Abstract
Recent investigations demonstrate increased Na/H exchanger-1 (NHE-1) activity and plasma levels of ouabain-like factor in spontaneously hypertensive rats. At nanomolar concentrations, ouabain increases Na-K-ATPase activity, induces cell proliferation, and activates complex signaling cascades. We hypothesize that the activity of NHE-1 and Na-K-ATPase are interdependent. To test whether treatment with picomolar ouabain regulates Na-K-ATPase through an NHE-1-dependent mechanism, we examined the role of NHE-1 in ouabain-mediated stimulation of Na-K-ATPase in kidney proximal tubule cell lines [opossum kidney (OK), HK-2, HKC-5, and HKC-11] and rat kidney basolateral membranes. Ouabain stimulated Na-K-ATPase activity and tyrosine phosphorylation in cells that express NHE-1 (OK, HKC-5, and HKC-11) but not in HK-2 cells that express very low levels of NHE-1. Inhibition of NHE-1 with 5 μM EIPA, a NHE-1-specific inhibitor, prevented ouabain-mediated stimulation of 86Rb uptake and Na-K-ATPase phosphorylation in OK, HKC-5, and HKC-11 cells. Expression of wild-type NHE-1 in HK2 cells restored regulation of Na-K-ATPase by picomolar ouabain. Treatment with picomolar ouabain increased membrane expression of Na-K-ATPase and enhanced NHE-1-Na-K-ATPase α1-subunit association. Treatment with ouabain (1 μg·kg body wt−1·day−1) increased Na-K-ATPase activity, expression, phosphorylation, and association with NHE-1 increased in rat kidney cortical basolateral membranes. Eight days' treatment with ouabain (1 μg·kg body wt−1·day−1) resulted in increased blood pressure in these rats. These results suggest that the association of NHE-1 with Na-K-ATPase is critical for ouabain-mediated regulation of Na-K-ATPase and that these effects may play a role in cardioglycoside-stimulated hypertension.
Keywords: cardioglycosides, phosphorylation
the basolateral Na-K-ATPase- or the sodium pump-mediated regulation of renal proximal tubule sodium reabsorption is essential for whole-body sodium homeostasis, regulation of extracellular fluid volume, and blood pressure control (8, 24, 65). Plant-derived cardioglycosides; including digitalis, digoxin, and ouabain, are well-established inhibitors of Na-K-ATPase activity that bind to an extracellular portion of the Na-K-ATPase α-subunit (34). Ouabain-like cardioglycosides, which are produced in the adrenal gland and hypothalamus, have been associated with salt-sensitive hypertension (19, 20, 36, 52, 55). Plant-derived cardiac glycosides have been exploited as drugs to treat congestive heart failure and atrial fibrillation (1). In cardiac cells, ouabain increases intracellular Ca2+ concentration and cardiac muscle contractility by inhibiting Na-K-ATPase activity, thus stimulating Na/Ca exchange (4). In some cells, however, ouabain at very low (pM to nM) concentrations increases Na-K-ATPase activity (23). Previously, we demonstrated that in opossum kidney (OK) cells, nanomolar concentrations of ouabain stimulate Na-K-ATPase-mediated ion transport through a Src kinase-, ERK1/2-, and Akt-mediated pathway (25, 26). Other investigators demonstrated that nanomolar ouabain also regulates the sodium pump by activating the Na-K-ATPase-associated Src, resulting in stimulation of protein tyrosine phosphorylation (32, 57). Multiple studies from numerous laboratories demonstrated that Na-K-ATPase complexes with membrane and cytoskeletal proteins in caveolae and converts ouabain-binding signaling into the activation of downstream signaling proteins, including phosphoinositide 3-kinase, tyrosine kinases, the Ras-Raf-MEK pathway, ERK, and protein kinase B (Akt) (20, 29, 30, 35, 36, 43, 52, 60, 62).
Like Na-K-ATPase, Na+/H+ exchanger-1 (NHE-1) is a ubiquitous integral membrane protein localized to the basolateral membrane (BLM) in polarized epithelial cells. NHE-1 regulates intracellular pH and cell volume via electroneutral exchange of intracellular H+ for extracellular Na+. It is also involved in cytoskeletal organization, cell growth, proliferation, and differentiation, heart disease, and cancer (9, 44, 61). NHE-1 is composed of an N-terminal membrane domain that functions in ion transport and a C-terminal cytoplasmic regulatory domain that regulates the activity and mediates cytoskeletal interactions (63, 64). Recent findings that link NHE-1 with the pathogenesis of hypertension (45) led us to investigate the possible role of NHE-1 in the mechanism of ouabain-stimulated Na-K-ATPase activation.
It has been well established that the activity of Na-K-ATPase can influence cardiac function and blood pressure because inhibition of Na-K-ATPase increases contractility of both cardiac and vascular smooth muscle cells (2, 5, 13). Additionally, several studies suggest that endogenous cardioglycosides directly interact with the Na-K-ATPase α-subunit and can play an important role in regulating cardiovascular function and blood pressure (2). Although the acute effects of nanomolar and micromolar concentrations of cardioglycosides on signal transduction pathways and Na-K-ATPase regulation in heart and vascular smooth muscle are well studied, the mechanisms of nanomolar or picomolar concentrations of cardioglycosides on Na-K-ATPase activity are largely unknown. We have previously demonstrated in OK cells that nanomolar ouabain increases Na-K-ATPase-mediated 86Rb uptake through a Src-ERK-Akt dependent mechanism. Based on this observation, we hypothesized that chronic stimulation with nanomolar ouabain will result in increased activity of Na-K-ATPase in kidney cortical BLMs through increased expression and tyrosine phosphorylation. To address this hypothesis, we treated rats with 1 μg/kg body wt ouabain for several days and measured blood pressure and renal cortical Na-K-ATPase expression, phosphorylation, and activity. Our data demonstrated an increase in Na-K-ATPase expression, phosphorylation, and activity in kidney cortical BLMs from ouabain-treated animals. Surprisingly, expression of NHE-1 was also increased in BLMs from ouabain-treated animals. This led us to hypothesize that NHE-1 may associate with Na-K-ATPase to increase its activity in ouabain-treated animals. The role of NHE-1 was investigated using human kidney proximal tubule cells. Our data demonstrate that treatment with picomolar ouabain increased association of the Na-K-ATPase α1-subunit with NHE-1. Inhibition of NHE-1 prevented the stimulation of Na-K-ATPase and expression and phosphorylation of Na-K-ATPase α1-subunit by picomolar ouabain.
EXPERIMENTAL PROCEDURES
Materials
Ouabain, 8-(diethyl amino) octyo-3,4,5-trimethoxybenzoate (TMB-8), EIPA, and ammonium chloride were purchased from Sigma (St. Louis, MO). Polyclonal antibodies against phosphotyrosine were purchased from Zymed Laboratories (Carlsbad, CA), and monoclonal antibodies against phosphotyrosine (clone 4G10) were purchased from Millipore (Billerica, MA). Antibodies against caveolin were purchased from Novus Biological. The monoclonal antibody against the Na-K-ATPase α1-subunit (α6F) developed by Dr. D. M. Fambrough was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of NIH CD and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). A rabbit polyclonal antibody against rat Na-K-ATPase α1 (RT-NKA) for immunoprecipitation was a gift from Dr. Thomas Pressley (Texas Tech University). Rabbit polyclonal antibodies against NHE-1 were previously characterized by J. R. Schelling (Case Western Reserve University, Cleveland, OH) (59), and monoclonal antibodies against NHE-1 were purchased from BD Biosciences. Horseradish peroxidase-linked secondary antibodies were purchased from Vector Laboratories. Streptavidin-agarose resins were purchased from Pierce Biotechnology (Rockford, IL). Phosphatase inhibitor cocktail-1 and protease inhibitor cocktail were purchased from Sigma. All other chemicals were purchased from Sigma, unless otherwise specified.
Animal Model
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Louisville. Sprague-Dawley rats, weighing 200–250 g, were stabilized on standard rat chow and water ad libitum for 1 wk before the experiments. Rats (n = 8 in vehicle or ouabain treated) were intraperitoneally injected with 1 μg/kg body wt ouabain (dissolved in sterile PBS) once daily for 4 (BLM preparation and Na-K-ATPase activity) or 8 days (blood pressure measurement). Blood pressure was measured in ketamine-anesthetized rats after a 4-day treatment with ouabain by placing a catheter in the right carotid artery, and data were analyzed by using customized Micro-Med software as described by Sen et al. (53). Blood was collected, and serum was separated and analyzed for ouabain levels. The animals were killed, and kidneys were removed and collected in ice-cold PBS. Kidneys were decapsulated for BLM preparation or for preparation of paraffin blocks for immunohistochemistry. Of note, blood pressure did not change significantly in animals treated with ouabain for 4 days. To detect changes in blood pressure, a separate group of animals was treated with either vehicle or ouabain (1 μg·kg body wt−1·day−1) for 8 days (n = 8 in each group), and blood pressure was measured as described above.
Determination of Ouabain Levels in Serum
Ouabain levels were measured in serum samples from rats treated with vehicle or ouabain (1 μg·kg body wt−1·day−1) for 4 or 8 days as described previously (16, 49). Briefly, ouabain concentration was measured by EIAs using antisera containing polyclonal antibodies to ouabain. Microtiter plate wells were coated for a minimum of 18 h at 4°C with 0.5 μg/well of BSA-conjugated ouabain diluted in carbonate-bicarbonate coating buffer containing 15 mM Na2CO3, 35 mM NaHCO3, and 3.1 mM NaN3 in water (pH 9.6). After coating, the plates were washed with 0.5 ml/l Tween 20 in PBS and then blocked with 10 g/l BSA solution in PBS for 1 h at 37°C. After washing, the standards and samples were added, followed by the addition of the appropriate antibody, and the plate was incubated at room temperature for 1 h. After another washing step, goat anti-rabbit horseradish peroxidase conjugate was added and allowed to bind to the primary antibody for an additional 2 h at room temperature. Finally, the plate was washed, and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) reagent as substrate was added to each well. Color development was monitored at 450 nm for a maximum of 30 min, after which the reaction was stopped with 100 μl of TMB stop buffer and the plate was read at 450 nm. The readings were blanked and adjusted for nonspecific binding. We used the plant-derived ouabain as a standard in the immunoassays. Therefore, all concentrations and amounts of measured ouabain refer to the respective immunoequivalences to the plant-derived ouabain.
BLM Isolation
Kidney cortical BLMs were prepared from rats treated with or without ouabain for 4 days by the method of Sacktor et al. (50) with slight modifications. All steps were performed at 4°C unless otherwise stated. Briefly, 3-mm slices of kidney cortex were carefully separated and homogenized in 250 mM sucrose, 1 mM PMSF, and 10 mM Tris·HCl, pH 7.4, by 20 strokes in a glass teflon homogenizer. The homogenate was subjected to high-speed homogenization in a polytron-type homogenizer at maximum speed for three pulses of 30 s each with a 30-s interval. The homogenates were incubated with 15 mM MgCl2 on ice with constant shaking for 20 min to precipitate other membrane organelles. The homogenate was centrifuged at 2,500 g for 10 min in a Sorvall centrifuge using a SS-34 rotor. The supernatant was centrifuged at 24,000 g in a Sorvall centrifuge using a SS-34 rotor. The pellet was resuspended in 32.2 ml of homogenization buffer and mixed vigorously with 2.8 ml Percoll (final concentration 8.23%). The samples were centrifuged at 30,000 g for 35 min, and the middle layer (8 ml) containing BLMs was diluted with KCl-mannitol buffer containing 100 mM mannitol, 100 mM KCl, and 10 mM Tris-HEPES buffer, pH 7.1, and centrifuged at 34,000 g for 30 min as above. The white fluffy BLMs were resuspended in the KCl-mannitol buffer and centrifuged again at 38,000 g for 30 min, and the final pellet was resuspended in 300 mM mannitol, and 5 mM Tris-HEPES, pH 7.4, at 1 ml/g starting tissue. The BLMs showed seven- to eightfold enrichment of Na-K-ATPase activity compared with homogenates (data not shown).
ATP Hydrolysis Assay
BLM vesicles were quickly frozen in liquid nitrogen and slowly thawed on ice to make them permeable to ATP before measurement of Na-K-ATPase activity. Na-K-ATPase activity in BLMs was assayed as ouabain (1 mM)-sensitive ATP hydrolysis as previously described (27). The inorganic phosphate released was measured as described previously (27).
Immunohistochemistry
Kidney slices (3 μm) from rats treated with or without ouabain for 4 days were cut using a microtome from paraffin-embedded kidneys and fixed on glass slides. Paraffin was removed, and samples were rehydrated by passing slides through xylene (twice) followed by 100, 90, and 70% ethanol. Antigens were unmasked, and endogenous peroxidase was quenched using 3% hydrogen peroxide. Slides were blocked by 5% horse serum in Tris buffer, in a humidified chamber for 30 min at room temperature. Slides were incubated with polyclonal antibodies against rat Na-K-ATPase α1-subunit, NHE-1, or appropriate isotype control IgG (negative control) in blocking solution overnight at 4°C. Slides were washed and incubated with anti-rabbit biotinylated secondary antibody for 30 min at room temperature in a humidified chamber. The slides were incubated with ABC reagent (Vector Laboratories) at room temperature for 30 min, and color was developed using DAB reagent and counterstained with hematoxylin. The slides were washed, dried, and mounted. Images were acquired using an Olympus BX51 microscope and analyzed by using Image-Pro Plus software exactly as described by Powell et al. (47).
Cell Culture
OK cells, a continuous cell line derived from Virginia opossum, and human kidney cells [HK-2 (ATCC) and HKC-5 and HKC-11 (a gift from Dr. Lorraine Racusen, Johns Hopkins University, Baltimore, MD)] were cultured as previously described (28). Cells were maintained in DMEM-F12 (1:1) supplemented with 10% FBS and 1% penicillin/streptomycin and cultured to 90–95% confluence. Cells were washed with serum-free medium 24 h before use.
Ouabain-Sensitive 86Rb Uptake
Ouabain-sensitive 86Rb uptake in HK and OK cells was measured at 37°C exactly as described previously (26, 28). OK or HK cells were pretreated at 37°C with 5 μM monensin for 30 min. One-half of the cells were treated at 37°C with 1 mM ouabain, and the other half of the cells were exposed to vehicle, 10 pM (HK cells), or 10 nM (OK cells) ouabain. After 5 min, a trace amount of 86Rb (∼1 μCi/ml 86Rb) in serum-free MEM was added and uptake was carried out for 10 min such that total ouabain treatment was for 15 min. The cells were washed five to six times with ice-cold PBS and lysed overnight in 0.5 N NaOH containing 0.1% Triton X-100 at 37°C. An aliquot (100 μl) of the lysate was used to measure radioactivity. The difference between 86Rb uptake measured in the presence of 1 mM and vehicle, 10 pM, or 10 nM ouabain was used as a measure of Na-K-ATPase-mediated transport activity. Uptake data are expressed as nanomoles rubidium accumulated per milligram of protein per 10 min (cells that received EIPA were pretreated with 5 μM EIPA for 30 min).
Measurement of Cell Sodium
Cell sodium was measured as described previously (28). Briefly, 6.3 × 105 (HK11) or 1.04 × 105 (HK2) cells were plated per well in six-well plates for the experiment. Confluent cells were serum starved for 4 h and treated with 5 μM monensin in DMEM (pH 7.4) for the chosen period of time at 37°C. Cell monolayers were washed with ice-cold isotonic magnesium chloride solution (100 mM MgCl2, adjusted to pH 7.4 with Tris base). The magnesium chloride solution was then removed, and the cells were lysed overnight by adding 500 μl of 30% nitric acid. After this, 1.5 ml of deionized water was added to each well, and the sodium content of the diluted cell lysates was measured by using an atomic absorption spectrophotometer (PerkinElmer, Norwalk, CT) at a wavelength of 566.5 nm.
Crude Membrane Isolation
Cells were washed twice with PBS and placed in ice-cold lysis buffer containing 50 mM mannitol, 5 mM Tris·HCl, pH 7.4, 10 μl/ml phosphatase inhibitor cocktail, and 10 μl/ml protease inhibitor cocktail. The lysates were homogenized using 27.5-g needle syringes, followed by centrifugation at 2,500 g at 4°C for 10 min to remove cell debris. The supernatant was centrifuged at 30,000 g for 45 min. The pellet was resuspended in buffer containing 300 mM mannitol, 5 mM Tris·HCl, pH 7.4, 10 μl/ml phosphatase inhibitor cocktail, and 10 μl/ml protease inhibitor cocktail.
Western Blot Analysis
Western blot analysis was performed exactly as described previously (26, 28).
Biotinylation
Surface biotinylation was performed as described previously (25). Briefly, cells were treated with ouabain for 15 min, washed with cold PBS, and incubated with N-hydroxysulfosuccinimidobiotin (10 μl /ml) in borate buffer, pH 9.0 (20 mM Tris, 150 mM NaCl, 10 mM boric acid, 7.2 mM KCl, 1.8 mM CaCl2) for 2 h at 4°C. Cells were then washed three times with cold PBS, quenched with 100 mM glycine in PBS for 15 min at 4°C, and then washed three times more. Next, crude membranes were isolated and incubated with streptavidin-agarose resins overnight on a rotator at 4°C. Proteins bound to the resins were eluted and then resolved on 10% SDS-PAGE followed by immunoblotting using monoclonal antibodies against the Na-K-ATPase α1-subunit (α6F).
Immunoprecipitation
Crude membranes from cells or BLMs from kidneys solubilized in immunoprecipitation (IP) buffer (20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 100 μl/ml phosphatase inhibitor cocktail, 100 μl/ml protease inhibitor cocktail, 1% Triton X-100, 0.5% NP-40, and 0.5% SDS) were centrifuged at 70,000 g for 1 h in a Beckman ultracentrifuge. One hundred micrograms protein from the supernatant was precleared with protein A-Sepharose beads for 2 h at 4°C. The beads were separated by centrifugation at 14,000 rpm for 1 min in a tabletop centrifuge (Spectrafuge, National Labnet, Edison, NJ). The supernatant was incubated overnight at 4°C with 50 ng/100 μg protein rabbit polyclonal anti-rat Na-K-ATPase α1-subunit antibodies or NHE-1. Protein A-Sepharose beads were added and incubated for 2 h at 4°C. The beads were washed three times with IP buffer by centrifugation at 14,000 rpm for 1 min in a tabletop centrifuge, and an equal volume of 2× Laemmli sample buffer was added and heated at 65°C for 10 min. The beads were centrifuged as above, and the proteins in the supernatant were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and probed with the indicated antibodies.
Identification of Ouabain-Stimulated Phosphorylation Sites
Na-K-ATPase phosphorylation sites, in ouabain-treated HKC-5 and HKC-11 cells, were identified following the method described by Bodenmiller et al. (6). Briefly, Na-K-ATPase was immunoprecipitated as described above using monoclonal anti-Na-K-ATPase α1-antibody α6F from the Developmental Studies Hybridoma Bank (Univ. of Iowa), except that in place of Triton X-100 and SDS only 1% n-octylglucoside was used as a detergent. The immunoprecipitated proteins were eluted using 0.5 M glycine, pH 2.7, and collected in 10 μl 1 M triethylammonium bicarbonate, pH 8.5. The eluted proteins were reduced, alkylated, and digested with trypsin. The second step of enrichment was performed by affinity purification of the phosphorylated peptides using a TiO2-packed nanobore HPLC column. Bound peptides were eluted in a single step online to a one-dimensional reverse phase HPLC column. Putative phosphopeptides were eluted in a gradient fashion onto a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) target. A peptide mass list was compiled by serial MALDI-TOF-MS survey scans. A theoretical phosphopeptide mass list was established using a Na-K-ATPase FASTA file submitted to the Protein Prospector tool MS-DIGEST. Tandem MS data were acquired for all matching peptides in an attempt to confirm candidate phosphorylated Na-K-ATPase peptides.
Protein Determination
Protein concentration was determined using a bicinchoninic acid protein assay kit (Sigma) using a BSA standard.
Statistics
Data are shown as means ± SE. The n values represent the number of independent experiments. Each experiment was performed in triplicate. P values were calculated using SigmaStat software utilizing Student's t-test or by one-way ANOVA, followed by Bonferroni analysis using GraphPad Prism software. A P value <0.05 was a priori considered statistically significant.
RESULTS
Role of Ouabain in Na-K-ATPase Regulation in Rat Kidney Cortical BLMs
Previous studies show that endogenous serum levels of cardiac glycosides like ouabain increase in animal models and in humans with salt-sensitive hypertension (4, 40, 55). We first confirmed that ouabain at nanomolar concentrations exerted hemodynamic effects in Sprague-Dawley rats (4). Circulating ouabain levels increased significantly in animals treated with ouabain (1 μg·kg body wt−1·day−1) for 4 days [baseline 0.52 ± 0.009 (∼0.89 nM) vs. 0.99 ± 0.156 ng/ml (∼1.69 nM) after ouabain treatment (P < 0.032 by t-test)]. The calculated ouabain values are slightly higher in both control and treated animals from the reported values (20). This may be due to the difference in the methods (RIA vs. EIA), the specificity of the anti-ouabain antibodies used, or the presence of endogenous circulating ouabain-like factors in serum (20). Sprague-Dawley rats treated with ouabain (1 μg·kg body wt−1·day−1) for 8 days showed a significant increase in blood pressure without a change in heart rate, as demonstrated in Table 1.
Table 1.
Effect of ouabain on blood pressure
| Vehicle | Ouabain (1 μg/kg body wt for 8 days) | |
|---|---|---|
| MAP. mmHg | 75.93 ± 4.13 | 94.93 ± 2.66* |
| SBP, mmHg | 92.27 ± 10.03 | 113.3 ± 2.598* |
| DBP, mmHg | 65.23 ± 3.56 | 86.1 ± 2.82* |
| Heart rate, beats/min | 257.1 ± 20.13 | 253.5 ± 3.5 |
Values are means ± SE. MAP, mean arterial pressure; SBP and DBP, systolic and diastolic blood pressure, respectively. Sprague-Dawley rats were treated intraperitoneally for 8 days with ouabain (1 μg/kg body wt). After 8 days, blood pressure was determined by inserting a PE catheter in ketamine-anesthetized rats as described in experimental procedures.
P < 0.05 by Student's t-test.
Effect of ouabain on Na-K-ATPase regulation in kidney cortical BLMs.
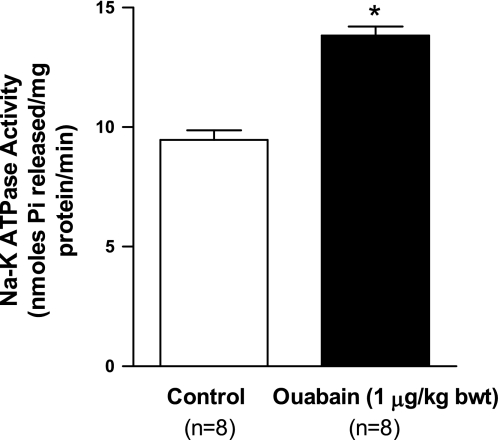
To determine the effects of nanomolar ouabain on Na-K-ATPase regulation, Na-K-ATPase activity in kidney cortex BLM vesicles was assayed according to previously described methods (27). As shown in Fig. 1 (top), treatment with ouabain (1 μg·kg body wt−1·day−1) for 4 days significantly increased Na-K-ATPase activity in kidney BLM.
Fig. 1.
Effect of nanomolar ouabain on Na-K-ATPase activity in kidney basolateral membranes (BLM). Sprague-Dawley rats, weighing 200–250 g, were treated for 4 days intraperitoneally with ouabain (1 μg·kg body wt−1·day−1). Kidneys were removed on day 4, decapsulated, and the cortex was carefully separated from the medulla. BLM were prepared from whole cortex as described in experimental procedures. Na-K-ATPase activity as ouabain (1 mM)-sensitive ATP hydrolysis was measured as described in experimental procedures. Values are means ± SE from BLM preparations from 8 separate animals (n = 8). All enzyme activity assays were run in triplicate; the values were averaged and considered as one value. *P < 0.05 by ANOVA followed by Bonferroni analysis.
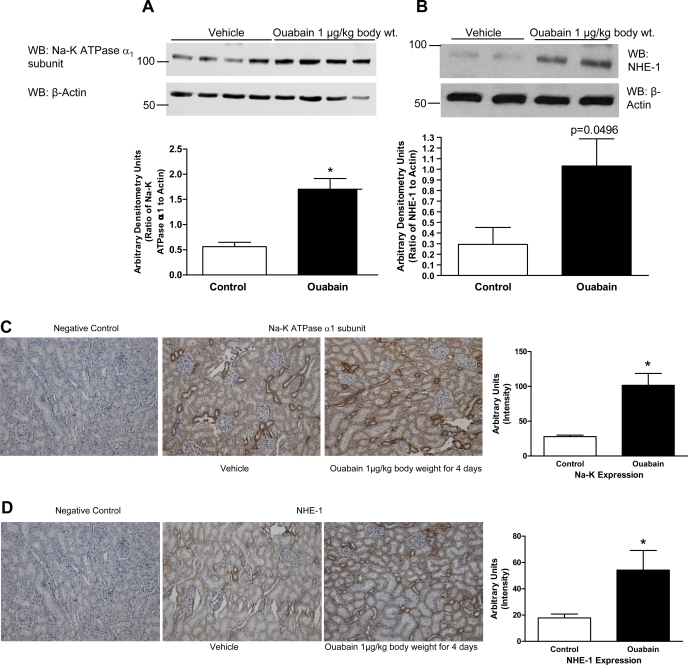
To determine the mechanism for the ouabain-stimulated increase in Na-K-ATPase activity, we examined Na-K-ATPase expression, NHE-1 expression, and tyrosine phosphorylation. As shown in Fig. 2, ouabain (1 μg·kg body wt−1·day−1) stimulated expression of the Na-K-ATPase α1-subunit (Fig. 2A) and NHE-1 (Fig. 2B) in kidney cortical BLM. To confirm the immunoblot data, we performed immunohistochemistry of kidney sections from the above animals. As shown in Fig. 2, C and D, expression of both the Na-K-ATPase α1-subunit (Fig. 2C) and NHE-1 (Fig. 2D) increased significantly with ouabain treatment in all cortical nephron segments.
Fig. 2.
Effect of nanomolar ouabain on Na-K-ATPase α1-subunit and sodium/ hydrogen exchanger-1 (NHE-1) expression in kidney BLM. Sprague-Dawley rats, weighing 200–250 g, were treated for 4 days intraperitoneally with ouabain (1 μg·kg body wt−1·day−1). Kidneys were removed on day 4, decapsulated, and the cortex was carefully separated from the medulla. BLM were prepared from whole cortex as described in experimental procedures. Proteins were separated and analyzed by immunoblotting using Na-K-ATPase α1-subunit (A) or NHE-1 (B) antibodies. Bottom: immunoblot for actin in the same blot. A representative blot from 4 (Na-K-ATPase α1-subunit) or 2 (NHE-1) BLM preparations from different animals is shown. WB, Western blot. Each bar represents data as arbitrary densitometry units (AU; ratio of Na-K-ATPase or NHE-1 to actin); values are means ± SE from 8 BLM preparations from different animals (n = 8). *P < 0.05 by ANOVA followed by Bonferroni analysis. C and D: Sprague-Dawley rats, weighing 200–250 g, were treated for 4 days intraperitoneally with ouabain (1 μg·kg body wt−1·day−1). Kidneys were removed on day 4, decapsulated, and fixed in paraffin. Kidney sections (3 μm) were cut and analyzed by immunohistochemistry as described in experimental procedures using Na-K-ATPase α1-subunit (C) or NHE-1 (D) antibodies. The color intensity was measured using Image Pro software. Values are means ± SE in AU from 8 different kidney sections from individual animals. Each section was analyzed at 5–7 different regions and averaged as one value.
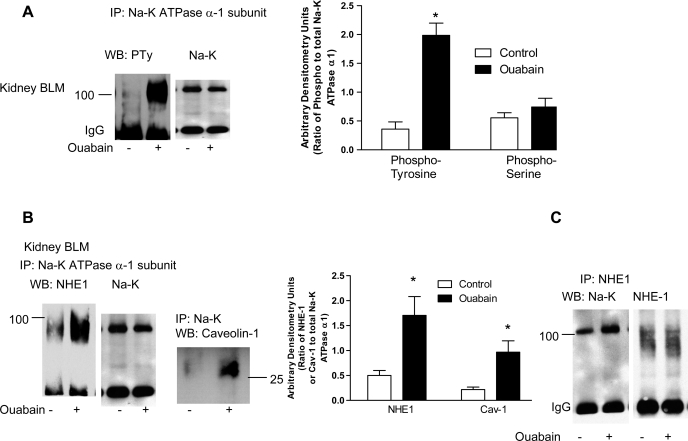
To determine whether ouabain increases tyrosine phosphorylation of the Na-K-ATPase α1-subunit in kidney BLM, the Na-K-ATPase α1-subunit was immunoprecipitated from BLM using polyclonal antibodies against the Na-K-ATPase α1-subunit and analyzed by immunoblotting using polyclonal phosphotyrosine antibodies. As shown in Fig. 3A, treatment with ouabain significantly increased Na-K-ATPase α1-subunit tyrosine phosphorylation in kidney BLM. In contrast, treatment with ouabain did not change serine phosphorylation of the Na-K-ATPase α1-subunit (Fig. 3A, bar diagram).
Fig. 3.
Effect of nanomolar ouabain on Na-K-ATPase α1-subunit phosphorylation and association with NHE-1 in kidney BLM. Sprague-Dawley rats, weighing 200–250 g, were treated for 4 days intraperitoneally with ouabain (1 μg·kg body wt−1·day−1). Kidneys were removed on day 4, decapsulated, and the cortex was carefully separated from the medulla. BLM were prepared from whole cortex as described in experimental procedures. Na-K-ATPase α1-subunit (A and B) or NHE-1 (C) was immunoprecipitated (IP) as described in experimental procedures. Immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting using phosphotyrosine or phosphoserine (A), NHE-1 or caveolin-1 (B), or Na-K-ATPase α1-subunit (C) antibodies. The blots were stripped and analyzed by immunoblotting for Na-K-ATPase α1-subunit (A and B) or NHE-1 (C) for equal loading. Each bar represents data as AU (ratio of phosphotyrosine or phosphoserine to total Na-K-ATPase α1-subunit or Na-K-ATPase α1-subunit or NHE-1 to total NHE-1 or Na-K-ATPase α1-subunit immunoprecipitated); values are means ± SE from 8 BLM preparations from different animals (n = 8). *P < 0.05 by ANOVA followed by Bonferroni analysis.
The above suggested an increase in both the Na-K-ATPase α1-subunit and NHE-1 in kidney BLM. Therefore, we reasoned that the two proteins may associate with each other. Several investigators have demonstrated that treatment with ouabain induces association of Na-K-ATPase with caveolin-1 in heart and LLC-PK1 cells (20, 21, 60). To determine whether the Na-K-ATPase α1-subunit, NHE-1, and caveolin-1 associate in BLM, we immunoprecipitated the Na-K-ATPase α1-subunit or NHE-1 and analyzed them by Western blotting for the Na-K-ATPase α1-subunit, caveolin-1, or NHE-1. As shown in Fig. 3B, treatment with ouabain increased association between the Na-K-ATPase α1-subunit and caveolin-1 as well as NHE-1. A reciprocal immunoprecipitation with NHE-1 confirmed the increased association between the Na-K-ATPase α1-subunit and NHE-1 (Fig. 3C).
Role of NHE-1 in Picomolar Ouabain-Mediated Effects in Human Kidney Proximal Tubular Cells
Effect of picomolar to nanomolar concentrations of ouabain on Na-K-ATPase activity in human kidney proximal tubule cells.
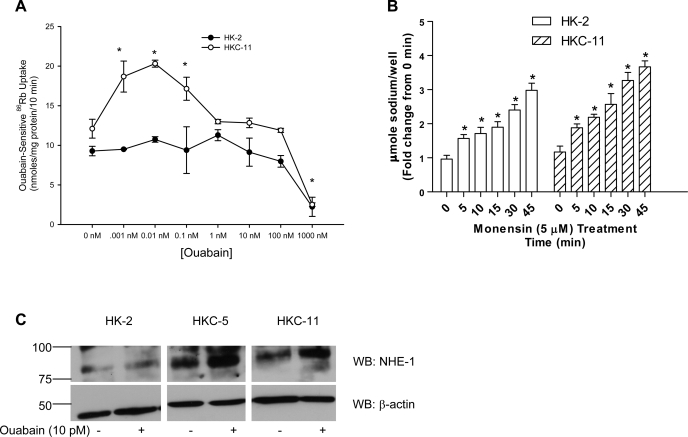
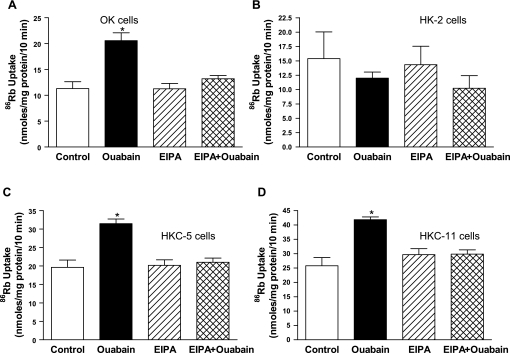
We first developed a dose-response relationship for the effect of ouabain on Na-K-ATPase-mediated 86Rb uptake in human kidney proximal tubule cell culture models. All 86Rb uptake experiments were carried out in the presence of 5 μM monensin to raise intracellular Na+ concentrations, and the assay is performed at Vmax for sodium. Of note, all cell culture studies employed acute ouabain treatment (15 min) in contrast to animal studies, where rats were chronically treated with ouabain (4–8 days). As shown in Fig. 4A, 10 pM ouabain maximally increased 86Rb uptake in HKC-11 cells, while higher concentrations of ouabain (1–1,000 nM) decreased Na-K-ATPase-mediated 86Rb uptake. In HKC-5 cells, ouabain stimulated 86Rb uptake maximally at 10 pM concentration, similar to HKC-11 cells (Supplemental Fig. 2A; all supplementary material for this article is avaialble on the journal web site). However, in OK cells ouabain increased 86Rb uptake maximally at 10 nM concentration (Supplemental Fig. 2B). In contrast, picomolar to nanomolar ouabain had no effect on 86Rb uptake in HK-2 cells. Higher concentrations of ouabain decreased 86Rb uptake similarly in all human kidney cell lines. Of note, treatment with 5 μM monensin increased intracellular sodium concentrations in a time-dependent manner in both HKC-11 and HK-2 cells (Fig. 4B). To determine the reason for the lack of a stimulatory ouabain response in HK-2 cells, we compared Na-K-ATPase α1-subunit expression in HK-2 and HKC-11 cells and found no difference (Supplemental Fig. 3A). Because both NHE-1 and Na-K-ATPase show similar activation in common cell functions including cell proliferation and physiological functions like blood pressure, we compared NHE-1 expression in the human kidney cell lines and found that NHE-1 expression was significantly less in HK-2 cells compared with OK, HKC-11, and HKC-5 cells (Supplemental Fig. 3B). Treatment of HKC-11 and HKC-5 cells with 10 pM ouabain increased NHE-1 expression in crude membranes but not in HK-2 cells (Fig. 4C). Expression of other major proximal tubule transporters NHE-3 and NBC-1 was not significantly different between the cell lines (Supplemental Fig. 3A).
Fig. 4.
Effect of different ouabain concentration on Na-K-ATPase-mediated 86Rb uptake in kidney proximal tubule cells. A: human kidney cells (HKC-11 or HK-2) were treated for 15 min with different concentrations of ouabain, and Na-K-ATPase-mediated 86Rb uptake was measured as described in experimental procedures. Values are means ± SE from 6 independent experiments performed in triplicate. Of note, all 86Rb experiments were performed in the presence of a sodium ionophore (monensin; 5 μM) such that the uptake was measured at Vmax for sodium. *P < 0.05 by 1-way ANOVA followed by Bonferroni analysis (GraphPad Prizm). B: cells were treated with 5 μM monensin for the indicated time and intracellular sodium was measured as described in experimental procedures. Each bar represents data from 6 individual experiments as fold-change from 0 min (means ± SE). *P < 0.05 by 1-way ANOVA followed by Bonferroni analysis (GraphPad Prizm). C: cells were treated for 15 min with 10 pM ouabain, and crude membranes were prepared as described in experimental procedures. NHE-1 expression was determined by immunoblotting as described in experimental procedures. The nitrocellulose membrane was cut below the 75-kDa marker, the top portion (above 75 kDa) was probed with anti-NHE-1 antibodies, and the bottom portion (below 75 kDa) was probed with anti-actin antibodies. A representative immunoblot from 3 individual experiments is shown.
To confirm that NHE-1 expression is required for Na-K-ATPase-mediated 86Rb uptake by ouabain, we treated OK, HK-2, HKC-5, and HKC-11 cells with either 10 nM (OK cells) or 10 pM (HK cells) ouabain in the presence or absence of 5 μM EIPA, a concentration that specifically inhibits NHE-1 (41, 46, 59), with minimal effect on NHE-3. Figure 5 shows that pretreatment with EIPA prevented stimulation of 86Rb uptake by ouabain in OK, HKC-5, and HKC-11 cells. EIPA alone had no effect on Na-K-ATPase-mediated 86Rb uptake in any of the cell culture models studied. Neither ouabain (10 pM) nor EIPA had any effect on Na-K-ATPase-mediated 86Rb uptake in NHE-1-deficient HK-2 cells.
Fig. 5.
Effect of NHE-1 inhibition on stimulation of Na-K-ATPase-mediated 86Rb uptake in kidney proximal tubular cells by ouabain. Intact cells were treated for 15 min with vehicle, 10 nM (OK cells, A) or 10 pM (HK cells, B–D) ouabain in the presence or absence of NHE-1 inhibitor EIPA (5 μM). Na-K-ATPase-mediated 86Rb uptake was measured as described in experimental procedures. Each bar represents means ± SE from 6 independent experiments performed in triplicate. Of note, all 86Rb experiments were performed in the presence of a sodium ionophore (monensin; 5 μM) such that the uptake was measured at Vmax for sodium. *P < 0.05 by 1-way ANOVA followed by Bonferroni analysis (GraphPad Prizm).
Effect of ouabain on membrane expression and phosphorylation of Na-K-ATPase α1-subunit.
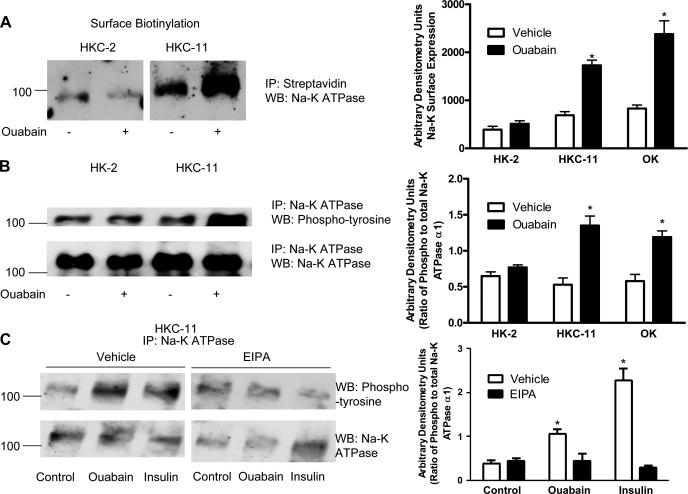
To determine whether the increase in 86Rb uptake is due to an increase in membrane expression of Na-K-ATPase, we examined the effect of 10 pM ouabain on plasma membrane expression of the Na-K-ATPase α1-subunit by surface biotinylation. As shown in Fig. 6A, 15-min treatment with 10 pM ouabain increased biotinylation of the Na-K-ATPase α1-subunit in HKC-11 cells compared with vehicle-treated cells but not in HK-2 cells. Ouabain increased surface expression of the Na-K-ATPase α1-subunit in OK cells similar to HKC-11 cells (Supplemental Fig. 4A).
Fig. 6.
Effect of ouabain on Na-K-ATPase α1-subunit expression and phosphorylation. A: cells were treated for 15 min with 10 pM ouabain, and cell surface was biotinylated as described in experimental procedures. Crude membrane proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting using Na-K-ATPase α1-subunit antibodies. Bar graph represents densitometry data from 3 independent experiments in AU. *P < 0.05 by 2-way ANOVA followed by Bonferroni analysis. B: cells were treated for 15 min with 10 pM ouabain, and the Na-K-ATPase α1-subunit was immunoprecipitated as described in experimental procedures. Immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting using phosphotyrosine antibodies (top). Bar graph represents densitometry data from 3 independent experiments (AU) as ratio of phosphotyrosine to total Na-K-ATPase band density. *P < 0.05 by 2-way ANOVA followed by Bonferroni analysis. C: cells were treated for 15 min with either 10 nM ouabain or 100 nM insulin in the continued presence or absence of 5 μM EIPA. The Na-K-ATPase α1-subunit was immunoprecipitated as above and analyzed by immunoblotting using phosphotyrosine antibodies. A representative immunoblot from 3 independent experiments is shown. The blots were stripped and probed with Na-K-ATPase α1-subunit antibodies for equal loading (bottom).
Tyrosine phosphorylation of the Na-K-ATPase α1-subunit has been identified as a mechanism for insulin-mediated stimulation of Na-K-ATPase and trafficking to the plasma membrane (18). To determine whether ouabain induced tyrosine phosphorylation of the Na-K-ATPase α1-subunit, HK-2 and HKC-11 cells were treated with 10 pM ouabain for 15 min and the Na-K-ATPase α1-subunit was immunoprecipitated using polyclonal antibodies against the Na-K-ATPase α1-subunit and analyzed by Western blotting using polyclonal antibodies against phosphotyrosine. As shown in Fig. 6B, ouabain stimulated tyrosine phosphorylation of the Na-K-ATPase α1-subunit in HKC-11 cells, but not in NHE-1-deficient HK-2 cells, suggesting a role for NHE-1 in tyrosine phosphorylation of the Na-K-ATPase α1-subunit. Similar to HKC-11 cells, ouabain also increased Na-K-ATPase α1-subunit phosphorylation in OK cells (Supplemental Fig. 4B). To confirm that ouabain induces tyrosine phosphorylation of the Na-K-ATPase α1-subunit, HK-2 and HKC-11 cells were treated with 10 pM ouabain for 15 min and the Na-K-ATPase α1-subunit was immunoprecipitated with monoclonal antibodies against Na-K-ATPase α1 (α6F antibody) and analyzed by Western blotting using monoclonal antibodies against phosphotyrosine (clone 4G10). As shown in Supplemental Fig. 5A, ouabain increased tyrosine phosphorylation of the Na-K-ATPase α1-subunit.
Potential tyrosine kinase-mediated phosphorylation sites on the Na-K-ATPase α1-subunit were identified by utilizing MALDI-TOF-MS and MALDI-TOF/TOF analysis of HKC-5 and HKC-11 cells stimulated with ouabain. Five candidate phosphopeptides were identified within the list of putative peptides purified using a TiO2 affinity chromatography approach. As shown in Table 2, we identified a previously described Tyr-10 phosphorylation (peptide 4) (18) and a unique tyrosine phosphorylation at tyrosine 260 of the Na-K-ATPase α1-subunit. The two putative phosphorylation sites (Y-10 and Y-260) on Na-K-ATPase were predicted as Src tyrosine kinase (32, 57) and enhanced green fluorescent protein kinase phosphorylation sites by web-based phosphorylation prediction software available on the EXPASY website (http://www.cbs.dtu.dk/services/NetPhosK/).
Table 2.
Identification of Na-K-ATPase phosphorylated peptides from ouabain-treated HKC-11 cells
| Peptide m/z | Putative Modifications | Start Sequence | End Sequence | Missed Cleavages | Peptide Amino Acid Sequence |
|---|---|---|---|---|---|
| 2,554.157 | 1Phospho | 662 | 683 | 1 | (K)ACVVHGSDLKDMTSEQLDDILK(Y) |
| 1Met-ox | |||||
| 2,093.914 | 3Phospho | 608 | 625 | 1 | (R)SAGIKVIMVTGDHPITAK (A) |
| 1,139.453 | 2Phospho | 256 | 264 | 0 | (R)GIVVYTGDR(T) |
| 1,453.528 | 2Phospho | 10 | 21 | 0 | (K)YEPAAVSEQGDK(K) |
| 2,537.037 | 3Phospho | 75 | 94 | 1 | (R)DGPNALTPPPTTPEWIKFCR(Q) |
Table shows peptide mass, phosphorylation site, and putative peptide sequences from the immunoprecipitated 100-kDa band. The peptide sequences that showed homology with the Na-K-ATPase α1-subunit are shown. Peptide 4 shows the known tyrosine phosphorylation of the Na-K-ATPase α1-subunit (Tyr-10). The other 4 peptides show putative unique serine/tyrosine phosphorylation sites in ouabain-treated samples. These peptides were not identified in control samples.
To examine the role of NHE-1 in ouabain-mediated tyrosine phosphorylation of the Na-K-ATPase α1-subunit, HKC-11 cells were treated with 10 pM ouabain in the presence and absence of 5 μM EIPA. As shown in Fig. 6C, treatment with either ouabain or insulin increased tyrosine phosphorylation of the Na-K-ATPase α1-subunit, and these effects were prevented by preincubation with EIPA.
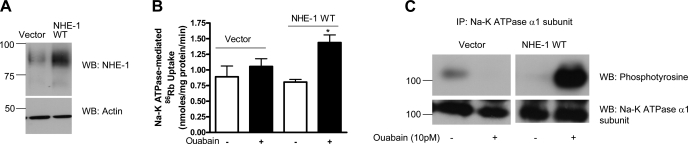
To confirm the role of NHE-1 in regulation of Na-K-ATPase by 10 pM ouabain in human kidney cells, we transfected HK-2 cells with either empty vector or human wild-type NHE-1. The cells were treated for 15 min with 10 pM ouabain. NHE-1 expression (Fig. 7A), Na-K-ATPase α1-subunit phosphorylation (Fig. 7B), and 86Rb uptake (Fig. 7C) were determined as described above. As shown in Fig. 7, ouabain increased Na-K-ATPase α1-subunit phosphorylation and 86Rb uptake in cells transfected with wild-type NHE-1, but not in vector-transfected cells.
Fig. 7.
Effect of NHE-1 transfection on ouabain-stimulated Na-K-ATPase α1-subunit phosphorylation in HK-2 cells. Wild-type (WT) heme agglutinin (HA)-tagged NHE-1 was transfected into HK-2 cells as described previously (14). Twenty-four hours after transfection, cells were treated for 15 min with 10 pM ouabain. A: NHE-1 expression in vector- or NHE-1 WT-transfected cells was determined by immunoblotting using anti-NHE-1 antibodies. Bottom: expression of actin as control for protein loading. C: Na-K-ATPase α1-subunit was immunoprecipitated from crude membranes of vector- or NHE-1-transfected HK-2 cells treated with 10 pM ouabain and analyzed by immunoblotting using phosphotyrosine antibodies. Blots were stripped and reprobed using Na-K-ATPase α1-subunit antibodies. A representative immunoblot from 2 independent experiments is shown. B: NHE-1 WT- or vector-transfected HK-2 cells were treated for 15 min with 10 pM ouabain, and Na-K-ATPase-mediated 86Rb uptake was measured as described in experimental procedures. Values are means ± SE from 6 independent experiments performed in triplicate. *P < 0.05 by 1-way ANOVA followed by Bonferroni analysis (GraphPad Prizm).
Effect of ouabain on NHE-1 phosphorylation and association with Na-K-ATPase α1-subunit.
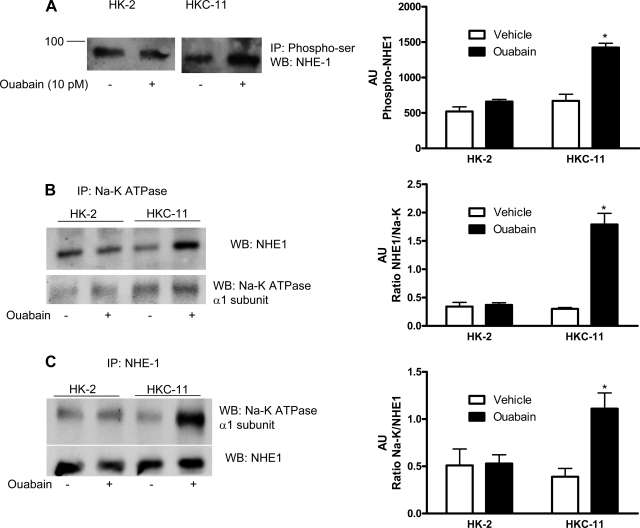
Several studies suggest that NHE-1 activity is modulated by changes in its phosphorylation state (38, 39, 51, 56). To assess NHE-1 phosphorylation, we immunoprecipitated phosphorylated proteins from human kidney cell lysates treated with 10 pM ouabain using phosphoserine/threonine antibodies and analyzed by them by immunoblotting using NHE-1 antibodies. As shown in Fig. 8A, treatment with 10 pM ouabain significantly increased phosphorylation of NHE-1 in HKC-11 cells but not in HK-2 cells. Ouabain increased serine phosphorylation of NHE-1 in OK cells similar to HKC-11 cells (Supplemental Fig. 4C).
Fig. 8.
Effect of picomolar ouabain on NHE-1 phosphorylation and association with Na-K-ATPase α1-subunit. A: cells were either treated or untreated for 15 min with 10 pM ouabain. Phosphorylated proteins from crude membranes were immunoprecipitated using a phosphoserine/threonine antibody as described in experimental procedures. Immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting using NHE-1 antibodies. It was not feasible to run a loading control because there is no reference protein that we can say with assurance remains unchanged. An equal amount of protein from control and treated cells was used. The assumption was made that the immunoprecipitation and immunoblotting efficiency was similar in the treated and control samples. Bar graph represents densitometry data from 3 independent experiments in AU of NHE-1 band density. *P < 0.05 by 2-way ANOVA followed by Bonferroni analysis. B and C: cells were treated for 15 min with 10 pM ouabain, and Na-K-ATPase α1-subunit (B) or NHE-1 (C) was immunoprecipitated as described in experimental procedures. Immunoprecipitated proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting for NHE-1 (C) or Na-K-ATPase α1-subunit (B). The immunoblots were stripped and reprobed with antibodies against Na-K-ATPase α1-subunit (B, bottom) or NHE-1 (C, bottom). A representative blot from 3 independent experiments is shown. Bar graphs represent densitometry data from 3 independent experiments (AU) as ratio of NHE-1 band density to total Na-K-ATPase (B), or as ratio of Na-K-ATPase band density to total NHE-1 immunoprecipitated (C). *P < 0.05 by 2-way ANOVA followed by Bonferroni analysis.
The above data suggest that 10 pM ouabain stimulated Na-K-ATPase regulation through NHE-1-dependent mechanisms. Both Na-K-ATPase and NHE-1 are localized to the BLMs in proximal tubular epithelial cells and may interact with each other. To determine whether the Na-K-ATPase α1-subunit associates with NHE-1, we treated HK-2 and HKC-11 cells with 10 pM ouabain. The Na-K-ATPase α1-subunit was immunoprecipitated and analyzed by immunoblotting. As shown in Fig. 8B, treatment with ouabain increased the association between the Na-K-ATPase α1-subunit and NHE-1 in HKC-11 cells but not in HK-2 cells. Reciprocal immunoprecipitation with NHE-1 and immunoblotting for the Na-K-ATPase α1-subunit (Fig. 8C) confirmed the ouabain-stimulated increase in association between NHE-1 and the Na-K-ATPase α1-subunit. Similar to HKC-11 cells, treatment with ouabain increased association between Na-K-ATPase and NHE-1 or caveolin-1 in OK cells (Supplemental Fig. 3D). To confirm that ouabain induces association of the Na-K-ATPase α1-subunit with NHE-1, HK-2 and HKC-11 cells were treated with 10 pM ouabain for 15 min and the Na-K-ATPase α1-subunit was immunoprecipitated with monoclonal antibodies against Na-K-ATPase α1 (α6F antibody) and analyzed by Western blottng using monoclonal antibodies against NHE-1 or the Na-K-ATPase α1-subunit (α6F antibody). As shown in Supplemental Fig. 5, A and B, ouabain increased association of the Na-K-ATPase α1-subunit with NHE-1. Reciprocal immunoprecipitation of NHE-1 using monoclonal antibodies and Western blotting with the Na-K ATPase α1-subunit (α6F antibody) confirmed the association (Supplemental Fig. 5B).
DISCUSSION
The present studies demonstrate a novel interaction between Na-K-ATPase and NHE-1 that is stimulated by ouabain in the nanomolar or picomolar concentration range. Na-K-ATPase interaction with NHE-1 was observed in intact rat kidney cells as well as in cultured human renal tubule cells. The ability of picomolar concentrations of ouabain to stimulate interaction between the two ion transporters is interesting in light of recent evidence that ouabain at picomolar and nanomolar concentrations, depending on species and tissue, activates a signaling cascade through its interaction with Na-K-ATPase.
In several models of salt-sensitive hypertension, endogenous ouabain-like cardiac glycoside levels are increased. Infusion of exogenous ouabain has been demonstrated to increase blood pressure through action on both α1- and α2-subunits of Na-K-ATPase, which could reflect alterations in cardiac output, vascular tone, central nervous system stimulation, or renal salt handling (11–14, 17, 37). Ferrari and colleagues (22) demonstrated increased Na-K-ATPase activity in kidneys from rats treated with ouabain (15 μg/kg body wt for 15 days). In a recent paper, Loreaux et al. (37) demonstrated that mice expressing a genetically modified α1-subunit that was sensitive to ouabain experienced a significant natriuresis in response to salt loading, while animals expressing a ouabain-resistant α1-subunit did not. Interestingly, the animals expressing the ouabain-sensitive α1-subunit showed an increase in mean arterial blood pressure with ouabain infusion for 30 min while the animals expressing the ouabain-resistant α1-subunit did not. These findings suggested to the authors that the hypertensive effect of ouabain was mediated by the effects of ouabain on the Na-K-ATPase expressed on the vasculature and did not support a role for ouabain-stimulated Na-K-ATPase activity in the kidney. In the present study, we demonstrate that infusion of ouabain at 1 μg/kg body wt for 4 days in rats caused a significant increase in serum ouabain levels within the nanomolar range, which resulted in a significant rise in blood pressure at 8 days. Our studies demonstrate that stimulation of Na-K-ATPase by low-dose ouabain occurs before an increase in blood pressure, suggesting a potential role of increased renal Na-K-ATPase activity in the genesis of ouabain-induced hypertension. However, further studies are required to confirm that the effects of cardiac glycoside on Na-K-ATPase activity in the kidney are primary and causal to the increase in blood pressure. In BLMs isolated from ouabain-treated rats, kidney cortex Na-K-ATPase expression, phosphorylation, enzyme activity, and association with NHE-1 increased. Our data demonstrated that there was an ∼47% increase in activity; however, there was about a threefold increase in expression and phosphorylation of the Na-K-ATPase α1-subunit. These findings are consistent with an increase in nonpumping Na-K-ATPase, as suggested by Liang et al. (33). Further experiments are required to confirm this conclusion. These findings are consistent with a hypertensive effect of nanomolar concentrations of ouabain through activation of renal Na-K-ATPase activity and enhanced proximal tubule Na+ reabsorption (19). Our study differs from the Loreaux study in that their studies examined only the acute effect of ouabain infusion on blood pressure and electrolyte homeostasis. The present study shows a more chronic effect of mildly elevated serum ouabain concentrations and suggests that the mechanism of hypertension stimulated by ouabain may be twofold, first through inhibition of the Na-K-ATPase in vascular tissue, resulting in enhanced contractility, and second through stimulation of the Na-K-ATPase in renal tissue, resulting in enhanced renal sodium reabsorption, as has been suggested by Ferrari et al. (22). The contrasting action of low cardiac glycoside concentration on the sodium pump in these two tissues is a reflection of the differences in sensitivity of Na-K-ATPase α-subunits in these two tissues. Specifically, in rodents, the α2-subunit which is expressed in the vascular smooth muscle and heart is relatively sensitive to the inhibitory effects of ouabain, while the α1-subunit which is expressed in the renal tubule cells is comparatively resistant to the inhibitory effects of ouabain. However, in humans, the different α-subunits of Na-K-ATPase are similarly sensitive to cardioglycosides. Whether the effects of picomolar or nanomolar increases in ouabain-like substances produce similar effects remains to be elucidated.
In a cell culture model of proximal tubule cells (OK cells), we previously demonstrated that treatment with 10 nM ouabain stimulates Na-K-ATPase-mediated 86Rb uptake and cell proliferation through activation of a signaling cascade involving Src kinase, ERK, and Akt (26, 28). In a recent report, Mandal et al. (39) demonstrated an increase in NHE-1 activity and phosphorylation in response to 1 μM ouabain in rat astrocytes. These findings, plus the numerous commonalities between the sodium pump and NHE-1, which include regulation of cell survival and proliferation through ERK- and Akt-dependent pathways, restricted localization to BLMs in polarized epithelial cells, and functional roles and scaffold properties to assemble signaling proteins like Src, ERK, and Akt (3, 15, 32, 57, 48), led us to hypothesize that the activity of NHE-1 and Na-K-ATPase may be linked. Our data suggest that Na-K-ATPase stimulation by ouabain in the picomolar (human kidney cells) and nanomolar (OK cells) concentration range requires NHE-1. In human kidney proximal tubule cells (HKC-11 and HKC-5) and in OK cells, the ability of picomolar or nanomolar concentrations of ouabain to stimulate Na-K-ATPase-mediated 86Rb uptake was prevented by the NHE-1 inhibitor EIPA. Inclusion of monensin in the medium used for our 86Rb uptake experiments creates a sodium entry pathway (10, 15) that effectively short-circuits sodium entry via NHE-1 and other sodium-dependent exchangers or cotransporters. Thus the observed rate of Na-K-ATPase-mediated 86Rb uptake was close to the Vmax for sodium and the increased rate in the presence of picomolar or nanomolar ouabain concentrations is not likely to be the result of an increase in NHE-1-mediated sodium entry. The results are consistent with an increase in Na-K-ATPase Vmax in cells exposed to picomolar or nanomolar concentrations of ouabain (data not shown).
Src kinase-dependent tyrosine phosphorylation of the Na-K-ATPase α1-subunit at Y-10 has been demonstrated to be crucial for insulin-dependent stimulation of Na-K-ATPase activity and trafficking to the plasma membrane (18). Our data demonstrating tyrosine phosphorylation of the Na-K-ATPase α1-subunit by picomolar concentrations of ouabain suggest that phosphorylation is a crucial step in Na-K-ATPase regulation. We identified that Y-10 and a unique tyrosine (Y-260) residue were phosphorylated by picomolar ouabain concentrations. Whether these two tyrosines play a role in the association of the Na-K-ATPase α1-subunit and NHE-1, trafficking to the plasma membrane and increased activity remain to be determined. Our data further demonstrate that inhibition of NHE-1 by EIPA prevents Na-K-ATPase α1-subunit phosphorylation by picomolar ouabain and insulin, suggesting that NHE-1 plays a critical role in regulation of Na-K-ATPase.
Our findings suggest that NHE-1 plays a critical role in the mechanism that stimulates Na-K-ATPase stimulation in the presence of nanomolar or picomolar concentrations of ouabain. We considered whether treatment with nanomolar or picomolar concentrations of ouabain stimulate NHE-1 activity. NHE-1 stimulation requires phosphorylation at serine 770 and 771 (38). In a recent report, Meima et al. (42) demonstrated that NHE-1 is a substrate for Akt and that Akt phosphorylates Ser648 of NHE-1. We have previously demonstrated that treatment of OK cells with 10 nM ouabain increases Na-K-ATPase-mediated 86Rb uptake in an Akt-dependent manner (26, 28). Taken together, it is possible that NHE-1 stimulation or NHE-1 phosphorylation through an Akt-mediated pathway is a required step in the chain of events leading to the increase in Na-K-ATPase activity. However, we cannot rule out the possibility that the scaffolding function of NHE-1 may be important for the Na-K-ATPase response (48).
In summary, the present studies demonstrate a novel interaction between NHE-1 and Na-K-ATPase that is triggered by ouabain in the nanomolar or picomolar range, depending on the species. The present studies also demonstrate that ouabain at nanomolar (OK cells and rat kidney BLMs) and at picomolar (HK cells) concentrations increase Na-K-ATPase activity and ion transport through a mechanism that involves tyrosine phosphorylation of the α1-subunit at positions 10 and 260 and association between NHE1 and the Na-K-ATPase α1-subunit. We speculate that an increase in endogenous ouabain-like factors may increase blood pressure through its cooperative effects on renal tubular NHE-1 and Na-K-ATPase activities.
GRANTS
This work was supported by a Veterans Affairs Merit Review (E. D. Lederer), a Grant-in-Aid, Great River Affiliate and a Scientist Development Grant from The American Heart Association (S. J. Khundmiri), National Institutes of Health Grant EY040414 (N. A. Delamere), and a Project Grant from the J. Graham Brown Cancer Center, University of Louisville (N. A. Delamere).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Lorraine Racusen (John Hopkins University School of Medicine, Baltimore, MD) for human kidney cells HKC-5 and HKC-11, Dr. Thomas Pressley (Texas Tech University, Lubbock, TX) for polyclonal antibodies against the rat Na-K-ATPase α1-subunit, and Dr. Diane Barber (University of California, San Francisco, CA) for HA-tagged NHE-1 constructs. We thank Nina Lesousky and Sarah Salyer for expert technical assistance.
REFERENCES
- 1.Bagrov A, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Prac Nephrol 4: 378–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrov AY, Roukoyatkina NI, Pinaev AG, Dmitrieva RI, Fedorova OV. Effects of two endogenous Na, K-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur J Pharmacol 274: 151–158, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol 287: C844–C850, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Blaustein M. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol Cell Physiol 264: C1367–C1387, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 290: R514–R523, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R. Reproducible isolation of distinct, overlapping segments of the phosphoproteome. Nat Methods 4: 231–237, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bullis BL, Li X, Singh DN, Berthiaume LG, Fliegel L. Properties of the Na+/H+ exchanger protein. Detergent-resistant aggregation and membrane microdistribution. Eur J Biochem 269: 4887–4895, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Caplan MJ. Ion pumps in epithelial cells: sorting, stabilization, and polarity. Am J Physiol Gastrointest Liver Physiol 272: G1304–G1313, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol 214: 810–819, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol 295: F1117–F1125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The α2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dostanic I, Schultz JJ, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the α1- and α2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 290: R524–R528, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA 102: 15845–15850, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28719–28726, 2003 [DOI] [PubMed] [Google Scholar]
- 16.El-Masri MA, Clark BJ, Qazzaz HM, Valdes R. Human adrenal cells in culture produce both ouabain-like and dihydroouabain-like factors. Clin Chem 48: 1720–1730, 2002 [PubMed] [Google Scholar]
- 17.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl loaded Dahl-S rats. J Hypertens 23:1515–1523, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Féraille E, Carranza ML, Gonin S, Béguin P, Pedemonte C, Rousselot M, Caverzasio J, Geering K, Martin PY, Favre H. Insulin-induced stimulation of Na+,K+-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol Biol Cell 10: 2847–2859, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrandi M, Brassi P, Molinari I, Torielli L, Tripodi G, Minotti E, Bianchi G, Ferrari P. Ouabain agonists as antihypertensive agents. Curr Pharm Des 11: 3301–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem 279: 33306–33314, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ferrandi M, Molinari I, Bianchi G, Ferrari P. Ouabain-dependent signaling in caveolae as a novel therapeutic target for hypertension. Cell Mol Biol (Noisy-le-grand) 52: 15–18, 2006 [PubMed] [Google Scholar]
- 22.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 290: R529–R535, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Wymore RS, Wang Y, Gaudette GR, Krukenkamp IB, Cohen IS, Mathias RT. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J Gen Physiol 119: 297–312, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Khundmiri SJ, Ameen M, Delamere NA, Lederer ED. PTH-mediated regulation of Na+-K+-ATPase requires Src kinase-dependent ERK phosphorylation. Am J Physiol Renal Physiol 295: F426–F437, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, Delamere NA. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol 293: C1171–C1180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khundmiri SJ, Asghar M, Banday AA, Khan F, Salim S, Levi M, Yusufi AN. Effect of ischemia reperfusion on sodium-dependent phosphate transport in renal brush border membranes. Biochim Biophys Acta 1716: 19–28, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 291: C1247–C1257, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie ZJ. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes: the role of Ras and mitogen-activated protein kinases. J Biol Chem 273: 15249–15256, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Kotova O, Al-Khalili A, Talia S, Hooke C, Feorova OV, Bagrov AY, Chibalin AV. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via Src- and ERK1/2-dependent mechanism. J Biol Chem 281: 20085–20094, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Li X, Donowitz M. Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep density gradient ultracentrifugation. Methods Mol Biol 440: 97–110, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem 281: 19709–19719, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Lingrel JB, Van Huysse J, O'Brien W, Jewell-Motz E, Askew R, Schultheis P. Structure-function studies of the Na, K-ATPase. Kidney Int 44: S32–S39, 1994 [PubMed] [Google Scholar]
- 35.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie ZJ, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284: C1550–C1560, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 293: C1489–C1497, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol 19: 1947–1954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malo ME, Li L, Fliegel L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids Ser770 and Ser771. J Biol Chem 282: 6292–6299, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Mandal A, Delamere NA, Shahidullah M. Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes. Am J Physiol Cell Physiol 295: C100–C110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol 290: R553–R559, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem 38: 547–554, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Meima ME, Webb BA, Witkowska HE, Barber DL. The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J Biol Chem 284: 26666–26675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi K, Kometiani P, Xie ZJ, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 276: 42050–42056, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Kamouchi M, Kitazono T, Kuroda J, Matsuo R, Hagiwara N, Ishikawa E, Ooboshi H, Ibayashi S, Iida M. Role of NHE1 in calcium signaling and cell proliferation in human CNS pericytes. Am J Physiol Heart Circ Physiol 294: H1700–H1707, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Orlov SN, Adarichev VA, Devlin AM, Maximova NV, Sun YL, Tremblay J, Dominiczak AF, Postnov YV, Hamet P. Increased Na+/H+ exchanger isoform 1 activity in spontaneously hypertensive rats: lack of mutations within the coding region of NHE1. Biochem Biophys Acta 1500: 169–180, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Orlowski J, Kandasamy RA. Delineation of transmembrane domains of the Na+/H+ exchanger that confer sensitivity to pharmacological antagonists. J Biol Chem 271: 19922–19927, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Powell DW, Bertram CC, Cummins TD, Barati MT, Zheng S, Epstein PN, Klein JB. Renal tubulointerstitial fibrosis in OVE26 type 1 diabetic mice. Nephron Exp Nephrol 111: e11–e19, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol 42: 527–552, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Qazzaz HM, El-Masri MA, Valdes R. Secretion of lactone-hydrogenated ouabain-like effector of sodium potassium-adenosine triphosphatase activity by adrenal cells. Endocrinology 141: 3200–3209, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sacktor B, Rosenbloom IL, Liang CT, Cheng L. Sodium gradient- and sodium plus potassium gradient-dependent l-glutamate uptake in renal basolateral membrane vesicles. J Membr Biol 60: 63–71, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Schelling JR, Abu-Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger 1. Am J Physiol Renal Physiol 295: F625–F632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac gycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 293: C509–C536, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Sen U, Rodriguez WE, Tyagi N, Kumar M, Kundu S, Tyagi SC. Ciglitazone, a PPARγ agonist, ameliorates diabetic nephropathy in part through homocysteine clearance. Am J Physiol Endocrinol Metab 295: E1205–E1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J 401:623–633, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stella P, Manunta P, Mallamac F, Melandri M, Spotti D, Tripepi G, Hamlyn JM, Malatino LS, Bianchi G, Zoccali C. Endogenous ouabain and cardiomyopathy in dialysis patients. J Int Med 263: 274–280, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem 274: 20206–20214, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 17: 317–326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The HNE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem 279: 26280–26286, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol 284: F829–F839, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem 269: 2434–2439, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 177: 1276–1284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie ZJ. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16: 4034–4045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yun CH, Tse CM, Nath SK, Levine SA, Donowitz M. Structure/function studies of mammalian Na-H exchangers—an update. J Physiol 482: 1S–6S, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol 276: F711–F719, 1999 [DOI] [PubMed] [Google Scholar]