Abstract
Conventional indicator dilution techniques for measuring body fluid volume are laborious, expensive, and highly invasive. Bioimpedance spectroscopy (BIS) may be a useful alternative due to being rapid, minimally invasive, and allowing repeated measurements. BIS has not been reported in mice; hence we examined how well BIS estimates body fluid volume in mice. Using C57/Bl6 mice, the BIS system demonstrated <5% intermouse variation in total body water (TBW) and extracellular (ECFV) and intracellular fluid volume (ICFV) between animals of similar body weight. TBW, ECFV, and ICFV differed between heavier male and lighter female mice; however, the ratio of TBW, ECFV, and ICFV to body weight did not differ between mice and corresponded closely to values in the literature. Furthermore, repeat measurements over 1 wk demonstrated <5% intramouse variation. Default resistance coefficients used by the BIS system, defined for rats, produced body composition values for TBW that exceeded body weight in mice. Therefore, body composition was measured in mice using a range of resistance coefficients. Resistance values at 10% of those defined for rats provided TBW, ECFV, and ICFV ratios to body weight that were similar to those obtained by conventional isotope dilution. Further evaluation of the sensitivity of the BIS system was determined by its ability to detect volume changes after saline infusion; saline provided the predicted changes in compartmental fluid volumes. In summary, BIS is a noninvasive and accurate method for the estimation of body composition in mice. The ability to perform serial measurements will be a useful tool for future studies.
Keywords: intracellular fluid volume, extracellular fluid volume, total body water
the assessment of body composition is extremely useful in studying the physiology of both humans and animals. While indicator dilution is the reference method for determination of body fluid volumes (BFV) in animals, this technique is time consuming, expensive, involves nonsurvival surgery, and requires bulky equipment. Bioimpedance spectroscopy (BIS) may be a useful alternative for BFV determination due to being rapid, minimally invasive, and allowing repeated measurements.
BIS measures the resistance of the body to the flow of an electrical current, which is related to the amount of water in the body [total body water (TBW)]. A constant current at low frequencies cannot pass through cell membranes; however, the current will travel through cell membranes at high frequencies. Therefore, over a range of frequencies (between 4 and 1,000 kHz), the impedance at low frequency is related to the extracellular fluid volume (ECFV), while the impedance at high frequencies is related to TBW (2). Values for intracellular fluid volume (ICFV) are derived by subtracting ECFV from TBW.
BIS has been extensively validated in humans (9, 14, 15) and in several animal species, including dogs, cats, sheep, and rats (8, 10, 11, 13). In particular, a recent study by Smith et al. (12) reported that BIS provided a precise and accurate means to determine TBW, fat mass, and fat free mass in rats compared with chemical carcass analysis. However, no previous studies have validated BIS in mice, a species in which BFV are especially difficult to assess. Consequently, the present study was designed to assess the utility of BIS in mice compared with the independent assessment of body composition by isotope dilution. We report that BIS accurately and reproducibly measures fluid compartment volumes in mice.
MATERIALS AND METHODS
Mice.
All experiments were performed with approval from the Institutional Animal Care and Use Committee at the University of Utah. Wild-type C57BL6 mice were studied at 3–4 mo of age.
Materials.
Tritiated water and 35SO4 were obtained from Perkin Elmer (Waltham, MA). All other reagents were obtained from Sigma Chemical (St. Louis, MO), unless stated otherwise.
Isotope dilution analysis of BFV.
TBW and ECFV were measured in mice by the distribution volumes of 3H2O and 35SO4, respectively. Values for ICFV were derived by subtracting ECFV from TBW. The mice were taken off feed and fluids in the morning before analysis. Two control mice were weighed and anesthetized, and blood samples (∼0.5–0.7 ml) were obtained by cardiac puncture. Twelve mice (6 male, 6 female) were weighed and orally gavaged with 0.25 ml of water containing 3H2O at 200 μCi/ml and 0.2 ml water containing 35SO4 at 100 μCi/ml. Following administration of the isotopes, the animals were returned to their cages. After 4 h to allow complete equilibration of the isotopes throughout the fluid compartments, a blood sample was collected by cardiac puncture under anesthesia. Plasma was frozen at −20°C until analysis in duplicate by liquid scintillation. The ratio of the initial concentration and volume of isotope administered to the final dispersed concentration allowed determination of the fluid compartment volume.
Bioimpedance measurement of BFV.
The ImpediVet Vet BIS1 system (ImpediMed, San Diego, CA) analyzes whole body bioimpedance data, providing determination of TBW, ECFV, and ICFV body composition estimates. Before analysis, animals were anesthetized and measured for length and weight. The animal details (length, weight, age, sex, and species) were then entered into the BIS software system. The animal was placed on its abdomen and shaved to allow good skin contact at the sites of needle placement. Four needles (25 g × 25 mm) bent at 90° ∼3 mm from the tip were inserted under the skin of the animal to be analyzed at each of the standard placement sites. The needle placement sites include the intercept between the front of the ears and the longitudinal midline, at the base of the tail, and 0.5 cm from these sites toward the nose and tip of the tail. After the leads were attached to the needles, measurements were begun.
Jugular cannulation and infusion.
Following induction of general anesthesia, the jugular vein was cannulated with PE-10 polyethylene tubing filled with 0.9% sodium chloride, containing 50 mg of EDTA disodium salt/ml. Saline was infused intravenously in increments of 0.1, 0.4, 0.5, 1, and a further 1 ml for accumulative totals of 0.1, 0.5, 1, 2, and 3 ml for each mouse. Impedance measurements were taken immediately after each infusion.
Calculations and statistics.
While 3H2O provides an accurate indication of TBW, no tracer provides an exact measure of ECFV. Results for ECFV obtained by 35SO4 dilution were multiplied by 0.90, 0.95, and 0.94, where 0.90 is the correction for nonextracellular distribution, 0.95 is the Donnan equilibration factor, and 0.94 is the correction factor for the water content of serum (1).
Saline infusion data were analyzed within a group between the different treatments using one-way ANOVA with the Bonferroni correction. Differences between groups were compared using one-way ANOVA or unpaired Student's t-test. P < 0.05 was taken as significant. Data are expressed as means ± SE.
RESULTS
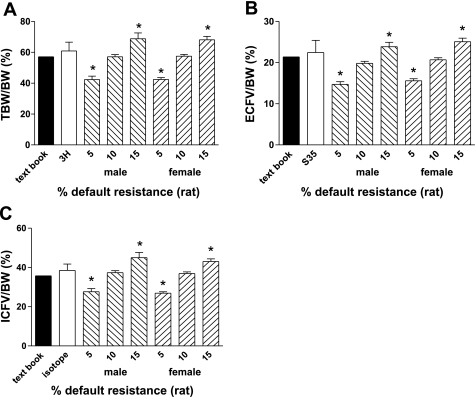
BIS values for TBW and ECFV are derived using a resistance coefficient that is specific for the particular species being analyzed. Since mice had not previously been studied using BIS, no such resistance coefficient existed. The resistance coefficient for rats, as supplied by the manufacturer, yielded body composition values for TBW that exceeded body weight (BW) in mice. Therefore, we initially empirically estimated the resistance coefficient for mice. To do this, we evaluated mice as a simple cylinder shape; in this case, impedance is proportional to length and inversely proportional to cross-sectional area. Therefore, since adult mice are approximately 1/10th the weight of adult rats, and, presuming a similar body proportionality between the species, resistance coefficients ranging from 5 to 15% of those defined for rats were assessed. Resistance values at 10% of those defined for rats provided TBW, ECFV, and ICFV ratios to BW with no difference from either textbook-published body composition data (7) or to fluid volumes measured by isotope dilution using 3H and 35S (Fig. 1). Consequently, a resistant coefficient that was 10% of that for rats was used in all subsequent studies.
Fig. 1.
Total body water (TBW)-to-body weight (BW) ratio (A), extracellular fluid volume (ECFV)-to-BW ratio (B), and intracellular fluid volume (ICFV)-to-BW ratio (C) in mice (n = 8) using isotope dilution (3H and 35SO4) and 5–15% default bioimpedance spectroscopy (BIS) system resistance constants (rats). Comparisons to textbook body fluid volume compartments in humans are shown. Values are means ± SE. *P < 0.05 vs. indication dilution and textbook values.
Bioimpedance demonstrated small intermouse variation in TBW, ECFV, and ICFV between mice of similar weight (Table 1). BWs were different (P < 0.05) between heavier male (N = 8, 28.6 ± 1.2 g) and lighter female (N = 8, 22.9 ± 0.3 g) mice. Similarly, TBW, ECFV, and ICFV differed between heavy and light mice. However, the ratios of TBW, ECFV, and ICFV to BW were not different between sexes and corresponded closely to textbook-published body composition values (7) (Table 1 and Fig. 1).
Table 1.
BW, TBW, ECFV, ICFV, TBW/BW, ECFV/BW, and ICFV/BW in male and female mice measured by bioimpedance spectroscopy
| Sex | BW, g | TBW, ml | ECFV, ml | ICFV, ml | TBW/BW, % | ECFV/BW, % | ICFV/BW, % |
|---|---|---|---|---|---|---|---|
| Male | 28.0 | 15.5 | 5.0 | 10.5 | 55.2 | 17.9 | 37.5 |
| 34.3 | 21.3 | 7.5 | 13.8 | 62.0 | 21.9 | 40.2 | |
| 31.9 | 17.5 | 6.0 | 11.5 | 54.8 | 18.8 | 36.1 | |
| 31.6 | 17.3 | 6.4 | 11.0 | 54.9 | 20.3 | 34.8 | |
| 25.4 | 15.1 | 5.5 | 9.6 | 59.5 | 21.7 | 37.8 | |
| 26 | 16.6 | 5.6 | 11.0 | 63.8 | 21.5 | 42.3 | |
| 26.2 | 14.2 | 5.0 | 9.2 | 54.3 | 19.5 | 35.1 | |
| 25.6 | 15.4 | 5.5 | 9.9 | 60.3 | 21.5 | 38.9 | |
| Mean ± SE | 28.6 ± 1.2* | 16.6 ± 0.8* | 5.8 ± 0.3* | 10.8 ± 0.5* | 58.1 ± 1.3* | 20.4 ± 0.5* | 37.8 ± 0.9* |
| Female | 21.1 | 11.9 | 3.9 | 8.0 | 56.5 | 18.5 | 37.9 |
| 23.2 | 13.4 | 4.6 | 8.9 | 58.0 | 19.8 | 38.4 | |
| 22.3 | 13.2 | 4.7 | 8.5 | 59.1 | 21.1 | 38.1 | |
| 23.1 | 14.0 | 5.1 | 8.9 | 60.7 | 22.1 | 38.5 | |
| 23.6 | 14.7 | 5.4 | 9.4 | 62.3 | 22.7 | 39.6 | |
| 23.1 | 12.2 | 4.5 | 7.7 | 52.8 | 19.5 | 33.3 | |
| 23.1 | 12.4 | 4.7 | 7.7 | 53.5 | 20.3 | 33.3 | |
| 23.7 | 13.6 | 5.1 | 8.5 | 57.4 | 21.6 | 35.8 | |
| Mean ± SE | 22.9 ± 0.3 | 13.2 ± 0.3 | 4.8 ± 0.2 | 8.5 ± 0.2 | 57.5 ± 1.2 | 20.7 ± 0.5 | 36.9 ± 0.9 |
BW, body weight; TBW, total body water; ECFV, extracellular fluid volume; ICFV, intracellular fluid volume; BW, body weight; TBW/BW, ECFV/BW, and ICFV/BW: ratio of TBW, ECFV, and ICFV to BW, respectively.
P < 0.05 between male and female groups.
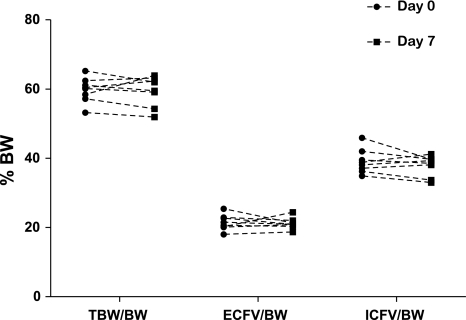
In the next study (Fig. 2), repeat measurements of TBW-, ECFV-, and ICFV-to-BW ratios were determined in the same mice 1 wk apart. Body fluid composition values did not differ in the same mice over the course of 1 wk (N = 8).
Fig. 2.
Comparisons of repeat measurement of TBW-, ECFV-, and ICFV-to-BW ratios in mice (n = 8 each data point) at days 0 and 7.
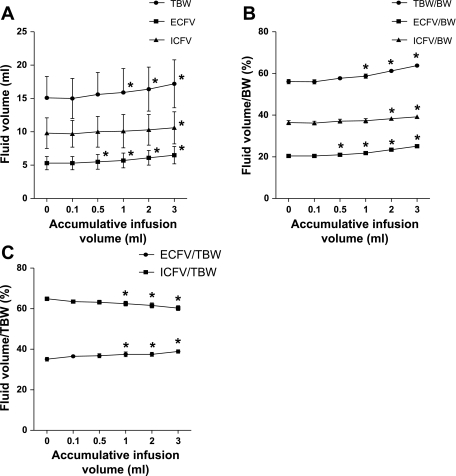
Finally, intravenous infusion of isotonic saline caused a proportional increase in TBW and TBW/BW in mice (Fig. 3). Infusion of 0.5 ml of saline produced an increase in ECFV, ECFV/BW, and ECFV/TBW that was detectable by BIS. BIS could detect an increase in TBW and TBW/BW after 1 ml of saline infusion. Changes in ICFV were more difficult to detect with BIS, wherein 3 ml of saline infusion were required to see changes in ICFV; however, changes in ICFV/TBW could be detected after 1 ml of saline infusion.
Fig. 3.
Effect of intravenous infusion of saline on body fluid compartment volumes measured by BIS in mice. A: TBW, ECFV, and ICFV. B: TBW-, ECFV-, and ICFV-to-BW ratio. C: ECFV/TBW and ICFV/TBW. Saline was infused intravenously with 0.1, 0.4, 0.5, 1, and 1 ml of normal saline to an accumulative total of 3 ml. Values are means ± SE; N = 12 each data point. *P < 0.05 compared with preinfusion volumes.
DISCUSSION
The present study demonstrates that BIS is an accurate method to assess BFV compartments in mice. A resistance coefficient that was 10% of that used for rats gave BIS values for TBW and ECFV in mice that compared closely with results obtained by conventional 3H2O and 35SO4 dilution methods. Furthermore, both BIS and isotope dilution techniques gave body fluid composition values in mice that compared closely with textbook values.
BIS has several advantages over indicator dilution techniques. First, while conventional indicator dilution methodologies are the present state of the art for determining BFV compartments, they are, in and of themselves, subject to errors (3). In addition, indicator dilution studies can only be done one time in a given mouse, as they require removal of large blood volumes. Furthermore, isotope dilution has an inherent variability that makes detection of small differences in BFV compartments problematic. In contrast, BIS is nondestructive and requires only about 5 min per mouse. This permits repeated measurements in the same animal. Importantly, our study demonstrated that BIS yielded highly reproducible values, i.e., very small intramouse variability. Furthermore, BIS is sufficiently sensitive to detect fluid compartment differences in mice of different weights, and changes in fluid compartment volumes following acute intravenous fluid administration, albeit changes in BFV compartments ≤0.5–1 ml cannot be reliably detected. Taken together, these findings indicated that BIS could be useful in studying longitudinal changes in fluid volume compartments. This utility would be particularly relevant to studies involving difficult-to-obtain mice, such as transgenic or knockout mouse lines. For example, the use of endothelin A receptor antagonists for the control of hypertension, or thiazolidinediones to treat type II diabetes mellitus, have lead to edema in humans (16, 17). Transgenic and knockout mice have been used to study these systems (4–6, 18); BIS could be useful in elucidating the underlying cause of fluid retention in these animals.
BIS appears to have relatively few disadvantages. One is that it requires purchasing the bioimpedance device and the necessary software. However, this expense would be offset by the ongoing costs of conventional techniques for measuring BFV. Furthermore, the ease and rapidity with which BIS can be performed will aid data collection and reduce operator training, while avoiding the use of radioisotopes. Another issue is that the BIS device measures body composition from impedance data using parameters calculated in rats; this required our having to empirically derive the resistance coefficients for mice. Hence, while it seems likely that our resistance coefficient values would likely be valid in all strains of mice with reasonably comparable body sizes, this has not been definitively determined.
In summary, the results of the present study suggest that BIS is a valid laboratory tool compared with conventional methods for estimating body fluid composition in mice. Its ability to noninvasively and longitudinally assess BFV compartments will likely make it useful in studying genetically engineered mice.
GRANTS
This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK96392 and by Gilead Sciences, Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Bell EF, Ziegler EE, Forbes GB. Corrected bromide space. Pediatr Res 18: 392–393, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Cornish BH, Thomas BJ, Ward LC. Improved prediction of extracellular and total body water using impedance loci generated by multiple frequency bioelectrical impedance analysis. Phys Med Biol 38: 337–346, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Culebras JM, Fitzpatrick GF, Brennan MF, Boyden CM, Moore FD. Total body water and the exchangeable hydrogen. II. A review of comparative data from animals based on isotope dilution and desiccation, with a report of new data from the rat. Am J Physiol Regul Integr Comp Physiol 232: R60–R65, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol 295: F1635–F1640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol 289: F692–F698, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med 11: 861–866, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Guyton A, Hall J. Textbook of Medical Physiology Philadelphia, PA: Saunders, 2006 [Google Scholar]
- 8.Jenkins T, Leymaster K, Turlington L. Estimation of fat-free soft tissue in lamb carcasses by use of carcass and resistive impedance measurements. J Anim Sci 66: 2174–2179, 1988 [Google Scholar]
- 9.Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr 11: 199–209, 1992 [PubMed] [Google Scholar]
- 10.Rutter K, Hennoste L, Ward LC, Cornish BH, Thomas BJ. Bioelectrical impedance analysis for the estimation of body composition in rats. Lab Anim 32: 65–71, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Scheltinga MR, Helton WS, Rounds J, Jacobs DO, Wilmore DW. Impedance electrodes positioned on proximal portions of limbs quantify fluid compartments in dogs. J Appl Physiol 70: 2039–2044, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Smith D, Johnson M, Nagy T. Precision and accuracy of bioimpedance spectroscopy for determination of in vivo body composition in rats. Int J Body Compos Res 7: 21–26, 2009 [PMC free article] [PubMed] [Google Scholar]
- 13.Stanton C, Hama D, Johnson D, Fettman M. Bioelectrical impedance and zoometry for body composition analysis in domestic cats. Am J Vet Res 53: 251–257, 1992 [PubMed] [Google Scholar]
- 14.Thomas BJ, Cornish BH, Ward LC. Bioelectrical impedance analysis for measurement of body fluid volumes: a review. J Clin Eng 17: 505–510, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Thomas BJ, Ward LC, Cornish BH. Bioimpedance spectrometry in the determination of body water compartments: accuracy and clinical significance. Appl Radiat Isot 49: 447–455, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Thomas ML, Lloyd SJ. Pulmonary edema associated with rosiglitazone and troglitazone. Ann Pharmacother 35: 123–124, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomized, double-blind, placebo-controlled trial. Lancet 374: 1423–1431, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci U S A 102: 9406–9411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]