Abstract
Aldosterone (Aldo) is a major sodium-retaining hormone that reduces renal sodium excretion and also stimulates sodium appetite. In the face of excess Aldo, the sodium-retaining action of this steroid is overridden by an adaptive regulatory mechanism, a phenomenon termed Aldo escape. The underlying mechanism of this phenomenon is not well defined but appeared to involve a number of natriuretic factors such prostaglandins (PGs). Here, we investigated the role of microsomal prostaglandin E synthase-1 (mPGES-1) in the response to excess Aldo. A 14-day Aldo infusion at 0.35 mg·kg−1·day−1 via an osmotic minipump in conjunction with normal salt intake did not produce obvious disturbances in fluid metabolism in WT mice as suggested by normal sodium and water balance, plasma sodium concentration, hematocrit, and body weight, despite the evidence of a transient sodium accumulation on days 1 or 2. In a sharp contrast, the 14-day Aldo treatment in mPGES-1 knockoute (KO) mice led to increased sodium and water balance, persistent reduction of hematocrit, hypernatremia, and body weight gain, all evidence of fluid retention. The escaped wild-type (WT) mice displayed a remarkable increase in urinary PGE2 excretion in parallel with coinduction of mPGES-1 in the proximal tubules, accompanied by a remarkable, widespread downregulation of renal sodium and water transporters. The increase in urinary PGE2 excretion together with the downregulation of renal sodium and water transporters were all significantly blocked in the KO mice. Interestingly, compared with WT controls, the KO mice exhibited consistent increases in sodium and water intake during Aldo infusion. Together, these results suggest an important role of mPGES-1 in antagonizing the sodium-retaining action of Aldo at the levels of both the central nervous system and the kidney.
Keywords: proximal tubule, Na/H exchanger, sodium appetite
aldosterone (aldo), an adrenocorticoid steroid hormone, is the key regulator of renal sodium excretion. Classically, Aldo is synthesized in the adrenal zona glomerulosa in response to angiotensin II (ANG II) or low potassium and binds to mineralocorticoid receptors in its target tissues. This steroid hormone is a key regulator of extracellular fluid volume. The primary site of Aldo action in the kidney is the distal nephron, comprising the distal convoluted tubule, connecting tubule, and collecting duct. Aldo increases the abundance of the thiazide-sensitive Na+-Cl− cotransporter (NCC) and the epithelial Na+ channel (ENaC), in part through transcriptional regulation of serum- and glucocorticoid-regulated kinase-1 (39, 40). The renal sodium-retaining action of Aldo is complemented by the central regulation of sodium appetite. Systemic mineralocorticoid administration selectively increases ingestive behavior for sodium but not water (4, 20, 54, 57, 58). Moreover, Aldo-sensitive neurons have been identified in the region of the nucleus tractus solitarius as a group of neurons that express the mineralocorticoid receptor (MR) and 11β-hydroxysteroid dehydrogenase type 2 (HSD2) and generate an output signal driving sodium ingestive behavior.
The sodium-retaining action of Aldo is balanced by inhibitory signals that promote renal sodium excretion and inhibit sodium appetite. The importance of the inhibitory mechanism is perhaps best evidenced by the escape phenomenon, a physiological adaptive response to high circulating levels of Aldo which are inappropriate to normal or increased extracellular volume. The mechanism responsible for Aldo escape is thought to be mediated by negative regulation of renal sodium transport by natriuretic factors such as atrial natriuretic factors, kinins, PGs, and nitric oxide (9, 23, 42, 44, 45). On the other hand, atrial natriuretic factors and another natriuretic factor, adrenomedullin, when administered via intracerebral injection, attenuate sodium appetite (1, 56). Interestingly, serum- and glucocorticoid-regulated kinase-1, a positive regulator of renal sodium transport, is reported to stimulate sodium intake during mineralocorticosteroid excess (54). Therefore, it is possible that common signaling pathways in the central nervous system and the kidney may be responsible for coordinating sodium-ingestive behavior and urinary sodium excretion.
PGE2, the major prostanoid produced in the kidney, has an established role in promoting salt and water excretion via integrated actions including inhibition of renal tubular transport, enhancement of renal hemodynamics, and reduction of vasopressin-dependent osmotic water flow (5, 25, 49–51). Aldo escape is associated with elevated levels of PGE2 that may participate in the natriuretic and diuretic responses during Aldo excess (13). To date, three major forms of PGE synthase (PGES) have been cloned and characterized: microsomal PGES (mPGES)-1, mPGES-2, and cytosolic PGES, with mPGES-1 being the best characterized PGES. Emerging evidence suggests a potential role for mPGES-1 in the regulation of sodium balance and blood pressure during salt loading and ANG II infusion (33). The first aim of the present study was to define the role of mPGES-1 in the renal response to excess Aldo. The second aim was to explore the role of mPGES-1 in the regulation of salt intake.
MATERIALS AND METHODS
Animals
mPGES-1 mutant mice were originally generated by Trebino et al. (52). This mouse colony was propagated at the University of Utah and maintained on a mixed DBA/1lacJ × C57BL/6 background. Genotypes were identified by PCR. All male mice at 3–4 mo of age were maintained under a 12:12-h light-dark cycle (lights on at 6:00 a.m. and lights off at 6:00 p.m.). All procedures were in accordance with guidelines approved by the University of Utah Institutional Animal Care and Use Committee.
Animal Experimental Protocols
Two different protocols were used to produce the Aldo escape model as described in the following.
Protocol 1.
Under anesthesia, male 3- to 4-mo-old mPGES-1 wild-type (WT) and knockout (KO) mice were subcutaneously implanted with a 14-day osmotic minipump delivering vehicle or 0.35 mg·kg−1·day−1 of Aldo. Following the procedure, animals were fed a gelled diet containing 0.09% Na+ (normal salt). This protocol provided Na+ intake at ∼0.25 meq/day. Immediately after the surgery, the mice were placed in metabolic cages (Hatteras Instruments) for determination of sodium and water intake and output using metabolic cages as previously described (63). A separate group of Aldo-infused WT mice were fed a gelled diet containing 0.01% Na+ to serve as a low-salt control. At the end of the experiment, the kidneys were harvested and blood samples were collected. One kidney was dissected into the cortex and the medulla (containing inner medulla and inner stripe of outer medulla), and half of the tissues were snap-frozen in liquid nitrogen and the other half stored in RNAlater; another kidney was fixed in 4% paraformaldehyde and embedded in paraffin. Urine and plasma osmolality was measured by using an osmometer (Osmett II, Precision Systems, Natick, MA).
Protocol 2.
mPGES-1 WT and KO mice were subcutaneously implanted with the minipump delivering 0.35 mg·kg−1·day−1 of Aldo as described in protocol 1. However, immediately after the surgery, the animals were placed on a low-salt diet for 3 days and then switched to a normal-salt diet to allow the escape to occur (55). Metabolic studies were started on the last day of the low-salt diet, and therefore the confounding influence of the surgical procedure on metabolic parameters could be minimized.
Blood Pressure Measurement
Systolic blood pressure was measured by the tail-cuff method using the Visitech BP2000 Blood Pressure Analysis System (Apex, NC) (37). All animals were habituated to the blood pressure measurement device for 7 days. They all underwent two cycles of 20 measurements recordered per day for a minimum of 3 days.
Measurement of Hematocrit
The sphenous vein was punctured using a no. 23-gauge needle and one drop of blood (∼5–10 μl) was collected using a 10-μl capillary glass (Idaho Technology). One side of the tube was sealed with Hemato-Seal and then centrifuged for 4 min in a Thermo IEC microcentrifuge machine.
Quantitative RT-PCR Analysis of Gene Expression in the Kidney
Total RNA isolation and reverse transcription were performed as previously described (41). Oligonucleotides were designed using Primer3 software (available at http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), and their sequences are shown in Table 1. Quantitative PCR (qPCR) amplification was performed using SYBR Green Master Mix (Applied Biosystems) and the Prism 7500 Real-Time PCR Detection System (Applied Biosystems). Cycling conditions were 95°C for 10 min followed by 40 repeats of 95°C for 15 s and 60°C for 1 min.
Table 1.
Sequences of oligonucleotides for qPCR
| Gene | Primer Sequence | Accession Number |
|---|---|---|
| GAPDH | Sense 5′-gtcttcactaccatggagaagg-3′ | M32599 |
| Antisense 5′-tcatggatgaccttggccag-3′ | ||
| α-ENaC | Sense 5′-gcttcatctttacctgtcgtttc-3′ | NM_011324 |
| Antisense 5′-ccagagattggagttgttcttgt-3′ | ||
| β-ENaC | Sense 5′-cagtggggagtcttcatcc-3′ | NM_011325 |
| Antisense 5′-tcctggtggtgttgctgt-3′ | ||
| γ-ENaC | Sense 5′-ctgcttcttcgatgggatg-3′ | NM_011326 |
| Antisense 5′-gacaccaggaaggggttgt-3′ | ||
| NCC | Sense 5′-gacaggcaccaacagtgaga-3′ | U61085 |
| Antisense 5′-tagagatggcggagatggag-3′ | ||
| NKCC2 | Sense 5′-gctcttcattcgcctctcct-3′ | NM_011389 |
| Antisense 5′-agcctattgacccaccgaac-3′ | ||
| NHE3 | Sense 5′-ctgaggaggaaccgagca-3′ | XM_993032 |
| Antisense 5′-aggcccagaacgatgagtag-3′ | ||
| AQP2 | Sense 5′-ggacctggctgtcaatgct-3′ | NM_009699 |
| Antisense 5′-atcggtggaggcaaagatg-3′ | ||
| V2R | Sense 5′-tcatcagccaccacacca-3′ | NM_019404 |
| Antisense 5′-agatagcagggccagttcag-3′ |
qPCR, quantitative PCR; ENaC, epithelial Na+ channel; NCC, Na+-Cl− cotransporter; NKCC2, Na+-K+-2 Cl− cotransporter; NHE3, type Na+/H+ exchanger; AQP2, aquaporin-2.
qRT-PCR Analysis of Gene Expression in Microdissected Proximal Tubules
mPGES-1 WT mice received subcutaneous infusion of vehicle or Aldo (n = 5/group) for 14 days in conjunction with a normal-sodium diet as described in protocol 1. Under anesthesia, the left kidney was perfused with 5 ml of cold saline followed by 5 ml of culture medium (DMEM, St. Louis, MO) containing 1 mg/ml of collagenase. Slices of renal cortex were incubated in the same DMEM-collagenase solution for 20 min at 37°C. Microdissection was performed at 4°C under a stereomicroscope as described elsewhere (60). Five millimeters of proximal tubules were microdissected, snap-frozen in liquid nitrogen, and stored at −80°C. Two to three specimens were collected from each animal. qRT-PCR for mPGES-1 and type 3 Na+/H+ exchanger (NHE3) was performed using a TaqMan MicroRNA Cells-to-CT kit from Applied Biosystems.
Immunoblotting
Renal cortices and medullas were lysed and subsequently sonicated in PBS that contained 1% Triton X-100, 250 μM PMSF, 2 mM EDTA, and 5 mM DTT (pH 7.5). Protein concentrations were determined by the use of Coomassie reagent. Forty micrograms of protein for each sample were denatured in boiling water for 10 min, separated by SDS-PAGE, and transferred onto nitrocellulose membranes. The blots were blocked overnight with 5% nonfat dry milk in Tris-buffered saline (TBS), followed by incubation for 1 h with the mPGES-1 polyclonal antibody (catalog no. 160140; Cayman Chemical, Ann Arbor, MI) or the γ-ENaC polyclonal antibody (catalog no. E4902; Sigma). After a washing with TBS, blots were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody and visualized using enhanced chemiluminescence.
Enzyme Immunoassay
Urine samples were centrifuged for 5 min at 10,000 rpm and diluted 1:1 with enzyme immunoassay (EIA) Buffer. Concentrations of PGE2 were determined by EIA according to the manufacturer's instructions (Cayman Chemical).
Immunohistochemistry
Kidneys were fixed with 3% paraformaldehyde and embedded in paraffin. Kidney sections (4-μm thickness) were incubated in 3% H2O2 for 10 min at room temperature to block endogenous peroxidase activity. The slides were boiled in antigen retrieval solution (1 mM Tris·HCl, 0.1 mM EDTA, pH = 8.0) for 15 min at high power in a microwave oven. The sections were incubated overnight at 4°C with primary antibodies at appropriate dilutions. Antibodies against mPGES-1 were from a commercial source (rabbit anti-mPGES-1; catalog no. 160140, Cayman Chemical). Antibodies against renal sodium and water transporters, including NHE3 (16) and aquaporin-2 (AQP2) (11), have been described previously. After a washing with PBS, the secondary antibody was applied and the signals were visualized using an ABC kit (Santa Cruz Biotechnology).
Statistical Analysis
All values are presented as means ± SE. Repeated measures ANOVA was used to analyze data from the time course studies (Fig. 1, A and C, and see Figs. 6, 9, and 10) with an unpaired Student's t-test to identify differences at a single time point. For the end-point studies of plasma and urine osmolality, renal expression of sodium and water transporters, an unpaired Student's t-test was used for comparisons between mPGES-1 WT and KO mice and a paired Student's t-test for comparisons within mPGES-1 WT and KO mice groups. Differences were considered to be significant when the P value was <0.05.
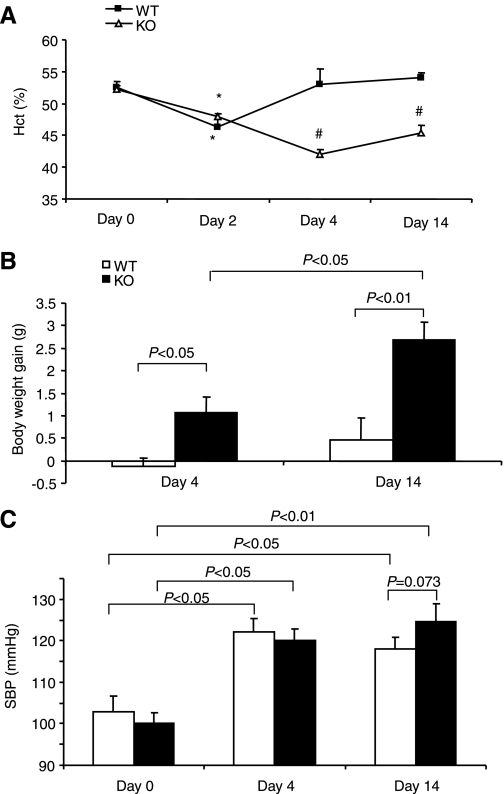
Fig. 1.
Evaluation of aldosterone (Aldo) escape in microsomal prostaglandin E synthase-1 (mPGES-1) wild-type (WT) and knockout (KO) mice. Animals were chronically infused for 14 days with Aldo at 0.35 mg·kg−1·day−1 via an osmotic minipump and fed a normal-salt diet throughout the entire experiment. Unless otherwise specified, the rest of the data were generated with the same protocol. Hematocrit (Hct; A), body weight gain (B), and systolic blood pressure (C) were monitored at the indicated time periods. *P < 0.01 vs. day 0 in the same strain. #P < 0.01 vs. WT at the corresponding period; n = 5–6/group (A and B) and n = 5–11/group (C).
Fig. 6.
Escape-related changes in urinary PGE2 output in mPGES-1 WT and KO mice. Urine PGE2 levels were determined by enzyme immunoassay. Values are means ± SE; n = 5–6/group.
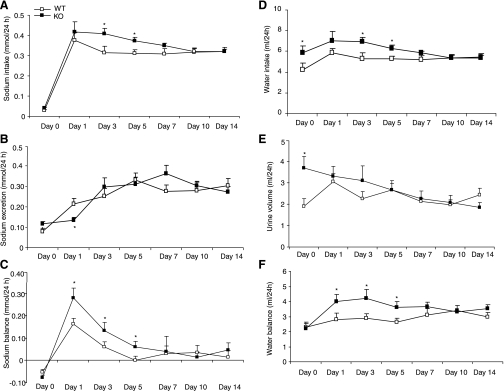
Fig. 9.
Sodium and water intake and output in mPGES-1 WT and KO mice over 14 days of treatment with vehicle (control) or Aldo. The protocol for this experiment was same as described in the legend for Fig. 1. Therefore, mice were placed in metabolic cages immediately after the surgical implantation of minipumps. A: sodium intake. B: sodium output. C: sodium balance (sodium intake subtracted by sodium output). D: water intake. E: urine output. F: water balance (water intake subtracted by urine output). Values are means ± SE; n = 5–7/group.*P < 0.05 vs. day 0 in the same group. #P < 0.05 vs. WT at the corresponding period.
Fig. 10.
Reevaluation of sodium and water intake and output in the Aldo-infused mPGES-1 WT and KO mice with a second protocol. Following implantation of the minipump driving Aldo infusion at 0.35 mg·kg−1·day−1, the animals were placed on a low-salt diet for 3 days and then switched to a normal-salt diet to allow escape to occur. At the indicated time points of the switch in diet, animals were placed in metabolic cages, and sodium and water intake and output were determined. Day 0 corresponds to the last day of low-salt treatment. A–F are defined as in the legend for Fig. 9. Values are means ± SE; n = 7/group. *P < 0.05 WT at the corresponding period.
RESULTS
Evaluation of Fluid Status and Blood Pressure
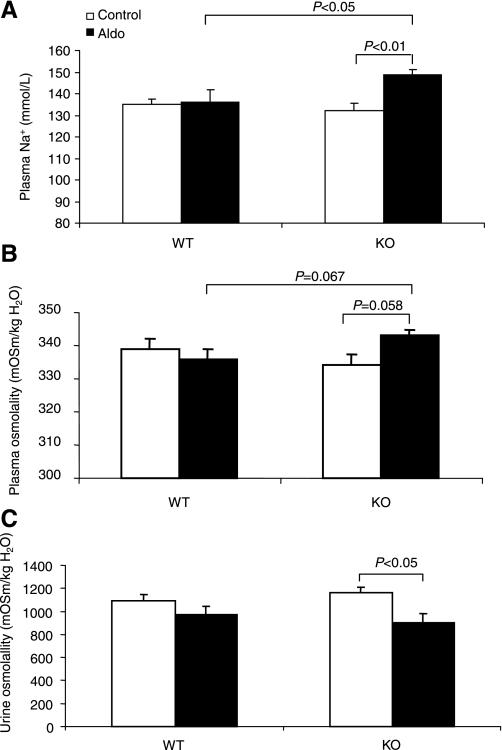
The escape model was generated in mPGES-1 WT and KO mice by chronic Aldo infusion (0.35 mg·kg−1·day−1) in conjunction with a gelled diet containing 0.09% Na+. Aldo infusion elevated plasma Aldo concentrations from 351.9 ± 41.1 to 1,458.0 ± 308.1 pg/ml in WT mice (n = 7–8, P < 0.05) and from 482.1 ± 59.4 to 1,801.8 ± 494.3 pg/ml in mPGES-1 KO mice (n = 6–11/group, P < 0.05). The values between the genotypes were not different after Aldo infusion (P > 0.05) despite a statistical difference in the basal condition (P < 0.05). Over a 14-day experimental period, hematocrit (Hct) was monitored to indirectly reflect the changes in plasma volume in Aldo escape (Fig. 1). On day 2, Aldo infusion in WT mice induced a transient fall in Hct (P < 0.05), followed by a rapid return to normal levels on day 4. While this treatment in mPGES-1 KO mice led to the steepest fall of Hct on day 4, it failed to return to normal levels on day 14, suggesting the lack of escape. Compared with WT controls, mPGES-1 KO mice exhibited a significant body weight gain that was noticeable on day 4 and maximal on day 14 (Fig. 1B). Following Aldo infusion, a modest increase in blood pressure was similarly detected in mPGES-1 WT and KO mice, and there was only a trend of difference between the genotypes (Fig. 1C). After a 14-day Aldo infusion, plasma sodium concentrations remained normal in WT mice but significantly elevated in the KO animals (Fig. 2A). The hypernatremia in the KO mice was associated with a trend of increased plasma osmolality (Fig. 2B). However, urinary osmolality in these mice was decreased but not increased, suggesting a urine concentrating defect (Fig. 2C).
Fig. 2.
Plasma sodium concentration (A), plasma osmolality (B), and urine osmolality (C) in mPGES-1 WT and KO mice following a 14-day treatment with vehicle or Aldo. All animals were fed a normal-salt diet. Values are means ± SE; n = 5–6/group.
Expression Profiles of Renal Sodium and Water Transporters
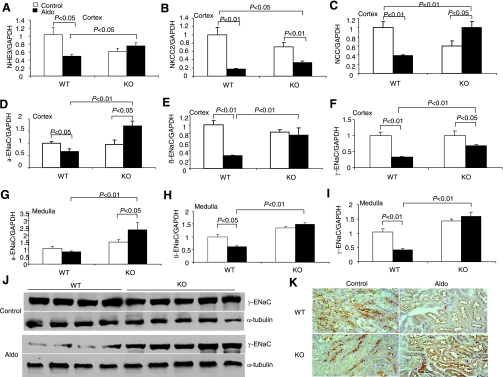
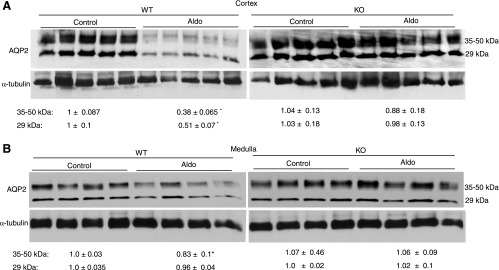
Downregulation of renal sodium transporters has been implicated in the development of Aldo escape. Therefore, we compared the expression profiles of renal sodium and water transporters between mPGES-1 WT and KO mice subjected to a 14-day Aldo infusion (Fig. 3). Aldo escape in WT mice was associated with a widespread inhibition of mRNA expression of all sodium transporters examined, including renal cortical NHE3, Na+-K+-2Cl− (NKCC2), NCC, α-ENaC, β-ENaC, and γ-ENaC, as well as renal medullary β-ENaC and γ-ENaC, except for a trend change for renal medullary α-ENaC. In contrast, the escape-associated downregulation of most of the transcripts was significantly attenuated or completely blocked in mPGES-1 KO mice; in these mice, the expression of α-ENaC and NCC was even upregulated by Aldo treatment. Some of these results were subsequently validated at protein levels. For example, immunoblotting detected an 80% reduction in renal protein abundance of γ-ENaC in the escaped WT mice that was significantly attenuated in mPGES-1 KO mice (Fig. 3J). Immunohistochemistry of NHE3 showed apical labeling in the proximal tubule that was reduced in the escaped WT mice. In contrast, this reduction of NHE3 immunoreactivity was not obvious in mPGES-1 KO mice (Fig. 3K). Renal AQP2 displayed a pattern of changes similar to that of the sodium transporters. Immunohistochemistry showed that Aldo infusion induced a consistent reduction of renal cortical and medullary AQP2 immunoreactivity in mPGES-1 WT but not KO mice (Fig. 4A, cortex; Fig. 4B, medulla). By qRT-PCR, renal cortical and medullary AQP2 mRNA in mPGES-1 WT mice was found to be reduced by 70 and 60', respectively, both of which were significantly attenuated in mPGES-1 KO mice (Fig. 4C, cortex; Fig. 4D, medulla). Renal mRNA expression of AVP V2 receptors displayed a pattern of changes almost analogous to that of AQP2 (Fig. 4E, cortex; Fig. 4F, medulla). Interestingly, the baseline expression of V2 receptors in the medulla but not in the cortex was significantly elevated in mPGES-1 KO mice compared with WT controls (Fig. 4E, cortex; Fig. 4F, medulla). Immunoblotting showed that Aldo infusion significantly downregulated AQP2 protein expression in the cortex and to a lesser degree in the medulla of WT mice (Fig. 5). In contrast, the downregulation was not seen either in the cortex or in the medulla of mPGES-1 KO mice (Fig. 5).
Fig. 3.
Escape-related changes in renal sodium transporters in mPGES-1 WT and KO mice. A–I: quantitative (q) RT-PCR analysis of various sodium transporters in the renal cortex and medulla. Values are means ± SE; n = 5–6/group. J: immunoblotting of γ-subunit of the epithelial sodium channel (γ-ENaC) and in the renal cortex. Immunobloting of α-tubulin served as a loading control. K: immunohistochemistry of type 3 Na+/H+ exchanger (NHE3) in the renal cortex.
Fig. 4.
Immunohistochemistry of aquaporin-2 (AQP2; A and B) and qRT-PCR of AQP2 (C and D) and V2 receptors (E and F) in the renal cortex (A, C, and E) and medulla (B, D, and F) of mPGES-1 WT and KO mice following a 14-day treatment with vehicle or Aldo. Values are means ± SE; n = 5–6/group.
Fig. 5.
Immunoblotting of AQP2 in the renal cortex (A) and medulla (B) of mPGES-1 WT and KO mice following a 14-day treatment with vehicle or Aldo. Immunobloting of α-tubulin serves as a loading control. Densitometry of 35- to 50- and 29-kDa bands of AQP2 was determined and normalized by α-tubulin. The relative values are shown underneath the immunoblot. Values are means ± SE; n = 5–6/group. *P < 0.05 vs. WT/control.
Evaluation of Urinary PGE2 Excretion and Expression of Key Enzymes in the PGE Synthesis Pathway
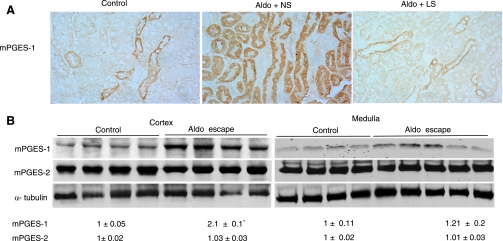
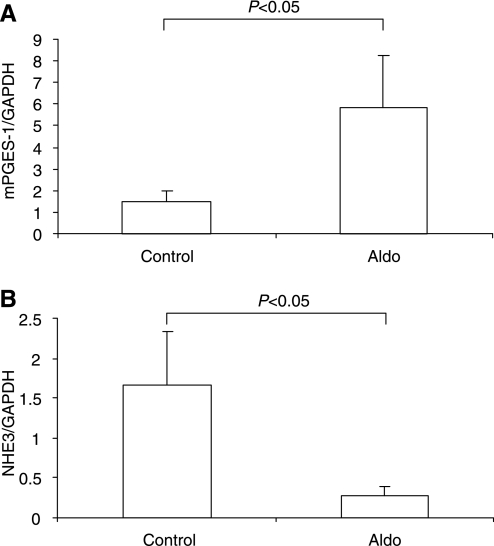
Aldo escape in mPGES-1 WT mice was associated with a time-dependent increase in urinary PGE2 output that was markedly attenuated in mPGES-1 KO mice (Fig. 6). Immunohistochemistry was performed to determine the intrarenal distribution of mPGES-1 WT mice in Aldo escape. As expected, under the baseline condition, a low level of expression of mPGES-1 was detected to in the collecting duct (Fig. 7, left). In the animals fed a normal-salt diet, Aldo infusion induced mPGES-1 expression in the proximal tubule (Fig. 7A, middle). To determine whether this induction was related to the escape phenomenon or to Aldo per se, we performed a parallel analysis in mice that received the same Aldo infusion but were fed a low-salt diet. In the salt-depleted animals, Aldo infusion no longer exhibited a stimulatory effect on expression of any of the enzymes (Fig. 7A, right). By immunoblotting, we detected mPGES-1 protein in the renal cortex as a 16-kDa band that exhibited a twofold increase in response to Aldo infusion (Fig. 7B). In contrast, the expression of mPGES-2 remained unchanged (Fig. 7B). Considering that the proximal tubule is not a typical site for the expression of PG synthesis enzymes, we performed qRT-PCR and a microdissection technique to validate the immunostaining results. As expected, the escaped WT animals exhibited a significant increase in mPGES-1 expression in the microdissected proximal tubules (Fig. 8A). In an opposite direction, the expression of NHE3 in these cDNA samples was remarkably downregulated (Fig. 8B). The predicted pattern of changes in NHE3 expression documented the validity of the technique.
Fig. 7.
Activation of mPGES-1 in the proximal tubule during Aldo escape. A: immunostaining of mPGES-1 in the kidneys of WT mice treated with vehicle+normal-salt diet (NS; control), Aldo+NS, or Aldo+low-salt diet (LS). Shown are representatives of 3 or 4 independent experiments. B: immunoblotting of mPGES-1 and -2 in the renal cortex of control and escaped mice. Immunobloting of α-tubulin serves as a loading control. The same blots were probed the with 3 different antibodies, and stripping was performed before probing with a second antibody. Densitometry of mPGES-1 and mPGES-2 was determined and normalized by α-tubulin. The relative values are shown underneath the immunoblot. Values are means ± SE; n = 5–6/group. *P < 0.05 vs. control.
Fig. 8.
qRT-PCR analysis of mRNA expressions of mPGES-1 (A) and NHE3 (B) in the microdissected proximal tubules from control and escaped WT mice. Values are means ± SE; n = 8/group.
Metabolic Studies
Sodium and water balance in mPGES-1 WT and KO mice during mineralocorticosteroid excess was initially evaluated with the above-described protocol. According to this protocol, animals were implanted with a minipump during Aldo infusion and immediately placed in metabolic cages. During the first 2 days of Aldo infusion, a persistent reduction of sodium and water intake was observed in both genotypes (Fig. 9) because of the confounding influence of pain and anesthesia associated with the surgical procedure. Starting from day 4 after recovery from the surgery, the two genotypes exhibited differences in the response to Aldo infusion. Sodium intake significantly increased in the KO mice from days 4–10 and returned to normal on day 14 (Fig. 9A), but urinary sodium excretion remained unchanged (Fig. 9B). Compared with WT controls, the KO mice exhibited increased sodium accumulation as a result of increased sodium intake from day 4 to day 10, and were able to reach sodium balance on day 14 (Fig. 9C). The changes in water output and intake and water balance followed almost exactly the same pattern as for sodium except that the KO mice were still not in water balance on day 14 (Fig. 9, D–F). The metabolic parameters were reevaluated using a different protocol which allowed for analysis of the early changes in escape-related natriuresis in the absence of the confounding influence of the surgical procedure (55). According to this protocol, a low-salt diet was implemented at the same time of Aldo treatment, and metabolic studies were not performed until the animals recovered from the surgery. Following the collection of baseline metabolic data, the escape was induced by treatment with a normal-salt diet. After the switch from a low- to normal-salt diet, WT mice exhibited a parallel increase in sodium intake and excretion and a transient positive sodium balance (Fig. 10, A–C). On day 1 of the normal-salt diet, WT mice exhibited a more than twofold increase in urinary sodium excretion (Fig. 10B). In contrast, the increase in urinary sodium excretion in the KO mice was not seen until day 3 of the normal-salt diet; at the subsequent time points, urinary sodium excretion in the KO mice was not different from their WT controls but was inappropriately low compared with increased salt intake. A greater increase in sodium intake was observed in the KO mice from day 3 to day 5 (Fig. 10A). Sodium balance in the KO mice was elevated from day 1 to day 5 and returned to normal afterward (Fig. 10C). At baseline, the KO mice had increased water intake and excretion but were in water balance following 3 days of Aldo infusion (Fig. 10, D–F). After the switch from a low- to normal salt diet, the KO mice exhibited increased water intake from day 3 and day 5 and increased sodium balance from day 1 to day 5 (Fig. 10D&F), a pattern similar to that of sodium.
DISCUSSION
COX-2-selective inhibitors, which include the coxibs celecoxib, rofecoxib, and valdecoxib, have dominated the prescription drug market for NSAIDs but are unfortunately associated with cardiovascular consequences, including fluid retention and hypertension (17, 27). As a result, rofecoxib has been withdrawn from the market. There is an ever-increasing demand for alternatives to COX-2 inhibitors. mPGES-1 has received much attention as a promising novel target for anti-inflammatory drugs (38). The lesson from COX-2 inhibitors highlights the importance of careful examination of possible physiological functions of mPGES-1. Our previous studies suggest potential roles of this enzyme in regulation of a number of physiological processes involving renal and vascular responses during salt loading and ANG II infusion (32, 33), despite some discrepancies with other studies (7, 19). The present study presents new evidence that mPGES-1 is an important determinant of the renal and central responses to excess Aldo.
A major goal of the present study was to investigate the role of mPGES-1 in Aldo escape by comparing the response of mPGES-1 WT and KO mice to a high dose of Aldo infusion in conjunction with normal salt intake. High doses of Aldo infusion in humans (65), dogs (10), and rats (55) are known to cause transient sodium retention followed by restoration of normal sodium balance. As expected, on day 2, Aldo infusion in WT mice produced a transient fall in Hct, a sign of plasma volume expansion, which was followed by a return to normal levels as early as day 4. Despite 14 days of Aldo infusion, WT mice displayed no signs of fluid retention as evidenced by normal sodium balance, plasma sodium concentration, Hct, and body weight. Moreover, renal expression of sodium transporters in these animals was downregulated, a phenomenon known to be associated with Aldo escape. Together, these results strongly support that the Aldo-infused WT mice had developed the escape phenomenon. In contrast with WT controls, the Aldo-infused KO mice exhibited the steepest fall in Hct on day 4, which failed to return to normal values on day 14, suggesting persistent plasma volume expansion. Along this line, Aldo-infused KO mice also developed hypernatremia and body weight gain. Furthermore, the escape-associated downregulation of renal transporters was attenuated in the KO mice. Renal excretory function was evaluated with two different protocols. As discussed in results, the first protocol is limited in that evaluation of urinary sodium excretion in the first 2 days of Aldo infusion was confoundingly influenced by the surgical procedure. At later time points after recovery from the surgery, urinary sodium excretion in the KO mice was not different from their WT controls but appeared to be inappropriate relative to increased intake, indirect evidence of impaired renal excretory function. In the second protocol, in which metabolic studies were conducted after recovery from the surgical procedure, we did observe that the KO mice displayed blunted natriuresis in the early phase of Aldo escape. Overall, the distinct responses of the two mouse strains during Aldo infusion demonstrated an important role of mPGES-1 in the escape phenomenon.
The role of mPGES-1 in Aldo escape is supported by the observation that renal mPGES-1 expression was significantly induced under this circumstance. Unexpectedly, the induction occurred in the proximal tubule, which is not a typical site for production or action of PGE2 in the kidney. PGE2 production in microdissected nephron segments has been measured in the presence of an excess of arachidonic acid by using radioimmunoassay or enzyme immunoassay (14, 15, 31, 35, 46). Despite differences in absolute values, the profiles were similar: low values in the proximal tubule, increasing in the thin limbs of the loop of Henle, low again in the thick ascending limb of the loop of Henle (TAL), and very high in the collecting duct (3). The lack of active PGE2 synthesis in the proximal tubule is commensurate with the absence of the key enzymes in the PGE synthesis pathway in this nephron segment. COX-1 and COX-2, which are rate-limiting enzymes in the PG synthesis pathway, are detected at substantially lower levels in the renal cortex than in the renal medulla (61). In the renal cortex, COX-1 is mainly expressed in the glomerulus and cortical collecting duct and COX-2 in the macula densa, and neither enzyme was found in the proximal tubule (28, 62). mPGES-1, which is the best-characterized PGES, is coexpressed with COX-1 in the distal nephron and with COX-2 in the macula densa and medullary interstitial cells, and, again, is absent in the proximal tubule (47). Tubular perfusion studies have demonstrated the direct effects of PGE2 on tubular transport in the TAL and the collecting duct (8, 22, 24, 29, 30, 49, 51), where active PGE2 synthesis takes place. A functional role for PGE2 in the proximal tubule has been less often described, although PGE2 is reported to antagonize the actions of parathyroid hormone in the perfused proximal straight tubule (12). In line with the renal activation of PGE synthesis enzymes in escaped WT mice, urinary PGE2 excretion in these animals was significantly elevated. The remarkable blockade of PGE2 excretion in the KO mice further suggests that mPGES-1 may represent a major source of enhanced renal PGE2 synthesis during Aldo escape.
A detailed mechanism responsible for the induction of mPGES-1 in the proximal tubule in Aldo escape remains elusive. However, the finding that Aldo infusion in conjunction with a low-salt diet failed to induce any of these enzymes strongly suggests that the induction was relevant to the escape process rather than Aldo per se. Along this line, DOCA treatment elevated renal medullary COX-2 expression in rats fed a normal-salt diet, but the stimulation was less in salt-depleted animals (64). Aldo escape is attributed to the activation of compensatory mechanisms secondary to the increases in renal perfusion pressure (26) or extracellular volume. It seems reasonable to speculate that these hemodynamic variables might be directly responsible for activation of the renal PGE synthesis pathway. Recently, Broadbelt et al. (6) reported that exposure of proximal tubular epithelial cells to pressures of 20–120 mmHg induces production of nitric oxide, another potential mediator of Aldo escape. This observation prompts future examination of PGE2 synthesis in pressurized proximal tubular epithelial cells.
The molecular targets of PGE2 during escape appear to be renal sodium and water transporters. In this regard, a 14-day Aldo infusion in WT mice induced a marked, widespread suppression of sodium transporters in virtually all nephron segments, including NHE3 (proximal tubule), NKCC2 (TAL), and NCC (distal convoluted tubule), as well as in α-, β-, and γ-ENaC (collecting duct). In contrast, in mPGES-1 KO mice, this suppression was significantly attenuated or completely blocked for most of the sodium transporters. Similarly, the escape in WT mice was associated with downregulation of AQP2 that was also attenuated in mPGES-1 KO mice. Together, these findings suggest that in Aldo escape, mPGES-1-derived PGE2 may broadly suppress the expression of various sodium and water transporters. In agreement with our findings, Bae et al. (2) recently reported a remarkable, parallel reduction of various sodium and water transporters in the kidney of DOCA-salt rats. Indeed, the majority of the functional studies show that both proximal and distal tubular responses are required in the escape (21, 36, 48, 59). However, our results disagree with the studies showing selective downregulation of NCC or NKCC2 but upregulation of NHE3 in Aldo-escaped rats (43, 53, 55). The reason for the discrepancy is unclear but may be related to differences in animal species or experimental protocols. For example, the length of Aldo infusion in these studies is shorter (4 days) than that in our study (14 days). It seems possible that the distal nephron response may be sufficient to produce escape from short-term Aldo treatment, whereas the proximal tubular response may be required in the face of long-term Aldo treatment.
Besides the renal action of mPGES-1 as discussed above, we have also discovered a central action of this enzyme in regulation of sodium and water intake during mineralocorticosteroid excess. With the two different protocols mentioned above, we consistently observed increases in sodium and water intake in the Aldo-infused mPGES-1 KO mice compared with their WT controls. A question arises as to whether mPGES-1 regulates sodium and water intake during changing sodium balance in the absence of meinrealocorticosteroid. Salt and water intake in the KO mice on normal- and high-salt diets has been examined in our previous study (33). This study shows that although the initial natriuretic response to high-salt loading is attenuated in the KO mice, sodium intake is not different between the genotypes. In the present study, we examined sodium and water intake in the KO mice on a low-salt diet. Again, we did not observe differences in sodium and water intake between the genotypes after salt depletion (data not shown). Therefore, the central regulation of sodium and water intake by mPGES-1 might be only operative in response to mineralocorticosteroid excess or other positive signals for sodium and/or water intake. Although no prior studies examine the role of PGE2 in the regulation of sodium appetite, exogenous PGE2 is reported to suppress drinking induced by ANG II when both were injected into rat cerebral ventricles (18) and, conversely, inhibition of endogenous PG synthesis with indomethacin augments water intake in response to intravenous infusion of ANG II (34). Future studies are needed to determine the molecular mechanism underlying the central action of mPGES-1.
In summary, in response to a 14-day Aldo infusion, mPGES-1 KO developed sustained plasma volume expansion, sodium retention, and body weight gain, accompanied by impaired renal excretory function and increased sodium intake. The escaped WT mice were associated with increased urinary PGE2 excretion and a parallel induction of mPGES-1, COX-1, and -2 in the proximal tubule. The increase in urinary PGE2 excretion was significantly blunted in mPGES-1 KO mice. These results suggest a novel role of mPGES-1 in the proximal tubular response in Aldo escape and also in the central regulation of sodium intake.
GRANTS
This work was supported by National Institutes of Health Grants HL079453 and DK066592, a Veterans Affairs Merit Review, and an American Heart Association Established Investigator Award (to T. Yang). T. Yang is an Established Investigator of the American Heart Association and a Research Career Scientist at the Department of Veterans Affairs.
ACKNOWLEDGMENTS
The authors thank John McNeish (Pfizer, Groton, CT) for providing breeder pairs of mPGES-1 KO mice, Mark A. Knepper (National Heart, Lung, and Blood Institute/National Institutes of Health) for providing antibodies to NHE3 and AQP2, and Shannon J. Odelberg and Ivor J. Benjamin (Cardiology, Univ. of Utah) for technical assistance.
REFERENCES
- 1.Antunes-Rodrigues J, McCann SM, Samson WK. Central administration of atrial natriuretic factor inhibits saline preference in the rat. Endocrinology 118: 1726–1728, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Bae EH, Kim IJ, Ma SK, Kim SW. Altered regulation of renal sodium transporters and natriuretic peptide system in DOCA-salt hypertensive rats. Regul Pept 157: 76–83, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bonvalet JP, Pradelles P, Farman N. Segmental synthesis and actions of prostaglandins along the nephron. Am J Physiol Renal Fluid Electrolyte Physiol 253: F377–F387, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Braun-Menendez E. Action of desoxycorticosterone on water exchange in the rat. Am J Physiol 163: 701, 1950 [Google Scholar]
- 5.Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol 63: 579–605, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Broadbelt NV, Stahl PJ, Chen J, Mizrahi M, Lal A, Bozkurt A, Poppas DP, Felsen D. Early upregulation of iNOS mRNA expression and increase in NO metabolites in pressurized renal epithelial cells. Am J Physiol Renal Physiol 293: F1877–F1888, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest 116: 1391–1399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culpepper RM, Andreoli TE. PGE2, forskolin, and cholera toxin interactions in modulating NaCl transport in mouse mTALH. Am J Physiol Renal Fluid Electrolyte Physiol 247: F784–F792, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Davis JO, Holman JE, Carpenter CC, Urquhart J, Higgins JT., Jr An extra-adrenal factor essential for chronic renal sodium retention in presence of increased sodium-retaining hormone. Circ Res 14: 17–31, 1964 [DOI] [PubMed] [Google Scholar]
- 10.Davis JO, Howell DS. Comparative effect of ACTH, cortisone and DCA on renal function, electrolyte excretion and water exchange in normal dogs. Endocrinology 52: 245–255, 1953 [DOI] [PubMed] [Google Scholar]
- 11.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez JH, Pitts TO, Brown T, Puschett DB, Schuler F, Chen TC, Puschett JB. Prostaglandin E2 and parathyroid hormone: comparisons of their actions on the rabbit proximal tubule. Kidney Int 26: 404–410, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Durr J, Favre L, Gaillard R, Riondel AM, Vallotton MB. Mineralocorticoid escape in man: role of renal prostaglandins. Acta Endocrinol (Copenh) 99: 474–480, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Farman N, Pradelles P, Bonvalet JP. Determination of prostaglandin E2 synthesis along rabbit nephron by enzyme immunoassay. Am J Physiol Renal Fluid Electrolyte Physiol 251: F238–F244, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Farman N, Pradelles P, Bonvalet JP. PGE2, PGF2α, 6-keto-PGF1α, and TxB2 synthesis along the rabbit nephron. Am J Physiol Renal Fluid Electrolyte Physiol 252: F53–F59, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper M. Concentrating defect in experimental nephrotic syndrone: altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int 54: 170–179, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med 351: 1709–1711, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fluharty SJ. Cerebral prostaglandin biosynthesis and angiotensin-induced drinking in rats. J Comp Physiol Psychol 95: 915–923, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Francois H, Facemire C, Kumar A, Audoly L, Koller B, Coffman T. Role of microsomal prostaglandin E synthase 1 in the kidney. J Am Soc Nephrol 18: 1466–1475, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Geerling JC, Loewy AD. Aldosterone-sensitive NTS neurons are inhibited by saline ingestion during chronic mineralocorticoid treatment. Brain Res 1115: 54–64, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Campoy JM, Kachelski J, Burnett JC, Jr, Romero JC, Granger JP, Knox FG. Proximal tubule response in aldosterone escape. Am J Physiol Regul Integr Comp Physiol 256: R86–R90, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Good DW. PGE2 reverses AVP inhibition of HCO3− absorption in rat MTAL by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 270: F978–F985, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Granger JP, Burnett JC, Jr, Romero JC, Opgenorth TJ, Salazar J, Joyce M. Elevated levels of atrial natriuretic peptide during aldosterone escape. Am J Physiol Regul Integr Comp Physiol 252: R878–R882, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Grantham JJ, Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3′,5′-monophosphate, and theophylline. J Clin Invest 47: 1154–1161, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan Y, Zhang Y, Breyer RM, Fowler B, Davis L, Hebert RL, Breyer MD. Prostaglandin E2 inhibits renal collecting duct Na+ absorption by activating the EP1 receptor. J Clin Invest 102: 194–201, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JE, Granger JP, Smith MJ, Jr, Premen AJ. Role of renal hemodynamics and arterial pressure in aldosterone “escape.” Hypertension 6: I183–I192, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70: 357–377, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F643–F650, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Imbert-Teboul M, Siaume S, Morel F. Sites of prostaglandin E2 (PGE2) synthesis along the rabbit nephron. Mol Cell Endocrinol 45: 1–10, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Jia Z, Guo X, Zhang H, Wang MH, Dong Z, Yang T. Microsomal prostaglandin synthase-1-derived prostaglandin E2 protects against angiotensin II-induced hypertension via inhibition of oxidative stress. Hypertension 52: 952–959, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kenney NJ, Moe KE. The role of endogenous prostaglandin E in angiotensin-II-induced drinking in rats. J Comp Physiol Psychol 95: 383–390, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Kirschenbaum MA, Lowe AG, Trizna W, Fine LG. Regulation of vasopressin action by prostaglandins. Evidence for prostaglandin synthesis in the rabbit cortical collecting tubule. J Clin Invest 70: 1193–1204, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohan DE, Knox FG. Localization of the nephron sites responsible for mineralocorticoid escape in rats. Am J Physiol Renal Fluid Electrolyte Physiol 239: F149–F153, 1980 [DOI] [PubMed] [Google Scholar]
- 37.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Kudo I, Murakami M. Prostaglandin e synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J Biochem Mol Biol 38: 633–638, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 40.McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 20: 134–139, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E2. Am J Physiol Renal Physiol 287: F152–F159, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Riazi S, Khan O, Hu X, Ecelbarger CA. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am J Physiol Renal Physiol 291: F597–F605, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Romero JC, Knox FG. Mechanisms underlying pressure-related natriuresis: the role of the renin-angiotensin and prostaglandin systems. Hypertension 11: 724–738, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Romero JC, Knox FG, Opgenorth TJ, Granger JP, Keiser JA. Contribution of sympathetic neural reflexes to mineralocorticoid escape. Fed Proc 44: 2382–2387, 1985 [PubMed] [Google Scholar]
- 46.Schlondorff D, Satriano JA, Schwartz GJ. Synthesis of prostaglandin E2 in different segments of isolated collecting tubules from adult and neonatal rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 248: F134–F144, 1985 [DOI] [PubMed] [Google Scholar]
- 47.Schneider A, Zhang Y, Zhang M, Lu WJ, Rao R, Fan X, Redha R, Davis L, Breyer RM, Harris R, Guan Y, Breyer MD. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int 65: 1205–1213, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Sonnenberg H. Proximal and distal tubular function in salt-deprived and in salt-loaded deoxycorticosterone acetate-escaped rats. J Clin Invest 52: 263–272, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stokes JB. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest 64: 495–502, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokes JB. Integrated actions of renal medullary prostaglandins in the control of water excretion. Am J Physiol Renal Fluid Electrolyte Physiol 240: F471–F480, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Stokes JB, Kokko JP. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest 59: 1099–1104, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA 100: 9044–9049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turban S, Wang XY, Knepper MA. Regulation of NHE3, NKCC2, and NCC abundance in kidney during aldosterone escape phenomenon: role of NO. Am J Physiol Renal Physiol 285: F843–F851, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Vallon V, Huang DY, Grahammer F, Wyatt AW, Osswald H, Wulff P, Kuhl D, Lang F. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am J Physiol Regul Integr Comp Physiol 289: R395–R401, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215–222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisinger RS, Blair-West JR, Denton DA, Tarjan E. Central administration of atrial natriuretic peptide suppresses sodium and water intake of sheep. Brain Res 579: 113–118, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Wolf G. Effect of deoxycorticosterone on sodium appetite of intact and adrenalectomized rats. Am J Physiol 208: 1281–1285, 1965 [DOI] [PubMed] [Google Scholar]
- 58.Wolf G. Sodium appetite elicited by aldosterone. Psychon Sci 1: 211–212, 1964 [Google Scholar]
- 59.Wright FS, Knox FG, Howards SS, Berliner RW. Reduced sodium reabsorption by the proximal tubule of Doca-escaped dogs. Am J Physiol 216: 869–875, 1969 [DOI] [PubMed] [Google Scholar]
- 60.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol Renal Physiol 277: F1–F9, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 102: 9406–9411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol 283: F509–F516, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Zierler K, Lilienthal J., Jr Sodium loss in man induced by desoxycorticosterone acetate. Am J Med 4: 186–192, 1948 [DOI] [PubMed] [Google Scholar]