Abstract
Diabetic nephropathy (DN) is the most common cause of end-stage renal failure. We previously demonstrated that a transcription factor called upstream stimulatory factor 2 (USF2) was upregulated in the kidneys from diabetic animals in vivo as well as in mesangial cells (MCs) exposed to high-glucose media in vitro. USF2 mediates glucose-induced thrombospondin 1 expression and transforming growth factor-β activity in MCs and plays a role in DN. Glycated proteins have been shown to accumulate in the kidneys of diabetic patients and contribute to DN. However, whether glycated proteins regulate USF2 expression in MCs and play a role in DN is unknown. In the present studies, we determined the effect of glycated albumin on UFS2 gene expression in primary rat MCs. We found that glycated albumin upregulated USF2 expression (mRNA and protein) in a dose- and time-dependent manner. We also demonstrated that glycated albumin stimulated USF2 gene expression at the transcriptional level. By using the luciferase-promoter deletion assay, site-directed mutagenesis, and transactivation assay, we identified a glycated albumin-responsive region in the USF2 gene promoter (−837 to −430, relative to the transcription start site) and demonstrated that glycated albumin-induced USF2 expression was mediated through NF-κB-dependent transactivation of the USF2 promoter. Furthermore, glycated albumin increased nuclear NF-κB subunit-p65 protein levels. siRNA-mediated p65 knockdown prevented glycated albumin-induced USF2 gene expression (promoter activity, mRNA, and protein levels). Taken together, these data suggest that glycated albumin upregulated USF2 gene transcription in MCs through NF-κB-dependent transactivation of the USF2 promoter.
Keywords: NF-κB
diabetes is associated with increased protein modifications. Glucose reacts nonenzymatically with amino groups of proteins to produce intermediate Amadori products (such as glycated albumin), which finally form a class of irreversibly cross-linked, fluorescent moieties termed advanced glycation end products (AGEs) (22). Glycated albumin is the major form of circulating glycated proteins in vivo and its levels are increased in diabetes (8, 16). Accumulating evidence suggests that elevated concentrations of glycated albumin or AGEs upregulate transforming growth factor-β (TGF-β) and stimulate collagen and fibronectin expression in mesangial cells (9, 13, 21, 39), contributing to the development of diabetic nephropathy (8, 14, 32).
Upstream stimulatory factor 2 (USF2) was initially characterized as a transcription factor implicated in the regulation of the adenovirus major late promoter (29). In mammals, USF2 is ubiquitously expressed with a molecular weight of 44 kDa. It belongs to the Myc family of transcription factors characterized by a basic/helix loop helix/leucine zipper domain responsible for dimerization and DNA binding. It can form homo- and heterodimers and recognize in vitro a CACGTG core sequence termed E box (31). Through binding to the E boxes of target genes, USF2 has been demonstrated to regulate expression of many genes (2, 4, 10, 20, 25–27, 33, 38). We demonstrated that USF2 binds to an 18-bp sequence in the thrombospondin1 (TSP1) gene promoter and regulates high glucose-induced TSP1 expression and TSP1-dependent TGF-β activity in mesangial cells (35). Moreover, overexpression of USF2 stimulates TSP1 expression, TGF-β activity, and extracellular matrix protein expression in the kidney and accelerates the development of diabetic nephropathy (23). All these studies suggest that USF2 is an important regulator of diabetic nephropathy. USF2 itself is upregulated by acute high-glucose exposure in mesangial cells (30, 35, 36, 38). However, whether glucose-modified proteins, e.g., glycated albumin, regulate USF2 expression in mesangial cells is unknown.
Therefore, the present studies were performed to investigate the effect of glycated albumin on USF2 expression in primary rat mesangial cells. Our studies suggested a transcriptional regulation of USF2 expression by glycated albumin treatment in mesangial cells. Moreover, we identified a glycated albumin-responsive region in the USF2 gene promoter and demonstrated that glycated albumin-induced USF2 expression is mediated through NF-κB-dependent transactivation of the USF2 promoter.
MATERIALS AND METHODS
Materials.
Antibodies against USF2, TATA-binding protein (TBP), p65, and p50 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). FBS, RPMI 1640 media, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). siRNA-p65 or control siRNA was purchased from Santa Cruz Biotechnology.
Glycated and nonglycated bovine serum albumin were purchased from Sigma (St. Louis, MO). Glycated albumin contained 3 mol of fructoselysine per mol albumin. The absence of AGE in the glycated albumin was assessed by measuring of AGE-related fluorescence at excitation maximum of 370 nm and emission maximum of 440 nm as described previously (24). Endotoxin (LPS) was not detectable in the glycated or nonglycated albumin by the use of Limulus Amebocyte Lysate test kit (Sigma).
Cell cultures.
Rat mesangial cells were the generous gift from Dr. A. Woods, Univ. of Alabama at Birmingham and cultured as described previously (34). Experiments in this study were performed on cells between passages 3 and 10.
Immunoblotting.
Rat mesangial cells were grown in growth medium containing 20% FBS for 2–3 days until cells reached 80% confluence and then rendered quiescent by culturing in serum- and insulin-free RPMI 1640 media for 48 h. Cells were treated with serum-free RPMI 1640 media (with 5 mM glucose) containing glycated bovine albumin or control bovine albumin at different concentrations for 24 h. After treatment, cells were harvested and cell lysates were prepared. The USF2 protein levels in the cell lysates were determined by immunoblotting using anti-USF2 antibody as described previously (35). Actin was used as a loading control.
For detection of NF-κB subunits p65 and p50 protein levels, nuclear proteins were extracted from mesangial cells after treatment by using NE-PER Nuclear and Cytoplasmic Extraction Reagents from Pierce (Rockford, IL). Equal amount of total nuclear protein was subjected to SDS-PAGE and the p65 or p50 protein levels were determined by immunoblotting using anti-p65 or anti-p50 antibody. TBP was used as a loading control.
Real-time PCR.
Rat mesangial cells were treated with glycated albumin or control albumin for different time periods. After treatment, total RNA from mesangial cells was extracted. The USF2 mRNA levels in each sample were determined by real-time PCR and normalized to β-actin. The primers used for PCR of USF2 were 5′-ACAGACCAGAGCCTACAGGC-3′ and 5′-AAGCCTTGGACAGGATGCCC-3′. The primers for β-actin were 5′-CACTGGCATTGTGATGGACT-3′ and 5′-TGGCATAGAGGTCTTTACGG-3′.
mRNA stability assay.
Rat mesangial cells were made quiescent in serum-free media for 48 h and then treated with glycated albumin or control albumin for 24 h. After treatment, media were removed and actinomycin D (5 μg/ml) was added (designated as t = 0). After different periods of incubation, cells were harvested. Real-time PCR analysis for USF2 mRNA was performed as described above. Measurement of the ratio of USF2/actin at t = 0 (from actinomycin D treatment) was assigned a relative value of 100%.
USF2 promoter-reporter constructs and luciferase assay.
The USF2 promoter-luciferase reporter constructs [USF2 (−2,400), USF2 (−1,742), USF2 (−1,620), USF2 (−1,209), USF2 (−837), and USF2 (−430)] were generated using pGL3-basic vector (Promega) as described previously (30). Mutated USF2 promoter constructs that carried mutations at the HSF, NF-κB, CREB, AP2, or SP1 sites within USF2 promoter region (−837 to −430) were generated by using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Mutations were confirmed by DNA sequencing. Mesangial cells were transiently transfected using Effectene transfection reagent (Qiagen) with 1 μg of USF2 promoter luciferase reporter plasmids or mutated USF2 promoter constructs. pRL-SV40 (0.02 μg; Promega) was used as an internal control for transfection. Transfected cells were treated with glycated or control albumin for 24 h, and the luciferase activities were analyzed using the dual-luciferase assay kit (Promega) according to the manufacturer's directions as described previously (30).
EMSA and supershift assay.
EMSA was performed using the LightShift Chemiluminescent EMSA kit following the instruction provided by the manufacturer (Pierce). Double-strand DNA fragments corresponding to the NF-κB binding site in the USF2 gene promoter (−712 to −692, relative to transcription start site) or DNA fragment containing the mutated NF-κB binding site were 3′-end labeled with biotin (Biotin 3′-end DNA labeling kit from Pierce) and used as probes to incubate with nuclear extracts. The sequence for the USF2 promoter containing NF-kB binding site (underlined) was −721-AACCGGTGGTGG TCCCG TGTAGAGTCCCC G-692. A mutation was introduced by substituting the “C” with “g” as indicated: −721-AACCGGTGGTGG TCCCG TGTAGAGTCgCC G-692. In competition experiments, a 100× excess amount of unlabeled competitior was mixed with the labeled probe before being added to the binding mixture for EMSA. For Supershift assay, control IgG or specific antibodies were incubated with nuclear extracts before being added to the binding mixture for EMSA.
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation assay was conducted as described previously (30) by using a kit from Upstate Biotechnology (Lake Placid, NY). In brief, cells were cultured and treated with glycated or control albumin for 24 h. After treatment, protein-DNA complexes were fixed by 1% formaldehyde, lysed, and sonicated on ice. The sonicated chromatins were incubated with anti-p65 or anti-p50 subunit of NF-κB antibodies (from Santa Cruz Biotechnology). The immunoprecipitated complexes of antibody-protein-DNA were collected using protein-A-agarose beads. The DNA isolated from the complex was subjected to PCR amplification using the following primers flanking the NF-κB site in the USF2 promoter: 5′-GTGGTCCCGTGTAGAG-3′ (forward) and 5′-CCTCCTTTACTGGAGC-3′ (reverse). The 174-bp PCR products were resolved by 2% agarose-ethidium bromide gel electrophoresis, visualized by UV.
Small interfering RNA-mediated p65 gene knockdown.
Mesangial cells were cultured, and NF-κB subunit p65 was knocked down by siRNA-p65 (Santa Cruz Biotechnology). siRNA-p65 or control siRNA was transiently transfected into mesangial cells with transfection reagents according to the instruction manual. After 48-h incubation, cells were harvested and cell lysates were prepared. The extent of knockdown of p65 was determined by immunoblotting.
Statistical analysis.
Data are expressed as means ± SE. Statistical evaluation of the data was performed by ANOVA or by t-test as appropriate, considering the P value of <0.05 as significant.
RESULTS
Glycated albumin treatment stimulated USF2 expression in mesangial cells at the transcriptional level.
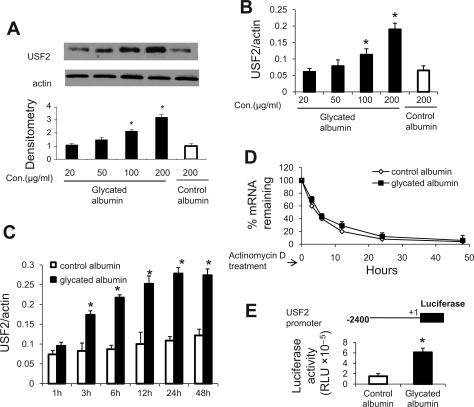
First, we determined the effect of glycated albumin on USF2 expression in rat mesangial cells. As shown in Fig. 1, A and B, glycated albumin treatment upregulated USF2 expression (mRNA and protein levels) in primary mesangial cells in a dose-dependent manner. Control albumin had no significant effect on USF2 expression. Based on this result and the reports from other investigators (7–9), the concentration of 200 μg/ml of glycated albumin is used for the following experiments. This concentration of glycated albumin (200 μg/ml) is similar to that found in clinical specimens and has been observed to stimulate extracellular matrix production in mesangial cells (39).
Fig. 1.
Glycated albumin upregulated upstream stimulatory factor 2 (USF2) expression in primary rat mesangial cells (RMCs) at the transcriptional level. A and B: primary RMCs were cultured, made quiescent in serum-free media for 48 h, and treated with different concentrations of glycated albumin for 24 h. After treatment, cells were harvested and USF2 protein levels or mRNA levels were analyzed by immunoblotting and real-time PCR, respectively. The blot was the representative of 3 independent experiments. Relative USF2 protein levels were determined by scanning the densitometry of immunoblots and normalized to β-actin levels. C and D: quiescent mesangial cells were treated with glycated or control albumin (200 μg/ml) in the absence or presence of actinomycin D (5 μg/ml) for indicated periods. After treatment, cells were harvested and total RNA was extracted. USF2 mRNA levels were determined by real-time PCR. The rate of decay of USF2 mRNA and the half-life were determined as described in materials and methods. Measurement of the ratio of USF2 at time = 0 was assigned a relative value of 100%. The results are expressed as means ± SE of 3 separate experiments. E: mesangial cells were transiently transfected with mouse USF2 promoter luciferase construct [USF2 (−2,400)] and then treated with control or glycated albumin (200 μg/ml) for 24 h. After treatment, cells were harvested and luciferase activity was measured and normalized to Renilla luciferase levels. Data are means ± SE of 3 separate experiments. *P < 0.05 vs. control albumin.
Next, we determined the transcriptional regulation of USF2 expression by glycated albumin treatment. We found that glycated albumin treatment increased USF2 mRNA levels in mesangial cells in a time-dependent manner (Fig. 1C). In addition, the effect of glycated albumin on USF2 mRNA stability was determined by adding actinomycin D to inhibit de novo RNA synthesis. As shown in Fig. 1D, USF2 mRNA stability was unaffected by glycated albumin treatment, suggesting that glycated albumin-mediated increase in USF2 mRNA levels is not due to the alteration in mRNA stability and further supporting the transcriptional mechanism. Thus, the effect of glycated albumin treatment on the transcriptional activity of the USF2 promoter was determined. With the transfection of a mouse full-length USF2 promoter-luciferase reporter construct to mesangial cells, we found that 24-h glycated albumin treatment significantly stimulated the transcriptional activity of the USF2 promoter (Fig. 1E). Taken together, these data suggest that glycated albumin treatment stimulated USF2 gene expression in mesangial cells at the transcriptional level.
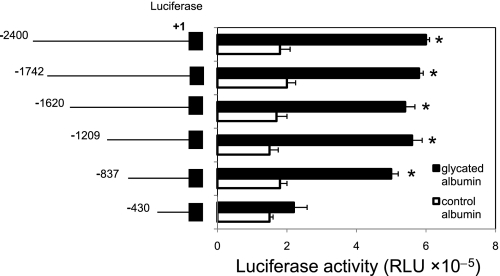
Glycated albumin-mediated increases in the USF2 promoter activity in mesangial cells were supported by the USF2 promoter region −837 to −430.
To identify the promoter elements that regulate USF2 gene transcription in mesangial cells in response to glyated albumin treatment, a series of USF2 promoter-luciferase reporter constructs were transiently transfected into mesangial cells. The promoter activity was measured and normalized to Renilla luciferase activity. As shown in Fig. 2, the longest construct, USF2 (−2,400), gave rise to a threefold increase in USF2 promoter activity in response to glycated albumin treatment. Deletion of a ∼400-bp region (−837 to −430) abolished glycated albumin responsiveness, suggesting that this 400-bp region is important for glycated albumin-induced USF2 gene transcription.
Fig. 2.
Glycated albumin stimulated the USF2 gene promoter activity and the region between −837 to −430 was involved. Quiescent rat mesangial cells were transiently transfected with mouse USF2 promoter luciferase construct [USF2 (−2,400)] and a series deletion of USF2 promoter constructs. Then, cells were treated with glycated or control albumin (200 μg/ml) for 24 h. After 24 h, cells were harvested and lysed. The luciferase activity was measured and normalized to Renilla luciferase levels. Data are means ± SE of triplicates of 3 separate experiments. *P < 0.05 vs. control albumin.
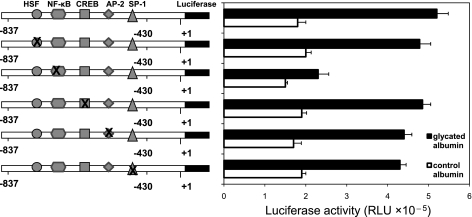
Glycated albumin stimulated the USF2 promoter activity in mesangial cells via NF-κB.
NF-κB, a dimeric transcription factor, plays important roles in kidney diseases (15). Glycated albumin has been shown to stimulate NF-κB activity in various cell types including mesangial cells, peritoneal mesothelial cells, and macrophages (1, 6, 24). Therefore, we tested the possibility that glycated albumin induced USF2 expression through activation of NF-κB. We performed a computer analysis to predict NF-κB binding site in the USF2 promoter region (−837 to −430) by using MATCH software (matrix search for transcription factor binding sites; Biological data bases: https://portal.biobase-international.com) and revealed one NF-κB binding site within this region. To determine whether this NF-κB binding site might be important for glycated albumin-induced USF2 gene transcription, we introduced point mutations to NF-κB binding site in the USF2 (−837) promoter-luciferase construct. In addition, binding sites for other transcription factors including HSF, CREB, AP2, and SP-1 within this USF2 promoter region (−837/−430) were also mutated. These mutated USF2 promoter-luciferase constructs were transiently transfected into mesangial cells. After glycated albumin or control albumin treatment, luciferase activity was measured. As shown in Fig. 3, mutation of binding sites for HSF, CREB, AP2, or SP1 had no effect on glycated albumin-induced USF2 promoter activity. However, mutation of NF-κB site abrogated glycated albumin-induced USF2 promoter activity, suggesting that NF-κB may involve glycated albumin-mediated increase in USF2 transcription in mesangial cells. This NF-κB site has high similarity between mouse, rat, and human USF2 promoter, suggesting that this sequence has important regulatory function.
Fig. 3.
Glycated albumin-stimulated increases in the USF2 promoter activity were mediated by a NF-κB site located between −837 and −430 of the USF2 promoter. Quiescent RMCs were transiently transfected with −837-bp USF2 promoter constructs bearing mutations of HSF, NF-κB, CREB, AP-2, and SP-1 sites in the presence of control or glycated albumin (200 μg/ml) for 24 h. The plasmid pRL-SV40 was used as internal control. After 24 h, cells were harvested and lysed. The luciferase activity was measured and normalized to Renilla luciferase level. Data are means ± SE of triplicates of 3 separate experiments.
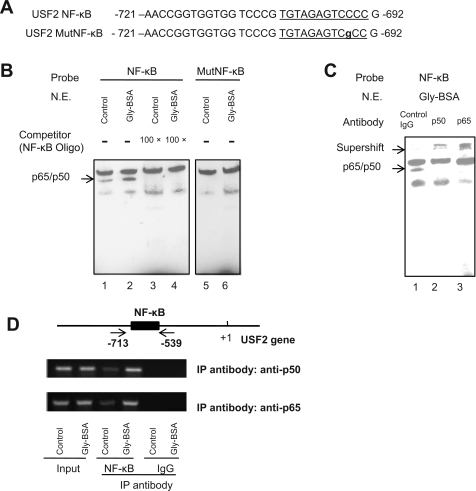
NF-κb bound to the USF2 promoter and mediated glycated albumin-induced USF2 expression.
We determined whether NF-κB binds to the NF-κB binding site located between −721 and −692 of the USF2 promoter by using both EMSA and chromatin immunoprecipitaton assay (ChIP). For EMSA, we used probes of biotin-labeled double-strand DNA corresponding to the NF-κB and its flanking sequence (−721/−692) or the mutated NF-κB as illustrated in Fig. 4A. The EMSA results (Fig. 4B) showed that the NF-κB probe formed a complex with nuclear proteins from mesangial cell nuclear extracts (lane 1), and this complex was enhanced by glycated albumin treatment (lane 2). The specificity of this protein-DNA complex was confirmed in the competition assays using unlabeled oligonucleotide (−721/−692) to arrogate the formation of this complex (lanes 3 and 4). No specific protein-DNA complex formed when we used the mutated USF2 NF-κB probe for EMSA (lanes 5 and 6). These results suggest that nuclear proteins that bind to the 30-bp sequence (−721/−629) of the USF2 promoter might be NF-κB. Thus, we immunologically probed the protein-DNA complex with antibodies against p65 or p50 (NF-κB subunits) in gel supershift assay. As shown in Fig. 4C, antibodies against p65 or p50 led to the formation of the supershifted complexes, indicating that the nuclear proteins binding to the 30-bp sequence of the USF2 promoter were p50/p65 NF-κB heterodimers.
Fig. 4.
Glycated albumin enhanced NF-κB binding to the USF2 promoter in mesangial cells. A: sequence of NF-κB binding site or mutated NF-κB site in the USF2 gene promoter region (−721 to −692). B: EMSA showed the specific binding of nuclear proteins to the USF2 NF-κB site through competition assay. Nuclear extracts isolated from control BSA or glycated BSA-treated mesangial cells were incubated with biotin-labeled NF-κB probe in the presence or absence of excess amount of unlabeled USF2 NF-κB oligo (lanes 1–4). In addition, EMSA was performed using the biotin-labeled mutant NF-κB probe (lanes 5–6). The blots shown are representative of 3 separate experiments. N.E., nuclear extracts. C: supershift assay was performed in the presence of control IgG, anti p65, or anti p50 antibodies. The representative blot is shown from 3 separate experiments. D: ChIP assay. Mesangial cells were treated with control or glycated albumin for 24 h. After treatment, formaldehyde cross-linked chromatin isolated from primary mesangial cells was immunoprecipitated (IP) with anti-NF-κB antibody (p65 and p50 subunits) and subjected to PCR as described under materials and methods. Two arrows indicate primers used for amplifying the region from −713 to −539 spanning the NF-κB site. PCR products were electrophoresed on 2% agarose gel. The experiments were repeated for 3 times, and the representative result is shown.
Having demonstrated the binding of NF-κB to the USF2 promoter in vitro, we further analyzed the in vivo binding of NF-κB to the USF2 promoter by ChIP. Chromatin immunoprecipitation with antibodies for NF-κB subunits p65 or p50 and subsequent PCR amplification, using primer pairs that cover the NF-κB site located between −713 and −539, demonstrated that NF-κB bound to the USF2 promoter. This binding was enhanced by glycated albumin treatment (Fig. 4D). Together, these results demonstrate that glycated albumin treatment enhanced NF-κB binding to the USF2 gene promoter in mesangial cells.
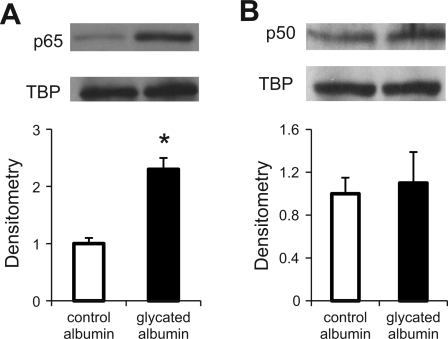
To determine whether the above changes in NF-κB binding activity reflect changes in nuclear accumulation of NF-κB subunits p65 and/or p50 protein levels, we performed immunoblotting analysis of nuclear extracts prepared from mesangial cells treated with either control albumin or glycated albumin. As shown in Fig. 5, treatment of mesangial cells with glycated albumin upregulated p65 (A) but not p50 (B) protein accumulation, suggesting that regulation of USF2 promoter NF-κB binding activity in mesangial cells by glycated albumin reflects in part the regulation of p65 protein accumulation.
Fig. 5.
Glycated albumin treatment increased nuclear NF-κB subunit-p65 protein levels. Quiescent RMCs were treated with glycated or control albumin (200 μg/ml) for 24 h. After treatment, cells were harvested and nuclear extracts were prepared. The protein levels of NF-κB subunits-p65 (A) and p50 (B) in the nuclear extracts were analyzed by immunoblotting. TATA-binding protein (TBP) was used as an internal control. The blots shown are representative of 3 separate experiments. Relative p65 or p50 levels were determined by scanning densitometry of blots. Data are expressed as means ± SE of 3 separate experiments. *P < 0.05 vs. control albumin.
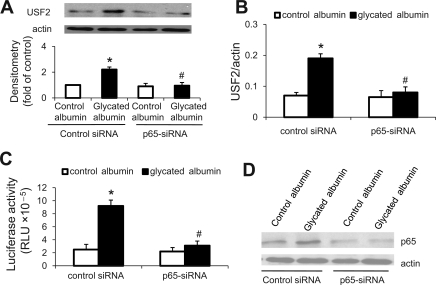
To examine the effects of NF-κB on USF2 gene expression, mesangial cells were transfected with siRNA-p65 to knock down NF-κB subunit-p65. Then, cells were transiently transfected with USF2-luciferase reporter construct [USF2 (−2,400)]. After 24 h of control or glycated albumin treatment, cells were harvested. Levels of USF2 protein, mRNA, and promoter activity were determined. As shown in Fig. 6, siRNA-mediated p65 gene knockdown prevented glycated albumin treatment-induced USF2 protein (A), mRNA levels (B), and promoter activity (C). The efficient knockdown of p65 was confirmed by immunoblotting (Fig. 6D). Taken together, these experiments suggest that NF-κB binds to the USF2 promoter and mediates glycated albumin-induced USF2 expression in mesangial cells.
Fig. 6.
NF-κB mediated glycated albumin-induced USF2 expression in mesangial cells. Mesangial cells were transiently transfected with siRNA-p65 (10 nM) or control-siRNA (10 nM) using siRNA transfection reagent for 48 h. Then, these cells were transiently cotransfected with USF2 (−837) promoter luciferase construct and internal control plasmid pRL-SV40 and treated with control or glycated albumin (200 μg/ml) for 24 h. After treatment, cells were harvested. A: USF2 protein levels in cell lysates were determined by immunoblotting. B: USF2 mRNA levels were analyzed by real-time PCR. The promoter activity (C) was quantified by assaying luciferase activity and normalized to Renilla luciferase activity. The experiments were repeated 3 times, and the representative result is shown. Data are represented as means of 3 replicates ± SE. *P < 0.05 vs. control albumin in control siRNA group. #P < 0.05 vs. glycated albumin treatment in control siRNA group. D: efficient knockdown of p65 was confirmed by immunoblotting to show the significantly reduced p65 protein levels in the whole cell lysates. The representative blot from 3 separate experiments is shown.
DISCUSSION
In the present studies, we demonstrated that glycated albumin upregulated USF2 expression in primary rat mesangial cells. Our data suggested a transcriptional regulation of USF2 expression by glycated albumin. Moreover, we identified a glycated albumin response region in the USF2 gene promoter and demonstrated that glycated albumin-induced USF2 expression is mediated through NF-κB-dependent transactivation of the USF2 promoter.
We previously demonstrated that USF2 was upregulated in the kidney from diabetic animals as well as in the mesangial cells after acute high-glucose exposure and plays an important role in the development of diabetic nephropathy through regulation of TSP1 and TSP1-dependent TGF-β activity (23, 30, 35). Our current studies revealed a new regulatory mechanism of USF2 expression in mesangial cells under diabetic conditions, e.g., glycated albumin-mediated upregulation of USF2. Our results are consistent with the reports from Weigert et al. (36) showing that elevated O-glycosylation increased USF2 expression (mRNA levels) in mesangial cells. Furthermore, we demonstrated a transcriptional mechanism of glycated albumin-induced USF2 expression in mesangial cells. It should be noted that the biological functions of glycated albumin described herein are operative under conditions of physiological glucose concentrations (5 mM glucose) and therefore cannot be ascribed to an influence of high-glucose conditions. It seems that high glucose and glycated albumin upregulate USF2 gene transcription through different mechanisms. Our previously identified glucose-responsive element in the USF2 gene promoter was located between −1,740 and −1,620 (relative to the transcription start site) (30); while current studies demonstrated that glycated albumin response region was located between −837 and −430 in the USF2 gene promoter. Furthermore, high-glucose levels upregulated USF2 gene transcription through CREB-dependent transactivation of the USF2 gene promoter, whereas glycated albumin increased nuclear NF-κB subunit-p65 accumulation and enhanced NF-κB binding to the USF2 gene promoter, resulting in increased USF2 gene transcription. At this time, the effect of glycated albumin on USF2 expression under high-glucose conditions is not known and needs to be further investigated.
Our results are in agreement with previous studies showing that glycated albumin activated NF-κB in a variety of cell types including vascular smooth muscle cells, monocytes, macrophages, and endothelial cells (3, 6, 11, 18, 19, 28). NF-κB, as a pleiotropic transcription factor, can be activated by many stimuli associated with stresses. NF-κB activation is involved in the development of renal disease (15). It mediates hyperglycemia-induced angiotensinogen production in the kidney as well as in the mesangial cells (12). It also regulates other proteins involved in diabetic nephropathy, such as MCP-1 (17). Our current studies showing that NF-κB regulates glycated albumin-induced USF2 expression in mesangial cells further added the importance for NF-κB in the development of diabetic nephropathy.
Glycated albumin is a predominant form of Amadori products generated by nonenzymatic glycation reaction between glucose and free animo groups in proteins, and associated with the development of diabetic macro and micro complications (8, 9, 16, 22, 39). Several studies demonstrated the binding sites for glycated albumin on endothelial and mesangial cells (5, 8). Glycated albumin has been shown to increase the expression of extracellular matrix proteins, activate protein kinase C, and stimulate the expression of TGF-β, contributing to the development of diabetic nephropathy (5, 8, 37). Glycated albumin binds to its cell surface receptors whose structures and functions remain to be clarified and induce cellular signaling. In endothelial cells, glycated albumin induced activation of NADPH oxidase and increased intracellular accumulation of reactive oxygen species, leading to activation of NF-κB and AP-1 (19). Glycated albumin activates ERK signaling pathway, leading to NF-κB activation in macrophages (6). Binding of glycated albumin to monocyte-like MonoMac6 cells leads to activation of MAPK p44/42 and p38 MAPK with subsequent translocation of NF-κB to nucleus (3). At this time, the cellular signaling pathways induced by glycated albumin that lead to NF-κB activation in mesangial cells are not known and need to be further investigated.
In summary, our present data provide the first evidence that glycated albumin stimulates USF2 expression in mesangial cells at the transcriptional level. A glycated albumin response region in the USF2 promoter (−837 to −430) is further identified, which is bound by NF-κB and mediates glycated albumin-induced USF2 expression in mesangial cells.
GRANTS
This work was supported in part by grants from Juvenile Diabetes Research Foundation and the National Institutes of Health (Grant DK-081555).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Berrou J, Tostivint I, Verrecchia F, Berthier C, Boulanger E, Mauviel A, Marti HP, Wautier MP, Wautier JL, Rondeau E, Hertig A. Advanced glycation end products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med 23: 513–520, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bidder M, Shao JS, Charlton-Kachigian N, Loewy AP, Semenkovich CF, Towler DA. Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J Biol Chem 277: 44485–44496, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Brandt R, Krantz S. Glycated albumin (Amadori product) induces activation of MAP kinases in monocyte-like MonoMac 6 cells. Biochim Biophys Acta 1760: 1749–1753, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Shen YH, Wang X, Wang J, Gan Y, Chen N, Wang J, LeMaire SA, Coselli JS, Wang XL. Human prolyl-4-hydroxylase alpha(I) transcription is mediated by upstream stimulatory factors. J Biol Chem 281: 10849–10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Cohen MP, Ziyadeh FN. Amadori-glycated albumin in diabetic nephropathy: pathophysiologic connections. Kidney Int Suppl 77: S40–S44, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med 141: 242–249, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cohen MP, Shea E, Shearman CW. ERK mediates effects of glycated albumin in mesangial cells. Biochem Biophys Res Commun 283: 641–643, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cohen MP, Ziyadeh FN. Role of Amadori-modified nonenzymatically glycated serum proteins in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol 7: 183–190, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Cohen MP, Ziyadeh FN, Lautenslager GT, Cohen JA, Shearman CW. Glycated albumin stimulation of PKC-β activity is linked to increased collagen IV in mesangial cells. Am J Physiol Renal Physiol 276: F684–F690, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Corre S, Galibert MD. Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Res 18: 337–348, 2005 [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira C, Colette C, Monnier L, Descomps B, Pares-Herbute N. Insulin alters nuclear factor-lambdaB and peroxisome proliferator-activated receptor-gamma protein expression induced by glycated bovine serum albumin in vascular smooth muscle cells. J Lab Clin Med 145: 144–150, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-κB pathway. Am J Physiol Renal Physiol 296: F1212–F1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, Imaizumi T, Cooper ME, Okuda S. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int 66: 2137–2147, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Fukami K, Yamagishi S, Ueda S, Okuda S. Role of AGEs in diabetic nephropathy. Curr Pharm Des 14: 946–952, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 59: 415–424, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Guthrow CE, Morris MA, Day JF, Thorpe SR, Baynes JW. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci USA 76: 4258–4261, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol 13: 894–902, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Banba N, Gross SS, Kasai K. Glycated serum albumin-induced nitric oxide production in vascular smooth muscle cells by nuclear factor kappaB-dependent transcriptional activation of inducible nitric oxide synthase. Biochem Biophys Res Commun 259: 128–132, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Higai K, Shimamura A, Matsumoto K. Amadori-modified glycated albumin predominantly induces E-selectin expression on human umbilical vein endothelial cells through NADPH oxidase activation. Clin Chim Acta 367: 137–143, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kingsley-Kallesen M, Luster TA, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 gene in glioblastoma cells. In Vitro Cell Dev Biol Anim 37: 684–690, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Lal MA, Brismar H, Eklof AC, Aperia A. Role of oxidative stress in advanced glycation end product-induced mesangial cell activation. Kidney Int 61: 2006–2014, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lapolla A, Fedele D, Reitano R, Arico NC, Seraglia R, Traldi P, Marotta E, Tonani R. Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom 15: 496–509, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Shi L, Wang S. Overexpression of upstream stimulatory factor 2 accelerates diabetic kidney injury. Am J Physiol Renal Physiol 293: F1727–F1735, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Nevado J, Peiro C, Vallejo S, El-Assar M, Lafuente N, Matesanz N, Azcutia V, Cercas E, Sanchez-Ferrer CF, Rodriguez-Manas L. Amadori adducts activate nuclear factor-kappaB-related proinflammatory genes in cultured human peritoneal mesothelial cells. Br J Pharmacol 146: 268–279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98: 8780–8785, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian J, Kaytor EN, Towle HC, Olson LK. Upstream stimulatory factor regulates Pdx-1 gene expression in differentiated pancreatic beta cells. Biochem J 341: 315–322, 1999 [PMC free article] [PubMed] [Google Scholar]
- 27.Rippe RA, Umezawa A, Kimball JP, Breindl M, Brenner DA. Binding of upstream stimulatory factor to an E-box in the 3′-flanking region stimulates alpha1(I) collagen gene transcription. J Biol Chem 272: 1753–1760, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Salazar R, Brandt R, Krantz S. Binding of Amadori glucose-modified albumin by the monocytic cell line MonoMac 6 activates protein kinase C epsilon protein tyrosine kinases and the transcription factors AP-1 and NF-kappaB. Glycoconj J 18: 769–777, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Sawadogo M, Roeder RG. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell 43: 165–175, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Liu S, Nikolic D, Wang S. High glucose levels upregulate upstream stimulatory factor 2 gene transcription in mesangial cells. J Cell Biochem 103: 1952–1961, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirito M, Lin Q, Maity T, Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res 22: 427–433, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D'Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol 11: 1656–1666, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Vallet VS, Henrion AA, Bucchini D, Casado M, Raymondjean M, Kahn A, Vaulont S. Glucose-dependent liver gene expression in upstream stimulatory factor 2 −/− mice. J Biol Chem 272: 21944–21949, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem 277: 9880–9888, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose upregulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem 279: 34311–34322, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Weigert C, Brodbeck K, Sawadogo M, Haring HU, Schleicher ED. USF proteins induce human TGF-beta1 gene activation via the glucose response element −1013/−1002 in mesangial cells–upregulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem 2: 2, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Yang YL, Chuang LY, Guh JY, Liu SF, Hung MY, Liao TN, Huang YL. Thrombospondin-1 mediates distal tubule hypertrophy induced by glycated albumin. Biochem J 379: 89–97, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Casado M, Vaulont S, Sharma K. Role of upstream stimulatory factors in regulation of renal transforming growth factor-beta1. Diabetes 54: 1976–1984, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ziyadeh FN, Han DC, Cohen JA, Guo J, Cohen MP. Glycated albumin stimulates fibronectin gene expression in glomerular mesangial cells: involvement of the transforming growth factor-beta system. Kidney Int 53: 631–638, 1998 [DOI] [PubMed] [Google Scholar]