Abstract
Nephronophthisis (NPHP) is the most frequent genetic cause of end-stage renal failure in the first three decades of life. It is characterized primarily by renal cysts with extrarenal involvements of the eye and brain. Ten recessive genes responsible for NPHP have been identified by positional cloning. This discovery supported a unifying theory of renal cystic disease, which states that all proteins mutated in cystic kidney diseases of human, mice, or zebrafish are expressed in primary cilia of renal epithelial cells. Mutations in nephrocystin-3 (NPHP3) are the cause of human nephronophthisis type 3 and polycystic kidney disease (pcy) mouse mutants. To study the functional role of NPHP3 in normal embryonic development and in the pathogenesis of cystic kidney disease, we characterized the zebrafish ortholog nphp3 by morpholino oligo (MO)-mediated knockdown. When nphp3 function was suppressed by either of the two MOs blocking the translation of the protein or the splicing of mRNA, zebrafish embryos displayed hydrocephalus and pronephric cysts. Knockdown of nphp3 also led to situs inversus phenotypes due to defective cilia at Kupffer's vesicle. We showed that nphp3 genetically interacts with nphp2/inversin and human NPHP3 localizes to primary cilia in Madin-Darby canine kidney cells. Like nphp2/inversin, nphp3 knockdown affected morphogenic cell movement during gastrulation, suggesting nphp3 is essential to regulate convergent extension. Thus nphp3, cooperating with nphp2/inversin, plays an essential role related to ciliary function, and the knockdown provides an animal model that may be used for studies of the pathogenesis and therapy for this disease.
Keywords: nephronophthisis, kidney cyst, situs inversus, cilium, Kupffer's vesicle
nephronophthisis (NPHP) is an autosomal recessive kidney disease that causes kidney cyst formation, fibrosis, and progressive renal failure. It is the most frequent genetic cause for end-stage renal disease (ESRD) in the first three decades of life. By positional cloning, we and others have identified 10 causative genes for NPHP, namely, NPHP1-6, Glis2/NPHP7, RPGRIPL1/NPHP8, NEK8/NPHP9, and MKS3/NPHP11. Notably, many of the NPHP gene products are localized at primary cilia, basal bodies, and centrosomes, implicating that abnormalities of these subcellular structures may be involved in the pathogenesis of NPHP (10, 11).
Recessive mutations in NPHP3, which encodes nephrocystin-3, were initially identified in a Venezuelan kindred as causing NPHP and tapetoretinal degeneration that developed ESRD at a median age of 19 yr (20). Subsequently, additional mutations in NPHP3 were identified in patients with a wide spectrum of symptoms, ranging from milder forms (isolated NPHP with late-onset ESRD) to severe forms [exhibiting Meckel syndrome (MKS)-like syndrome with malformation of multiple organs] (5, 22). The genotype-phenotype correlation suggests that hypomorphic mutations in NPHP3 are associated with degenerative diseases including isolated NPHP, retinal degeneration, and liver fibrosis, while loss-of-function mutations lead to more severe diseases with dysplasia and malformation of multiple organs in a MKS-like syndrome combined with severe congenital cystic kidney disease (29, 34). This genotype-phenotype correlation is paralleled by mouse models of Nphp3 deficiency: pcy mouse mutants that carry a missense mutation in Nphp3 develop polycystic kidney disease, whereas Nphp3 knockout mice develop more severe phenotypes including situs inversus, congenital heart defects, cystic kidney, and embryonic lethality (5, 20).
Zebrafish have been widely used in studies of nephronophthisis and other cystic kidney diseases. Morpholino-mediated knockdown of many NPHP, Bardet-Biedl syndrome (BBS), and cystic kidney disease genes in zebrafish have produced phenotypes consistent with symptoms observed in human patients (9, 14, 19, 23–27, 36). Here, we report the functional characterization of zebrafish nphp3 ortholog via morpholino oligo-mediated knockdown. Knockdown of zebrafish nphp3 reproduced the human disease phenotype and led to hydrocephalus, cystic pronephric kidney, and left-right asymmetry defects, which have been found in cilium-deficient diseases in human (1). The motile cilia at Kupffer's vesicle, a zebrafish laterality organizer (7, 15), were abnormal following nphp3 knockdown, demonstrating that nphp3 is required for normal ciliary development. Consistent with previous reports in Xenopus laevis (5), knockdown of nphp3 also affected morphogenic cell movement during gastrulation, a process known to be dependent on proper planar cell polarity.
MATERIALS AND METHODS
Fish breeding and maintenance.
Zebrafish (Danio rerio) were reared and maintained as described (35). Embryos were collected after natural spawns, kept at 28.5°C, and staged according to hours postfertilization (hpf). A laboratory-inbred wild-type strain and AB* wild-type strain were used for morpholino-mediated knockdown experiments. All animal procedures have been reviewed and approved by the University Committee on the Use and Care of Animals at the University of Michigan (protocol 09737).
RT-PCR and cDNA cloning.
A BLAST search for the zebrafish nphp3 gene was performed using the protein sequence of human NPHP3 (GenBank accession NP_001008880). The zebrafish genome database (http://www.ensembl.org/Danio_rerio/) was searched for genomic sequences, and the acquired genomic sequences were analyzed with Genscan software (http://genes.mit.edu/GENSCAN.html) to determine the potential coding exons. Based on the sequence analysis results, primers were designed for RT-PCR.
Total RNA was isolated from 24- to 72-hpf embryos using Tri-reagent (Invitrogen, Carlsbad, CA) and reserve-transcribed with oligo-dT primers and Superscript II reverse transcriptase (Invitrogen) following the manufacturer's protocol (Superscript II manual, version 11-11-203). RT-PCR was carried out following standard protocol, and the PCR products were gel-purified, cloned into the Promega T-easy vector (Promega, Madison, WI) and sequenced at the University of Michigan Sequencing Core. Sequence alignment (Clustal W method) was done with Lasergene software (DNAStar, Madison, WI). Primer pairs for cloning the cDNA of zebrafish nphp3 were 5′-TTTTTCGCTTGGGTGGAGTATTT (forward) and 5′ CCCAGTGGCAGTGAAGATTAGATT (reverse).
In situ hybridization.
In situ hybridization was carried out as previously described (37). To prevent pigmentation for expression analysis after 24 hpf, embryos were transferred to water containing 0.2 mM 1-phenyl-2-thiourea at 20 hpf and fixed at appropriate stages. The digoxigenin-labeled probes for zebrafish nphp3, krox20, and myoD were in vitro synthesized from the cloned cDNAs. The probes for cmlc2, ins, and fkd2 are gifts from Dr. R. D. Burdine (27).
Morpholino oligo-mediated knockdown.
To knock down zebrafish nphp3, two morpholino oligos were synthesized (Gene-Tools). The translation-blocking morpholino oligo (AUG-MO; 5′-TGCCGTACCCATAACAACTGATAAG) is 3′-labeled with FITC. The splice-blocking morpholino oligo (SP-MO; 5′-GCCGTGTGTGTACCTGAATGAAAGT) is targeted to the junction of exon 5 and intron 5. The morpholino oligo for zebrafish nphp2/inversin (invs-MO) is as previously reported (23). A standard control morpholino oligo (control-MO; 5′-CCTCTTACCTCAGTTACAATTTATA) is used as a negative control. The morpholino oligos were dissolved in nuclease-free water and injected into zebrafish embryos at one- to four-cell stages in 0.1 M KCl at the specified dosage. The injection volume is estimated to be 1–2 nl. A χ2 test was used to determine the statistical significance of the laterality defects resulting from the morpholino oligo injection.
Histology.
Zebrafish embryos were fixed with 4% paraformaldehyde overnight, serial-dehydrated with 25, 50, 75, and 95% ethanol, and then equilibrated with JB-4 solution (Polysciences) overnight at 4°C. The embryos were embedded in JB-4 resin and sectioned with a Leica R2265 microtome. The sections were stained with methylene blue as previously described (12). The images were captured with an upright compound microscope.
Immunohistochemistry.
Zebrafish embryos were fixed with Dent's fixative (80% methanol, 20% DMSO), rehydrated with 1× PBS, and blocked at room temperature for 2 h with the incubation buffer (2 mg/ml BSA, 1% DMSO, 0.2% Triton-X 100, 1× PBS, pH 7.4) containing 10% serum. After blocking, the embryos were incubated with anti-acetylated tubulin antibody (1:500, Sigma) and Alex 594-conjugated anti-mouse IgG (1: 1,000, Invitrogen), respectively, at 4°C overnight. The confocal images were captured with a Zeiss 510 laser scanning microscope and analyzed with LSM510 software (Zeiss). A two-tail Student's t-test was used to analyze the ciliary abnormality and the convergent extension defect.
Madin-Darby canine kidney (MDCK-II) cells were obtained from the American Type Culture Collection and were cultured as recommended. Cells were grown on coverslips in DMEM (GIBCO) supplemented with 10% FBS (Atlanta Biologicals) in the presence of penicillin G (10 U/ml), streptomycin sulfate (10 mg/ml), and amphotericin B (0.25 mg/ml, GIBCO). At confluence, cells were washed in 1× PBS, fixed with 4% formaldehyde for 15 min, permeabilized with 0.1% Triton for 5 min, and blocked with 2% goat serum (Abcam) for 2 h. After immunostaining, coverslips were mounted using ProLong antifade with 4,6-diamidino-2-phenylindole (Molecular Probes). Confocal laser microscopy was performed using a Zeiss LSM 510 confocal microscope mounted on a Zeiss Axiovert 100M inverted microscope (Zeiss). Images were acquired using a ×63 Plan Neofluor water-immersion objective (Zeiss). Images were edited using Adobe Photoshop 7.0. Polyclonal antibody N-18 against NPHP3 (Santa Cruz Biotechnology) was used at 1:500 and the monoclonal antibody against acetylated tubulin (Sigma) at 1:1,000. Fluorescently labeled Alexa-Fluor secondary antibodies (Molecular Probes) were used at the concentrations suggested by the manufacturer.
RESULTS
Morpholino-mediated knockdown of zebrafish nphp3 leads to body curvature, hydrocephalus, and pronephric cysts.
We identified the zebrafish nphp3 ortholog (ENSDARG00000016184) by searching zebrafish genome database. The zebrafish nphp3 gene is predicted to encode a protein of 1,303 amino acids that is highly conserved compared with human and mouse NPHP3, with a sequence identity of 66–68% and a sequence similarity of 82–83%. Like mammalian nephrocystin-3 (20), zebrafish nephrocystin-3 contains eight conserved protein-interacting tetratricopeptide repeats (TPR) (Fig. 1).
Fig. 1.
Sequence alignment of nephronophthisis type 3 (NPHP3) proteins (NPHP3 encodes nephrocystin-3). Zebrafish (Danio rerio), Homo sapiens, Mus musculus, and Xenopus laevis NPHP3 protein sequences were aligned with ClustalW. Zebrafish nephrocystin-3 shares significant amino acid sequence similarity and identity with its mammalian counterparts. Like mammalian nephrocystin-3, zebrafish nephrocystin-3 encodes a coiled-coil (CC) domain near its N terminus and 8 TRP (tetratricopeptide repeat) domains in its C-terminal half. (Reference sequences are H. sapiens: NP_694972; M. musculus: NP_082997; and X. laevis: NP_001086695).
To study the function of nphp3 during zebrafish embryonic development, we injected a morpholino oligo (AUG-MO) targeting the translation initiation site of nphp3 mRNA. Injection of this morpholino into fertilized eggs resulted in phenotypes resembling those observed in other knockdown models of zebrafish nephrocystin orthologs (23, 26): body curvature was observed in 51% (n = 125) of the morphant embryos injected with 8 ng of AUG-MO as early as 24 hpf (Fig. 2, A and D); after 36 hpf, morphants started to develop hydrocephalus and by 48 h, 98% (n = 154) of embryos showed hydrocephalus (Fig. 2, B and E); 6.7% (n = 105) of the embryos showed cysts in the pronephric tubules at 60 hpf (Fig. 2, C and F). To confirm that these phenotypes are specifically due to the loss of nphp3 function, we injected another morpholino oligo (SP-MO) targeting the splice junction of exon 5 and intron 5. Two nanograms of SP-MO produced two aberrant RT-PCR products by altering the splicing of nphp3 mRNA and led to the phenotypes observed in AUG-MO-injected embryos (Fig. 2, G and H). Sequencing revealed that the aberrant mRNAs skipped exon 5 and included exon 6, resulting in a premature stop-codon in the coding sequence. Thus knockdown of nphp3 function in zebrafish embryos produced phenotypes presented in other zebrafish models of NPHP, BBS, and ciliary genes, suggesting nphp3 is involved in ciliary function.
Fig. 2.
Disruption of zebrafish nphp3 function during zebrafish embryonic development results in body curvature, hydrocephalus, and pronephric cysts. Sixty hours postfertilization (hpf)-zebrafish embryos injected with 4 ng control-morpholino oligo (MO) has normal morphology (A), but injection with 4 ng of nphp3 targeting the translation initiation site of nphp3 mRNA (AUG-MO) led to downward body curvature and hydrocephalus (D). Similar phenotypes are seen in embryos injected with 2 ng of splice-blocking (SP-MO; H). Sections through the hindbrain region, as indicated by white dashed lines in A and D showed the ventricle (star) was of normal size in embryos injected with control MO (B) but was enlarged in nphp3 morphant embryo (E). Cross sections through the pronephros revealed cysts (asterisks) in nphp3 morphants (F), whereas control-MO injected embryos have normal pronephric tubules (arrows; C). RT-PCR shows that SP-MO altered nphp3 RNA processing (G). Two shorter RT-PCR products (stars) were detected solely in SP-MO morphants. *1 indicates the cDNA fragment that skips exon 5, whereas *2 indicates the cDNA fragment that skips exon 5 but includes intron 6. The normal RT-PCR product (arrow) is absent in morphants at 24 hpf. At later stages, the normal product is detected in morphants due to diminishing efficacy of MO knockdown.
Knockdown of zebrafish nphp3 leads to left-right asymmetry defects.
NPHP2/inversin is the causal gene of infantile nephronophthisis, and loss of function of NPHP2 causes situs inversus in mouse mutants and human patients (23). Recently, NPHP2 has been shown to physically interact with NPHP3 (5), suggesting a functional relationship between these two genes. Thus we examined the left-right asymmetry patterning in zebrafish embryos after knocking down nphp3. At 55 hpf, zebrafish embryos injected with control-MO displayed normal heart jogging, with the ventricle on the left and atrium on the right (Fig. 3A). However, 22.5% (n = 322) of AUG-MO morphant embryos showed inverted heart jogging (Fig. 3B), and 7.3% showed a heterotaxia phenotype (Fig. 3C). The abnormal heart jogging was also present in SP-MO morphant embryos in a dose-dependent manner (Fig. 3C). In addition, the laterality of other visceral organs, such as the pancreas and liver, was also affected by knockdown of nphp3 (Fig. 3, D–F). Furthermore, to investigate the genetic interaction between nphp2/inversin and nphp3, we coinjected subphenotypic dosages of nphp3 AUG-MO and invs-MO. Coinjection of 1 ng invs-MO and 2 ng nphp3 AUG-MO led to 11.5% inverted heart jogging and 13% heterotaxia phenotype (n = 164), a significantly higher penetrance than caused by knockdown of nphp3 (6.2% inverted heart jogging and 6.2% heterotaxia phenotype, n = 183) or inversin alone (7.9% inverted heart jogging and 9.1% heterotaxia phenotype, n = 252) (χ2 < 0.001) (Fig. 3G).This enhancement of the penetrance of the laterality defects indicated a genetic interaction between nphp2/inversin and nphp3.
Fig. 3.
Disruption of zebrafish nphp3 results in abnormal laterality. Ventral view of 55-hpf embryos with in situ hybridization of cmlc2 showed normal heart jogging in control-MO-injected embryos (A) and inverted jogging in nphp3 morphants (B). C: quantification in percentage of the heart jogging phenotype using 2 different MOs and negative control MO. nphp3 morphants (E and F) also developed abnormal positioning of the pancreas (P) and liver (L) compared with control-MO injected embryos (D). Coinjection of MOs against nphp3 and nphp2/invs led to a higher penetrance of situs inversus phenotype (G). H: dorsal view of 23-hpf embryos showed the left-sided expression of lefty1/2 in the midbrain and in the developing heart of control-MO injected embryos. In nphp3 morphants, lefty1/2 expression was inverted to the right side (I) or to the midline (J). K: quantification of the lefty1/2 expression pattern. L: dorsal view of 19- to 20-hpf embryos showed the left-sided expression of southpaw in the control-MO-injected embryos. Southpaw expression was inverted to the right side (M) or bilateral (N) in nphp3 morphants. O: quantification of the southpaw expression pattern.
Lefty1/2 are asymmetric markers expressed in the cardiac field and the midbrain during early embryonic development (6, 27). We found that the lefty1/2 expression pattern was affected by knockdown of nphp3, with a penetrance comparable to the heart jogging phenotype at 23 hpf (Fig. 3, H–K). Sounthpaw is one of the earliest markers that display asymmetric expression in embryonic development (17). We found that its expression pattern was also affected in nphp3 morphants at 19–20 hpf (Fig. 3, L–O). Thus nphp3 function is required before the establishment of the asymmetric expression pattern of southpaw.
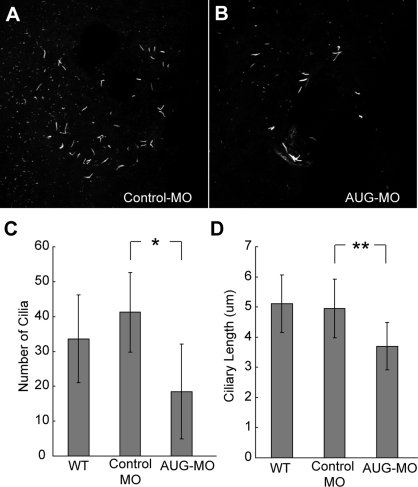
Kupffer's vesicle is a ciliated organ that transiently exists during zebrafish embryonic development, and the motile cilia at Kupffer's vesicle play an essential role in establishing the left-right asymmetry patterning (7, 15). Thus we examined these cilia within Kupffer's vesicle in nphp3 morphants by immunohistochemistry with an anti-acetylated tubulin antibody. Comparison of the ciliary number and ciliary length revealed that morphant embryos have significantly fewer cilia with shorter length in Kupffer's vesicle (Fig. 4, A and B). Wild-type embryos and control-MO-injected embryos have 33.6 ± 12.6 (n = 8) or 41.2 ± 11.4 (n = 6) cilia, respectively. However, nphp3 morphants have a reduced number of cilia (18.5 ± 13.6, n = 10, P < 0.001) (Fig. 4C). The length of cilia in nphp3 morphants (3.7 ± 0.79 μm, n = 76, P < 0.005) is also shorter than in wild-type (5.11 ± 0.96 μm, n = 102) or control-MO injected embryos (4.95 ± 0.98 μm, n = 90) (Fig. 4D). Thus we concluded that nphp3 function is essential for the normal development of cilia at Kupffer's vesicle.
Fig. 4.
Disruption of zebrafish nphp3 results in abnormal cilia at Kupffer's vesicle. Shown is anti-acetylated tubulin staining of cilia at Kupffer's vesicle in control-MO-injected embryos (A) and nphp3 morphants (B) at 14 hpf. C: quantification of ciliary length at Kupffer's vesicle. D: quantification of the number of cilia at Kupffer's vesicle. WT, wild-type. **P < 0.001, *P < 0.005 by Student's t-test.
Nephrocystin-3 is localized at primary cilia in renal epithelial cells.
To further define the role of nephrocystin-3 in the ciliary function, we characterized the subcellular localization of nephrocystin-3 in cultured MDCK cells with an antibody against human nephrocystin-3 (Supplementary Fig. S. 1). We found nephrocystin-3 was colocalized with acetylated tubulin at primary cilia in MDCK cells (Fig. 5). This localization suggests that nephrocystin-3 is required for the building and the maintenance of primary cilia. Furthermore, the subcellular location of nephrocystin-3 is similar to that of NPHP2/INVERSIN (18), supporting that NPHP3 and NPHP2 are present within a protein complex.
Fig. 5.
Nephrocystin-3 is localized at primary cilia in Madin-Darby canine kidney (MDCK) cells. Nephrocystin-3 (green) is colocalized with acetylated tubulin (red) in the primary cilia of MCDK cells.
Knockdown of nphp3 causes convergent extension defects.
Besides situs inversus and cystic kidney, knockdown of nphp2/inversin in zebrafish and X. laevis resulted in the inhibition of noncanonical Wnt signaling that regulates planar cell polarity and hence defective convergent extension cell movements during gastrulation (5, 28). We thus examined whether nphp3 morphants displayed any abnormality in convergent extension cell movements. Since defective convergent extension movements will lead to a shortened and widened embryo (32), the length/width ratio can quantitatively represent the outcome of cell movements during gastrulation. We defined the length/width ratio dividing the length of somites by the width of rhombomere 5 based on the staining of myoD and krox20, respectively (Fig. 6A). We found that nphp3 morphants have a significantly smaller length/width ratio (3.56 ± 0.87, n = 23, P < 0.001) than the control group (5.57 ± 0.46, n = 12), indicating a convergent extension defect due to the disruption of nphp3 function (Fig. 6B). This indicates that nphp3 function is required for proper convergent extension cell movements.
Fig. 6.
Disruption of D. rerio nphp3 function caused convergent extension defects. Dorsal view of 14- to 15-hpf embryos with in situ hybridization staining of krox20 and myoD in control-MO-injected embryos (A) and nphp3 morphants (B). Horizontal bar represents the width of krox20 staining in hindbrain rhombomere 5. Vertical bar represents the total length of somatic segments. C: length/width ratio is significantly lower in nphp3 morphants than in control-MO-injected embryos, indicating a convergent extension defect. *P < 0.001 by Student's t-test.
DISCUSSION
To model nephronophthisis type 3 in zebrafish and study the functional role of nephrocystin-3 during embryonic development, we characterized the zebrafish ortholog of NPHP3 by morpholino oligo-mediated knockdown. We found that disruption of zebrafish nphp3 led to many defects that have been shared by several zebrafish models of NPHP, BBS and MKS (2, 3, 23, 24, 26, 33, 36). These phenotypes, including body curvature, hydrocephalus, situs inversus, and pronephric cysts, have also been reported for zebrafish morphants of ciliary genes (14, 15, 19, 27, 30, 31), suggesting an essential role of nphp3 in ciliary function. Indeed, we showed that the motile cilia in Kupffer's vesicle in zebrafish embryos are defective in length and quantity following knockdown of nphp3, directly pointing out the involvement of nphp3 in ciliogenesis. Recently, a large-scale screening for genes involved in ciliary development using small interfering RNA-mediated knockdown identified NPHP3 as a potential regulator of ciliogenesis in human retinal pigmented epithelial cells (13). Consistently, nephrocystin-3 is highly expressed at the node in mouse embryos, and this expression is reduced in mouse mutants of Noto, which regulates ciliogenesis (4, 20). In addition, we found that human nephrocystin-3 is localized to primary cilia of MDCK3 cells, where other ciliary proteins, including nephrocystins, BBS, and MKS gene products, are present (9, 16, 18, 20, 21, 23, 25). Thus our data corroborate the unifying “ciliopathy” theory for renal cystic diseases (11).
Intriguingly, the nonmotile primary cilia in renal tubular epithelial cells are longer in Nphp3 knockout mice compared with their normal siblings, which suggests Nphp3 is involved in the regulation of ciliary length (5). As the cilia at Kupffer's vesicle of zebrafish embryos are motile with “9+2” structure (7, 15), which differs from that of nonmotile primary cilia, it is possible that the length regulation of different types of cilia might employ different mechanisms. To address this question, it will be worthwhile examining the nodal cilia in the knockout model of Nphp3 in mice.
Nephrocystin-3 has been shown to physically interact with NPHP2/INVERSIN (5), and overexpression of NPHP2/inversin or NPHP3 can inhibit canonical Wnt signaling activity (5, 28). Consistently, we showed the genetic interaction between nphp2/inversin and nphp3 by co-knockdown of these two genes in zebrafish. It has been shown that knockdown of nphp2/inversin in zebrafish enhances canonical Wnt signaling and led to defective convergent extension cell movement (28). Our finding that knockdown of nphp3 leads to similar defective cell movement during gastrulation demonstrates that nphp3 is required together with nphp2 in regulating this process. Therefore, it is possible that nphp3 is involved in controlling a balanced Wnt signaling. However, noncanonical Wnt signaling may be playing a permissive rather than instructive role in polarized cell movement during vertebrate gastrulation. Multiple downstream effectors, such as Ca2+/calmodulin, PKC, and cell-cell adhesion, and many other signaling pathways are involved in controlling cell movement during gastrulation. Whether nphp3 participates in any of these aspects is an open question.
Zebrafish nephrocystin-3 shares a significant amino acid sequence similarity and protein domain structure with its mammalian counterparts. Accordingly, the phenotypes of zebrafish nphp3 morphants are similar and consistent with the disease phenotype in human patients and in the knockout mouse model, suggesting the functional role of nephrocystin-3 is highly conserved through evolution. Given recent reports that several pharmacological agents have been able to effectively ameliorate renal phenotypes in hypomorphic mutant mice of Nphp3 (8, 33), the zebrafish model of NPHP3 may be used to further explore the therapeutic approaches for this disease. Since the knockout mice of Nphp3 are embryonically lethal whereas pcy mice develop merely the renal phenotype, the zebrafish model could be particularly useful to search for therapeutical interventions for extrarenal abnormalities.
GRANTS
F. Hildebrandt is the Frederick G. L. Huetwell Professor, an Investigator of the Howard, Hughes Medical Institute, Doris Duke Distinguished Clinical Scientist and is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK069274 and RO1-DK068306.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors appreciate Dr. Iain A. Drummond for help with histological analyses and Dr. Rebecca D. Burdine for sharing the riboprobes.
REFERENCES
- 1.Afzelius BA. Cilia-related diseases. J Pathol 204: 470–477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet 72: 650–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature 439: 326–330, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc Natl Acad Sci USA 104: 15765–15770, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, Nurnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nurnberg P, Gretz N, Lo C, Lienkamp S, Schafer T, Walz G, Benzing T, Zerres K, Omran H. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82: 959–970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development 126: 3253–3262, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132: 1247–1260, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 39: 1350–1360, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol 20: 23–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol 18: 1855–1871, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Humphrey CD, Pittman FE. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol 49: 9–14, 1974 [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464: 1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14: 954–961, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132: 1907–1921, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet 38: 155–157, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development 130: 2303–2316, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Nurnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell 13: 3096–3106, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol 17: 2706–2718, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 34: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Otto E, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O′Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F. A novel ciliary IQ domain protein, NPHP5, is mutated in Senior-Loken syndrome (nephronophthisis with retinitis pigmentosa), and interacts with RPGR and calmodulin. Nat Genet 37: 282–288, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Otto EA, Helou J, Allen SJ, O′Toole JF, Wise EL, Ashraf S, Attanasio M, Zhou W, Wolf MT, Hildebrandt F. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat 29: 418–426, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Otto EA, Schermer B, Obara T, O′Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413–420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sayer JA, Otto EA, O′Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38: 674–681, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Schafer T, Putz M, Lienkamp S, Ganner A, Bergbreiter A, Ramachandran H, Gieloff V, Gerner M, Mattonet C, Czarnecki PG, Sayer JA, Otto EA, Hildebrandt F, Kramer-Zucker A, Walz G. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Hum Mol Genet 17: 3655–3662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134: 1605–1615, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson MA, Cross HE, Cross L, Helmuth M, Crosby AH. Lethal cystic kidney disease in Amish neonates associated with homozygous nonsense mutation of NPHP3. Am J Kidney Dis 53: 790–795, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev Biol 314: 261–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol 13: 251–260, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol 23: 2095–2099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tory K, Rousset-Rouviere C, Gubler MC, Moriniere V, Pawtowski A, Becker C, Guyot C, Gie S, Frishberg Y, Nivet H, Deschenes G, Cochat P, Gagnadoux MF, Saunier S, Antignac C, Salomon R. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int 75: 839–847, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Westfield M. The Zebrafish Book Eugene, OR: University of Oregon Press, 1995 [Google Scholar]
- 36.Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet 15: 667–677, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Hildebrandt F. Molecular cloning and expression of phospholipase C epsilon 1 in zebrafish. Gene Expr Patterns 9: 282–288, 2009 [DOI] [PubMed] [Google Scholar]