Abstract
Atherosclerotic renal artery stenosis (ARAS) is increasingly identified in patients with end-stage renal disease. Renal function in ARAS patients deteriorates more frequently than in nonatherosclerotic renal artery stenosis (RAS). This study was designed to test the hypothesis that atherosclerosis modifies the relationship between single-kidney hemodynamics and function and the severity of stenosis. The degree of unilateral RAS in domestic pigs (4 normal, 26 RAS, and 22 ARAS) was correlated with renal function and hemodynamics evaluated by 64-slice multidetector computerized tomography before and after endothelium-dependent challenge with ACh. The degree of stenosis and increase in mean arterial pressure were similar in RAS and ARAS. Stenotic single-kidney volume, blood flow, glomerular filtration rate, and cortical perfusion were lower than normal in both RAS and ARAS, but only in RAS correlated inversely with increasing degree of stenosis (r = −0.62, r = −0.49, r = −0.51, and r = −0.46, respectively, P < 0.05 for all). Basal tubular fluid concentration capacity and stenotic cortical perfusion response to ACh were both blunted only in ARAS. This study shows that atherosclerosis modulates the impact of a stenosis in the renal artery on stenotic kidney hemodynamics, function, and tubular dynamics. These observations underscore the direct intrarenal effect of atherogenic factors on the kidneys.
Keywords: renovascular hypertension, multidetector CT
atherosclerotic renal artery stenosis (ARAS) is a common manifestation of generalized atherosclerosis and is the predominant renal arterial lesion in patients over 50 years old (9). ARAS is present in up to 50% of those with atherosclerotic disease elsewhere (10). Moreover, ARAS is an independent risk factor for aggravation of cardiovascular disease (8) and may lead to renovascular hypertension and ischemic nephropathy.
While the severity of parenchymal damage in the ischemic ARAS kidney is an important prognostic factor for renal function (24), the degree of the stenosis does not necessarily predict renal hemodynamics and function distal to renal artery stenosis (RAS) (13, 20). It is recognized that high-grade atherosclerotic lesions in the renal artery may decrease renal perfusion and impair renal function. Therefore, therapeutic strategies have focused on restoring renal blood supply. However, improvement in blood pressure control or recovery of renal function after renal revascularization are achieved only in selected ARAS patients (22, 23), whereas repair of renal arterial lesions uncomplicated by atherosclerosis often achieves better outcomes (14). These observations suggest that deleterious factors beyond the stenosis, probably triggered by the atherogenic process, play dominant roles in compromising the function and recovery of the ischemic kidney in ARAS.
Endothelial dysfunction occurs early in the process of atherogenesis and has been shown to be associated with adverse cardiovascular outcomes (21). The residual glomerular filtration function of the kidney before revascularization is an important determinant of its recovery potential (17, 19). Similarly, it is likely that vascular function in the kidney also reflects the severity of microvascular disease, a hallmark of atherosclerosis.
We have previously shown in a swine model of ARAS, achieved by superimposing hypercholesterolemia on RAS, that both RAS and ARAS induce a reduction in renal blood flow (RBF) and glomerular filtration rate (GFR) of the ischemic kidney (1, 4). Furthermore, ARAS impaired tubular function, reflected in decreased intratubular fluid concentration (ITC) of the stenotic kidney. Compared with RAS, the ARAS kidney exhibits greater fibrosis, which is accompanied by and probably driven by increased oxidative stress and tissue injury (1). However, whether the severity of stenosis imposed a greater burden on renal and microvascular function and reactivity in the presence of atherosclerosis remains unclear.
Therefore, this study was designed to test the hypothesis that atherosclerosis modulates the relationships between the hemodynamics and function of ARAS kidneys and the degree of stenosis in the main renal artery. To test this hypothesis, we compared in a swine model of unilateral RAS and ARAS the relationship between the degree of stenosis and severity of individual kidney hemodynamics, function, and tubular functions.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee. Fifty-four female domestic pigs (40–65 kg) were studied in vivo after 10 wk of observation (normal, n = 4), unilateral RAS and normal pig chow feeding (n = 28), or unilateral RAS fed an atherogenic high-cholesterol diet (ARAS, n = 24). The high-cholesterol diet included 2% cholesterol and 15% lard by weight (TD93296; Harlan Teklad, Madison, WI). After 4 wk of corresponding diet, RAS was induced by placing a local-irritant coil in the main renal artery of the RAS and ARAS pigs. We have previously shown that this approach led to development of unilateral RAS within several days (1, 12). For this purpose, the animals were anesthetized with intramuscular ketamine (20 mg/kg) and xylazine (2 mg/kg), intubated, and ventilated. Anesthesia was maintained with ketamine (0.2 mg·kg−1·min−1) and xylazine (0.03 mg·kg−1·min−1). An intravenous bolus of heparin (5,000 units) was followed by continuous infusion (1,000 U/h). A telemetry catheter was then secured in the femoral artery for monitoring daily mean arterial pressure (MAP) (5), subsequently averaged from 2 wk before in vivo studies.
Later (6 wk), the degree of stenosis was assessed by renal angiography (1), and all pigs were studied using a 64-slice multidetector computerized tomography (MDCT, SOMATOM Definition 64; Siemens, Forcheim, Germany) to assess single-kidney hemodynamics and function and tubular dynamics of the stenotic kidneys before and after administration of ACh. The presence of visually appreciable collateral vessels was also determined.
MDCT scanning.
After angiography, in vivo MDCT flow studies were performed for assessment of basal regional renal perfusion, RBF, GFR, and tubular ITC, as previously detailed (7, 11). Briefly, this involved sequential acquisition of 140 consecutive scans after a central venous injection of iopamidol (0.5 ml·kg−1·2 s−1), which were repeated during suprarenal infusion of the vasodilator and diuretic ACh (5 μg·kg−1·min−1), to test endothelium-dependent responses and tubular function. A renal volume study was then performed in the spiral mode (7).
Images from the flow and volume studies were reconstructed, and time-attenuation curves were obtained from the aorta, bilateral renal cortex, and medulla. The parameters obtained from each regional curve were used to calculate cortical and medullary perfusion, ITC, and tubular fluid mean transit time (MTT) (11) along the nephron (proximal tubule, Henle's loop, and distal tubule), and normalized GFR (ml·min−1·cm−3 tissue) (3, 7). Single kidney volume, RBF (ml/min), GFR (ml/min), and renal vascular resistance (RVR) were subsequently calculated as previously described (1, 4).
Histology.
After completion of all in vivo studies (3 days), animals were killed with IV Sleepaway (100 mg/kg iv; Fort Dodge Laboratories). The kidneys were harvested and prepared for histology. For fibrosis and microvascular remodeling (media-to-lumen ratio), kidney samples were embedded in paraffin, and 5-μm-thick sections were stained with trichrome and α-smooth muscle actin (DakoCytomation) and analyzed using a computer-aided image analysis program (MetaMorph; Molecular Devices, Sunnyvale, CA).
Statistical analysis.
Results are expressed as means ± SE. Comparisons within and between groups were performed with ANOVA or paired and unpaired Student's t-tests, with the Bonferroni correction. Least-square regression was used to assess the relationship between renal angiography and renal function. Multiple-variable linear regression analysis was done to assess differences between groups’ correlations. Statistical significance was accepted if P ≤ 0.05.
RESULTS
Animals developing total occlusion with collaterals vessels (2 RAS, 2 ARAS) were excluded from the study. The remaining RAS (n = 26) and ARAS (n = 22) pigs developed an increase in MAP compared with normal by the day of the in vivo study (Table 1). The degree of stenosis determined by renal angiography was similar in both groups, and the increase in PRA did not reach statistical significance in either group. As per protocol design, cholesterol levels were higher in ARAS compared with normal and RAS (Table 1).
Table 1.
Systemic and stenotic kidney function in pigs with unilateral RAS and ARAS
| Normal | RAS | ARAS | |
|---|---|---|---|
| No. of kidneys | 8 | 26 | 22 |
| Body wt, kg | 48.3 ± 1.0 | 46.6 ± 1.3 | 48.6 ± 5.3 |
| Degree of stenosis, % | 0 | 64.7 ± 4.5* | 63.8 ± 4.2* |
| Total cholesterol, mmol/l | 2.2 ± 0.1 | 2.1 ± 0.1 | 10.2 ± 0.2*† |
| PRA, pg/ml | 0.3 ± 0.5 | 2.85 ± 1.4 | 2.4 ± 1.5 |
| Mean arterial pressure, mmHg | 98.8 ± 2.3 | 117 ± 4.3* | 117.6 ± 4.5* |
| GFR, ml/min | 80.7 ± 5.4 | 51.0 ± 3.5* | 56.4 ± 4.0* |
| Renal blood flow, ml/min | 584 ± 13 | 347.7 ± 30* | 324.1 ± 30* |
| Renal volume, ml/kg | 2.31 ± 0.07 | 1.86 ± 0.07* | 1.60 ± 0.08*† |
| Perfusion, ml·min−1·ml−1 | |||
| Cortex | 4.7 ± 0.2 | 3.8 ± 0.2* | 3.8 ± 0.2* |
| Medulla | 3.1 ± 0.3 | 2.2 ± 0.3 | 2.0 ± 0.3* |
| RVR, mmHg·ml−1·min−1 | |||
| Basal | 0.17 ± 0.01 | 0.39 ± 0.05* | 0.34 ± 0.03* |
| ACh | 0.14 ± 0.02 ‡ | 0.30 ± 0.03*‡ | 0.33 ± 0.02* |
| ITC basal | |||
| Proximal | 3.94 ± 0.3 | 4.74 ± 0.3 | 4.30 ± 0.4 |
| Henle's | 5.04 ± 0.4 | 5.38 ± 0.7 | 5.22 ± 0.4 |
| Distal | 6.82 ± 0.7 | 6.92 ± 0.3 | 5.85 ± 0.3*† |
| ITC ACh | |||
| Proximal | 3.05 ± 0.2‡ | 3.32 ± 0.1‡ | 3.53 ± 0.3‡ |
| Henle's | 3.99 ± 0.7‡ | 3.75 ± 0.4‡ | 4.80 ± 0.6 |
| Distal | 4.72 ± 0.6‡ | 4.86 ± 0.1‡ | 5.10 ± 0.6 |
Values are means ± SE. RAS, renal artery stenosis; ARAS, atherosclerotic renal artery stenosis; PRA, plasma renin activity; GFR, glomerular filtration rate, RVR, renal vascular resistance; ITC, intratubular fluid concentration (arbitrary units). P < 0.05 vs. normal (*), vs. RAS (†), and vs. baseline (‡).
Hemodynamics and function and tubular dynamics of the stenotic kidney.
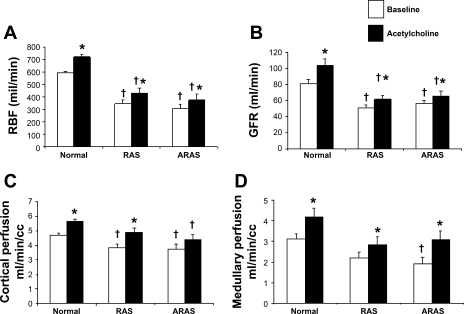
Stenotic kidney RBF, GFR, and cortical perfusion were all lower in RAS and ARAS compared with normal (Table 1 and Fig. 1), confirming hemodynamically significant stenoses. Furthermore, renal volume was smaller in ARAS than in RAS (P = 0.03), and both were smaller than normal (Table 1). Moreover, only in ARAS medullary perfusion was lower than normal (Table 1 and Fig. 1). Vascular MTT in the cortex was slightly but not significantly increased in RAS kidneys compared with normal (9.1 ± 0.6 and 8.52 ± 0.2 s, respectively, P = 0.2) but prolonged in ARAS compared with normal (10.1 ± 0.5 s, P = 0.036 vs. normal). Basal ITC in RAS was slightly but not significantly higher compared with normal in the proximal tubule. In contrast, in ARAS, distal tubule ITC was significantly lower than normal and RAS (Table 1), suggesting decreased fluid reabsorption. In addition, tubular fluid MTT were prolonged along the nephron (proximal, Henle's, and distal tubules) in ARAS (39 ± 1, 67 ± 2, and 111 ± 2 s, respectively,) compared with normal (34 ± 2, 59 ± 4, and 101 ± 6 s, respectively, P < 0.05 for all), but in RAS tubular MTT (43 ± 1, 77 ± 2, and 118 ± 3 s, respectively, P < 0.01 vs. normal, P < 0.05 vs. ARAS) were increased compared with both normal and ARAS, indicating slower fluid transit in RAS.
Fig. 1.
Renal blood flow (RBF, A), glomerular filtration rate (GFR, B), and cortical (C) and medullary (D) perfusion at baseline (open bars) and in response to ACh (filled bars) in normal, renal artery stenosis (RAS), and atherosclerotic renal artery stenosis (ARAS) kidneys. P < 0.05 vs. baseline (*) and vs. normal (†).
ACh infusion increased RBF, GFR, and medullary perfusion in all three groups, but the GFR response that was not different in RAS compared with normal (19.6 ± 5 vs. 33.2 ± 3.1%, respectively, P = 0.08) was attenuated in ARAS (13.3 ± 5.6%, P < 0.05 vs. normal). Cortical perfusion significantly and similarly increased in response to ACh in normal and RAS, but in ARAS it did not increase and remained lower than normal (Fig. 1), suggesting a slightly exacerbated cortical microvascular endothelial dysfunction in ARAS. Basal RVR was elevated in both RAS and ARAS compared with normal. During ACh infusion, RVR remained higher than normal in both RAS and ARAS, but in ARAS RVR did not decrease compared with baseline (Table 1). The diuretic ACh also induced a significant decrease in ITC along the nephron in normal and RAS pigs, suggesting decreased tubular fluid reabsorption. However, at the loop of Henle and distal tubule of ARAS, ITC did not decrease (Table 1), indicating impaired tubular response to ACh.
Relationship between stenotic kidney function and the degree of stenosis.
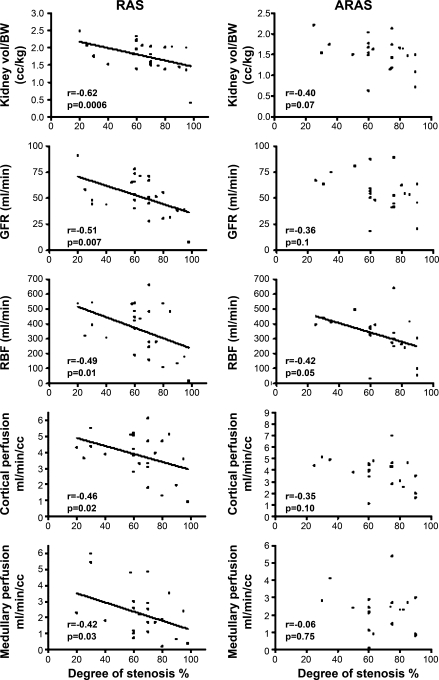
RAS renal volume, cortical and medullary perfusion, RBF, and GFR declined with increasing degree of stenosis (Fig. 2). In ARAS, only RBF modestly correlated with the degree of stenosis in the renal artery. In contrast, neither kidney volume nor GFR, cortical, or medullary perfusion in ARAS correlated with the degree of stenosis (Fig. 2), suggesting that factors other than the stenosis were also involved in the renal parenchymal damage in ARAS.
Fig. 2.
Correlation of the renal volume per body wt (vol/BW), GFR, RBF, and cortical and medullary perfusion with the severity of the stenosis in RAS (left) and ARAS (right). All of these functional and hemodynamic parameters correlated better in RAS than in ARAS.
Basal ITC in the proximal tubule of RAS correlated directly with the severity of the stenosis (r = 0.54, P = 0.006), but in ARAS ITC tended to correlate inversely with the severity of stenosis in the proximal (r = −0.44, P = 0.06) and distal (r = −0.39, P = 0.09) tubules. Furthermore, in RAS kidneys, proximal and distal tubular fluid MTT significantly and directly correlated with the severity of the stenosis (r = 0.50, P = 0.008 and r = 0.52, P = 0.007, respectively), suggesting slower cortical tubular flow with increasing degree of stenosis, whereas in ARAS no such correlation was observed (r = −0.16, P = 0.60 and r = −0.30, P = 0.2, respectively).
Multiple-variable linear regression analysis did not detect significant differences in the correlations between RAS and ARAS.
Renal tissue.
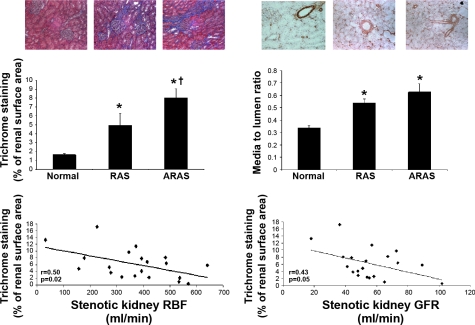
Trichrome staining showed more extensive regions of interstitial fibrosis in the stenotic ARAS kidneys compared with normal and RAS (Fig. 3). Of note, for both RAS and ARAS stenotic kidneys, the degree of renal fibrosis correlated inversely with GFR and RBF (Fig. 3), suggesting that fibrosis contributes to the impairment in renal hemodynamics and function in the stenotic kidney.
Fig. 3.
Representative renal staining for trichrome and α-smooth muscle actin (top) and quantitation (middle) in normal, RAS, and ARAS stenotic kidneys. Atherosclerosis increased fibrotic changes in ARAS. Bottom: correlation of trichrome staining (indicative of fibrosis content) with RBF (left) and GFR (right) in the stenotic kidney of RAS and ARAS. P < 0.05 vs. normal (*) and vs. RAS (†).
The microvascular media/lumen was similarly increased in the stenotic kidney in RAS and ARAS compared with normal (Fig. 3). No significant correlations were observed between media/lumen and renal hemodynamics and function.
DISCUSSION
Powerful tomographic imaging tools such as MDCT, which provide measurements of individual kidney cortical and medullary blood flow, volume, and tubular dynamics, allow precise assessment of single-kidney hemodynamics as a function of the severity of stenosis. Our results demonstrated that coexisting atherosclerosis induced greater kidney volume reduction, significantly more tubular impairment, and slightly greater vascular dysfunction in the stenotic ARAS kidney compared with nonatherosclerotic RAS. Therefore, our study suggests that the main impact of atherosclerosis on the stenotic kidney manifests in tubular dysfunction and interstitial fibrosis, which exceed its effect on the renal vascular or glomerular compartments.
ARAS is being increasingly identified in patients with end-stage renal disease (15), and clinical studies demonstrate unfavorable outcomes in ARAS compared with other causes of RAS (18), implying that mechanisms beyond the stenosis contribute to the deleterious effect of hypoperfusion on the kidneys and augment renal damage. Atherosclerosis might interact with renal hypoperfusion imposed by the stenosis via several shared pathophysiological pathways to cause vascular injury and renal dysfunction. We had previously shown that ARAS aggravates oxidative stress, inflammation, and fibrosis in the stenotic kidney compared with RAS alone (1, 2). These mechanisms may impair basal or stimulated vascular and tubular function and intensify renal injury in ARAS compared with RAS.
The current study showed that RBF, GFR, and cortical perfusion were similarly decreased in ARAS and RAS. On the other hand, medullary perfusion declined only in ARAS compared with normal. This might exacerbate medullary ischemia and contribute to the unfavorable outcomes observed in ARAS (1) because the renal medulla is particularly vulnerable to hypoperfusion and ischemia (6). ARAS kidney volume was also smaller than normal and RAS, possibly reflecting renal shrinkage as is manifested in exacerbated fibrosis in ARAS kidneys.
Interestingly, we observed that, in ARAS, only RBF modestly correlated while stenotic single-kidney GFR, volume, cortical, or medullary perfusion did not correlate with the degree of stenosis. Conversely, the hemodynamics and function of the stenotic kidney showed a significant linear relationship with the degree of stenosis in RAS. The reason behind the moderate, albeit significant, relationship observed in RAS may be twofold. First, within the range of RBF autoregulation, renal function changes minimally until the stenosis is >60–70%. Second, assessment of the degree of stenosis from two-dimensional projection images is know to be associated with inaccuracies. Nevertheless, we observed significant linear relationship between these parameter in RAS. Although these relationships did not differ statistically significantly between RAS and ARAS, their attenuation in ARAS suggests that atherosclerosis itself alters the effects of the stenotic lesion on many of the impairments in renal hemodynamics and function compared with those observed in nonatherosclerotic RAS. Therefore, this study suggests that atherosclerosis modulates the effect of a stenosis in the renal artery on the stenotic kidney.
Some of these outcomes might be attributable to endothelial dysfunction distal to the stenosis, which was only modestly greater in ARAS than RAS. Although RBF response to ACh was similar in ARAS and RAS, only in ARAS the drop in RVR was blunted, and cortical perfusion failed to respond to the endothelium-dependent vasodilator and remained lower than normal. This might be because of a more pronounced decrease in nitric oxide bioavailability in this group compared with RAS. In the stenotic RAS kidney, an increase in oxidative stress secondary to local activation of the renin-angiotensin system and NAD(P)H oxidase might inactivate nitric oxide and impair endothelial function. Atherosclerosis also induces oxidative stress and vascular inflammation, which may exacerbate endothelial dysfunction in ARAS kidneys, and in turn contribute to impairment in renal hemodynamics and functions. In addition to endothelial dysfunction, it is not unlikely that the fixed upstream stenosis interferes with the ability of RBF to increase in response to ACh, although this effect should be similar in both RAS and ARAS, which has a comparable degree of stenosis. Interestingly, despite the attenuated cortical perfusion response in ARAS, overall RBF response to ACh was similar to that in RAS, probably because medullary perfusion showed a slightly greater increase. Importantly, tomographic imaging techniques enabled detection of distinct effects of atherosclerotic renal artery stenosis on renal regional perfusion, which do not necessarily manifest in measures of RBF.
The most prominent effects of atherosclerosis on the stenotic kidney in this study were observed in the tubulointerstitial compartment. One of the earliest manifestations of renal injury, and the closest correlate with renal outcome, is tubular injury (16). However, few methods can assess in vivo renal tubular function in the intact kidney. We have previously demonstrated using MDCT that, in RAS kidneys, ITC increased, suggesting increased tubular fluid reabsorption secondary to the decrease in renal perfusion pressure. The current study demonstrates that, in RAS, tubular fluid MTT is also prolonged and that the increase in ITC and cortical tubular transit time is related to the severity of the stenosis. Contrarily, in ARAS, the stenosis induced tubular dysfunction, reflected in impaired tubular fluid reabsorption and decreased ITC (1). This study shows that ARAS ITC tends to decrease and tubular transit time was significantly lower than normal and RAS and did not increase with the severity of the stenosis, underscoring the remarkable tubular damage and dysfunction elicited by coexistence of renal hypoperfusion and early atherosclerosis (1).
In addition, the severity of histopathological injury is an important predictor of renal outcomes in patients with atherosclerotic nephropathy (24). Notably, the current study extends our previous observations (1, 2) and shows that not only ARAS kidneys have more fibrosis than RAS but renal fibrosis correlated inversely with stenotic kidney hemodynamics and function. Furthermore, kidney volume was significantly smaller in ARAS. Hence, adverse tissue remodeling is likely an important mechanism by which atherosclerosis modulates adaptive responses to RAS.
This study does have some limitations. In the present study, we used relatively young pigs, with short-term preexisting disease and exposure to atherosclerosis and renovascular disease. While the differences between the hemodynamics and function of the ARAS and RAS kidneys after this relatively short duration were subtle, longer duration of ARAS might lead to pathophysiological implications for evolution of kidney injury. Additionally, in contrast to our previous observations (usually employing 6–7 animals/group), in this study, using a large number of pigs, ACh induced a small increase in GFR, RBF, and intrarenal perfusion in RAS and ARAS. Nevertheless, the lower responses of GFR to ACh in both groups, and the inadequate response of cortical perfusion and RVR in ARAS, imply that endothelial function is impaired in RAS and further exacerbated in ARAS.
In summary, taking advantage of unique imaging methods, the present study demonstrates that atherosclerosis intensifies renal tubular and vascular dysfunction and modulates the impact of RAS on single-kidney hemodynamics and function. This might be mediated by significant aggravation of renal fibrosis and modest accentuation of endothelial dysfunction in the stenotic kidneys. The prominent tubular and subtle vascular and glomerular disturbances that develop independent of the degree of stenosis partly may account for the frequent failure to resolve renal injury by renal revascularization and for the increased propensity for chronic kidney disease observed in patients with ARAS. Additional studies are needed to determine which of these alterations portend the success of renal revascularization. Furthermore, this study supports a role for interventions that aim to directly decrease injury in the kidney distal to the stenosis to ensure renal viability and capacity to recover.
GRANTS
This study was partly supported by National Institutes of Health Grants DK-73608, DK-77013, HL-77131, and PO1HL-085307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Current address for R. Lavi: Dept. of Anesthesia and Perioperative Medicine, London Health Care Science Centre, University of Western Ontario, London, Ontario, Canada.
REFERENCES
- 1.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chade AR, Rodriguez-Porcel M, Herrmann J, Krier JD, Zhu X, Lerman A, Lerman LO. Beneficial effects of antioxidant vitamins on the stenotic kidney. Hypertension 42: 605–612, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 15: 958–966, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med 165: 207–213, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 112: 1362–1374, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Hansen KJ, Cherr GS, Dean RH. Dialysis-free survival after surgical repair of ischemic nephropathy. Cardiovasc Surg 10: 400–404, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int 49: 846–854, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Marekovic Z, Mokos I, Krhen I, Goreta NR, Roncevic T. Long-term outcome after surgical kidney revascularization for fibromuscular dysplasia and atherosclerotic renal artery stenosis. J Urol 171: 1043–1045, 2004 [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin K, Jardine AG, Moss JG. ABC of arterial and venous disease. Renal artery stenosis. Br Med J 320: 1124–1127, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson RJ. Tubulointerstitial disease: role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens 12: 233–241, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ramos F, Kotliar C, Alvarez D, Baglivo H, Rafaelle P, Londero H, Sanchez R, Wilcox CS. Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 63: 276–282, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 344: 431–442, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Singer GM, Remetz MS, Curtis JP, Setaro JF. Impact of baseline renal function on outcomes of renal artery stenting in hypertensive patients. J Clin Hypertens (Greenwich) 11: 615–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suresh M, Laboi P, Mamtora H, Kalra PA. Relationship of renal dysfunction to proximal arterial disease severity in atherosclerotic renovascular disease. Nephrol Dial Transplant 15: 631–636, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Trivedi HS, Pang MM, Campbell A, Saab P. Slowing the progression of chronic renal failure: economic benefits and patients’ perspectives. Am J Kidney Dis 39: 721–729, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant 16: 765–770, 2001 [DOI] [PubMed] [Google Scholar]