Abstract
The fine regulation of Na+ and K+ transport takes place in the cortical distal nephron. It is well established that K+ secretion occurs through apical K+ channels: the ROMK and the Ca2+- and voltage-dependent maxi-K. Previously, we identified the voltage-gated Kv1.3 channel in the inner medulla of the rat kidney (Escobar LI, Martínez-Téllez JC, Salas M, Castilla SA, Carrisoza R, Tapia D, Vázquez M, Bargas J, Bolívar JJ. Am J Physiol Cell Physiol 286: C965–C974, 2004). To examine the role of Kv1.3 in the renal regulation of K+ homeostasis, we characterized the effect of dietary K+ on the molecular and functional expression of this channel. We performed real-time-PCR and immunoblot assays in kidneys from rats fed a control (CK; 1.2% wt/wt) or high-K+ (HK; 10% wt/wt) diet for 5–15 days. Kv1.3 mRNA and protein expression did not change with HK in the whole kidney. However, dietary K+ loading provoked a change in the cellular distribution of Kv1.3 from the cytoplasm to apical membranes. Immunolocalization of Kv1.3 detected the channel exclusively in the intercalated cells. We investigated whether Kv1.3 mediated K+ transport in microperfused cortical collecting ducts (CCDs). The HK diet led to an increase in net K+ transport from 7.4 ± 1.1 (CK) to 11.4 ± 1.0 (HK) pmol·min−1·mm−1. Luminal margatoxin, a specific blocker of Kv1.3, decreased net K+ secretion in HK CCDs to 6.0 ± 1.6 pmol·min−1·mm−1. Our data provide the first evidence that Kv1.3 channels participate in K+ secretion and that apical membrane localization of Kv1.3 is enhanced in the intercalated cells by dietary K+ loading.
Keywords: potassium diet, potassium transport, distal nephron, intercalated cell, rat kidney, urine pH
kv1.3 is a voltage-gated potassium channel (Kv), and its activation results in outward K+ currents that suppress excitability in neurons. Kv1.3 is widely distributed in diverse cell types: lymphocytes (7, 9), neurons (63), kidney (11, 84), liver, skeletal muscle, testis, spermatozoa (29), and osteoclasts (3). Kv1.3 participates in the stabilization of the cell resting membrane potential in nonexcitable cells. In addition, Kv1.3 participates in several cell functions that range from apoptosis (64), cell volume regulation (9), to T cell stimulation (50). Kv1.3 activity is regulated by phosphorylation: PKC enhances Kv1.3 channel activity by shifting its voltage dependence in human lymphocytes (8), while tyrosine phosphorylation at multiple sites in the amino and carboxyl terminus of Kv1.3 channel suppresses channel activity (13, 14). Kv1.3 contributes also to glucose homeostasis: the Kv1.3-deficient mice (Kv1.3−/−) weigh less than control littermates and are protected from diet-induced obesity (82). Furthermore, inhibition of Kv1.3 channel activity improves insulin sensitivity by upregulation of the glucose transporter GLUT4 at the plasma membrane (83). Finally, Kv1.3 activity is upregulated by the serum-glucocorticoid-activated kinase (SGK), one of the main mediators of aldosterone's action at the renal distal tubule (77).
K+ channels fulfill a critical role in K+ recycling and K+ secretion in the kidney. The mechanisms underlying and regulation of renal K+ secretion have been studied mainly in the mammalian cortical collecting duct (CCD), a segment that can be isolated for patch-clamp analysis and in vitro microperfusion (2, 4, 10, 12, 16, 19, 21, 42, 56, 57, 62, 65, 66, 79). A high-K+ diet (HK) for days to weeks stimulates net K+ secretion in the CCD (39, 41, 42, 61). The K+ adaptation exhibited by the distal tubule in response to chronic dietary K+ loading is due in part to an increase of the basolateral Na+-K+-ATPase activity (39), and to an upregulation of the apical ROMK (19) and maxi-K (42) channels in CDs.
It is well established that the ROMK inwardly rectifying K+ channel corresponds to the low-conductance or secretory K+ channel (SK) of the CCD (46). Due to its voltage independence and weak inward rectification, ROMK allows K+ ions to exit the cell at voltages above the potassium equilibrium potential. ROMK has been recognized as the major route for K+ secretion in the distal nephron under baseline conditions (75). Intriguingly, patients with Bartter's syndrome due to loss of function of ROMK (59) and ROMK knockout mice (37, 38) still show net K+ secretion in the urine, without the hyperkalemia expected from the absence of ROMK. The maxi-K channel has been proposed to mediate K+ secretion in the absence of ROMK (3, 37, 38, 42); however, iberiotoxin, a specific inhibitor of the former channel, fails to completely inhibit urinary K+ excretion in ROMK knockout mice (4). These observations suggest the presence of other K+-secretory pathways in the kidney.
In a previous study, we recorded an outward K+-selective conductance in a primary cell culture of the rat inner medullary collecting duct. We identified three members of the Kv1 subfamily, i.e., Kv1.1, Kv1.3, and Kv1.6, by immunofluorescence and immunoblot assays (11). In the present study, we examined the expression of Kv1.3 in cortical and medullary CDs of kidneys from rats fed CK and HK diets.
METHODS
Animals.
Male Wistar rats (200 g) were used for all studies. Animals were anesthetized by intraperitoneal administration of pentobarbital sodium and killed by cervical dislocation. The animal protocol for microperfusion studies was approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine.
Membrane protein extraction from rat kidney.
Brain and kidney [cortex (Cx); outer (OM) and inner (IM) medulla] were homogenized (0.1 g tissue/1 ml) in a buffer solution containing 250 mM sucrose, 1 mM EDTA, 10 mM Tris·HCl buffer, pH 7.6, and a protease cocktail inhibitor complete mini 1 tablet/10 ml (Roche Diagnostics, Rotkreuz, Switzerland). Large tissue debris and nuclear fragments were removed by two low-speed spins (1,000 g, 10 min, 4°C). The supernatant (crude membranes) was collected and centrifuged (17,000 g, 20 min, 4°C). The pellet enriched in plasma membranes was resuspended in buffer solution (300 μl). The protein concentration was measured with the Bio-Rad D C protein assay (Bio-Rad, Hercules, CA).
Immunoblotting.
Crude or plasma membrane samples (50 μg) were electrophoretically separated in 6% SDS-PAGE and electroblotted to a nylon membrane (Amersham Biosciences, Freiburg, Germany). Nonfat dry milk (5%, Bio-Rad) in TBS-T (20 mM Tris·HCl, 136 mM NaCl, 0.1% Tween 20, pH 7.6) was added for 1 h. The nylon membrane was incubated overnight with rabbit polyclonal antibodies against Kv1.3 (1:300, Alomone, Jerusalem, Israel) diluted in TBS-T at 4°C. The membrane was rinsed three times (10 min each) with TBS-T and incubated with the secondary antibody donkey anti-rabbit IgG coupled to horseradish peroxidase (1:5,000; Amersham Biosciences) in TBS-T for 1 h at room temperature (RT). Immunoblots were detected using ECL plus detection reagents (Amersham Biosciences) and exposed to autoradiographic film for 1 min (Kodak). A brain sample and the primary antibody preincubated with its antigen control served as the positive and negative control, respectively (6).
Real-time RT-PCR.
Cx and medulla sections were isolated from both kidneys and snap frozen in liquid nitrogen. Total RNA was isolated from each kidney following the TRIzol method (Invitrogen, Carlsbad, CA) as previously reported (11). To avoid DNA contamination, all total RNA samples were treated with DNAase I (Invitrogen). Reverse transcription (RT) was carried out with 2.5 μg of total RNA using 200 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen). Kv1.3 mRNA was quantified by real-time RT-PCR on the ABI Prism 7300 Sequence Detection System (TaqMan, Applied Biosystems ABI, Foster City, CA). Primers and probes were ordered as kits: Rn01521807_s1 (Assays-on-Demand, ABI). Eukaryotic 18S rRNA (predesigned assay reagent Applied by ABI, external run) was used as an endogenous control. Relative quantification of the Kv1.3 expression was performed using the comparative CT method (36).
K diet and functional parameters.
Male Wistar rats (200–250 g) were fed a HK (10%) or CK (1.2%) diet (Harlan-Teklad, Madison, WI) for 15 days (n = 6/group), and sterile distilled water was made freely available. At the end of the study, rats were placed in metabolic cages and urine spontaneously voided during 24 h was collected. Na+ and K+ concentrations were measured in plasma and urine samples with a NOVA4 electrolyte analyzer (NOVA Biomedical). Absolute and fractional rates of Na+ and K+ excretion were calculated. In addition, serum aldosterone levels were quantitatively determined by radioimmunoassay (DiaSorin). Rats were anesthetized, and kidneys were removed and processed as above. Thirty micrograms of protein from Cx, OM, and IM were assayed for Western blot analysis.
Urinary pH and Pco2.
Twenty-four hours before death, rats were housed individually in metabolic cages with free access to tap water. Individual 12-h urine samples were collected under mineral oil. Urinary pH was measured immediately using a pH microelectrode connected to an Orion 410A pH meter (Orion Research), whereas urinary Pco2 concentrations were measured with a gas analyzer (model 5395, Roche Diagnostics).
Immunohistochemistry.
Tissues were retrograde perfused via the aorta with ice-cold PBS (20 mM NaH2PO4/Na2HPO4, pH 7.4 buffer, 149 mM NaCl, 2 mM KCl) for 5 min and then with 4% paraformaldehyde in PBS (PFAS) for 5 min. Kidneys were removed and fixed overnight. After fixation, tissues were cryoprotected with 30% sucrose in PFAS. Sagittal sections (μm) were cut in a cryostat (CM1100, Leica), collected on slides, and hydrated in PBS. Antigen retrieval was performed incubating the slides in 10 mM sodium citrate buffer, pH 6, for 30 min at 80°C. The samples were preincubated for 1 h at RT with 0.3% H2O2 in PBS and permeabilized with 0.3% Triton X-100 (USB, Cleveland, OH) in PBS (10 min). Blocking was performed with 1% BSA, 5% fetal bovine serum, 5% donkey serum, and 0.1% Triton X-100 in PBS for 1 h at RT. The samples were then incubated with rabbit anti-Kv1.3 antibody (1:100; Alomone) in the same solution for 24 h at 4°C. Incubation without anti-Kv1.3 antibody or preincubation of the antibody with the control peptide served as a negative control. After three washes with 0.1% Triton X-100 in PBS (PBS-TX), sections were incubated with the secondary antibody coupled to horseradish peroxidase (donkey anti-rabbit, 1:200 Amersham) for 1 h at RT. Samples were then washed with PBS-TX for 10 min, three times and incubated with 0.03% diaminobenzidine (Sigma), 0.1% H2O2, in PBS during 5 min. The samples were washed for 10 min in PBS, dehydrated in an ethanol-xylol train, i.e., 30% EtOH (in tap water); 70% EtOH; 90% EtOH; 100% EtOH; 100% EtOH; 50% EtOH-50% xylol; 100% xylol, 100% xylol (5 min each), and mounted on coverslips with Permount mounting media (Electron Microscopy Sciences). Slides were observed under ×20 and ×40 objectives with a bright light Nikon eclipse 80i microscope, and images were captured with a Coolpix-4300 Nikon digital camera with four megapixels of resolution.
Immunofluorescence.
Tissues were processed as described above but without preincubation with H2O2 after the antigen retrieval. To identify specific cell types in CDs, double-immunofluorescence staining was performed using rabbit anti-Kv1.3 (1:100), a secondary antibody coupled to Alexa 594 (goat anti-rabbit, 1:200; Molecular Probes, Eugene, OR), and the specific CD principal cell marker Dolichus biflorus aggutinin (DBA), coupled to 5 μg/ml fluorescein (DBA-F; Sigma), or cells were immunostained with the goat anti-B1-H-VATPase (1:200, Santa Cruz Biotechnology) as a marker of acid-secreting intercalated cells in DBA-positive segments, and donkey anti-goat secondary antibodies coupled to Alexa 488 (1:500; Molecular Probes). Tissues were mounted on microscope coverslips with Vectashield (Vector Laboratories). Slides were examined and images were obtained with a ×40 oil-immersion objective mounted on a confocal inverted microscope (Leica TCS-SP5). Image analysis was performed with the Leica Application Suite Advance Fluorescence Lite program.
In vitro microperfusion assays.
Kidneys from CK and HK rats were removed via a midline incision, and single CCDs were dissected freehand in cold (4°C) Ringer solution containing (in mM) 135 NaCl, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 lactate, 6.0 l-alanine, 5.0 HEPES, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O. A single tubule was studied from each animal. Isolated CCDs were microperfused in vitro as previously described (42, 56, 80). Briefly, each isolated tubule was immediately transferred to a temperature- and O2/CO2-controlled specimen chamber, mounted on concentric glass pipettes, and perfused and bathed at 37°C with Burg's perfusate containing (in mM) 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 Na lactate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O. During the 30-min equilibration period and thereafter, the perfusion chamber was continuously suffused with a gas mixture of 95% O2-5% CO2 to maintain the pH of Burg's solution at 7.4 at 37°C. The bathing solution was continuously exchanged at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT).
Transport measurements were performed in the absence of transepithelial osmotic gradients, and thus water transport was assumed to be zero. Three to four samples of tubular fluid were collected under water-saturated light mineral oil by timed filling of a calibrated ∼15-nl volumetric constriction pipette before and at least 20–30 min after addition and in the continued presence of margatoxin (MgTX; 1 nM) in the luminal perfusate. CCDs were perfused at a rate of ∼2.5 nl·min−1·mm−1; the flow rate was maintained by adjusting the height of the perfusate reservoir. To determine the concentration of K+ and Na+ delivered to the tubular lumen, ouabain (200 μM) was added to the bath at the conclusion of each experiment to inhibit active transport, and an additional three to four samples of tubular fluid were obtained for analysis. The cation concentrations of perfusate and collected tubular fluid were determined by helium glow photometry, and the rates of net transport (Jx; in pmol·min−1·mm tubular length−1) were calculated using standard flux equations (56, 80). The calculated ion fluxes were averaged to obtain a single mean rate of ion transport for the CCD under each condition.
Statistics.
The commercially available SigmaStat 3.5 program was used for statistical analysis. All values were expressed as means ± SE and evaluated to determine a normal distribution. Statistical comparisons of differences were performed using one-way ANOVA combined with the Bonferroni test. P < 0.05 was considered for statistical significance. In each analysis, n refers to the number of animals in each group.
RESULTS
Expression of Kv1.3 in rat kidney Cx and medulla.
Immunoblotting analysis of Kv1.3 in crude and plasma membrane samples from rat brain, kidney Cx, OM, and IM revealed a band at ∼70 kDa (Fig. 1). The amount of Kv1.3 protein in plasma membranes was lower than in crude membranes from the three renal sections. Therefore, Kv1.3 is expressed predominantly in the cytoplasm of the Cx and medulla, according to our previous observations in the rat IM collecting duct (11).
Fig. 1.
Immunoblot analysis of Kv1.3 protein in rat kidney. Immunoreactive bands corresponding to Kv1.3 protein were detected at ∼70 kDa in crude membranes (cm) and plasma membranes (pm). Samples were obtained from brain, renal cortex (Cx), outer (OM), and inner (IM) medulla. Bands disappeared when primary antibodies were preincubated with their control peptide (CP+).
Effect of high K+ load on serum and urine electrolytes.
It is well established that an increase in dietary K+ enhances the rate of K+ secretion in the distal nephron (39, 41, 42, 61). We fed rats a HK (10%) or CK (1.2%) diet for 15 days. A summary of Na+ and K+ concentrations in plasma and urine as well as that of serum aldosterone in rats adapted to the above diets is shown in Table 1. As expected, serum Na+ concentration was not affected by dietary K+, although a kaliuretic effect of HK was observed. The urinary K+ concentration was fivefold greater (0.277 ± 0.02 meq K+/ml) in HK compared with CK rats (0.055 ± 0.005 meq K+/ml). Plasma K+ was similar in HK and CK rats (4.3 ± 0.3 and 4.2 ± 0.2 meq/l, respectively), consistent with renal K+ adaptation (81). The serum aldosterone concentration in CK (213.8 ± 20) rats was significantly less than that measured in HK (1,097.0 ± 200.1 pg/ml) rats, in accordance with other reports (34, 48, 61).
Table 1.
Effect of a high-K+ diet on electrolytes and aldosterone levels
| Diet | n | Serum Na, meq/l | Serum K, meq/l | Urinary Na, meq/ml | Urinary K, meq/ml | Serum Aldosterone, pg/ml |
|---|---|---|---|---|---|---|
| Control K+ | 4 | 147.0 ± 0.6 | 4.3 ± 0.3 | 0.022 ± 0.001 | 0.055 ± 0.005 | 213.8 ± 20 |
| High K+ | 6 | 145.6 ± 0.8 | 4.2 ± 0.2 | 0.014 ± 0.003 | 0.277 ± 0.02* | 1,097.0 ± 200.1* |
Values are means ± SE; n = no. of rats. Rats were fed for 15 days.
P < 0.05 vs. control K+.
Effect of HK diet on Kv1.3 mRNA and protein expression in rat kidney.
First, we quantified the effect of the HK diet on Kv1.3 mRNA expression in the whole kidney by real-time RT-PCR. The steady-state abundance of the Kv1.3 transcript in rats fed a HK diet for 15 days was similar to control animals (Fig. 2A). We acknowledge that analysis of steady-state mRNA expression after 15 days of a HK diet, once adaptation has occurred, may have missed an earlier increase, as has been described for genes regulated during metabolic acidosis (43). Therefore, we proceeded to determine whether a HK diet modified the channel protein expression. The relative amount of Kv1.3 protein in crude membranes from the kidney sections was not changed by the HK (Fig. 2B). In contrast, immunoblot analysis of plasma membranes showed that Kv1.3 was significantly increased in all kidney sections by the HK diet (Fig. 3A). Specifically, quantification of Kv1.3 protein in plasma membranes identified increases of 2.5-fold in Cx, 4-fold in OM, and 2-fold in IM (Fig. 3B) in response to dietary K+ loading. Therefore, we conclude that the HK diet upregulated Kv1.3 protein in plasma membranes.
Fig. 2.
Dietary K+ intake has no effect on Kv1.3 channel transcript and protein expression. A: real-time PCR assays were performed to detect the relative abundance of Kv1.3 transcript in Cx, OM, and IM from rats fed control K+ (CK) and high-K+ (HK) diets. There was no significant difference in the Kv1.3 mRNA expression in any region between the CK and HK groups (n = 6). B: representative Western blot showing Kv1.3 in crude membranes from Cx, OM, and IM from rats CK and HK diets. Crude protein (30 μg) was loaded on each lane. No differences were observed in Kv1.3 protein expression between the CK and HK groups.
Fig. 3.
Effect of dietary K+ intake on Kv1.3 expression in plasma membranes. A: representative immunoblot of Kv1.3 in plasma membranes from Cx, OM, and IM of rats fed CK and HK diets. Thirty micrograms of protein was loaded on each lane. B: significant relative increase was detected in the 3 regions of kidney studied in response to dietary K+ loading, suggesting increased trafficking or stability of Kv1.3 protein to/on plasma membranes. Values are mean ± SE; n = 6/dietary group. *P < 0.05.
Kv1.3 is localized in intercalated cells.
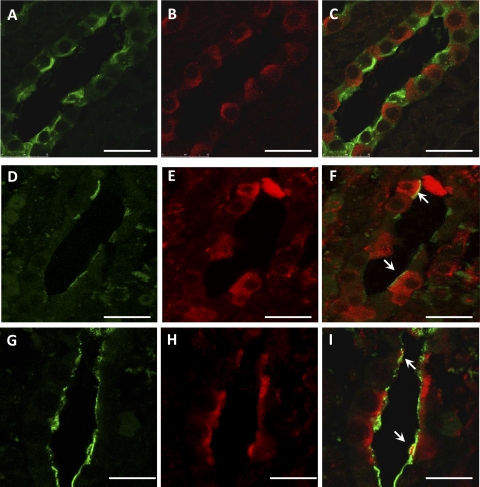
Immunohistochemistry for Kv1.3 revealed significant changes in channel distribution after the HK diet. Kv1.3 staining of CDs was intracellular under the CK diet (Fig. 4, A and C). Remarkably, the HK diet provoked the trafficking of Kv1.3 to the apical membranes of CDs (Fig. 4, B and D). This finding suggested that to enhance urinary excretion of the dietary K+ load, Kv1.3 is directed to luminal membranes of CDs to accomplish, at least in part, this task. CDs include both principal and intercalated cells (25). Coincident double-labeling of tissue sections with anti-Kv1.3 and DBA for principal cells or B1-H-VATPase for intercalated cells revealed localization of Kv1.3 exclusively in intercalated cells (Fig. 5).
Fig. 4.
Immunohistochemistry of Kv1.3 in the kidney Cx in rats fed CK (A) and HK diets (B). Cortical collecting ducts (CCDs) are observed with positive cytoplasmic (A) and polarized (B, arrows) immunoreactivity. Higher magnification shows a cytoplasmic distribution in all CCD cells of Kv1.3 in CK rats (C); the inset at the bottom left shows a negative control (preincubation of the anti-Kv1.3 antibody with the control peptide). The HK diet enhances apical expression of Kv1.3 in a few cells (D, arrows). Bars = 50 μm.
Fig. 5.
Kv1.3 expression in collecting duct intercalated cells from kidneys of HK-fed rats. Shown is detection of principal cells with Dolichus biflorus aggutinin coupled to fluorescein (DBA-F; A; green), intercalated cells with goat-anti-B1-H-VATPase antibody (D and G; green), and Kv1.3 with an anti-Kv1.3 antibody (B, E, and H; red) in collecting ducts of HK-fed rats by indirect immunofluorescence confocal microscopy. Note that Kv1.3 is localized only to cells lacking DBA-F staining (C; merged) and in presumably intercalated cells expressing apical H+-VATPase (F and I; merged). Arrows point to apical Kv1.3-H+-VATPase colocalization (yellow). Bars = 25 μm.
Kv1.3 is a secretory channel during high dietary K+.
The observations of apical localization of Kv1.3 in intercalated cells and its upregulation in the plasma membrane by HK strongly suggested that Kv1.3 participates in K+ secretion under HK conditions. To test this possibility, we examined the contribution of Kv1.3 to net K+ secretion in isolated, microperfused CCDs. Net K+ secretion was 7.4 ± 1.1 pmol·min−1·mm−1 in CK CCDs and was not affected by luminal MgTX, a specific Kv1.3 blocker (18) (Fig. 6A). In rats fed a HK diet, net K+ secretion was 54% greater than that measured in CK tubules, averaging 11.4 ± 1.0 pmol·min−1·mm−1. This K+-secretory flux was significantly inhibited to 6.0 ± 1.6 pmol·min−1·mm−1 by luminal addition of MgTX (Fig. 6A). Net Na+ absorption was similar in the absence and presence of MgTX in both CK and HK CCDs.
Fig. 6.
Margatoxin (MgTX) reduces net K+ secretion in CCDs isolated from HK-fed rats. Net transport of K+ (JK; A) and Na+ (JNa; B) in single CCDs from CK (n = 4) and HK (n = 3; 5 days) rats is shown before and after 1 nM MgTX is added to the luminal perfusate. In CK rats, MgTX failed to have any significant effect on net K+ secretion. K+ secretion, significantly increased in response to dietary K+ loading, was inhibited by MgTX. Na+ absorption was not affected by MgTX. #P < 0.05 compared with CK diet tubule. *P < 0.05 compared with HK tubules perfused without MgTX.
Urinary alkalinization is produced by chronic HK loading.
An increase in Kv1.3 activity at the apical membrane would tend to hyperpolarize the intercalated cell, which could affect the activity of the H-ATPase pump. A more positive lumen membrane voltage would decrease the electrochemical driving force for H+ secretion. To our knowledge, measurements of total CO2 concentration and/or urine pH and Pco2 have not been reported in rats fed a HK diet. Therefore, we measured urine pH and Pco2 at 3, 7, and 10 days of HK. Remarkably, urine pH and Pco2 changed significantly with HK (Table 2). Urine pH increased from 6.27 ± 0.05 (CK) to 7.55 ± 0.14 (10 days of HK) and Pco2 (mmHg) quadrupled from 33.96 ± 3.3 (CK) to 131.6 ± 7.5 (10 days of HK). This HK-induced alkalinization of the urine is consistent with either enhanced net HCO3− secretion or net inhibition of HCO3− absorption in the rat CD by dietary K+ loading, possibilities that remain to be examined.
Table 2.
Effect of a high-K+ diet on urine pH and Pco2 levels
| Day 0 | Day 3 | Day 7 | Day 10 | |
|---|---|---|---|---|
| pH | 6.27 ± 0.05 | 7.03 ± 0.23 | 7.66 ± 0.15 | 7.55 ± 0.14 |
| Pco2, mmHg | 33.96 ± 3.3 | 67.04 ± 9.3* | 107.7 ± 9.1* | 131.6 ± 7.5* |
Values are means ± SE. Rats were fed for 15 days.
P < 0.05 vs. control K+.
DISCUSSION
In this work we explored the regulation of Kv1.3 channels in cortical and medullary CDs. Kv1.3 was localized mostly in the cytoplasm under basal metabolic conditions. Kv1.3 protein abundance is higher in the IM > OM > Cx.
ROMK and maxi-K channels are also localized in the cytoplasm, and their trafficking to apical membranes in distal tubules is regulated by dietary K+ (26, 42, 76). Thus, for example, the density of apical ROMK, estimated by patch-clamp recordings, and apical maxi-K channels, assessed as iberiotoxin-sensitive flow-stimulated net K+ secretion, increases in the CCD in response to a HK diet (16, 42, 45, 48, 71, 72, 73). Based on these reports, we examined the effect of dietary K+ loading on Kv1.3 mRNA and protein expression in the whole kidney, as well as its cellular localization. As expected, chronic K+ loading led to enhanced K+ urinary excretion and an increase in plasma aldosterone levels, thereby maintaining the physiological K+ levels in plasma (Table 1) (34, 48, 61, 81). Kv1.3 mRNA abundance in kidneys of animals adapted to a HK diet was similar to that in CK rats; whether an increase in transcript abundance occurred within the first week of dietary K+ loading was not examined. It should be noted that abundance of the maxi-K α- and β-subunit mRNAs and maxi-K α-subunit protein (42), as well as ROMK mRNA (16) and protein (78), is not modified in the rabbit and rat whole kidney, respectively; this may reflect the analysis of an admixture of multiple nephron segments and cells, “diluting” a response that might be localized to a single tubule. However, upregulation of maxi-K α- and β2-4-subunit mRNAs by HK diet has been detected in single CCDs (42).
Kv1.3 protein was upregulated in plasma membrane fractions from Cx, OM, and IM by HK diet. Importantly, HK loading led to a change in immunodetectable channel cellular localization from cytoplasmic to apical plasma membranes of CDs. We concluded at this stage that an intracellular pool of Kv1.3 exists under normal metabolic conditions and that K+ loading stimulates the trafficking of Kv1.3 in the CDs to the apical membrane, as has been reported for ROMK and maxi-K channels.
Kv1.3 trafficking has been explored in other cell types such as pyramidal neurons, where the T1 domain of Kv1.3 mediates targeting of the channel to the axonal surface (52). The cellular distribution of Kv1.3 is also determined by different Kv1 α-subunit associations to form a heterotetramer. Since Kv1.3 may associate with caveolin, heteromeric Kv1.3/Kv1.x channels target to distinct surface microdomains (67). To complicate the picture further, Kv1.3 trafficking, targeting and activity are also altered by the presence of the β-subunit KCNE4. KCNE4 associates with Kv1.3 in the endoplasmic reticulum (ER) and reduces the number of Kv1.3 channels at the cell surface (60). KCNE4 mRNA is abundant in the kidney (24), but its protein localization in the distal nephron has not as yet been explored. Finally, HK loading stimulates the expression of apical K+ channels in rat CCD by aldosterone-dependent and -independent signaling pathways. An HK diet suppresses the inhibitory action of WNK4 on ROMK channels through a SGK1 pathway (28). SGK1 and PKA stimulate renal K+ secretion by promoting the export of ROMK channels from the ER (85). Signaling pathways involved in the activation of Kv1.3 include the phosphatidylinositol-3 (PI3) protein kinase, 3-phosphoinositide-dependent protein kinase PDK1, and SGK1 (17, 77). In addition, Kv1.3 is a target of the ubiquitin ligase Nedd4–2, which leads to downregulation of the channel (27). Experiments aimed at testing whether similar pathways regulate Kv1.3 activity in intercalated cells, while important, remain beyond the scope of this paper.
Double-immunofluorescence labeling experiments with DBA-F and anti-B1-V-H-ATPase antibodies revealed apical membrane localization of Kv1.3 in acid-secretory intercalated cells. Morphological studies of intercalated cells in animals subjected to different experimental conditions have been interpreted as pointing to a role for these cells not in K+ secretion but rather in K+ reabsorption and in H+ or NH4+ secretion (30). Our detection of MgTX-sensitive and thus Kv1.3-mediated net K+ secretion in microperfused CCDs isolated from HK, but not CK rats, provides strong evidence that Kv1.3 is present in the apical membrane of rat CD and participates in K+ secretion during HK.
Although MgTX is relatively specific for Kv1.3 homomultimers, a related issue is the possible existence of other Kv1 homomultimers and/or Kv1.3/Kv1.x heteromultimers in the distal nephron. First, MgTX IC50 for homomultimers of Kv1.1, Kv1.2, and Kv1.3 is 144, 675, and 230 pM, respectively (32), and Kv1.3/Kv1.x heteromultimers have less inhibitor sensitivity. Therefore, 1 nM MgTX in the luminal perfusate solution will block apical homo- and/or heteromultimeric Kv1.1, Kv1.2, and Kv1.3 channels. Whether these channel combinations participate is still an open question. In any case, it will mean that we are underestimating the contribution of Kv1 channels to K+ secretion in dietary HK. Therefore, the data presented in this paper indicate the important role of Kv1 channels in K+ secretion during HK loading. Furthermore, since the Kv1.3 knockout mice do not develop obesity and no kidney failure has been reported so far, we can speculate that K+ secretion in HK diets is compensated not only by the upregulation of ROMK and maxi-K channels, but in addition by other homo- and/or heteromultimeric Kv1 channels (Kv1.1–Kv1.7) in the distal tubule. In addition, the fact that ROMK knockout mice secrete K+ and that K+ secretion in these animals is not completely eliminated by iberiotoxin, a specific inhibitor of Ca2+-activated maxi-K channels (37, 38), suggests now that Kv1.3 is an alternative pathway to accomplish this task.
Many patch-clamp studies have clearly identified conducting ROMK and maxi-K channels in distal tubules (45, 46, 47, 71, 74). An immediate question is why Kv1.3 channels have not as yet been functionally identified in these segments. While few electrophysiological analyses of intercalated cells have been performed, maxi-K channels have been characterized in intercalated cells (44), where their single-channel conductance (γ = 100–200 pS) (15, 44) is an order of magnitude higher than the corresponding Kv1.3 conductance (γ = 7–14 pS) (3, 84). Since both Kv channels activate in the same voltage range, we believe that large maxi-K currents mask the corresponding Kv1.3 currents in intercalated cells. Furthermore, Kv1.3 is blocked also by the nonspecific blockers Ba2+ and TEA, commonly used in electrophysiological assays of maxi-K currents in distal tubules (44, 45, 49, 79).
Another unresolved question is how intercalated cells could mediate net K+ secretion. It is well established that the driving force for K+ secretion through ROMK channels in principal cells is determined by electrogenic Na+ absorption through apical Na+ channels (ENaC) and enhanced basolateral Na+-K+-ATPase activity. Intercalated cells are generally considered to lack significant basolateral pump activity. However, immunocytochemical studies in the rat kidney performed with monoclonal antibodies directed against the Na+-K+-ATPase α-subunit and an antigen-retrieval technique revealed little labeling in intercalated cells of the CCD and outer medullary collecting duct (54). Although ouabain-sensitive currents were not detected in intercalated cells in rat connecting tubule and CCD (49), functional measurements of Na+-K+-ATPase pump activity have been reported (5).
It is now accepted that maxi-K channel-mediated, flow-stimulated net K+ secretion in the CCD is mainly due through intercalated cells, as the density of conducting (33, 44, 49, 57) and immunodetectable (12, 22, 23, 42, 51) channels in these cells is greater than that in principal cells. So how do intercalated cells take up K+ at their basolateral membranes to sustain K+ secretion? Immunodetectable NKCC1 is found along the basolateral membrane of type A H+-secreting intercalated cells (but not principal cells) in the rat outer medullary collecting duct (20), where it participates in the transepithelial transport of Na+, K+, and Cl− (68, 69). Mice with genetic disruption of NKCC1 exhibit increased plasma renin (but not aldosterone) and a higher serum K+ concentration, with inappropriately low urinary K+ excretion compared with wild-type mice (40, 70). The sensitivity of maxi-K channel-mediated, flow-stimulated but not basal JK to basolateral bumetanide (35), measured in microperfused rabbit CCDs and without an effect on flow-stimulated net Na+ absorption (JNa), suggests that basolateral NKCC1 may participate in K+ uptake into intercalated cells to sustain K+ secretion. In addition, we now have evidence that a cationic nonselective HCN channel (activated by cAMP and hyperpolarization) is present in intercalated cells that can mediate basolateral Na+ and K+ uptake (Escobar LI, unpublished observations). HCN channels in intercalated cells will allow Na+ (basal conditions) or K+ (in HK load) uptake. Passive Na+ uptake by HCN channels will explain basal JK by K+ channels present in the luminal membrane of intercalated cells. In fact, a recent preliminary patch-clamp study identified a Na+-activated high-conductance K channel in intercalated cells in the rat CCD, which notably expresses mRNA encoding Slo2 K+ channels (35). Remarkably, in microperfused rat CCDs, flow-stimulated K+ secretion was IBX insensitive (35), suggesting that K+-secretory pathways other than those mediated by ROMK and the maxi-K channel exist in the rat CCD. In conclusion, NKCC1 and HCN2 channels may allow Na+ and K+ influx into intercalated cells, thereby providing a means of continuous substrate (K+) to be secreted into the urinary fluid. We propose that ROMK and Kv1.3 channels mediate net K+ secretion in rat CCD under basal and HK dietary conditions, respectively, in rat CCD.
HCO3− secretion in the CCD of both rats and rabbits is mediated by apical Cl−/HCO3− exchange (58). The apical anion exchanger pendrin is involved in HCO3− secretion in type B intercalated cells (53). However, pendrin is also present in type non-A-non-B intercalated cells (31). The majority of filtered HCO3− is reabsorbed by the proximal tubule. Consistent with this, high rates of urinary HCO3− excretion during alkali loading or in response to metabolic alkalosis have largely been attributed to inhibition of HCO3− reabsorption in the proximal tubule (1). However, the final regulation of urine acidification takes place in the collecting duct, which is a site of both reabsorption and secretion of HCO3−. Based in our results, alkalinization of the urine during HK load may result from inhibition of either HCO3− reabsorption in the distal nephron and/or a metabolic alkalosis promoted by suppressed vacuolar H-ATPase activity in CCD. We speculate that in the CCD, in which a lumen negative potential is generated by Na+ transport, total CO2 flux is altered when the potential is altered: absorption of CO2 in the CCD will fall in parallel with a fall in lumen negative transepithelial potential, with increased K+ secretion. Therefore, our results suggest that K+ secretion during a HK diet produces alkalinization of the urine due to inhibition of the H-V-ATPase pump. However, the possibility that HK regulates acid-base homeostasis at the level of the intercalated cell in the distal nephron remains to be further explored.
In conclusion, our findings contribute importantly to the understanding of kidney K+ homeostasis and reveal that Kv1.3 is a novel K+-secretory channel in the rat kidney.
GRANTS
This work was supported by Dirección General de Asuntos del Personal Académico (DGAPA) under its research program (PAPIIT IN224406 and IN201110 to L. I. Escobar) at the Universidad Nacional Autónoma de México, Conacyt, Grant 48483 (to N. A. Bobadilla), and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-038470 (to L. M. Satlin) and P30 DK079307 (The Pittsburgh Center for Kidney Research). Rolando Carrisoza-Gaytán and Joyce Trujillo are PhD students supported by fellowships grants from Conacyt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to the Facultad de Medicina, PhD program “Programa de Doctorado en Ciencias Biomédicas,” Universidad Nacional Autónoma de México.
REFERENCES
- 1.Alpern RJ. Renal acidification mechanisms. In: The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2000, p. 455–519 [Google Scholar]
- 2.Amorim JB, Musa-Aziz R, Mello-Aires M, Malnic G. Signaling path of the action of AVP on distal K+ secretion. Kidney Int 66: 696–704, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arkett SA, Dixon J, Yang JN, Sakai DD, Minkin C, Sims SM. Mammalian osteoclasts express a transient potassium channel with properties of Kv1.3 Receptors Channels 2: 281–293, 1994 [PubMed] [Google Scholar]
- 4.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JBO, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Carrisoza R, Salvador C, Bobadilla NA, Trujillo J, Escobar LI. Expression and immunolocalization of ERG1 potassium channels in the rat kidney. Histochem Cell Biol 133: 189–199, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci 25: 280–289, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung I, Schlichter LC. Native Kv1.3 channels are upregulated by protein kinase C. J Membr Biol 156: 73–85, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Deutsch C, Chen LQ. Heterologous expression of specific K+ channels in T lymphocytes: functional consequences for volume regulation. Proc Natl Acad Sci USA 90: 10036–10040, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Escobar LI, Martínez-Téllez JC, Salas M, Castilla SA, Carrisoza R, Tapia D, Vázquez M, Bargas J, Bolívar JJ. A voltage-gated K+ current in renal inner medullary collecting duct cells. Am J Physiol Cell Physiol 286: C965–C974, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci 18: 6126–6137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadool DA, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol 83: 2332–2348, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol Renal Fluid Electrolyte Physiol 252: F458–F467, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Frindt G, Zhou H, Sackin H, Palmer LG. Dissociation of K channel density and ROMK mRNA in rat cortical collecting tubule during K adaptation. Am J Physiol Renal Physiol 274: F525–F531, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Gamper N, Fillon S, Feng Y, Friedrich B, Lang PA, Henke G, Huber SM, Kobayashi T, Cohen P, Lang F. K+ channel activation by all three isoforms of serum- and glucocorticoid-dependent protein kinase SGK. Pflügers Arch 445: 60–66, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem 268: 18866–18874, 1993 [PubMed] [Google Scholar]
- 19.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Grantham JJ, Burg MB, Orloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest 49: 1815–1826, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunnet M, Rasmussen HB, Hay-Schmidt A, Rosenstierne M, Klaerke DA, Olesen SP, Jespersen T. KCNE4 is an inhibitory subunit to Kv1.1 and Kv13 potassium channels. Biophys J 85: 1525–1537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamm LL, Alpern RJ, Preisig PA. Cellular mechanisms of renal tubular acidification. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. Amsterdam: Elsevier, 2008, p. 1539–1585 [Google Scholar]
- 26.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 199: 194–199, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Huang DY, Wulff P, Völkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Jacob A, Hurley IR, Goodwin LO, Cooper GW, Benoff S. Molecular characterization of a voltage-gated potassium channel expressed in rat testis. Mol Hum Reprod 6: 303–313, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Kaissling B. Structural aspects of adaptive changes in renal electrolyte excretion. Am J Physiol Renal Fluid Electrolyte Physiol 243: F211–F226, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Koschak A, Bugianesi RM, Mitterdorfer J, Kaczorowski GJ, Garcia ML, Knaus HG. Subunit composition of brain voltage-gated potassium channels determined by hongotoxin-1, a novel peptide derived from Centruroides limbatus venom. J Biol Chem 273: 2639–2644, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linas SL, Peterson LN, Anderson RJ, Aisenbrey GA, Simon FR, Berl T. Mechanism of renal potassium conservation in the rat. Kidney Int 15: 601–611, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Zavilowitz B, Wanderling S, Levy DI, Satlin LM, Wei Y. A novel and functional Na+-activated K channel is present in distal nephron of rat kidney (Abstract). J Am Soc Nephrol 20: 127A, 2009 [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem 277: 37871–37880, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Lu M, Wang T, Yan Q, Dong K, Knepper MA, Wang W, Giebisch G, Schull GE, Hebert SC. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice. J Biol Chem 277: 37881–37887, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malnic G, Muto S, Giebisch G. Regulation of potassium secretion. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. Amsterdam: Elsevier, 2008, p. 1301–1347 [Google Scholar]
- 40.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol 283: H1846–H1855, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Muto S. Potassium transport in the mammalian collecting duct. Physiol Rev 81: 85–114, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Nowik M, Lecca MR, Velic A, Rehrauer H, Brändli AW, Wagner CA. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Pácha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer LG, Choe H, Frindt G. Is the secretory K channel in the rat CCT ROMK? Am J Physiol Renal Physiol 273: F404–F410, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int 57: 1324–1328, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Panyi G, Varga Z, Gáspár R. Ion channels and lymphocyte activation. Immunol Lett 92: 55–66, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Rivera JF, Chu PJ, Arnold DB. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur J Neurosci 22: 1853–1862, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Sampaio MS, Bezerra IP, Peçanha FL, Fonseca PH, Capella MA, Lopes AG. Lack of Na+,K+-ATPase expression in intercalated cells may be compensated by Na+-ATPase: a study on MDCK-C11 cells. Cell Mol Life Sci 65: 3093–3099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol 55: 267–288, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Solé L, Roura-Ferrer M, Pérez-Verdaguer M, Oliveras A, Calvo M, Fernández-Fernández JM, Felipe A. KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating. J Cell Sci 122: 3738–3748, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Stanton BA, Giebisch G. Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol Renal Fluid Electrolyte Physiol 243: F487–F493, 1982 [DOI] [PubMed] [Google Scholar]
- 62.Stokes JB. Ion transport by the collecting duct. Semin Nephrol 13: 202–212, 1993 [PubMed] [Google Scholar]
- 63.Stühmer W, Ruppersberg JP, Schröter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J 8: 3235–3244, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szabó I, Bock J, Grassmé H, Soddemann M, Wilker B, Lang F, Zoratti M, Gulbins E. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci USA 105: 14861–14866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabei K, Muto S, Furuya H, Sakairi Y, Ando Y, Asano Y. Potassium secretion is inhibited by metabolic acidosis in rabbit cortical collecting ducts in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 268: F490–F495, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Vicente R, Villalonga N, Calvo M, Escalada A, Solsona C, Soler C, Tamkun MM, Felipe A. Kv1.5 association modifies Kv1.3 traffic and membrane localization. J Biol Chem 283: 8756–8764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter NKCC1 to Cl− secretion in rat OMCD. Am J Physiol Renal Physiol 280: F913–F921, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Wall SM, Fischer MP. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to transepithelial transport of H+, NH4+, K+, and Na+ in rat outer medullary collecting duct. J Am Soc Nephrol 13: 827–835, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 72.Wang W, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol 278: F165–F171, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol 66: 547–569, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Hebert SC. The molecular biology of renal potassium channels in: Seldin and Giebisch's the Kidney: Physiology and Pathophysiology (4th ed.), edited by Alpern RJ, Hebert SC. Amsterdam: Elsevier, 2008, p. 1249–1267 [Google Scholar]
- 76.Wang W, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflügers Arch 458: 157–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wärntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermüller N, Witzgall R, Mack AF, Wagner HJ, Wagner A, Bröer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflügers Arch 443: 617–624, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol 221: 437–448, 1971 [DOI] [PubMed] [Google Scholar]
- 82.Xu J, Koni PA, Wang P, Li G, Kaczmarek L, Wu Y, Li Y, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol Genet 12: 551–559, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, Koni PA, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc Natl Acad Sci USA 101: 3112–3117, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao X, Chang AY, Boulpaep EL, Segal AS, Desir GV. Molecular cloning of a glibenclamide-sensitive, voltage-gated potassium channel expressed in rabbit kidney. J Clin Invest 97: 2525–2533, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1 and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]