Abstract
Systemic infusion of TNF-α exerts renal vasoconstriction but caused marked natriuresis in mice. Similar renal responses were also observed during systemic infusion of nitric oxide (NO) synthase inhibitors as opposed to their usual antinatriuretic responses when administered intrarenally. In the present study, we examined the hypothesis that acute NO blockade systemically induces TNF-α generation. which induces this natriuretic response. Renal responses to intravenous infusion of the NO synthase inhibitor nitro-l-arginine methyl ester (l-NAME; 0.2 μg·min−1·g body wt−1 for 85 min) and its impact on the plasma level of TNF-α were evaluated in anesthetized mice. Plasma TNF-α was undetected in untreated mice (n = 7) but was elevated in l-NAME-treated mice (109 ± 22 pg/ml; P < 0.01 vs. untreated group; n = 7) along with an increase in TNF-α protein expression in kidney tissue. l-NAME infusion caused a usual increase in mean arterial pressure (MAP; 98 ± 3 to 122 ± 3 mmHg; P < 0.01) and decreases in renal blood flow (RBF; 8.6 ± 0.3 to 4.4 ± 0.2 ml·min−1·g−1; P < 0.01) and glomerular filtration rate (GFR; 1.14 ± 0.07 to 0.77 ± 0.04 ml·min−1·g−1; P < 0.01) with a marked increase in sodium excretion (UNaV; 0.48 ± 0.10 to 3.52 ± 0.85 μmol·min−1·g−1; P < 0.01). Interestingly, in mice (n = 7) pretreated with the TNF-α blocker etanercept (5 mg/kg sc), the UNaV response to l-NAME infusion was markedly blunted (0.58 ± 0.08 to 1.22 ± 0.28 μmol·min−1·g−1; P = NS) although responses for MAP, RBF, and GFR were mostly unchanged. However, pretreatment with the superoxide scavenger tempol in mice (n = 7) did not alter the UNaV response to l-NAME. These data demonstrate that l-NAME-induced natriuresis is mediated, at least in part, by concomitant generation of TNF-α during NO blockade.
Keywords: l-NAME, renal hemodynamics, sodium excretion
nitric oxide (NO), an endogenous vasodilator, is synthesized from the amino acid l-arginine by the action of the enzyme NO synthase (NOS). NO production in the kidney contributes to the regulation of renal hemodynamics and excretory function. Inhibition of NOS in the kidney markedly reduces renal blood flow (RBF), urine flow, and sodium excretion with minimal or no reduction in glomerular filtration rate (GFR) (29, 30, 39). Administration of a NO donor compound directly into the renal artery reversed the effects of NO blockade and caused increases in urine flow and in sodium excretion, confirming that NO serves as a diuretic and natriuretic agent (32). It is known that these diuretic and natriuretic effects of NO are due to both its direct action on epithelial cells to inhibit tubular reabsorption (41) as well as indirectly by the associated changes in peritubular hemodynamics (29).
On the other hand, acute systemic administration of NOS inhibitors in rodents has been reported to cause marked diuresis and natriuresis in many studies although it reduces RBF or glomerular filtration (3, 16, 18). The exact mechanism of these diuretic and natriuretic responses to systemic infusion of NOS inhibitors still remains unclear. An associated increase in systemic arterial pressure during administration of NOS inhibitors was previously suggested to play a role in this natriuretic response (18). However, it has been shown in many studies that an increase in sodium excretion that occurs in response to an acute increase in arterial pressure (pressure-natriuresis) is actually dependent on intact NO production, and the inhibition of NOS locally in the kidney has been consistently shown to abolish this response (11, 29, 33). Therefore, the observed natriuresis in the condition of NOS blockade cannot be explained fully by the associated increase in arterial pressure. The possibility that an inhibition of renal sympathetic nerve activity due to baroreflex modulation during increases in arterial pressure in response to systemic NO inhibition was also suggested as the mechanism of this natriuretic response (13, 20). However, other studies (17, 37) using direct measurement of sympathetic nerve activity during administration of NOS inhibitors do not support any compelling role for renal nerves in this associated natriuretic response.
Recently, we have reported that the systemic infusion of TNF-α exerted renal vasoconstriction but caused marked natriuresis in mice (40). These findings generally resemble those with acute systemic infusion of NOS inhibitors, as mentioned earlier (3, 16, 18). Previously, it had been demonstrated that a high-salt (8%) diet induced increases in the production of various inflammatory cytokines including TNF-α in Dahl salt-sensitive rats (41). Pretreatment with a superoxide (O2−) scavenger, tempol, attenuated this high salt-induced increase in TNF-α production in these Dahl salt-sensitive rats (41) and indicated that oxidative stress was involved in the mechanism of cytokine production. Acute systemic administration of a NOS inhibitor in experimental models of rats that were subjected to hepatic ischemia-reperfusion or acute lung injury also caused a marked increase in the plasma level of TNF-α (24–26). Since NOS inhibition has been reported to cause an increase in O2− activity (22, 31), it might be possible that a systemic infusion of a NOS inhibitor induces an increase in TNF-α production from its primary sources, mainly from circulatory leukocytes and macrophages (24–26). A direct relationship between NO deficiency and the generation of inflammatory cytokines including TNF-α has also been suggested in many recent studies (1, 5, 27). In addition, in vitro studies using monocytes (49) or cardiomyocytes (46) showed an increased production of TNF-α in the deficient condition of NOS activity. Thus it is possible that acute systemic blockade of NOS may induce a primed condition that leads to generation of TNF-α from its primary sources of cells/organs that are prone to the production of inflammatory mediators.
In the present study, we examine the hypothesis that a concomitant increase in TNF-α production is involved in exerting a natriuretic response induced by acute systemic infusion of NOS inhibitors. In these experiments in anesthetized mice, we have measured the plasma TNF-α level in response to acute intravenous infusion of a NOS inhibitor, nitro-l-arginine methyl ester (l-NAME), and evaluated the renal hemodynamic and excretory responses to such l-NAME infusion in the presence or absence of etanercept, an agent that blocks the actions of TNF-α (40). To examine further the role of O2− activity in influencing TNF-α production and its renal actions during l-NAME infusion, experiments have also been conducted in mice pretreated with an O2− scavenger, tempol (40).
MATERIALS AND METHODS
Animal preparations.
All the experimental procedures described in this study were performed in accordance with the guidelines and practices established by the Tulane University Animal Care and Use Committee. C57BL6 mice (Jackson Laboratories, Bar Harbor, ME), weighing between 22–25 g, were housed in a temperature- and light-controlled room and allowed free access to a standard diet (Ralston Purina, St. Louis, MO) and tap water. On the day of experiments, the animals were anesthetized with a combination of Inactin (thiobutabarbital sodium, 100 mg/kg ip, Sigma, St. Louis, MO) and ketamine (6 mg/kg ip, Vedco, St. Joseph, MO) to conduct acute renal clearance studies as described before (40). The mice were placed on a servo-controlled surgical table that maintained body temperature at 37°C, and a tracheostomy was performed. The animals were allowed to breathe air enriched with O2 by placing the exterior end of the tracheal cannula inside a small plastic chamber into which humidified 95% O2-5% CO2 was continuously passed. The use of this humidified gas mixture for breathing was found to be effective in stabilizing arterial blood pressure of pentobarbital sodium-anesthetized rats (35) as well as mice (8, 14, 37). The right carotid artery was cannulated with polyethylene tubing (PE-10) connected to a pressure transducer (AcqKnowledge data acquisition system, Biopac) for continuous recording of arterial pressure. The right jugular vein was catheterized with a PE-10 tube for fluid infusion at a rate of 3 μl/min with the help of an infusion pump (CMA). During surgery, an isotonic saline solution containing 6% albumin (bovine serum, Calbiochem, La Jolla, CA) was infused. The bladder was catheterized with a PE-90 tube via a suprapubic incision for urine collection. After surgery, the infusion fluid was replaced with isotonic saline containing 1% albumin, 7.5% inulin (Inutest, Laevosan, Linz/Donau, Austria), and 1.5% PAH (Merck Sharpe & Dohme, West Point, PA). Inulin and PAH clearances were measured to determine GFR and RBF in these experimental animals.
Experimental mice were grouped as follows: 1) control mice (n = 7), in which only l-NAME was infused; 2) etanercept-pretreated mice (n = 7), in which l-NAME was infused in mice pretreated with the TNF-α blocker etanercept to determine the contribution of TNF-α to the responses to l-NAME; and 3) tempol-pretreated mice (n = 7), in which l-NAME was infused in mice pretreated with the O2− scavenger tempol to determine the role of O2− production in the responses to l-NAME. Etanercept (Immunex) was administered (5 mg/kg body wt sc) in conscious mice 1 day before the experiment. A second dose was also given to the anesthetized mice 3 h before l-NAME infusion on the day of experiment. This dose of etanercept was standardized in a previous study from our laboratory (40), which was shown to completely block the acute TNF-α-induced renal responses in mice. Tempol (Sigma) was administered at a rate of 2 μg·min−1·g body wt−1 (40) starting 2 h before l-NAME infusion and was continued until the end of the experiment.
Experimental protocol.
After a 60-min equilibration period following completion of surgical procedures, the experimental protocol was started with two consecutive 30-min control urine collections (basal period). An arterial blood sample (100 μl) was then taken for measurements of basal hematocrit and plasma PAH, inulin, and sodium/potassium concentrations. An infusion of l-NAME (0.2 μg·g−1·min−1, Sigma), dissolved in isotonic saline containing 1% albumin, 7.5% inulin, and 1.5% PAH, was then initiated and continued until the end of the experiment. The dose of l-NAME used in this study was selected on the basis of a previous study with l-NAME from our laboratory (16). Twenty-five minutes after the initiation of l-NAME infusion (stabilization period), another two 30-min urine clearance collections were made (l-NAME treatment period). After the final collection period, another arterial blood sample (100 μl) was taken for measurements of hematocrit, plasma PAH, inulin, and sodium/potassium concentrations.
For the measurement of plasma TNF-α level in these experimental animals, another larger blood sample of 200 μl was also collected from the carotid cannula after the completion of the experimental protocol. To maintain a stable experimental preparation without any hemodynamic complications, this larger sample collection was not made during the basal period. However, to assess the plasma TNF-α level in untreated animals, a separate set of time control experiments (n = 5) was also conducted with a similar protocol with saline (vehicle) infusion only without any treatment. In these time control experiments in vehicle-treated animals, a blood sample of 200 μl was collected from the carotid cannula after the completion of the experimental protocol. This collected blood was immediately centrifuged to separate the plasma, which was then snap-frozen in liquid nitrogen and stored at −80°C until analyzed. Portions of the collected urine samples during basal and experimental periods were also separated for the measurement of 8-isoprostane concentration (oxidative stress marker) (22). These separated samples were also snap-frozen in liquid nitrogen immediately after collection and stored at −80°C until analyzed. At the end of the experiment, the animals were killed with a high dose of ketamine (60 mg/kg body wt iv) and the kidneys were removed and weighed.
Immunoprecipitation and Western blot analysis.
Kidneys were dissected out and cut into two pieces and then homogenized in lysis buffer (20 mM Tris·HCl, pH 7.5, 5 mM EGTA, 150 mM NaCl, 20 mM glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.1% Tween 20, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM N-tosyl-l-phenylalanine chloromethyl ketone, and 0.5 mmol/l N-p-tosyl-l-lysine chloromethyl ketone). Extracts were incubated on ice for 30 min and then centrifuged (12,000 g for 15 min at 4°C). The supernatant fractions were retained, and protein concentrations in samples were determined using a BCA protein assay kit (Pierce, Rockford, IL). Two hundred micrograms of protein were immunoprecipitated with the specific antibody anti-TNF-α (Abcam, Cambridge, MA) overnight and then subjected to immunoblotting with the same antibody, as previously described (4).
Analysis of samples.
Urine volume (V) was measured gravimetrically. Blood and urine samples collected during systemic experiments were analyzed for inulin, PAH, and sodium/potassium concentrations as previously reported (40). The concentrations of sodium in urine and blood were used to calculate the urinary sodium excretion rate (UNaV) and fractional excretion of sodium (FENa), respectively. Renal vascular resistance (RVR) was calculated by dividing the value of mean systemic arterial pressure (MAP) with the value of RBF. Inulin and PAH concentrations were determined by spectrophotometry, and sodium/potassium concentrations were determined by flame photometry. The value for inulin clearance was considered as GFR, and the value for PAH clearance was considered as renal plasma flow. RBF is calculated from renal plasma flow and hematocrit value. 8-Isoprostane concentration in the urine was measured by ELISA (Cayman Chemical, Ann Arbor, MI) (40). TNF-α concentration in the plasma was measured by ELISA using a TNF-α ELISA kit (R&D Systems, Minneapolis, MN).
Calculations and statistical analysis.
The mean values obtained during the first two control collection periods were considered as the “basal value,” while the mean of the values collected during the two l-NAME infusion periods was taken as the “treatment value.” The differences in the values between the basal and the treatment periods were considered as the responses to l-NAME treatment. All values were normalized per gram of kidney weight. Results are expressed as means ± SE of the absolute or percent change of the responses. Differences between basal and treatment values in the same set of experiments were analyzed by a paired Student's t-test. Comparison of the data among the different sets of experiments were made by one-way ANOVA, followed by the Student-Newman-Keuls post hoc test for multiple comparisons. Differences were considered significant at P < 0.05. In a case of the analysis of TNF-α values, the nondetected concentration of plasma TNF-α level in time control animals was considered zero for the purpose of statistical analysis.
RESULTS
Plasma TNF-α levels in response to l-NAME infusion.
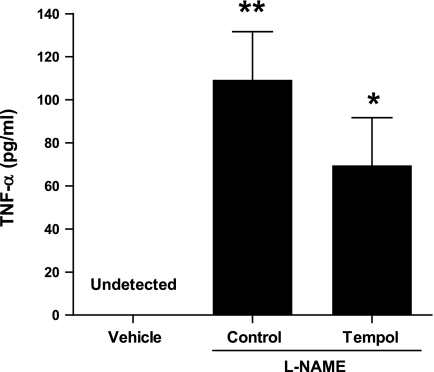
Results are depicted in Fig. 1. TNF-α levels were not detectable in the plasma obtained from the vehicle-treated animals in time control experiments. However, when l-NAME was infused for 1 h and 25 min in a control group of animals, a significant rise in plasma TNF-α levels (109 ± 22 pg/ml, P < 0.01) was observed compared with vehicle-treated animals (as stated above in Calculations and statistical analysis, the nondetected value of plasma TNF-α level in time control animals was considered zero for the purpose of statistical analysis). Similarly, when l-NAME was infused in tempol-pretreated animals, a significant rise in plasma TNF-α levels (70 ± 22 pg/ml, P < 0.05) was observed compared with vehicle-treated animals. However, this plasma TNF-α level induced by l-NAME observed in tempol-pretreated mice was slightly lower but not statistically different from that observed in control mice (Fig. 1). It is to be mentioned here that due to a cross-reactivity of TNF-α antibody with the etanercept compound in the plasma samples, TNF-α measurements in etanercept-treated mice showed abnormally high and inconsistent values and were erroneous and thus were not reported in this study.
Fig. 1.
Plasma TNF-α levels in response to nitro-l-arginine methyl ester(l-NAME) infusion (0.2 μg·g−1·min−1 for 85 min) in mice. In vehicle-treated mice, TNF-α in the plasma was considered as the zero level, as it was undetected. Values are means ± SE; n = 7 animals/group. *P < 0.05, **P < 0.01 vs. zero value in vehicle-treated mice.
TNF-α protein levels in the kidney in response to l-NAME infusion.
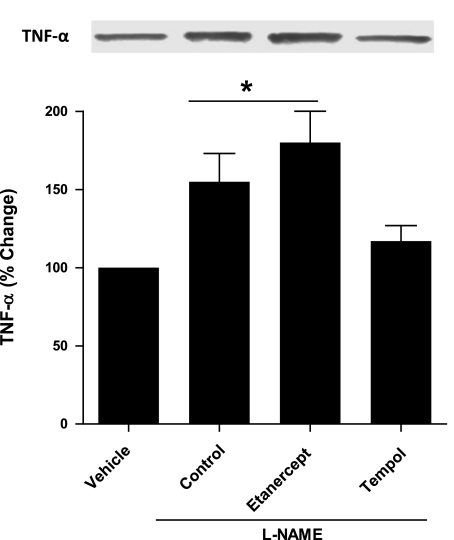
To determine the effects of systemic l-NAME administration on renal TNF-α expression, renal tissues were collected at the end of the experiments and examined for TNF-α protein expression using immunoprecipitation and Western blot analysis. As depicted in Fig. 2, renal TNF-α protein expression level was significantly greater in the control group of animals infused with l-NAME (1.5-fold, P < 0.05) than that observed in vehicle-treated animals. The TNF-α expression level was also increased when l-NAME was administered to etanercept-pretreated mice (1.8-fold, P < 0.05). Etanercept is a soluble receptor which binds TNF-α and prevents its interaction with its endogenous receptor and thereby inhibits its action. The soluble fusion proteins reportedly inhibited the bioactivity of TNF-α by neutralizing it and not by reducing its level. They function simultaneously both as TNF-α carriers and antagonists of TNF-α bioactivity. This phenomenon therefore may result in an increase in biologically inactive TNF-α in serum as previously reported (36). Thus the increase in the renal TNF-α protein expression level in response to l-NAME observed in etanercept-pretreated mice may be due to the cross-reactivity of TNF-α antibody with etanercept-bound TNF-α, which is no longer biologically active. However, when l-NAME was infused in tempol-pretreated animals, no significant rise in renal TNF-α expression level [1.15-fold, P = not significant (NS)] was observed compared with vehicle-treated animals.
Fig. 2.
TNF-α expression in kidney tissue lysate in response to l-NAME infusion (0.2 μg·g−1·min−1 for 85 min) in mice. Values are means ± SE; n = 4 animals/group. Control group, l-NAME given alone in mice; etanercept group, l-NAME given in etanercept-pretreated mice; tempol group, l-NAME given in tempol-pretreated mice; vehicle group, only vehicle treatment without l-NAME. *P < 0.05 vs. vehicle-treated mice.
Effects of etanercept and tempol pretreatment on the renal hemodynamic responses to l-NAME infusion.
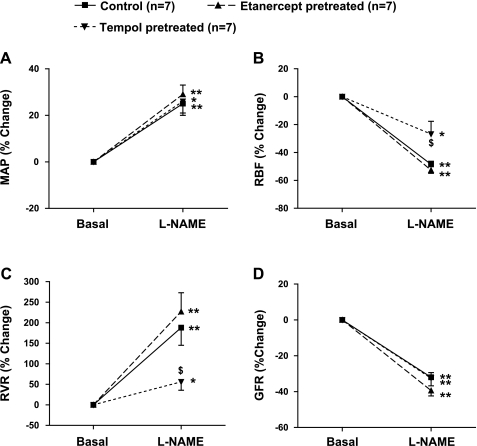
The absolute changes in MAP, RBF, RVR, and GFR in response to l-NAME infusion are given in Table 1, and percent responses are illustrated in Fig. 3, A–D. l-NAME infusion resulted in marked increases in systemic blood pressure among all the groups. In response to l-NAME, there were 25 ± 4 (P < 0.01), 29 ± 4 (P < 0.01), and 26 ± 6% (P < 0.05) increases in MAP in the control, etanercept-pretreated, and tempol-pretreated mice, respectively. As illustrated in Fig. 3B, l-NAME caused a marked reduction in RBF from the basal values in control (49 ± 2%; P < 0.01) and etanercept-pretreated (53 ± 2%; P < 0.01) mice. A significant decrease in RBF by l-NAME from the basal values (27 ± 9%; P < 0.05) was also observed in tempol-pretreated animals; however, the magnitude of the l-NAME-induced decrease in RBF in tempol-pretreated animals was found to be significantly attenuated (P < 0.05) compared with that observed during l-NAME infusion in control and in etanercept-pretreated mice.
Table 1.
Absolute responses to l-NAME infusion (0.2 μg·g−1·min−1 for 85 min) on blood pressure, renal hemodynamics, and excretory function in mice
| Control (n = 7) |
Etanercept-Pretreated (n = 7) |
Tempol-Pretreated (n = 7) |
||||
|---|---|---|---|---|---|---|
| Basal | l-NAME | Basal | l-NAME | Basal | l-NAME | |
| MAP, mmHg | 98 ± 3 | 122 ± 3† | 94 ± 5 | 122 ± 4† | 87 ± 5 | 109 ± 8* |
| RBF, ml·min−1·g−1 | 8.6 ± 0.3 | 4.4 ± 0.2* | 7.8 ± 1.0 | 3.7 ± 0.6† | 7.3 ± 1.2 | 5.4 ± 1.2* |
| RVR, mmHg·ml−1·min−1·g | 11.2 ± 0.9 | 30.9 ± 2.4† | 13 ± 1.9 | 42.0 ± 7.6† | 10.3 ± 1.4 | 15.2 ± 1.5* |
| GFR, ml·min−1·g−1 | 1.14 ± 0.07 | 0.77 ± 0.04† | 1.01 ± 0.1 | 0.60 ± 0.04† | 1.36 ± 0.13 | 0.92 ± 0.1† |
| V, μl·min−1·g−1 | 6.8 ± 1.3 | 24.6 ± 5.0* | 5.2 ± 0.6 | 10.7 ± 2.3 | 6.5 ± 0.9 | 27.0 ± 5.3* |
| UNaV, μmol·min−1·g−1 | 0.48 ± 0.10 | 3.52 ± 0.85* | 0.58 ± 0.08 | 1.22 ± 0.28 | 0.63 ± 0.10 | 4.06 ± 0.66† |
| FENa, % | 0.37 ± 0.12 | 2.88 ± 1.05* | 0.43 ± 0.08 | 1.21 ± 0.31 | 0.31 ± 0.04 | 2.95 ± 0.68* |
| UKV, μmol·min−1·g−1 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 |
| UIsoV, pg·min−1·g−1 | 8.1 ± 0.9 | 10.7 ± 0.7† | 7.8 ± 0.6 | 9.4 ± 0.5* | 6.3 ± 0.6 | 6.2 ± 0.4 |
Values are means ± SE; n = no. of animals. l-NAME, nitro-l-arginine methyl ester; MAP, mean arterial pressure; RBF, renal blood flow; RVR, renal vascular resistance; GFR, glomerular filtration rate; V, urine flow; UNaV, urinary sodium excretion rate; UKV, urinary potassium excretion rate; FENa, fractional excretion of sodium; UIsoV, urinary 8-isoprostane excretion rate.
P < 0.05,
P < 0.01 vs. corresponding basal value.
Fig. 3.
Percent responses to l-NAME infusion (0.2 μg·g−1·min−1 for 85 min) on mean arterial pressure (MAP; A), renal blood flow (RBF; B), renal vascular resistance (RVR; C), and glomerular filtration rate (GFR; D) in mice. Groups are defined as in Fig. 2. Values are means ± SE; n = 7 animals/group. *P < 0.05, **P < 0.01 vs. basal values. $P < 0.05 vs. corresponding values in control mice.
Figure 3C illustrates the percent response of RVR to l-NAME treatment. In control and etanercept-pretreated mice, l-NAME markedly increased RVR by 188 ± 43 (P < 0.01) and 227 ± 46% (P < 0.01) from their respective basal values. However, in mice pretreated with tempol, l-NAME increased RVR only by 56 ± 20% (P < 0.05) from the basal value, which was significantly less (P < 0.05) than the responses observed in control or etanercept-pretreated mice. On the other hand, the magnitude of l-NAME-induced decreases in GFR in control (32 ± 3%), in etanercept-pretreated (39 ± 3%), and in tempol-pretreated mice (33 ± 4%) were similar, as illustrated in Fig. 3D.
Effects of etanercept and tempol pretreatment on renal excretory responses to l-NAME infusion.
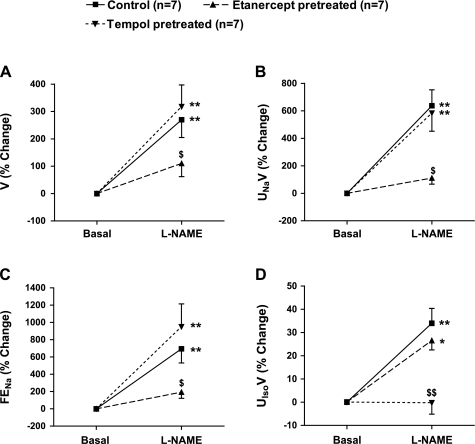
The absolute changes in the excretory function in response to l-NAME infusion in the three groups of mice are given in Table 1 and, percent responses are illustrated in Fig. 4, A–D. l-NAME infusion in control mice caused marked increases in V (270 ± 65%; P < 0.01), UNaV (637 ± 116%; P < 0.01), and FENa (694 ± 163%; P < 0.01) from their respective basal values (Fig. 4, A–C). Interestingly, when l-NAME was infused in etanercept-pretreated mice, there was marked attenuation of the responses in V (110 ± 48%; P = NS), UNaV (111 ± 44%; P = NS), and FENa (193 ± 71%; P = NS) which were not statistically significant from their respective basal values (Fig. 4, A–C). However, l-NAME caused marked increases in V (318 ± 79%; P < 0.01), UNaV (585 ± 132%; P < 0.01), and FENa (950 ± 265%; P < 0.01) in tempol-pretreated mice which were similar to those observed in control mice (Fig. 4, A–C). The percent change in urinary excretion rate of 8-isoprostane (UIsoV) in response to l-NAME infusion is illustrated in Fig. 4D. l-NAME infusion in control mice resulted in a 34 ± 6% increase in UIsoV (P < 0.01) from the baseline value. Similarly, in the etanercept-pretreated mice, l-NAME caused a significant rise in UIsoV (22 ± 4%; P < 0.05) from the baseline value. However, when l-NAME was infused in tempol-pretreated mice, no significant change was observed in UIsoV (0.2 ± 4%; P = NS) compared with the baseline value.
Fig. 4.
Percent responses to l-NAME infusion (0.2 μg·g−1·min−1 for 85 min) on urine flow (V; A), absolute (UNaV; B) and fractional excretion of sodium (FENa; C), as well as urinary 8-isoprostane excretion rate (UIsoV; D) in mice. Groups and n are as defined in Fig. 3. *P < 0.05, **P < 0.01 vs. basal values. $P < 0.05 vs. corresponding values in control mice. $$P < 0.01 vs. values in control mice.
In time control (vehicle treated) experiments (n = 7), no significant difference was observed in values of systemic MAP and other renal parameters between the basal and treatment collection periods, which were as follows: MAP, 100 ± 3 to 99 ± 4 mmHg; RBF, 8.0 ± 0.9 to 7.7 ± 0.6 ml·min−1·g−1; RVR, 13.8 ± 1.3 to 13.6 ± 1.3 mmHg·ml·min−1·g; GFR, 1.00 ± 0.04 to 1.07 ± 0.06 ml·min−1·g−1; V, 8.4 ± 1.7 to 7.8 ± 1.6 μl·min−1·g−1; UNaV, 0.69 ± 0.17 to 0.65 ± 0.15 μmol·min−1·g−1; FENa, 0.53 ± 0.15 to 0.52 ± 0.17%; UKV, 1.55 ± 0.25 to 1.59 ± 0.06 μmol·min−1·g−1; and UIsoV 7.31 ± 0.90 to 7.50 ± 0.65 pg·min−1·g−1.
DISCUSSION
In the present investigation, we have demonstrated that acute systemic infusion of a NOS inhibitor, l-NAME, in anesthetized mice results in an increase in plasma TNF-α level which is actually not detectable in the vehicle-treated time control mice. TNF-α protein expression in the renal tissues, collected at the end of experiments, is also increased significantly due to l-NAME infusion. Such an increase in TNF-α protein expression as well as its production rate due to systemic NOS inhibition within the short period of l-NAME administration (∼90 min) are interesting and uniquely reported in the present study. However, previous studies also reported a significant increase in TNF-α mRNA and protein synthesis in a time period as short as 30–60 min in response to different stimuli like ANG II (19), coronary artery occlusion (12), hindlimb ischemia-reperfusion (28), or TNF-α itself (42). An increase in plasma TNF-α levels occurred in response to systemic l-NAME administration either chronically (6, 45, 48) or acutely (24–26) in many pathological conditions. Of course, the appearance of TNF-α in the plasma in the present study implies excessive overall tissue production of this cytokine in response to systemic NOS inhibition. Furthermore, it is a well-known property of the proinflammatory cytokines to amplify their own secretion in a feed-forward manner. Thus it may be possible that increased TNF-α synthesis in response to systemic NOS inhibition in the present study is also a contributing factor in augmenting its own synthesis further, as suggested elsewhere (40, 42).
As reported previously (16), l-NAME infusion in mice in the present study also results in marked diuresis and natriuresis along with decreases in RBF and GFR. Interestingly, it is observed that these diuretic and natriuretic responses to l-NAME administration are markedly inhibited in mice pretreated with a TNF-α blocker, etanercept. We previously demonstrated (40) that an intravenous infusion of TNF-α, at a dose that may achieve plasma concentrations resembling active inflammatory conditions, resulted in marked increases in urine flow and sodium excretion which could be blocked by etanercept treatment at the dose used in the current study. Collectively, these results demonstrate that an induction of TNF-α production occurs during systemic or global NOS inhibition, which is involved in the mediation of diuretic and natriuretic responses observed during acute l-NAME administration systemically.
It is well established that NO is a natriuretic agent that exerts a direct inhibitory effect on epithelial sodium transport (41). However, the mechanism involved in the contrasting findings that systemic NOS blockade induces natriuresis is a subject of long-standing debate (18, 29, 33). As many previous studies (32, 33) convincingly demonstrated that NO inhibition directly in the kidney markedly attenuates the arterial pressure-induced changes in sodium excretion, it is unlikely that a concomitant increase in arterial pressure in response to systemic NOS blockade is directly involved in this mechanism (18). It is also unlikely that the factor(s) related to the blockade of intrarenal NO directly is involved in this natriuretic response as agents that release NO, such as acetylcholine and bradykinin, as well as the direct infusion of NO donors exert primary action as natriuretic agents (32, 39). Thus it is conceivable that such a natriuretic effect to l-NAME infusion is due to the influence of some other factors that are released extrarenally during systemic NOS blockade. The finding in the present investigation that the l-NAME-induced natriuresis is attenuated in etanercept-treated mice clearly demonstrates a link between systemic NO inhibition and the generation of TNF-α, which can influence renal excretory function at least in this acute experimental setting.
A blockade of NO synthesis by l-NAME administration in experimental animals leads to development of an oxidative stress condition, as indicated by an associated increase in UIsoV in many previous experiments in vivo (16, 22, 31) and an increase in vascular O2− production in vitro (38). It was also suggested that the production of inflammatory molecules such as TNF-α may be induced by the condition of oxidative stress (34, 44). However, as in the present study, the diuresis and natriuresis as well as the increase in plasma TNF-α level in response to systemic NOS blockade remain persistent in tempol-pretreated mice, although the development of an oxidative stress condition was minimized (Fig. 3D) in these animals. These findings indicate that the generation of TNF-α in response to systemic NOS inhibition occurred mostly independently of an oxidative stress condition. It should also be noted that systemic NOS blockade resulted in similar changes in blood pressure and GFR in tempol-pretreated mice compared with those in control animals. However, the magnitude of changes in RBF and RVR in response to systemic NOS blockade was found to be attenuated in tempol-pretreated mice, indicating that l-NAME-induced renal vasoconstriction was partly mediated by enhanced oxidative stress, as we previously reported (16).
It is known that NO has powerful anti-inflammatory properties and endogenous production of NO plays an important role in the regulation of various inflammatory cytokines including TNF-α (9, 43). However, the exact source of TNF-α release during systemic NOS blockade is yet to be determined. Our present findings suggest that endogenous NO production exerts a tonic inhibition on the production of TNF-α, possibly from its primary sources. It has been reported in a recent in vitro study (49) that application of asymmetric dimethylarginine (ADMA; an endogenous NOS inhibitor compound) induced TNF-α production in the circulating monocytes. Inhibition of NOS by Nω-nitro-l-arginine in rat cardiomyocytes also induced TNF-α production in a dose-dependent manner (46). A direct correlation between NO and inflammatory cytokines was indeed emphasized by the observation of increased expression of TGF-β and TNF-α in isolated endothelial NOS-deficient murine cardiomyocytes under basal condition (46). Thus it is possible that during systemic blockade of NOS, TNF-α is released from its systemic sources and exerts its effects in various organs, including the kidney. Although the underlying molecular mechanism by which NOS deficiency induces TNF-α expression was not studied in the present study, a specific role of NF-κB and p38 MAPK pathways has been implicated in this phenomenon recently (46, 49). Future studies are warranted to identify the mechanistic link between NOS activity and its inhibitory action in cells/organs that are prone to the production of inflammatory mediators.
The present findings indicate that TNF-α exerts natriuresis via a direct action on the renal tubules as noted in the marked increase in FENa (Table 1 and Fig. 4C). Our previous study (40) also demonstrated such direct tubular action of TNF-α, when infused systemically in anesthetized mice. It has been reported that TNF-α reduced the activity and expression of renal Na+K+-ATPase and the Na+K+-2Cl− cotransporter in the isolated cells in vitro originated from the renal cortex and medulla (23). In another in vitro study (2), TNF-α was shown to inhibit the renal epithelial sodium channel (ENaC) activity which was mediated by a PKC-dependent pathway. In a preliminary study in our laboratory (7), we have also observed that the natriuretic responses to acute TNF-α infusion could be blocked in mice pretreated with amiloride (ENaC blocker) and bendroflumethiazide (inhibitor for Na-Cl cotransport pathway). Collectively, these studies suggest that TNF-α produces natriuresis by its direct action on renal tubules.
An association of NO deficiency and an increased level of plasma TNF-α have been suggested in many pathophysiological conditions such as chronic l-NAME-induced hypertension (6) as well as many other models of hypertension (10, 14, 15, 45). However, the exact role of increased TNF-α in the NO-deficient form of hypertension in terms of its natriuretic effect is not yet clear. The present data together with our previous findings (40) suggest that the concomitant generation of TNF-α and its natriuretic effect may be a counterregulatory mechanism to limit the hypertensive response to generalized NO inhibition. Further studies are required to explain the implications of inflammatory responses during NO deficiency.
Although a direct relationship between constitutive NOS (eNOS) deficiency and increased TNF-α production has been reported in many recent in vivo (1, 5, 27) as well as in vitro studies (46, 49), it has also been reported in experimental models of sepsis that TNF-α stimulates the inducible form of NOS (iNOS) or a blockade of iNOS actually suppressed TNF-α production (21, 47). This discrepancy in the findings associated with two different conditions is not yet clearly understood. However, it should be noted here that a condition like septicemia (or LPS-induced sepsis) is largely mediated by the iNOS isoform that would normally be induced only in some pathological conditions (21, 47). Thus the mechanism of TNF-α production and its interactions with NOS activity may vary depending on the underlying physiological and pathological conditions. These mechanisms need to be clarified in future studies.
In summary, it has been demonstrated in the present investigation that acute systemic administration of the NOS inhibitor l-NAME causes a marked increase in the plasma TNF-α level in mice. The results show that the natriuresis induced by acute systemic NOS blockade is mediated, at least in part, by the concomitant generation of TNF-α. These data also indicate that such induction of TNF-α generation in response to systemic l-NAME administration is largely related to NO blockade per se, rather than to its associated increase in O2− activity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-66432 and the Tulane Enhancement Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are grateful to Alexander Castillo for excellent technical assistance and to Dr. Zakariya Abdel Mageed for help with Western blot analysis of renal tissue samples in this study.
Present address of M. Shahid: Wellman Center for Photomedicine, Department of Dermatology, and Anesthesia Center for Critical Care Research, Department of Anesthesia and Critical Care, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
REFERENCES
- 1.Alayan J, Ivanovski S, Gemmell E, Ford P, Hamlet S, Farah CS. Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol Immunol 21: 360–365, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-alpha through protein kinase C. Am J Physiol Renal Physiol 293: F1178–F1186, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Harton P, Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol 1: 875–881, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol 295: H69–H76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bougaki M, Searles RJ, Kida K, DeYu J, Buys ES, Ichinose F. NOS3 protects against systemic inflammation and myocardial dysfunction in murine polymicrobial sepsis. Shock In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourraindeloup M, Adamy C, Candiani G, Cailleret M, Bourin MC, Badoual T, Su JB, Adubeiro S, Roudot-Thoraval F, Dubois-Rande JL, Hittinger L, Pecker F. N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation 110: 2003–2009, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Castillo A, Shahid M, Islam MT, Majid DS. Tumor necrosis factor-alpha (TNF-alpha) induced natriuresis is prevented by blockade of distal tubular sodium transport in mice. FASEB J 23: 805, 2009 [Google Scholar]
- 8.Cervenka L, Mitchell KD, Navar LG. Renal function in mice: effects of volume expansion and angiotensin II. J Am Soc Nephrol 10: 2631–2636, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol 129: 4–10, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenoy FJ, Ferrer P, Carbonell L, Garcia-Salom M. Role of nitric oxide on papillary blood flow and pressure natriuresis. Hypertension 25: 408–414, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gabbai FB, Thomson SC, Peterson O, Wead L, Malvey K, Blantz RC. Glomerular and tubular interactions between renal adrenergic activity and nitric oxide. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1004–F1008, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque MZ, Majid DS. Reduced renal responses to nitric oxide synthase inhibition in mice lacking the gene for gp91phox subunit of NAD(P)H oxidase. Am J Physiol Renal Physiol 295: F758–F764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res 72: 511–516, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RA, Freeman RH. Pressure natriuresis in rats during blockade of the l-arginine/nitric oxide pathway. Hypertension 19: 333–338, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Kalra D, Sivasubramanian N, Mann DL. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation 105: 2198–2205, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Khraibi AA. Role of renal nerves in natriuresis of l-NMMA infusion in SHR and WKY rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F17–F21, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol Scand 43: 275–288, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568–572, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kreydiyyeh SI, Markossian S. Tumor necrosis factor alpha down-regulates the Na+-K+ ATPase and the Na+-K+2Cl- cotransporter in the kidney cortex and medulla. Cytokine 33: 138–144, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Xu B, Hock CE. Inhibition of nitric oxide synthesis by l-name exacerbates acute lung injury induced by hepatic ischemia-reperfusion. Shock 16: 211–217, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Xu B, Spokas E, Lai PS, Wong PY. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-repefusion. Shock 13: 217–223, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Liu YC, Kao SJ, Chuang IC, Chen HI. Nitric oxide modulates air embolism-induced lung injury in rats with normotension and hypertension. Clin Exp Pharmacol Physiol 34: 1173–1180, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Livonesi MC, Rossi MA, de Souto JT, Campanelli AP, de Sousa RL, Maffei CM, Ferreira BR, Martinez R, da Silva JS. Inducible nitric oxide synthase-deficient mice show exacerbated inflammatory process and high production of both Th1 and Th2 cytokines during paracoccidioidomycosis. Microbes Infect 11: 123–132, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Hamilton JA, Shen J, Pang T, Jones DL, Potter RF, Arnold JM, Feng Q. Role of tumor necrosis factor-alpha in myocardial dysfunction and apoptosis during hindlimb ischemia and reperfusion. Crit Care Med 34: 484–491, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Majid DS, Navar LG. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol Renal Fluid Electrolyte Physiol 262: F40–F46, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 14: 74S–82S, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Majid DS, Williams A, Kadowitz PJ, Navar LG. Renal responses to intra-arterial administration of nitric oxide donor in dogs. Hypertension 22: 535–541, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Fluid Electrolyte Physiol 264: F79–F87, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol 293: H2726–H2737, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell KD, Navar LG. Modulation of tubuloglomerular feedback responsiveness by extracellular ATP. Am J Physiol Renal Fluid Electrolyte Physiol 264: F458–F466, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 151: 1548–1561, 1993 [PubMed] [Google Scholar]
- 37.Ramchandra R, Barrett CJ, Guild SJ, McBryde F, Malpas SC. Role of renal sympathetic nerve activity in hypertension induced by chronic nitric oxide inhibition. Am J Physiol Regul Integr Comp Physiol 292: R1479–R1485, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Rauchova H, Pechanova O, Kunes J, Vokurkova M, Dobesova Z, Zicha J. Chronic N-acetylcysteine administration prevents development of hypertension in N(omega)-nitro-l-arginine methyl ester-treated rats: the role of reactive oxygen species. Hypertens Res 28: 475–482, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Romero JC, Lahera V, Salom MG, Biondi ML. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol 2: 1371–1387, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Shahid M, Francis J, Majid DS. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol 295: F1836–F1844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoos BA, Garvin JL. Actions of nitric oxide on renal epithelial transport. Clin Exp Pharmacol Physiol 24: 591–594, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Taishi P, Churchill L, De A, Obal F, Jr, Krueger JM. Cytokine mRNA induction by interleukin-1beta or tumor necrosis factor alpha in vitro and in vivo. Brain Res 1226: 89–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomassen MJ, Buhrow LT, Connors MJ, Kaneko FT, Erzurum SC, Kavuru MS. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol 17: 279–283, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tsukimori K, Komatsu H, Fukushima K, Kaku T, Nakano H, Wake N. Inhibition of nitric oxide synthetase at mid-gestation in rats is associated with increases in arterial pressure, serum tumor necrosis factor-alpha, and placental apoptosis. Am J Hypertens 21: 477–481, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Wenzel S, Rohde C, Wingerning S, Roth J, Kojda G, Schlüter KD. Lack of endothelial nitric oxide synthase-derived nitric oxide formation favors hypertrophy in adult ventricular cardiomyocytes. Hypertension 49: 193–200, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H, Kidachi Y, Umetsu H, Ryoyama K. l-NAME inhibits tumor cell progression and pulmonary metastasis of r/m HM-SFME-1 cells by decreasing NO from tumor cells and TNF-alpha from macrophages. Mol Cell Biochem 312: 103–112, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Yang HY, Yang SC, Chen ST, Chen JR. Soy protein hydrolysate ameliorates cardiovascular remodeling in rats with l-NAME-induced hypertension. J Nutr Biochem 19: 833–839, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Zhang GG, Bai YP, Chen MF, Shi RZ, Jiang DJ, Fu QM, Tan GS, Li YJ. Asymmetric dimethylarginine induces TNF-alpha production via ROS/NF-kappaB dependent pathway in human monocytic cells and the inhibitory effect of reinioside C. Vascul Pharmacol 48: 115–121, 2008 [DOI] [PubMed] [Google Scholar]