Abstract
Increased expression of the facilitative glucose transporter, GLUT1, leads to glomerulopathy that resembles diabetic nephropathy, whereas prevention of enhanced GLUT1 expression retards nephropathy. While many of the GLUT1-mediated effects are likely due to mesangial cell effects, we hypothesized that increased GLUT1 expression in podocytes also contributes to the progression of diabetic nephropathy. Therefore, we generated two podocyte-specific GLUT1 transgenic mouse lines (driven by a podocin promoter) on a db/m C57BLKS background. Progeny of the two founders were used to generate diabetic db/db and control db/m littermate mice. Immunoblots of glomerular lysates showed that transgenic mice had a 3.5-fold (line 1) and 2.1-fold (line 2) increase in GLUT1 content compared with wild-type mice. Both lines showed similar increases in fasting blood glucose and body weights at 24 wk of age compared with wild-type mice. Mesangial index (percent PAS-positive material in the mesangial tuft) increased 88% (line 1) and 75% (line 2) in the wild-type diabetic mice but only 48% (line 1) and 39% (line 2) in the diabetic transgenic mice (P < 0.05, transgenic vs. wild-type mice). This reduction in mesangial expansion was accompanied by a reduction in fibronectin accumulation, and vascular endothelial growth factor (VEGF) levels increased only half as much in the transgenic diabetic mice as in wild-type diabetic mice. Levels of nephrin, neph1, CD2AP, podocin, and GLUT4 were not significantly different in transgenic compared with wild-type mice. Taken together, increased podocyte GLUT1 expression in diabetic mice does not contribute to early diabetic nephropathy; surprisingly, it protects against mesangial expansion and fibronectin accumulation possibly by blunting podocyte VEGF increases.
Keywords: metabolism, glucose, vascular endothelial growth factor, type 2 diabetes, diabetic complications
diabetic nephropathy has a complex and prolonged pathogenesis. Multiple factors play important roles in the development and progression of this most common cause of end-stage renal disease, yet many of the critical determinants of progressive nephropathy remain a mystery. The earliest manifestations of the disease occur in the kidney glomerulus and, consequently, most attention to mechanisms has focused on altered responses of diabetic glomerular cells, especially mesangial cells and, more recently, podocytes. Podocytes appear to be quite susceptible to injury in diabetes (33), and this injury leads to loss of podocytes in part via apoptosis (23, 40). This podocyte depletion appears to be a relatively early event in the evolution of diabetic nephropathy and predicts clinical progression of the disease (31). The factors that lead to podocyte loss in diabetes are being actively investigated and include enhanced oxidative stress (39, 40) and activation of the renin-angiotensin-aldosterone system (14).
Until recently, little attention has been paid to the potential pathogenic role of the molecules that transport glucose into these cells, the facilitative glucose transporters. While several of these transporters have been identified in kidney glomerular cells, it appears that GLUT1 and GLUT4 comprise the major transporters in both mesangial cells (4, 16, 18, 30) and podocytes (9), although GLUT2 (29) and GLUT8 (36) have also been detected in cultured podocytes. We and others demonstrated that expression of the GLUT1 glucose transporter in mesangial cells is enhanced by high glucose (20) and is increased in glomeruli of diabetic animal models (17). Elevated GLUT1 levels in mesangial cells lead directly to activation of PKC isoforms and aldose reductase, and enhanced fibronectin and collagen IV accumulation (6, 18, 19, 22, 42), typical features of early diabetic nephropathy. However, there are no studies of the effects of enhanced GLUT1 in podocytes. We speculated that increased GLUT1 levels in podocytes would similarly enhance glucose uptake with similar effects on cell signaling as in mesangial cells as well as induction of reactive oxygen species as has been documented in adipocytes from high fat-fed diabetic mice (41). Such derangements in podocyte signaling and oxidative stress could lead to podocyte dysfunction, injury, and loss.
To test this hypothesis most directly, we generated diabetic and control mouse lines that specifically overexpressed GLUT1 only in podocytes. Surprisingly, these diabetic podocyte-specific GLUT1 transgenic mice did not consistently develop enhanced proteinuria or show any reduction in total podocyte numbers but in fact manifested decreased mesangial expansion and glomerular levels of fibronectin and vascular endothelial growth factor (VEGF) compared with diabetic wild-type mice. These studies suggest that increased GLUT1 expression has no pathogenic effect on podocytes and in fact induces a response that ameliorates diabetic glomerulopathy.
METHODS
Podocyte-specific GLUT1 transgenic mice.
Protocols for animal use were approved by the University Committee on Use and Care of Animals of the University of Michigan and all animals were monitored by the veterinarian staff of the Unit for Laboratory Animal Medicine. Mice were housed in a pathogen-free, temperature-controlled environment on a 12-h (0600–1800) light-dark cycle and had access to standard chow (LabDiet 5001, PMI, Richmond, IN) and water ad libitum. All mice were weaned at 21 days.
The transgene construct included a 2.5-kb fragment of the human NPHS2 (podocin) promoter (32) followed by the coding sequence of the rat GLUT1 cDNA. Superovulation in 42 C57BLKS/J-Leprdb/m egg donors (25–30 days old; Jackson Laboratory Stock Number 000662, Bar Harbor, ME) was induced with 5 IU pregnant mare's serum gonadotropin (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) in 0.1 ml PBS (Invitrogen, Carlsbad, CA) by intraperitoneal injection and 46–50 h later with 5 IU human chorionic gonadotropin (Sigma, St. Louis, MO) in 0.1 ml PBS. After mating to C57BLKS/J (BKS) males, a total of 1,696 eggs was collected, of which 1,065 were injected (67% fertilization rate). It was observed during microinjection with the NPHS2/GLUT1 sequence that the eggs were more prone to lysis than C57BL/6J eggs. Intact microinjected eggs were transferred to pseudopregnant females and 116 pups were born (18% birth rate) of which 30 were transgenic founders. Thus, the transgenic efficiency was 2.8 transgenic founders produced per 100 microinjected eggs. The transgenic efficiency of C57BL/6NTac and C57BL/6J mice was reported to be 1.2 and 1.0, respectively (2, 12), thus the efficiency of producing NPHS2-Glut1 transgenic founders in BKS mice was at least twofold more efficient.
Two lines of mice with significant glomerular GLUT1 overexpression were generated. The resulting lines were denoted as C57BLKS/J-Tg(Nphs2-Slc2a1) Leprdb/m lines 1 and 2. Genotype of the mice was verified by PCR analysis of genomic DNA isolated from tail biopsies (High Pure PCR Template Preparation Kit, Roche Diagnostics, Pleasanton, CA) as previously described (24) with the following primers that produced a positive PCR product of ∼500 bp in Nphs2 GLUT1 (pGT1) mice: transgenic forward primer: 5′-ACAGCTCCACCAAGACACA-3′ and transgenic reverse primer: 5′-CAGCAGGTTCATCATCAGC-3′. The db/db and db/m genotype was confirmed with the following primers: db forward primer: 5′-CCAACAGTCCATACAATATTAGAAGATCTTTACATTTT-3′ and db reverse primer: 5′-CCTAATGGAATCTAATATGGAAGCT-3′.
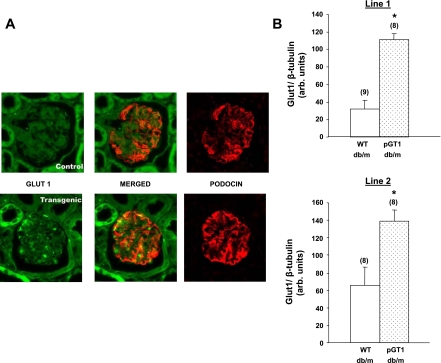
PCR products were digested with Hpy81 (MjaIV) which recognizes the sequence mutation in the db leptin receptor allele, but not the sequence in the misty allele, and then separated on agarose gels. Overexpression of GLUT1 was confirmed in glomeruli by immunoblotting and immunofluorescence microscopy (Fig. 1).
Fig. 1.
Podocyte-specific overexpression of GLUT1 in 2 transgenic mouse lines. A: GLUT1 and podocin immunohistochemistry of adjacent histologic sections of a representative glomerulus from a wild-type (WT) mouse (control; top) and pGT1 mouse (transgenic; bottom) showing increased GLUT1 expression in the pGT1 glomerulus in a distribution that is similar to that of the podocyte protein, podocin, suggesting that the enhanced GLUT1 expression is podocyte-specific. B: GLUT1 quantitation in immunoblots from glomerular lysates of WT and pGT1 nondiabetic (db/m) animals in both of the transgenic lines. Data are means ± SE with total number of animals in parentheses. *P < 0.01 vs. wild-type db/m.
Physiologic measurements.
Fasting blood glucose and body weight were recorded every 4 wk until the end of the trials. A small drop of tail blood was collected after fasting and analyzed using an Accu-Chek, Advantage glucometer (Roche Diagnostics, East Sussex, UK). Twenty-four-hour urine collections were obtained at 24 wk of age using Hatteras metabolic cages (Hatteras Instruments, Cary, NC). Urine volume and creatinine and albumin excretion in 24 h were measured (Creatinine Companion and Albuwell M; Exocell, Philadelphia, PA) and then used to calculate the urinary albumin-creatinine ratio. Glycosylated hemobglobin (GHb) was measured by the Michigan Diabetes Research and Training Center Chemistry Laboratory Core using the Helena Laboratories Test kit, Glyco-Tek Affinity column Method (catalog no. 5351; Helena Laboratories, Beaumont, TX). This test measures any stable form of glycosylated hemoglobin. Interassay variations are 8.8 at 6.0% GHb and 3.8 at 19.5% GHb.
Histologic assessment.
At 24 wk of age, mice were deeply anesthetized with pentobarbital sodium (Abbott Laboratories, North Chicago, IL). The abdominal aorta was cannulated with a 23-gauge catheter and a small sample of blood was withdrawn for GHb and cholesterol measurements. Each mouse was perfused via the aorta with PBS containing 50 U/ml of heparin (American Pharmaceutical Partners, Schaumburg, IL) at 100 mmHg with the liver nicked to allow blood to exit. Once cleared of blood, the left kidney from each mouse was ligated and the right kidney was perfused with ferric oxide slurry in PBS via the abdominal aorta. The left kidney was removed, weighed, and fixed overnight in a solution of 2% paraformaldehyde in PBS. Iron-containing glomeruli from the right kidney were then isolated over a magnet as previously published (45). Isolated glomeruli were placed in lysis buffer and used for immunoblotting.
For quantification of mesangial extracellular matrix, 3-μm sections from paraformaldehyde-fixed, paraffin-embedded kidney slices were stained using Periodic Acid-Schiff's reagent (PAS) (1). Mesangial area was expressed quantitatively by calculating the percentage of the total glomerular area that was PAS positive. Fifteen glomerular tufts per animal were chosen randomly for analysis.
For podocyte counts, paraffin-embedded, paraformaldehyde-fixed tissue was sectioned at 3 and 9 μm and was performed as previously published (39, 45) following the methods of Sanden et al. (34). Briefly, podocyte nuclei were detected with a rabbit polyclonal anti-WT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 20 μg/ml. Images of all glomeruli from both thick (9 μm) and thin (3 μm) kidney cortical cross sections were collected using the MetaMorph Image System (version 6.1, Molecular Devices, Downingtown, PA) by a blinded observer (JS). Glomerular volume/podocyte (GV/P) was calculated as previously reported (39, 45). GV/P is a variable that incorporates the relationship between both podocyte number and glomerular basement membrane surface area, is the reciprocal of podocyte density, and is a useful measure of the degree of podocyte reserve (34).
Immunoblotting.
Glomerular samples were sonicated and/or mechanically disrupted in lysis buffer [0.1% SDS, 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 50 mM NaF, 1 mM phenylmethylsulphonyl fluoride, and complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) in 10 mM Tris·HCl, pH 7.4] and used for SDS-PAGE as previously reported (37, 45). Lysates were run on 7.5 or 10% SDS-PAGE and immunoblotted with antibodies for fibronectin and β-tubulin (mab1926 and 05–661, respectively; Millipore, Temecula, CA), GLUT1 (a gift from Dr. Carter-Su, University of Michigan), nephrin or Neph1 (15) (gifts from Dr. L. Holzman, University of Michigan), VEGF-A (cat. no. MS-1467-R7, VEGF Ab-7, Clone VG1, Neomarkers, Labvision, Fremont, CA), or β-actin (β-actin Ab, cat. no. ab8226; Abcam, Cambridge, MA). Blots were exposed to film after incubation in Roche LumiLight Western Blotting Substrate. All exposures were within the linear range of the film and normalized to β-actin or β-tubulin content.
Statistical analysis.
All data are expressed as means ± SE. The differences between groups were analyzed by a one-factor ANOVA, followed by a Tukey-Kramer test for multiple comparisons of the means. When comparing two groups, a Student's t-test was used. All tests were assessed at the 0.05 level of significance. Data analyses were performed with the statistical package StatView 5 (SAS Institute, Cary, NC).
RESULTS
Nondiabetic pGT1 mice demonstrated 3.5-fold (line 1; P < 0.0001) and 2.1-fold (line 2; P < 0.02) higher levels of glomerular GLUT1 protein compared with levels in wild-type mice (Fig. 1). GLUT1 immunohistochemistry showed enhanced glomerular staining in pGT1 mice compared with wild-type mice consistent with podocyte-specific overexpression (Fig. 1). Glomerular GLUT1 levels were increased in both pGT1 and wild-type mice compared with nondiabetic controls, but the relative increase in GLUT1 expression was maintained in pGT1 diabetic mice compared with wild-type diabetic mice at 24 wk of age (data not shown).
At 24 wk of age, both lines of pGT1 and wild-type diabetic BKS mice showed substantial weight gain and severe fasting hyperglycemia compared with db/m controls but there were no significant differences between the pGT1 transgenic and wild-type BKS db/db mice (Table 1). While the diabetic pGT1 mice had slightly lower glycosylated hemoglobin levels, these were not statistically significant. Lipid levels were not different between the diabetic pGT1 and wild-type mice (Table 1).
Table 1.
Physiological data
| Line 1 | Line 2 | |
|---|---|---|
| Body wt, g | ||
| WT db/m | 27.3 ± 1.3 (11) | 26.7 ± 0.8 (12) |
| WT db/db | 50.4 ± 1.3 (17)*† | 43.3 ± 1.4 (10)*† |
| pGT1 db/m | 28.2 ± 0.9 (13) | 27.5 ± 1.37 (12) |
| pGT1 db/db | 48.2 ± 2.3 (13)*† | 43.5 ± 1.7 (12)*† |
| Left kidney wt, g | ||
| WT db/m | 0.21 ± 0.02 (6) | 0.19 ± 0.01 (11) |
| WT db/db | 0.27 ± 0.01 (9) | 0.24 ± 0.01 (12) |
| pGT1 db/m | NA | 0.22 ± 0.02 (14) |
| pGT1 db/db | 0.25 ± 0.02 (8) | 0.23 ± 0.01 (11) |
| Fasting blood glucose, mg/dl | ||
| WT db/m | 139.9 ± 5.8 (11) | 134.0 ± 8.7 (12) |
| WT db/db | 450.8 ± 29.2 (16)*† | 487.7 ± 38.0 (11)*† |
| pGT1 db/m | 132.0 ± 6.6 (13) | 117.4 ± 8.0 (12) |
| pGT1 db/db | 441.8 ± 36.6 (13)*† | 476.0 ± 47.6 (7)*† |
| Glycosylated hemoglobin, % | ||
| WT db/m | 5.6 ± 0.3 (12) | 5.7 ± 0.2 (12) |
| WT db/db | 13.1 ± 0.5 (16)*† | 12.4 ± 0.6 (12)*† |
| pGT1 db/m | 5.3 ± 0.2 (14) | 6.0 ± 0.2 (10) |
| pGT1 db/db | 11.7 ± 0.8 (13)*† | 11.5 ± 1.0 (11)*† |
| Cholesterol, mg/dl | ||
| WT db/m | 66 ± 6 (9) | 60 ± 3(11) |
| WT db/db | 92 ± 4 (15)* | 95 ± 6(10)* |
| pGT1 db/m | 76 ± 4 (7) | 67 ± 4 (11) |
| pGT1 db/db | 112 ± 8 (12)*† | 93 ± 5 (9)*† |
| HDL cholesterol, mg/dl | ||
| WT db/m | 43 ± 5 (9) | 42 ± 2 (10) |
| WT db/db | 62 ± 3 (16)*† | 58 ± 2 (9)*† |
| pGT1 db/m | 53 ± 4 (7) | 43 ± 3 (11) |
| pGT1 db/db | 72 ± 5 (12)*† | 61 ± 2 (9)*† |
Data are means ± SE with total number of animals in parentheses. WT, wild-type mice; pGT1, podocyte-specific GLUT1 transgenic mice; HDL, high-density lipoprotein.
P < 0.05 vs. WT db/m,
vs. pGT1 db/m.
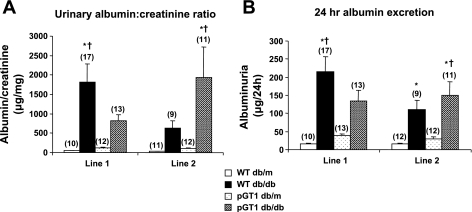
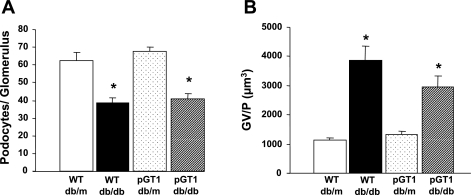
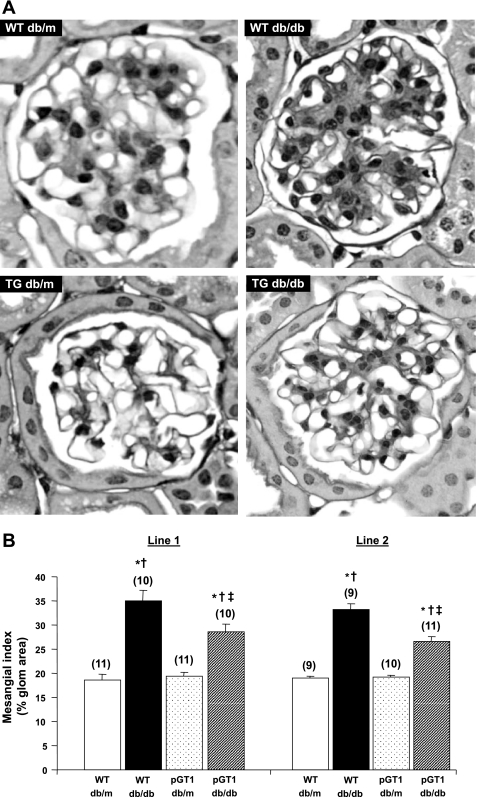
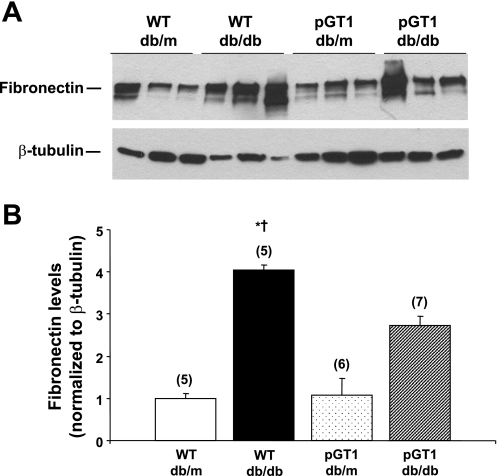
Albuminuria was variable (Fig. 2). While the diabetic pGT1 line 1 mice developed less albuminuria at 24 wk than did diabetic wild-type mice, this reduction in albuminuria was not found in line 2 mice (Fig. 2). Moreover, although diabetes induced a reduction in the number of WT-1-positive podocytes, there was no difference in the degree of podocyte reduction in the pGT1 line 1 mice compared with the diabetic wild-type mice (Fig. 3). Interestingly, there was a substantial and significant reduction in mesangial expansion in diabetic pGT1 mice in both lines compared with diabetic wild-type mice. The mesangial index (percent PAS-positive material in the mesangial tuft) increased 88% (line 1) and 75% (line 2) in the wild-type diabetic mice but increased only 48% (line 1) and 39% (line 2) in pGT1 diabetic mice (Fig. 4). Thus, overexpression of GLUT1 in podocytes reduced mesangial expansion in diabetes by approximately half. Similarly, fibronectin levels were elevated about fourfold in the diabetic wild-type mice but only around twofold in pGT1 diabetic mice compared with controls (Fig. 5).
Fig. 2.
Albuminuria in control (db/m) and diabetic (db/db) WT and pGT1 mice at 24 wk of age expressed as albumin:creatinine ratios (A) or as 24-h albuminuria (B). Values for each transgenic line and littermate controls are shown. Data are means ± SE with total number of animals in parentheses. *P < 0.05 vs. WT db/m, † vs. pGT1 db/m.
Fig. 3.
Podocyte number in control (db/m) and diabetic (db/db) WT and pGT1 line 1 mice at 24 wk of age. A: average number of podocytes per glomerulus. B: degree of podocyte reserve as measured in glomerular volume per podocyte (GV/P). Data are means ± SE. Total number of animals in each group was 7. *P < 0.05 vs. WT db/m and pGT1 db/m.
Fig. 4.
Mesangial extracellular matrix area in control (db/m) and diabetic (db/db) WT and pGT1 mice. A: representative examples of glomerular PAS staining. B: mesangial area was expressed quantitatively by calculating the percentage of the total glomerular area that was PAS positive at 24 wk of age. Data are means ± SE, with total number of animals in parentheses. *P < 0.05 vs. WT db/m, † vs. pGT1 db/m, ‡ vs. WT db/db. TG, transgenic.
Fig. 5.
Glomerular fibronectin levels in control (db/m) and diabetic (db/db) WT and pGT1 line 1 mice. A: representative immunoblot of glomerular lysates from control and diabetic mice at 24 wk of age. β-Tubulin was used as a loading control. B: relative intensities as determined by densitometry. Data are means ± SE. Number of animals for each group is in parenthesis. *P < 0.05 vs. WT db/m, † vs. pGT1 db/m.
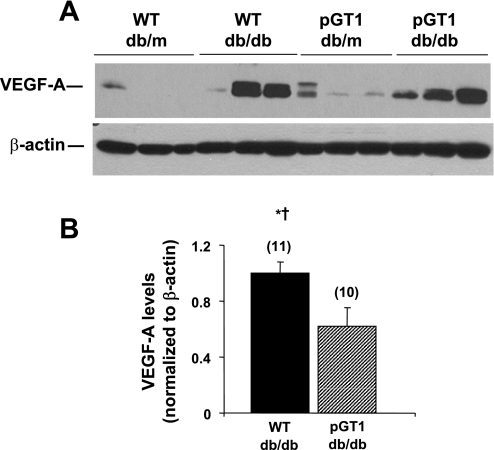
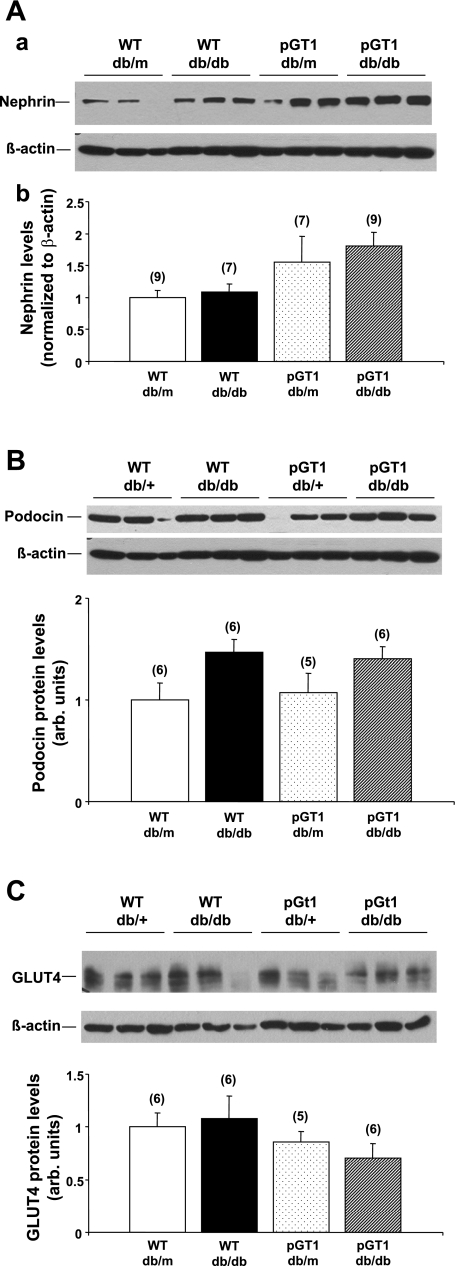
Levels of a number of podocyte proteins (VEGF, nephrin, neph1, CD2AP, podocin, and GLUT4) were assessed in glomerular lysates of transgenic and wild-type line 1 mice. VEGF levels increased substantially with diabetes (Fig. 6). Because the glomerular VEGF levels in nondiabetic animals in this model were so low, we were unable to validly quantitate glomerular VEGF in those mice. However, glomerular VEGF levels were reduced in pGT1 diabetic animals (line 1) to ∼60% of those in diabetic wild-type mice (Fig. 6). Similar VEGF reductions were found in line 2 animals. Nephrin levels appeared to be higher in both diabetic and control transgenic mice than in wild-type mice. The increase in the diabetic transgenic mice approached, but did not reach, statistical significance (the ANOVA had a P value of 0.053 and the mean difference between diabetic transgenic and diabetic wild-type mice was 0.71-fold which approached the critical difference of 0.90-fold; Fig. 7). Variance in nephrin levels in the four groups made it difficult to detect the less than twofold differences given the sample size. There was no change in glomerular podocin or GLUT4 levels with diabetes or in the transgenic animals (Fig. 7). Neph1 and CD2AP levels were compared in nondiabetic transgenic and wild-type line 1 and 2 animals and showed no differences (not shown; n = 5–7 in each group).
Fig. 6.
Glomerular vascular endothelial growth factor (VEGF)-A levels in control (db/m) and diabetic (db/db) WT and pGT1 line 1 mice. A: representative immunoblot of glomerular lysates from control and diabetic mice at 24 wk of age. β-Actin was used as a loading control. B: relative protein levels as determined by densitometry. Data are means ± SE. Number of animals for each group is in parenthesis. *P < 0.05 vs. WT db/m, † vs. pGT1 db/m.
Fig. 7.
Glomerular nephrin (A), podocin (B), and GLUT4 (C) levels in control (db/m) and diabetic (db/db) WT and pGT1 line 1 mice. Top: representative immunoblots of glomerular lysates from control and diabetic mice at 24 wk of age. β-Actin was used as a loading control. Bottom: relative protein levels as determined by densitometry. Data are means ± SE. Number of animals for each group is in parenthesis.
DISCUSSION
Diabetic nephropathy is the most common cause of end-stage renal disease in the United States (3, 44). Many factors have been implicated in the pathogenesis of diabetic nephropathy; however, the mechanisms remain incompletely understood. In virtually all mammalian cells, glucose uptake occurs via members of the family of facilitative glucose transporters. Glomerular expression of GLUT1 is increased in animal models of diabetes including db/db BLKS mice (17) used in the experiments reported here. Overexpression of GLUT1 in glomerular mesangial cells in the absence of hyperglycemia results in glomerular hypertrophy and glomerulosclerosis, similar to that seen in diabetic nephropathy (5, 20), whereas prevention of elevated GLUT1 expression inhibits mesangial matrix expansion in diabetic mice (17). Although multiple studies identified a role for GLUT1 in the pathogenesis of diabetic nephropathy, the precise mechanisms by which GLUT1 promotes nephropathy remain to be identified. Moreover, it is not clear which glomerular cells overexpress GLUT1 in diabetic glomerulopathy. Whereas most previous experimental studies focused on mesangial cells, we wished to determine whether enhanced expression of GLUT1 in podocytes could also contribute to diabetic glomerulopathy, specifically by enhancing podocyte dysfunction or loss. To do so, we used a GLUT1 construct driven by a podocyte-specific 2.5-kb human NPHS2 (pdodicin) promoter that has been demonstrated to drive podocyte-specific gene expression in both mice and rats in several previous publications (11, 32, 38, 43).
Surprisingly, we found no consistent enhancement of a podocyte phenotype either in terms of worsening albuminuria or reduction in podocyte number in the pGT1 mice. Even more surprisingly, we found a substantial reduction in mesangial matrix expansion in diabetic pGT1 mice from both lines compared with diabetic wild-type db/db BLKS mice. These data imply that overexpression of GLUT1 in podocytes signals in some manner to glomerular mesangial cells, and potentially other cells in the mesangium, that are responsible for matrix synthesis and degradation. While the nature of such a signal is not known, we did find that VEGF levels were not as markedly increased in the pGT1 diabetic mice as they were in wild-type diabetics.
Others showed that blockade of VEGF signaling by administration of blocking antibodies (10, 13) or overexpression of the endogenous VEGF inhibitor, sFlt-1, either specifically in podocytes (26) or systemically via intramuscular injection of an adeno-associated viral vector (25) limits albuminuria and can reduce glomerular and mesangial matrix expansion (13, 26) in early diabetic nephropathy. Why our model, with substantial reductions in VEGF, was not associated with a consistent reduction in albuminuria is unclear but could be due to less profound inhibition of VEGF receptor signaling than that found in the other models. Indeed, the diabetic pGT1 line 1 animals that had a more profound increase in podocyte GLUT1 as well as a more consistent reduction in glomerular VEGF levels did develop less albuminuria than diabetic wild-type mice, while the line 2 animals with a smaller increase in GLUT1 showed no statistically significant change in albuminuria with diabetes compared with wild-type mice. These variant data are consistent with a possible VEGF dose effect. In addition, the duration of diabetes was longer in our experiments than those reported by the VEGF inhibition studies and this could have contributed to some of the different findings, as albuminuric factors other than VEGF may be more important at later stages of nephropathy. Finally, it is possible that the ameliorative effects of GLUT1 expression on mesangial matrix expansion in diabetes occurred via an independent mechanism or mechanisms. The causal link between enhanced podocyte GLUT1, reduced glomerular VEGF levels, and reduced nephropathy awaits formal testing. Moreover, the mechanism by which enhanced GLUT1 expression results in reduced VEGF expression is yet unknown.
GLUT1 overexpression also appeared to be associated with an increase in glomerular nephrin levels. This difference did not quite reach statistical significance given a relatively large variability in expression levels (in nephrin and in several other proteins). Since reduced nephrin levels have been implicated (7), albeit inconclusively, in the progression of diabetic nephropathy and since higher nephrin levels may be protective, an increase in nephrin levels in association with enhanced podocyte GLUT1 could be of interest if it is verified in future studies. Nephrin clearly augments GLUT1 trafficking to the plasma membrane of podocytes (8) and it is possible that there is a reciprocal effect maintaining higher levels of nephrin at the slit diaphragm when GLUT1 levels increase. Determination of whether such a relationship exists will require further testing.
It is unclear why we found such a variability in nephrin (and other) protein levels in our glomerular lysates, which was somewhat greater than what we found in a type 1 diabetic model on a different genetic background (45). The mice in this study were all genetically identical and the glomerular preparations were monitored for purity. It is possible that modest environmental differences (degree of hyperglycemia, weight gain, etc.) could affect expression of some glomerular proteins.
Some indirect support of the surprisingly protective role of GLUT1 in podocytes has been recently reported by Saleem's group (28). In their report, the authors show that the PPAR-γ agonist, rosiglitazone, enhances glucose uptake in cultured podocytes by increasing plasma membrane GLUT1 expression. We (45) and others (for a review, see Ref. 35) showed that treatment with rosiglitazone reduces diabetic glomerulopathy via a PPAR-γ-dependent mechanism and that rosiglitazone and other thiazolidinediones reduce glomerular VEGF levels in diabetes (27). Although thiazolidinediones have pleiotrophic effects, one mechanism for protection could be via their effects on GLUT1 and VEGF.
Polymorphisms in the GLUT1 gene have been found to be associated with a higher risk of diabetic nephropathy in several human populations with both type 1 and type 2 diabetes (for the most recent review, see Ref. 17). While we and others generally assumed that such risk was determined by polymorphisms that led to enhanced GLUT1 expression or action in mesangial cells, the results in the current study also suggest that polymorphisms that reduce podocyte GLUT1 expression or function may contribute to enhanced nephropathy.
GRANTS
These studies were supported by grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK-076139 to F. C. Brosius III). This research used the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center supported by NIH Grant DK-20572 and the Basic Science Core of the George O'Brien Kidney Center at the University of Michigan supported by NIH Grant P30-DK-081943.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.American Registry of Pathology. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology New York: McGraw-Hill, 1968 [Google Scholar]
- 2.Auerbach AB, Norinsky R, Ho W, Losos K, Guo Q, Chatterjee S, Joyner AL. Strain-dependent differences in the efficiency of transgenic mouse production. Transgenic Res 12: 59–69, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Bottinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Brosius FC, 3rd, Briggs JP, Marcus RG, Barac-Nieto M, Charron MJ. Insulin-responsive glucose transporter expression in renal microvessels and glomeruli. Kidney Int 42: 1086–1092, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Brosius FC, Heilig CW. Glucose transporters in diabetic nephropathy. Pediatr Nephrol 20: 447–451, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chen SHK, Brosius FC, III, Heilig CW. Diabetes increases glomerular GLUT1 and antisense-GLUT1 protects against diabetic glomerulosclerosis. J Am Soc Nephrol 14: 581A, 2003 [Google Scholar]
- 7.Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol 22: 393–398, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavare JM, Mathieson PW, Saleem MA. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavare JM, Mathieson PW, Saleem MA. The human glomerular podocyte is a novel target for insulin action. Diabetes 54: 3095–3102, 2005 [DOI] [PubMed] [Google Scholar]
- 10.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 12: 993–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 11.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17: 1334–1344, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Filipiak WE, Saunders TL. Advances in transgenic rat production. Transgenic Res 15: 673–686, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 51: 3090–3094, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Fredersdorf S, Weil J, Ulucan C, Birner C, Buttner R, Schubert T, Boger CA, Debl K, Muders F, Riegger GA, Luchner A. Vasopeptidase inhibition attenuates proteinuria and podocyte injury in Zucker diabetic fatty rats. Naunyn Schmiedebergs Arch Pharmacol 375: 95–103, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilig C, Zaloga C, Lee M, Zhao X, Riser B, Brosius F, Cortes P. Immunogold localization of high-affinity glucose transporter isoforms in normal rat kidney. Lab Invest 73: 674–684, 1995 [PubMed] [Google Scholar]
- 17.Heilig CW, Brosius FC, Cunningham C. Role for GLUT1 in diabetic glomerulosclerosis. Expert Rev Mol Med 8: 1–18, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, Cortes P. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest 96: 1802–1814, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilig CW, Kreisberg JI, Freytag S, Murakami T, Ebina Y, Guo L, Heilig K, Loberg R, Qu X, Jin Y, Henry D, Brosius FC., 3rd Antisense GLUT-1 protects mesangial cells from glucose induction of GLUT-1 and fibronectin expression. Am J Physiol Renal Physiol 280: F657–F666, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Heilig CW, Liu Y, England RL, Freytag SO, Gilbert JD, Heilig KO, Zhu M, Concepcion LA, Brosius FC. D-glucose stimulates mesangial cell GLUT1 expression and basal IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 46: 1030–1039, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Henry DN, Busik JV, Brosius FC, 3rd, Heilig CW. Glucose transporters control gene expression of aldose reductase, PKCα, and GLUT1 in mesangial cells in vitro. Am J Physiol Renal Physiol 277: F97–F104, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377: 151–155, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Kosugi T, Nakayama T, Li Q, Chiodo VA, Zhang L, Campbell-Thompson M, Grant M, Croker BP, Nakagawa T. Soluble-Flt-1 gene therapy ameliorates albuminuria, but accelerates tubulointerstitial injury in diabetic mice. Am J Physiol Renal Physiol 298: F609–F616, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Ku CH, White KE, Dei Cas A, Hayward A, Webster Z, Bilous R, Marshall S, Viberti G, Gnudi L. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes 57: 2824–2833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MY, Lee EY, Lee BJ, Won CS, Koh JH, Shin JY, Shin YG, Cho BP, Chung CH. Beneficial effects of thiazolidinediones on diabetic nephropathy in OLETF rats. Yonsei Med J 48: 301–307, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon R, Welsh GI, Singh A, Satchell SC, Coward RJ, Tavare JM, Mathieson PW, Saleem MA. Rosiglitazone enhances glucose uptake in glomerular podocytes using the glucose transporter GLUT1. Diabetologia 52: 1944–1952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewko B, Bryl E, Witkowski JM, Latawiec E, Golos M, Endlich N, Hahnel B, Koksch C, Angielski S, Kriz W, Stepinski J. Characterization of glucose uptake by cultured rat podocytes. Kidney Blood Press Res 28: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Marcus RG, England R, Nguyen K, Charron MJ, Briggs JP, Brosius FC., 3rd Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 267: F816–F824, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms' tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol 14: 2484–2493, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int 70: 1223–1233, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Schiffer M, Susztak K, Ranalletta M, Raff AC, Bottinger EP, Charron MJ. Localization of the GLUT8 glucose transporter in murine kidney and regulation in vivo in nondiabetic and diabetic conditions. Am J Physiol Renal Physiol 289: F186–F193, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol Renal Physiol 276: F658–F665, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC., 3rd Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC Nephrol 7: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 41.Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-Δ activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab 285: E295–E302, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Weigert C, Brodbeck K, Brosius FC, 3rd, Huber M, Lehmann R, Friess U, Facchin S, Aulwurm S, Haring HU, Schleicher ED, Heilig CW. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes 52: 527–535, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wolf G, Chen S, Ziyadeh FN. Perspectives in diabetes: from the periphery of the glomerular capillary wall toward the center of disease, podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, Kretzler M, Feldman EL, Pennathur S, Brosius FC., 3rd Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol 295: F1071–F1081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]