD than in the juxtamedullary region. Also, Rhbg expression in the collecting duct was heterogeneous, with some cells expressing high levels of Rhbg and some not expressing detectable Rhbg. This pattern is consistent with differential expression in intercalated cells and principal cells.
During the early postnatal period there was rapid transformation of Rhbg expression from the fetal to the adult pattern. P1 showed continued differentiation of the kidney (Fig. 3H). In the cortex, a subpopulation of convoluted tubular structures expressed Rhbg, but expression was increased compared with E20. In general, almost all cortical cells that expressed Rhbg were in convoluted segments. In the medulla, Rhbg immunolabel was greatest in the papillary tip region, with minimal labeling in the region adjacent to the corticomedullary junction. P7 was characterized by an increase in the number of Rhbg-positive cells in the base of the medulla and adjacent to the corticomedullary junction and decreased numbers in the distal MCD (Fig. 3I), and by P14 there was almost complete disappearance of labeling from the papillary tip, and preferential Rhbg expression in the base of the IMCD and in the inner stripe of the OMCD, a pattern approaching Rhbg's expression in the adult kidney (Fig. 3J).
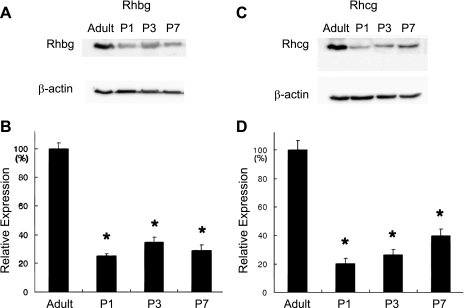
These immunohistochemistry studies suggest that Rhbg expression is decreased in the developing kidney relative to the adult kidney. Immunoblot analysis identified that Rhbg expression was substantially less in the P1, P3, and P7 kidneys compared with the adult kidney (Fig. 4).
Fig. 4.
Rhbg and Rhcg protein expression in the postnatal kidney. A: immunoblot analysis of Rhbg expression in the adult kidney and in the P1, P3, and P7 kidney. β-Actin is used as a loading control. B: quantitative analysis of Rhbg expression relative to β-actin expression. Rhbg expression is significantly less in the postnatal kidney at each time point than in the adult kidney. Results are shown as expression relative to mean adult kidney expression (100%). C: immunoblot analysis of Rhcg expression in adult kidney and in P1, P3, and P7 kidney. β-Actin is again used as a loading control. D: quantitative analysis of Rhcg expression relative to β-actin expression. Results are shown as expression relative to mean adult kidney expression (100%). Rhcg expression is significantly less in the postnatal kidney at each time point than in the adult kidney. *P < 0.05 by Student's 2-tailed t-test; n = 3 in each group.
To determine whether the Rhbg-positive cells in the developing kidney were A-type intercalated cells, non-A, non-B intercalated cells, or both, we used double-immunolabeling with the intercalated cell-specific markers H+-ATPase, pendrin, and AE1. In preliminary studies, we determined that low abundance of Rhbg and these intercalated cell marker proteins precluded successful double-immunolabeling earlier than P4 (data not shown). At P4, as described above, Rhbg-positive cells were present in the cortex and in the distal medulla (Fig. 5). In connecting tubule segments, both pendrin-positive and pendrin-negative cells exhibited intense basolateral Rhbg immunolabel (Fig. 5, A–D), thereby identifying these cells as non-A, non-B intercalated cells and A-type intercalated cells, respectively. In CCD segments, only very rare cells expressed basolateral Rhbg (Fig. 5D). These cells had an immature appearance, with a flat epithelial profile and expressed substantially less Rhbg immunolabel than did Rhbg-positive connecting segment cells. These Rhbg-positive CCD cells did not express detectable pendrin, thereby identifying these cells as A-type intercalated cells. In the medulla, Rhbg-positive cells were present only in the very distal region of the MCD. These Rhbg-positive cells did not express a detectable pendrin immunolabel, identifying these cells as A-type intercalated cells. In the MCD, Rhbg-positive cells expressed apical H+-ATPase immunolabel, consistent with these cells being A-type intercalated cells (Fig. 5E). Pendrin-positive cells in the MCD expressed either very low levels or did not express detectable Rhbg immunolabel, thereby identifying these cells as B-type intercalated cells. Thus the Rhbg-positive intercalated cells in the cortex of the developing kidney are A-type and non-A, non-B intercalated cells, and in the MCD are almost exclusively A-type intercalated cells.
Fig. 5.
Double-immunolabeling, Rhbg and pendrin, at P4. A: composite micrograph showing entire kidney, from cortex to medullary tip. Rhbg immunolabel is present in a subset of cells in the cortex and the medullary tip, and is absent from intermediate regions. B: high-power micrograph of inset b in A showing convoluted epithelial tubule structure from the outer cortex. Cells with intense basolateral Rhbg immunolabel (arrow) do not express detectable pendrin and are identified as A-type intercalated cells. Pendrin-positive cells (arrowhead) express very low levels of basolateral Rhbg and are identified as B-type intercalated cells. C: high-power micrograph of inset c in A showing CNT closer to the corticomedullary junction that demonstrates that both A-type intercalated cells, with intense basolateral Rhbg but not apical pendrin (arrow) immunolabel, and non-A, non-B cell cells, with apical pendrin and basolateral Rhbg immunolabel (arrowhead), are present. D: high-power micrograph of inset d in A showing collecting duct from the deep cortex showing that only rare A-type intercalated cells, with basolateral Rhbg and without detectable pendrin immunolabel (arrow), are present. Rhbg immunolabel intensity is low, consistent with an immature phenotype of intercalated cells at this time point. E: micrograph from the deep medulla. Cells with intense basolateral Rhbg immunolabel (brown) express apical H+-ATPase immunolabel (blue), identifying these cells as A-type intercalated cells (arrow).
Rhcg expression during development.
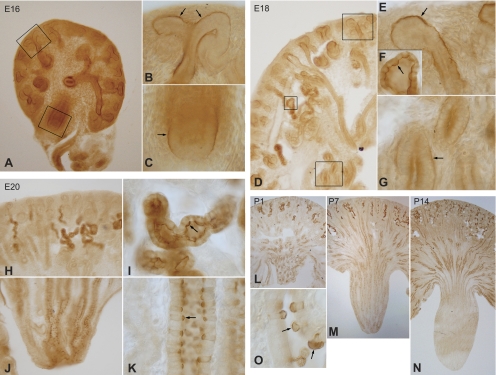
Rhcg expression during kidney development exhibited both parallels with and differences from Rhbg expression. In contrast to Rhbg, we detected Rhcg expression as early as E16. In contrast to Rhbg, which was expressed first in the cortex and only later in the developing medulla, Rhcg immunolabel was detectable simultaneously in the cortex and the medulla (Fig. 6, A–C). Importantly, at this early time point Rhcg immunolabel was exclusively basal, which contrasts with its apical and basolateral expression in the adult kidney. In the cortex, Rhcg expression was concentrated at sites of branching morphogenesis. In the medulla, basal Rhcg was present in MCD segments. In contrast to Rhbg, where only a subset of cells expressed immunolabel at the early time points, Rhcg expression at E16 did not exhibit cell-specific differences at this early time point.
Fig. 6.
Rhcg expression in developing rat kidney. A: low-power micrograph of Rhcg immunolabel in the E16 kidney. Distinct Rhcg immunolabel is present in both cortical and medullary structures. B: basal Rhcg immunolabel that is concentrated in collecting duct segments at sites of branching morphogenesis (arrow). C: basolateral Rhcg immunolabel (arrow) in the medulla of the E16 kidney. Apical Rhcg immunolabel is not detectable in either cortical or medullary sites at E16. D: low-power micrograph of E18 kidney. Rhcg immunolabel is present in both the cortex and the medulla. E: high-power micrograph of a CCD segment. Basolateral, but not apical, Rhcg immunolabel is present. F: apical Rhcg immunolabel in CNT cells (arrow), the first site of apical Rhcg expression. G: MCD at E18 that demonstrates continued basal Rhcg immunolabel without significant apical Rhcg expression. H: low-power micrograph of cortex of E20 kidney. Intense Rhcg immunolabel is present in a subpopulation of convoluted tubule structures, but not in collecting duct segments. I: high-power micrograph of CNT demonstrating strong apical Rhcg immunolabel (arrow). J: low-power micrograph of medulla at E20. Numerous Rhcg-positive cells are present, particularly in the distal MCD. K: high-power micrograph of MCD. Intense apical Rhcg immunolabel with less-intense basolateral Rhcg immunolabel are present (arrow). L: low-power micrograph of Rhcg expression at P1. In the cortex, there is continued preferential expression of Rhcg in convoluted tubule structures, but absence of Rhcg expression in CCD segments. In the medulla, the predominance of Rhcg expression is in the distal MCD. M: low-power micrograph of Rhcg expression at P7. There is continued development of Rhcg expression in convoluted tubule segments and the onset of expression in the CCD. Rhcg expression in the CCD tends to be more intense in the deep CCD, closer to the corticomedullary junction. In the medulla, there are fewer Rhcg-expressing cells in the tip of the MCD, with a relative increase in numbers at the base of the papilla and the appearance of labeled cells in the OMCD near the corticomedullary junction. N: low-power micrograph at P14. In the cortex there is a nearly adult phenotype of Rhcg expression, with Rhcg present in both convoluted tubule cells and in CCD. In the medulla, there is near complete removal of Rhcg-expressing cells from the papilla, with rare cells present in the base of the papilla and greater expression in the outer medulla. O: region of the papillary surface epithelium at P7. Cells with intense apical Rhcg immunolabel are in the process of being extruded from the basement membrane into the papillary space, a known mechanism of intercalated cell deletion during development.
At E18 there was a significant change in Rhcg expression compared with E16. Expression was increased and easily evident in both the cortex and medulla (Fig. 6D). The first appearance of apical Rhcg immunolabel was in cortical connecting segment cells (Fig. 6F). Basal, but not apical, Rhcg immunolabel, continued to be present in collecting duct segments in both the cortex and the medulla (Fig. 6, E and G). Thus apical Rhcg is first expressed in the connecting segment, not the collecting duct.
E20 showed further evolutions in Rhcg immunolabel compared with E18. In the cortex, an increased number of convoluted tubule segments expressed intense apical Rhcg immunolabel (Fig. 6, H and I). There was also the development of cellular heterogeneity in the intensity of Rhcg immunolabel. In the medulla, only a subset of cells expressed Rhcg immunolabel, with both apical and basolateral immunolabel being identified in these cells (Fig. 6, J and K). The homogenous basal Rhcg immunolabel present at earlier time points was no longer observed in either the cortex or medulla.
In the postnatal period, there was a rapid transition from the embryonic to the adult phenotype of Rhcg immunolabel. At P1 there was continued intense Rhcg immunolabel in convoluted tubule structures in the cortex and in MCD segments, particularly in the distal medulla (Fig. 6L). Relatively few Rhcg-positive cells were present in the corticomedullary junction region. At P7 there was increased Rhcg immunolabel in connecting tubule segments, a decrease in the number of Rhcg-expressing cells in the distal MCD, and an increase in the number of Rhcg-positive MCD cells in the midmedullary region (Fig. 6M). At P14 there was almost complete loss of Rhcg-expressing cells from the papilla, and increased expression of Rhcg-positive cells in the nascent outer medulla (Fig. 6N). In the cortex, there was continued development of expression in convoluted tubule segments and increased expression in CCD segments. In papillary surface epithelial cells, a subset of cells expressed apical and basolateral Rhcg immunolabel (Fig. 6O). On occasion Rhcg-expressing cells papillary surface epithelial cells appeared to be in the process of being extruded into the papillary space.
These immunohistochemistry studies suggest that Rhcg expression is decreased in the developing kidney relative to the adult kidney. Immunoblot analysis identified that Rhcg expression was substantially less in the P1, P3, and P7 kidneys compared with the adult kidney (Fig. 4).
To characterize further the Rhbg- and Rhcg-positive cell types during development, we used double-immunolabeling of Rhbg, Rhcg, pendrin, and AE1 in serial sections. As described previously, P4 was the earliest time at which Rhbg, Rhcg, pendrin, and AE1 immunolabel was detectable by immunohistochemistry (Fig. 7). These studies confirmed that Rhbg and Rhcg were expressed in A-type intercalated cells and in non-A, non-B cells in the cortex, whereas in the MCD pendrin-positive cells either expressed very low levels or nondetectable levels of Rhbg and Rhcg, identifying these cells as B-type intercalated cells. A-type intercalated cells, identified by basolateral Rhbg, apical Rhcg, basolateral AE1, and the absence of apical pendrin immunolabel, were also prevalent in the MCD at P4.
Fig. 7.
Double-immunolabel of Rhbg with pendrin and Rhcg with AE1 in serial sections. In the cortex (A and B), Rhbg-positive, pendrin-negative cells (arrow, A) expressed basolateral AE1 and apical Rhcg immunolabel (arrow, B), identifying these cells as A-type intercalated cells. Rhbg-positive, pendrin-positive cells (arrowhead, A) were Rhcg-positive and AE1-negative (arrowhead, B) in serial sections. Thus these are non-A, non-B cell cells. In the medulla (C and D), pendrin-positive cells in general did not express detectable Rhbg immunolabel (arrowhead, C), nor did they express detectable Rhcg or AE1 immunolabel (arrowhead, D). These cells are identified as B-type intercalated cells. Rhbg-positive cells were pendrin negative (arrow, C) and expressed apical Rhcg and basolateral AE1 immunolabel (arrow, D). Thus these are A-type intercalated cells. E–G: Rh glycoprotein expression in the papillary surface epithelium of the P4 kidney. E: low-power micrograph demonstrating Rhcg (blue) and AE1 (brown) immunolabel in occasional cells in papillary surface epithelium. F: high-power micrograph of A-type intercalated cell, identified by expression of both Rhcg and AE1. G: double-immunolabel of serial section for pendrin (blue, not detectable) and Rhbg (brown) in the same cell as in F.
Because some studies have suggested that intercalated cells are present in the papillary surface epithelium in the developing kidney, we examined Rhbg and Rhcg expression in these cells at P4. Using double-labeling with AE1, we found that both Rhbg and Rhcg were expressed in A-type intercalated cells in papillary surface epithelium. Non-A, non-B cells and B-type intercalated cells were not observed in developing papillary epithelium (Fig. 7, E–G).
DISCUSSION
The current study is the first demonstrating expression of Rhbg and Rhcg, nonerythroid members of the recently identified mammalian ammonia transporter family (55, 57), in the developing kidney. Rhbg expression is detectable first in the CNT at E18, followed at E20 by expression in intercalated cells in the MCD and by an increase in the number of Rhbg-positive cells in the CNT. Rhcg expression is first detectable at E16, where its expression is almost exclusively basal. At E18 the first apical Rhcg immunolabel can be observed in the CNT, and at E20 apical Rhcg immunolabel is also expressed in intercalated cells in the MCD. Thereafter, there is a pattern of increased expression of both Rhbg and Rhcg in the CNT and a gradual removal of Rhbg- and Rhcg-positive cells from the distal MCD and subsequently from the mid- and then initial IMCD. In the CCD, Rhbg and Rhcg expression does not become routinely identifiable until the early postnatal period. Thus these studies indicate patterned embryological origin of Rhbg- and Rhcg-positive cells in two distinct locations, in the CNT and the MCD. These observations have important implications for understanding renal development and the development of renal ammonia metabolism.
The first major finding in this study is that Rhbg and Rhcg expression develops essentially simultaneously in both the CNT and the MCD and changes in parallel through the remainder of development and in the early postnatal period. This pattern of expression is similar to that described for intercalated cells (27) and for other proteins predominantly expressed in intercalated cells, including H+-ATPase, carbonic anhydrase isoform II (CA II), and pendrin (4, 28, 52). Moreover, our study confirms the observations by Song et al. (52) that in the embryonic kidney B-type intercalated cells arise in both the cortex and medulla, whereas non-A, non-B cells are found only in the cortex and supports the thesis that these are fundamentally distinct cell types. Thus the essentially simultaneous expression of Rhbg and Rhcg in two different sites, in the cortex and the distal MCD, supports the thesis that intercalated cells develop simultaneously at two separate sites from two distinct precursor cells, one in the cortex in the site of the CNT and another in the MCD.
During development, there was a loss of Rhbg- and Rhcg-expressing cells in the developing inner medulla, with subsequent increasing expression in the outer medulla and, later, the appearance of expression in the CCD. This is similar to the observation regarding intercalated cell development (28). This parallel development of intercalated cells and of ammonia transporter family member, Rhbg and Rhcg, expression supports a coordinated involvement of Rhbg and Rhcg in intercalated cell-mediated acid-base and ammonia transport. Moreover, the observation of Rhcg-positive and Rhbg-positive cells undergoing extrusion into the tubule lumen was similar to the previous observation that collecting duct A-type intercalated cells undergo programmed cell deletion in the medulla through extrusion into the tubule lumen (27).
Although the most widely recognized membrane location for Rhcg is in the apical membrane, the current study demonstrates the development of basal Rhcg expression before apical Rhcg expression, followed by parallel subsequent development of apical and basolateral expression in intercalated cells. This identification of basolateral Rhcg expression in the developing rat kidney is consistent with its expression in the rat, mouse, and human kidney (12, 25, 48, 49). Thus simultaneous expression of apical and basolateral Rhcg during renal development is consistent with the adult expression pattern and with Rhcg mediating an important role in both apical and basolateral plasma membrane ammonia transport. The identification of basal Rhcg expression at E16, before the onset of other proteins involved in intercalated cell function, its expression in all collecting duct cells, without differentiation between intercalated cells and principal cells, and its rapid disappearance at E18, and its later reappearance with intercalated cell-predominant expression in the postnatal period, raise the possibility that basal Rhcg expression at E16 mediates a role other than in ammonia transport. Interestingly, other ammonia transporter family members, particularly the yeast protein, Mep2, have been shown to have “sensor” functions (38). However, Rhcg expression does not appear to be essential for collecting duct development as there is normal collecting duct development in mice with either global or collecting duct-specific Rhcg deletion (2, 30).
Immature Rhbg and Rhcg expression at birth is consistent with findings that renal ammonia metabolism is incompletely developed at the time of birth. In the human fetus, between 20 and 38 wk of gestation, urinary ammonia levels average <1 mmol/l (43), whereas in adults urinary ammonia levels are typically 20–40 mmol/l. Studies performed in human infants shortly after birth have shown an intact ability to acidify urine but impaired ability to excrete ammonia (40–43), and premature infants have further impairment of renal ammonia excretion compared with full-term infants (10). Intact ability to acidify urine but impaired ammonia excretion suggests defects in ammonia transport, metabolism, or both and are thereby consistent with the observation in the current study of immature Rhbg and Rhcg expression at birth. In particular, recent studies show that both global and collecting duct-specific Rhcg deletion results in an intact ability to acidify urine but impaired basal and acidosis-stimulated ammonia excretion (2, 30). Animal models demonstrate both low levels of basal ammonia excretion in newborn animals and that the response to acid loads is impaired. In the fetal lamb, urinary ammonia excretion in response to an acid load are impaired (53). In 9- to 14-day-old rats, a time at which the current studies show that Rhbg and Rhcg expression is immature, urinary ammonia excretion rates are suppressed compared with adult rats and do not increase significantly in response to an acid load (1, 8, 9). Importantly, acid loads increase urinary acidification, indicating that the defect in ammonia secretion cannot be ascribed to defective H+ secretion (1, 8). Studies in newborn, 2-day-old dogs demonstrate an inability to increase urinary ammonia excretion in response to an acid load despite an intact ability to increase urinary acidification (5). These consistent observations in multiple species of impaired ammonia excretion despite intact urine acidification may be due, at least in part, to immature expression of the ammonia transporter family members, Rhbg and Rhcg.
Impaired ammonia metabolism in the fetus and newborn appears to involve immature expression of many of the proteins involved in renal ammonia metabolism in addition to Rhbg and Rhcg. Renal glutamine, the substrate for renal ammoniagenesis, is present at only 55% of adult levels at P9 (9). Phosphate-dependent glutaminase, the rate-limiting enzyme for proximal tubule ammonia production, exhibits decreased expression in early fetal development, followed by gradual increases in expression over the initial few weeks following birth (22, 23, 34, 46). Similar findings have been observed for phosphoenolpyruvate carboxykinase (21, 60). Ammonia produced from glutamine in the proximal tubule undergoes preferential secretion in the proximal tubule through mechanisms that appear to involve type 3 Na+/H+ exchanger (NHE3) and reabsorption by the thick ascending limb of the loop of Henle that appear to involve Na+-K+-2Cl− cotransporter (NKCC2) (6, 11, 29). Expression of both NHE3 and NKCC2 is immature at the time of birth and undergoes maturational increases in the postnatal period (31, 32). Thus immature expression of Rhbg and Rhcg at the time of birth in conjunction with incomplete development of other components of renal ammonia metabolism and transport likely contribute to impaired ammonia excretion in the newborn.
Immature urinary ammonia excretion mechanisms in the newborn may be physiologically appropriate. In normal weanling rats and in human infants, careful metabolic balance studies demonstrate a net positive alkali balance (24, 50, 51). This limits the renal need for net acid excretion, of which ammonia is the primary component. The limited need, and capacity, to excrete urinary ammonia may also be beneficial in that early development requires a positive nitrogen balance; limiting urinary ammonia excretion and nitrogen losses contributes to a positive nitrogen balance.
A secondary finding in the current study is increased information about the cell-specific expression of the nonerythroid Rh glycoproteins in intercalated cells in the mammalian kidney. We reported previously that Rhbg is expressed in high levels in the A-type intercalated cell and the non-A, non-B intercalated cell, but not in the B-type intercalated cell (54). More recently, studies using immunogold electron microscopy showed high levels of apical and basolateral Rhcg in the A-type intercalated cell whereas the non-A, non-B intercalated cell expressed high levels of only apical Rhcg (25). The current study adds to these findings by confirming expression of Rhcg in the A-type intercalated cell and the non-A, non-B intercalated cell, but not in the B-type intercalated cell. Moreover, the differential expression pattern of Rhbg and Rhcg in the three major intercalated cell types, A-type intercalated cell, B-type intercalated cell, and the non-A, non-B intercalated cell, supports the finding that these different intercalated cell types have differing physiological functions.
The coordinated developmental regulation of Rhbg and Rhcg along with other acid-base transporters suggests the possibility that there might similar developmental regulatory pathways. One possible regulatory pathway involves the forkhead transcription factor Foxi1. Foxi1 is localized to the distal nephron of mice beginning at E16 (44), and genetic deletion of Foxi1 is associated with altered cellular composition of the collecting duct and failure of collecting duct intercalated cells to express several acid-base transport proteins, including pendrin, H+-ATPase, AE1, and AE4 (3, 20). Another possible transcription factor that may be involved is the grainyhead-related transcription factor CP2-like1 (CP2L1); mice lacking CP2L1 lack normal developmental expression of the intercalated cell proteins H+-ATPase and pendrin (59).
In summary, the current studies define the developmental renal expression of the ammonia transporter family members, Rhbg and Rhcg. These proteins are expressed initially in two different sites at the time of intercalated cell differentiation. Moreover, the incomplete expression of these ammonia transporter family members in the fetal and early neonatal period likely contributes to incomplete ability to excrete urinary ammonia. These observations have important implications for the development of renal ammonia and net acid excretion.
GRANTS
Funds from the National Institutes of Health (NIH; R01-DK045788), the Department of Veterans Affairs Merit Review Program, and the National Research Foundation of Korea (2009-0073733) supported this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH, or the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Gina Cowsert for secretarial assistance.
REFERENCES
- 1.Benyajati S, Goldstein L. Renal glutaminase adaptation and ammonia excretion in infant rats. Am J Physiol 228: 693–698, 1975 [DOI] [PubMed] [Google Scholar]
- 2.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnici B, Wagner CA. Postnatal expression of transport proteins involved in acid-base transport in mouse kidney. Pflügers Arch 448: 16–28, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cort JH, McCance RA. The renal response of puppies to an acidosis. J Physiol 124: 358–369, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol 1: 1193–1203, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein L. Renal ammonia and acid excretion in infant rats. Am J Physiol 218: 1394–1398, 1970 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein L. Ammonia metabolism in kidneys of suckling rats. Am J Physiol 220: 213–217, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Gordon HH, McNamara H, Benjamin HR. The response of young infants to ingestion of ammonium chloride. Pediatrics 2: 290–302, 1948 [PubMed] [Google Scholar]
- 11.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han KH, Jung JY, Cha JH, Kim H, Madsen KM, Kim J. 1,25-Dihydroxyvitamin D3 stimulates osteopontin expression in rat kidney. Nephron Physiol 93: 76–86, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B glycoprotein and Rh C glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297: L153–L163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han KH, Woo SK, Kim WY, Park SH, Cha JH, Kim J, Kwon HM. Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol 287: F878–F885, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol 293: F1342–F1354, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerbäck S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development 130: 2013–2025, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Jones CT, Ashton IK. The development of some enzymes of gluconeogenesis in the liver and kidney of the foetal guinea pig. Biochem J 130: 23P–24P, 1972 [PMC free article] [PubMed] [Google Scholar]
- 22.Katunuma N, Katsunuma T, Tomino I, Matsuda Y. Regulation of glutaminase activity and differentiation of the isozyme during development. Adv Enzyme Regul 6: 227–242, 1968 [DOI] [PubMed] [Google Scholar]
- 23.Katunuma N, Tomino I, Sanada Y. Differentiation of organ specific glutaminase isozyme during development. Biochem Biophys Res Commun 32: 426–432, 1968 [DOI] [PubMed] [Google Scholar]
- 24.Kildeberg P, Engel K, Winters RW. Balance of net acid in growing infants. Endogenous and transintestinal aspects. Acta Paediatr Scand 58: 321–329, 1969 [DOI] [PubMed] [Google Scholar]
- 25.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Cha JH, Tisher CC, Madsen KM. Role of apoptotic and nonapoptotic cell death in removal of intercalated cells from developing rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 270: F575–F592, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Tisher CC, Madsen KM. Differentiation of intercalated cells in developing rat kidney: an immunohistochemical study. Am J Physiol Renal Fluid Electrolyte Physiol 266: F977–F990, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Knepper MA. NH4+ transport in the kidney. Kidney Int 40: S95–S102, 1991 [PubMed] [Google Scholar]
- 30.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HW, Kim WY, Song HK, Yang CW, Han KH, Kwon HM, Kim J. Sequential expression of NKCC2, TonEBP, aldose reductase, and urea transporter-A in developing mouse kidney. Am J Physiol Renal Physiol 292: F269–F277, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Li XX, Albrecht FE, Robillard JE, Eisner GM, Jose PA. Gβ regulation of Na/H exchanger-3 activity in rat renal proximal tubules during development. Am J Physiol Regul Integr Comp Physiol 278: R931–R936, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linder-Horowitz M. Changes in glutaminase activities of rat liver and kidney during pre- and post-natal development. Biochem J 114: 65–70, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Huang CH. The mouse Rhl1 and Rhag genes: sequence, organization, expression, and chromosomal mapping. Biochem Genet 37: 119–138, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Lorenz MC, Heitman J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J 17: 1236–1247, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000 [DOI] [PubMed] [Google Scholar]
- 40.McCance RA, Hatemi N. Control of acid-base stability in the newly born. Lancet 1: 293–297, 1961 [DOI] [PubMed] [Google Scholar]
- 41.McCance RA, Widdowson EM. Renal function before birth. Proc R Soc Lond 141: 488–497, 1953 [PubMed] [Google Scholar]
- 42.McCance RA, Widdowson EM. Renal aspects of acid-base control in the newly born. 1. Natural development. Acta Paediatr 49: 409–414, 1960 [Google Scholar]
- 43.Muller F, Dommergues M, Bussieres L, Lortat-Jacob S, Loirat C, Oury JF, Aigrain Y, Niaudet P, Aegerter P, Dumez Y. Development of human renal function: reference intervals for 10 biochemical markers in fetal urine. Clin Chem 42: 1855–1860, 1996 [PubMed] [Google Scholar]
- 44.Overdier DG, Ye H, Peterson RS, Clevidence DE, Costa RH. The winged helix transcriptional activator HFH-3 is expressed in the distal tubules of embryonic and adult mouse kidney. J Biol Chem 272: 13725–13730, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Radde IC, McCance RA. Glutaminase activity of the foetal membranes and kidneys of pigs. Nature 183: 115–116, 1959 [DOI] [PubMed] [Google Scholar]
- 47.Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int 20: 353–358, 1982 [DOI] [PubMed] [Google Scholar]
- 48.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter, Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Shohl AT, Sato A. Acid-base metabolism. I. Determination of base-balance. J Biol Chem 58: 235–255, 1923 [Google Scholar]
- 51.Slater JE. Retentions of nitrogen and minerals by babies one week old. Br J Nutr 15: 83–97, 1961 [Google Scholar]
- 52.Song HK, Kim WY, Lee HW, Park EY, Han KH, Nielsen S, Madsen KM, Kim J. Origin and fate of pendrin-positive intercalated cells in developing mouse kidney. J Am Soc Nephrol 18: 2672–2682, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Vaughn D, Kirschbaum TH, Bersentes T, Dilts PV, Assali NS. Fetal and neonatal response to acid loading in the sheep. J Appl Physiol 24: 135–141, 1968 [DOI] [PubMed] [Google Scholar]
- 54.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hyper 13: 533–540, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Weiner ID. Expression of the non-erythroid Rh glycoproteins in mammalian tissues. Transfus Clin Biol 13: 159–163, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi Y, Yonemura S, Takada S. Grainyhead-related transcription factor is required for duct maturation in the salivary gland and the kidney of the mouse. Development 133: 4737–4748, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Yeung D, Oliver IT. Gluconeogenesis from amino acids in neonatal rat liver. Biochem J 103: 744–748, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]