Abstract
To probe further the contributions of the rostral pons to eupneic respiratory rhythm and pattern, we tested the hypothesis that ibotenic acid (IA) injections in the pontine respiratory group (PRG) would disrupt eupneic respiratory rhythm and pattern in a site- and state-specific manner. In 15 goats, cannulas were bilaterally implanted into the rostral pontine tegmental nuclei (RPTN; n = 3), the lateral (LPBN; n = 4) or medial parabrachial nuclei (MPBN; n = 4), or the Kölliker-Fuse nucleus (KFN; n = 4). After recovery from surgery, 1- and 10-μl injections (1 wk apart) of IA were made bilaterally through the implanted cannulas during the day. Over the first 5 h after the injections, there were site-specific ventilatory effects, with increased (P < 0.05) breathing frequency in RPTN-injected goats, increased (P < 0.05) pulmonary ventilation (V̇i) in LPBN-injected goats, no effect (P < 0.05) in MPBN-injected goats, and a biphasic V̇i response (P < 0.05) in KFN-injected goats. This biphasic response consisted of a hyperpnea for 30 min, followed by a prolonged hypopnea and hypoventilation with marked apneas, apneusis-like breathing patterns, and/or shifts in the temporal relationships between inspiratory flow and diaphragm activity. In the awake state, 10–15 h after the 1-μl injections, the number of apneas was greater (P < 0.05) than during other studies at night. However, there were no incidences of terminal apneas. Breathing rhythm and pattern were normal 22 h after the injections. Subsequent histological analysis revealed that for goats with cannulas implanted into the KFN, there were nearly 50% fewer neurons (P < 0.05) in all three PRG subnuclei than in control goats. We conclude that in awake goats, 1) IA injections into the PRG have site-specific effects on breathing, and 2) the KFN contributes to eupneic respiratory pattern generation.
Keywords: pons, respiratory rhythm generation, sleep
the role of the pons in respiratory rhythm and pattern generation is controversial. In the 1920s, Lumsden (19–22) built on the work of Marckwald (24, 25) and proposed that a pontomedullary network, including the pneumotaxic center, is required to control respiratory rhythm and pattern generation. Decades later, St. John and Bledsoe (38) proposed in decerebrate cats that the pons alone is capable of producing a respiratory rhythm in the myohyoid branch of the trigeminal nerve and that these mechanisms are within the pneumotaxic center and “may underlie the neurogenesis of eupnea.” Studies over the last few decades have localized the pneumotaxic center to the parabrachial region in the rostral dorsolateral pons, and combined with the Kölliker-Fuse nucleus (KFN), this region has been given the more contemporary name “pontine respiratory group” (PRG) (12).
Studies over the last 20 years have resulted in different concepts regarding the sites and mechanisms of respiratory rhythm and pattern generation. In 1991, Smith et al. (33) published data indicating that the pre-Bötzinger complex (pre-BötzC) was critical for respiratory rhythmogenesis in a neonatal rat brain stem in vitro preparation. These and subsequent data led to the idea of medullary pacemaker neurons and/or a network of rhythmogenic neurons generating the respiratory rhythm and pattern. However, St. John (37) refuted the interpretation of these in vitro data, instead concluding that “the rhythmic activities of these preparations are like the rapidly peaking activity of gasping and not the ramplike rise of eupnea.” Indeed, Abdala et al. (1) recently found, by examining precise sequential transections through the pons and medulla in rats, that the pons is necessary for the full, eupneic three-phase (inspiration, postinspiration, and expiration) respiratory pattern and that the pre-BötzC in isolation mediates a gasp-like breathing pattern. Further studies by Abdala et al. (2) and Rubin et al. (30) provide support for the concept that the rostral pons provides an excitatory input necessary for the three-phase pattern, including the active expiration in rats during hypercapnia. A current concept is that this excitatory effect is to a “core circuit of components that constitute the neural machinery for respiratory rhythm and shaping inspiratory and expiratory motor patterns,” and this core includes the pre-BötzC and surrounding medullary nuclei (34). Supporting the importance of this “core” are recent findings in awake rats (41) and goats (44) that abrupt destruction of the pre-BötzC causes cessation of phasic diaphragm activity and terminal apnea.
Most data supporting views on PRG contributions were obtained from reduced preparations, emphasizing the need for studies under in vivo physiological conditions (28, 29, 33), because they “may be the ultimate testing ground for insights derived from the in vitro research” (28). To probe further and address the ongoing controversies, we designed this study to determine the effects of neurotoxic lesions in the PRG on respiratory rhythm and pattern in an in vivo awake/asleep animal. We hypothesized 1) that neurotoxic lesion of the PRG would disrupt eupneic respiratory rhythm and pattern in a site- and state-specific manner and 2) that goats would recover from acute effects of lesioning the PRG. The rationale for the hypotheses was based on evidence that 1) multiple brain stem sites are capable of generating rhythmic respiratory activity (15, 18, 38), 2) there is heterogeneity in function and topography among PRG subnuclei (5, 7, 13) and state-dependent effects of rostral pontine perturbations (3, 14, 17), and 3) there is time-dependent plasticity after lesioning the medial parabrachial nucleus in cats (31) and pre-BötzC of goats (16).
METHODS
For our studies, we used adult goats, because their large size permitted chronic implantation of stainless steel cannulas into the brain stem, enabling microinjection into target sites during the awake state. Furthermore, our laboratory has considerable background data concerning respiratory rhythm and pattern generation in goats (16, 26, 44). Physiological data acquired were from 15 female adult goats, weighing 46.4 ± 1.9 kg. Six additional goats were used for histological purposes only (44.1 ± 2.9 kg). Goats were housed and studied in an environmental chamber with a fixed ambient temperature and photoperiod. All goats were allowed free access to hay and water, except during periods of study. All aspects of the study were reviewed and approved by the Medical College of Wisconsin Animal Care Committee before the studies were initiated.
Experimental design.
The goats we studied were part of a larger study (Fig. 1) that also included determining the effect of attenuating cholinergic modulation through microdialysis of atropine (data reported elsewhere). Each goat underwent two surgeries: 1) an initial surgery in which instrumentation necessary for monitoring of physiological signals was implanted and 2) a craniotomy in which cannulas were bilaterally implanted into the rostral pons. After both surgeries, the animals were allowed to recover for at least 2 wk. The first set of studies (Fig. 1) aimed to establish control values for all ventilatory parameters. The second set of studies, 2 wk after cannula implantation, aimed to establish whether the physical lesion of the implantation affected physiological variables (Fig. 1). The third set was to detect whether atropine had chronically affected the physiological variables (Fig. 1). Studies thereafter aimed to determine the effect of injection of ibotenic acid (IA) into the PRG (Fig. 1). IA is an excitotoxin (irreversible glutamate receptor agonist) and was accordingly administered last in the protocol. As the aforementioned studies progressed, microinjection of N-methyl-d-aspartate (NMDA) was added to the experimental design in some goats before and after the IA studies in an attempt to aid in the interpretation of the IA injection response. One week after the last IA injection, all studies were complete, the animal was euthanized, and histological analyses were performed on the brain stem (Fig. 1). The entire experimental protocol lasted ∼2 mo in duration.
Fig. 1.
Experimental design of the present study. Each goat underwent 2 surgeries, and on full recovery and training of goats, studies were conducted to establish the normal eupneic ventilatory parameters. These studies were repeated as shown to ascertain whether any perturbation affected the ventilatory parameters. MD, microdialysis; IA inj, ibotenic acid injection.
Surgical procedures.
Goats were anesthetized with a mixture of ketamine and xylazine (15 and 1.25 mg/kg, respectively), intubated, and mechanically ventilated with 2.5% isoflurane in 100% oxygen. The two surgeries were performed under sterile conditions. In the first surgery, a 5-cm segment of each carotid artery was isolated from the vagi and elevated subcutaneously to facilitate future placement of chronic catheters for sampling of arterial blood and monitoring of heart rate and blood pressure. In this same surgery, bipolar electromyographic (EMG) electrodes were implanted into the genioglossus (GG), thyroid arytenoid (TYA), posterior cricoarytenoid, and thyropharyngeus (TP) muscles through an anterior midline incision in the neck and were exteriorized through the skin. EMGs were also implanted in duplicate into both the diaphragm (DIA) and abdominal (ABD) muscles. The activity of these muscles was recorded to determine the normal, eupneic activation pattern of respiratory muscles. Finally, for monitoring and scoring of sleep state, we also implanted electroencephalographic (EEG) and electrooculographic (EOG) electrodes in the midline cranium and superior orbital ridge, respectively. Ceftiofur sodium (Naxcel; 4 mg/kg im) was administered once daily (QD) for 1 wk postoperatively to minimize infection. Buprenorphine hydrochloride (Buprenex; 0.005 mg/kg im) was administered twice daily (BID) for 48 h to mitigate pain.
After ≥3 wk, the second surgery was performed to chronically implant stainless steel cannulas (70-mm length, 1.27-mm outer diameter, 0.84-mm inner diameter) bilaterally into the rostral pons. On full recovery, these cannulas were used for focal administration of IA into target brain areas. The bilateral cannula implantations required a single occipital craniotomy created through a posterior midline incision, after which the dura mater was excised to expose the posterior cerebellum and medulla and for visualization of obex. To standardize stereotaxic coordinates, the orientations of the dorsal medullary surface, obex, and midline were used to determine the dorsoventral, rostrocaudal, and mediolateral planes, respectively. In our first few goats, cannulas were implanted perpendicular to the brain stem, rostral to the confluence of the sinuses, lateral to the superior sagittal sinus, and through the rostral cerebellum. The tip of the cannula in these goats was rostral to the PRG in pontine tegmental nuclei. Nevertheless, these goats were useful in providing physiological control data for comparison to the PRG-implanted goats. To target the PRG in general and to target specific subnuclei within the PRG, an “angled” approach was adopted whereby cannulas were inserted caudal to the confluence of the sinuses, through the midcerebellum, at angles of 10.5–24° from normal (relative to the dorsal medullary surface), depending on the desired rostrocaudal coordinates. Given the subnucleus of choice, cannula implantation coordinates were adjusted within a range of coordinates: 0–2 mm ventral, 4–5 mm lateral, and 20–24 mm rostral to respective reference points. Using different coordinates in this way, we implanted cannulas at different sites within the PRG in 12 goats. After placement, cannulas were anchored to the surrounding bone using screws and dental acrylic, and a stainless steel stylet was inserted to maintain patency.
Experienced laboratory personnel continuously monitored the goats for at least 24 h after brain surgery until stable conditions were achieved. Buprenorphine was administered for pain as previously explained. Chloroamphenicol (20 mg/kg iv) was administered three times daily (TID) for 3 days to minimize infection, as was dexamethasone (0.4 mg/kg decreasing to 0.05 mg/kg iv, TID) for 7 days to minimize swelling. Thereafter, until death, centiofur sodium and gentamycin (Gentamax 100; 6 mg/kg im, QD) were administered.
Physiological measurements.
For all studies, a custom-fitted mask was secured to the goat's muzzle, with a two-way breathing valve attached to the mask. A pneumotach was attached in series with the inspiratory side to measure flow via a Windaq computer data acquisition system. The expiratory side was connected to a spirometer, allowing determination of expired volume and concentrations of CO2 and O2 to calculate metabolic rate. A chronically placed catheter in the elevated carotid artery was used to continuously record arterial blood pressure (BP) and heart rate and for collection of blood samples to obtain arterial pH (pHa), Pco2 (PaCO2), and Po2 (PaO2) values (model 248; Bayer Diagnostics). Rectal temperature was measured at regular intervals. Respiratory muscle activity was recorded via Windaq using the chronically implanted electrodes.
Control studies.
Control values for ventilatory parameters during the day and at night were assessed before and after cannula implantation surgery, after atropine microdialysis studies, and after IA injection studies. Daytime eupneic respiratory rhythm and pattern were recorded for at least 30 min while goats were breathing room air, and blood samples were collected for analysis of blood gases and pH. Nighttime eupneic respiratory rhythm and pattern were recorded for at least 5 h while goats were breathing room air, with hourly arterial blood samples taken.
IA injections (day and night protocols).
For daytime studies, animals were loosely tied in a stanchion so that they could not assume sternal recumbancy. IA injection protocols consisted of a 30-min control period followed by two unilateral (ipsilateral, then contralateral) microinjections of IA through the chronically implanted cannulas. Small injection tubes were preloaded with IA, and then these tubes were inserted into the cannula to its distal tip, which enabled the injection to be made directly into the tissue without penetrating it. These injections were separated by 1–2 h, to assess acute unilateral effects. Goats were monitored and recorded for at least 5 h after the first injection. Thus total study duration was ≥5.5 h. To progressively lesion target areas, IA (50 mM) injections of 1 and then 10 μl were made, separated by 1 wk (Fig. 1).
On the same night given an injection, goats were monitored and recorded for 5 h (9:00 PM-2:00 AM) to assess any effects of IA injection on non-rapid eye movement (NREM) and/or rapid eye movement (REM) sleep. Before studies conducted at night, goats were not allowed to assume sternal recumbancy (4:00 PM-9:00 PM) in an effort to consolidate sleep. Goats were still allowed to eat and drink ad libitum during this time. On commencement of the nighttime study, the goats were allowed to assume sternal recumbancy within the stanchion. The goats were also studied in this same manner 3–6 days after each injection (1–2 days before the next injection).
NMDA injections.
To aid in the interpretation of IA injection responses, in the last three goats we also injected 1 μl of NMDA (100 mM), an NMDA receptor agonist, the week before the first IA injection and the week after the last IA injection. Ipsi- and contralateral injections were made on 2 separate days. Injections were preceded by a 30-min control period and were succeeded by at least 1 h of monitoring.
Data and statistical analyses.
Pulmonary ventilation (V̇i; l/min), breathing frequency (f; breaths/min), tidal volume (Vt; l/min), expiratory (Te; s) and inspiratory (Ti; s) times, and respiratory muscle activity (mV) were analyzed on a breath-by-breath basis. In addition, for night studies, the EEG and EOG activities were analyzed to established awake and sleep states. At 5-min intervals during day studies, O2 uptake (V̇o2; l/min) and CO2 production (V̇co2; l/min) were also determined. The BP and airflow signals were calibrated against known values, whereas EMG signals were zeroed at the baseline. Calibrated BP and airflow values and EMG signals were rectified and time averaged. During the experimental control periods, these rectified and time-averaged signals were used to recalibrate the EMG signals with the assignment of arbitrary peak (1) and baseline values (0). These recalibrated EMG signals were again rectified and time averaged. From the calibrated values for each breath, Vt for each breath was computed. The BP, airflow, and EMG signals were converted to a .txt file and input into a custom-designed program that output all parameters on a breath-by-breath basis. In addition, for each muscle we computed the onset and offset times relative to the onset and offset of inspiratory airflow, and these values were expressed as a percent of inspiratory airflow time.
The EEG and EOG activities were used to visually distinguish between states of consciousness. Wakefulness was characterized by a high-frequency, low-voltage EEG, with variable EOG activity. NREM sleep was characterized by a low-frequency (<2 Hz), high-voltage (2–3 times greater than that found in wakefulness) EEG, with absent EOG activity. REM sleep was characterized by a high-frequency, low-voltage EEG, similar to wakefulness but concurrent with frequent and distinct bursting activity (>30 μV) in the EOG. For night studies, each breath was categorized as occurring during awake, NREM sleep, or REM sleep states. The breaths were then further categorized as “quiet,” regular breaths or those that were “irregular/disrupted,” characterized by respiratory muscle activity caused by swallowing, coughing, mastication, and eructation, as well as any abnormal patterns that could not be identified as a specific behavior. The rationale for this second categorization was as stated by Bolser et al. (4): “the presence of elements that are normally silent in close proximity to spontaneously active respiratory neurons raises the possibility that some interventions that are intended to study the neurogenesis of breathing might instead be influencing components of the holarchical system that are only conditionally active;” thus it was essential to make a delineation between the two types of breaths (quiet vs. disrupted).
Breath-by-breath values were averaged into 30-min bins and expressed in either their raw form or as a percentage of the control bin. The breath-to-breath coefficient of variation (CV) (SD/mean × 100) was also calculated for each 30-min bin. For night studies, respective states were reported as a percentage of the total time per 30-min bin. The number of apneas (apnea Te ≥ 2.5 × average Te) and augmented breaths (augmented breath Vt ≥ 2.5 × average Vt) were tabulated for each study. Post-sigh apneas were not included. To test whether eupneic breathing significantly changed (P < 0.05) over the course of the several weeks of the experimental protocol and whether there were differences between goats with different sites of cannula implantation, we used two-way analysis of variance (ANOVA) and Tukey's post hoc analysis on all control values for all ventilatory variables, including blood gases. The same statistical tests were used to test 1) whether ventilatory variables were significantly altered (P < 0.05) in the 5 h subsequent to IA injections compared with control levels and whether there were differences between goats with different sites of cannula implantation, 2) whether the CV for all variables were significantly altered (P < 0.05) in the 5 h subsequent to IA injections compared with control levels and whether there were differences between 5-h controls and the 1- and 10-μl IA injections, 3) whether the amount of NREM and REM sleep, the duration of sleep epochs, and the number of apneas and augmented breaths were significantly altered (P < 0.05) over the course of the experimental protocol and whether there were differences between goats with different sites of cannula implantation, and 4) whether the number of neurons and remaining area per nucleus were significantly altered (P < 0.05) over the rostrocaudal distance in the rostral pons and whether there were differences between goats with different sites of cannula implantation.
Histological analyses.
One week after completion of the experimental protocol, all goats (as well as the control goats) were euthanized (Beuthansia, intravenously), and the brain was perfused with physiological buffer solution (PBS) and fixed with 4% paraformaldehyde in PBS. The brain stem was excised and placed in 4% paraformaldehyde in PBS for 24 h, and then sequentially in 20 and 30% sucrose solutions. The brain stem was frozen and serially sectioned (25 μm) from obex to the superior colliculi in a transverse (n = 20) or sagittal plane (n = 1), and the sections were adhered to gelatin-chrome-alum-coated slides. Sections were acquired such that every fourth section was contained within a respective “series.” Thus there were four series in total; within a series, each section was 100 μm from the next section in sequence, allowing for high-resolution neuronal and anatomical profiling. One series was stained for Nissl substance to profile the total number of neurons. A second series was stained for muscarinic type-2 immunoreactivity by complexing anti- muscarinic type-2 receptor primary antibody (Millipore; 1:200 dilution) with biotinylated anti-mouse secondary antibody (Vector; 1:100 dilution). After the antibody-antigen complex was incubated, it was localized by avidin (Vector ABC Elite) and developed with diaminobenzene. A third series was stained with hematoxylin and eosin to identify living and dead neurons. Dead neurons were stained pink and were round in shape, whereas living neurons were stained dark purple and were amorphous in shape (8). A fourth series was stained for reason unrelated to purposes of this report. Target nuclei were identified using all these stains, and neuron counts and area quantifications were made (every 200 μm) on remaining intact tissue using Metamorph software and standardized procedures.
RESULTS
Morphology of PRG in goats.
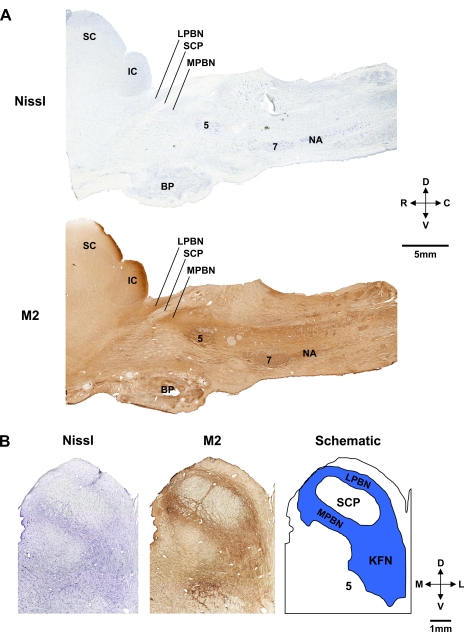
Sagittal (Fig. 2A) and transverse images (Fig. 2B) from control goats show the orientation of the PRG within the brain stem. The dark brown regions in Fig. 2 reflect positive staining for the muscarinic type-2 receptor, which is characteristic of the PRG sites flanking the superior cerebellar peduncle (23). In goats, the morphology of the KFN is distinctly different from that of the LPBN and MPBN. The KFN is quite confined, spanning only 2–3 mm rostrocaudally, and is more ventral and lateral than the lateral (LPBN) or medial parabrachial nuclei (MPBN) (Figs. 3 and 4). The LPBN and MPBN extend over 6–8 mm rostrocaudally; thus the total number of neurons and total area of these nuclei are greater than in the KFN. As a result of these differences, we were only able to lesion a portion of the LPBN and MPBN, whereas a larger percentage of the KFN was lesioned.
Fig. 2.
Histochemical and immunohistochemical staining for Nissl substance and muscarinic type-2 (M2) receptors provided anatomic characterization of the pontine respiratory group (PRG) in goats. A: sagittal sections 4.9 mm lateral to the midline showing the location in the rostral dorsal pons of the lateral (LPBN) and medial parabrachial nuclei (MPBN) and the Kölliker-Fuse nucleus (KFN) relative to the superior cerebellar peduncle (SCP). B: transverse Nissl and M2 sections and a schematic to further illustrate the location of the LPBN, MPBN, and KFN. Locations of the superior (SC) and inferior colliculus (IC), trigeminal motor nucleus (5), and basal pons (BP), facial nucleus (7), and nucleus ambiguus (NA) are indicated. D, dorsal; V, ventral; R, rostral; C, caudal; M, medial; L, lateral.
Fig. 3.
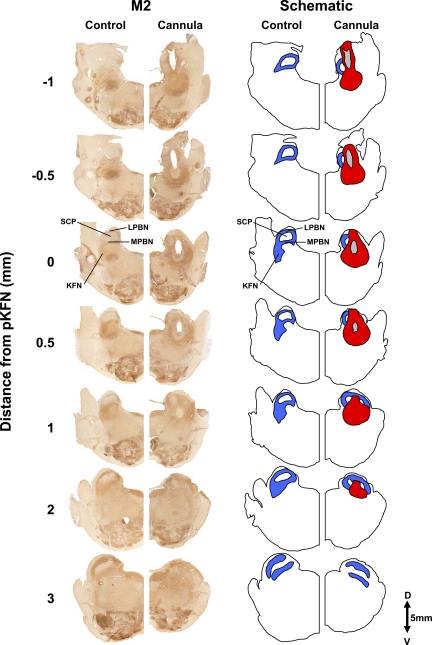
Immunohistochemical staining for M2 receptors of hemisections from 1 control goat and 1 goat that had a cannula implanted into the KFN. These hemisections illustrate the rostral-caudal changes in the LPBN, MPBN, and KFN beginning 1 mm caudal to the peak in number of KFN neurons (pKFN) and extending 3 mm rostral to the peak. The blue area in the schematic illustrates the orientation of the LPBN, MPBN, and KFN relative to the SCP. The tract of the cannula (gray area in schematic) extends over 2 mm, and the area devoid of neurons (red area in schematic) extends over 3 mm in the rostral-caudal direction. Note the clear presence of the KFN at its peak number of neurons (0 mm) and its absence at a more rostral distance (3 mm).
Fig. 4.
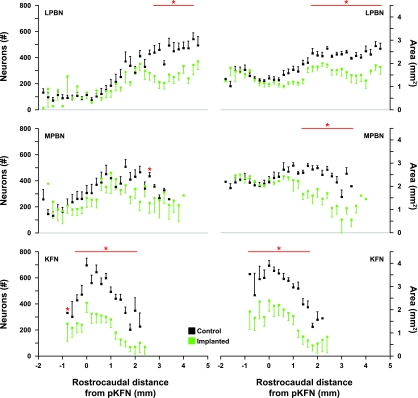
Number of neurons and intact area of control and lesioned (implanted) goats in the LPBN, MPBN, and KFN caudal and rostral to the pKFN. The red horizontal bars indicates values where neuron counts and the area of intact nuclei were reduced significantly (*P < 0.05) in the cannula-implanted goats. In the lesioned groups, the distal edge of the cannula was just dorsal to the LPBN, MPBN, or KFN. Note the 6-mm rostral-caudal extent of the LPBN and MPBN, whereas the KFN only extends over 3 mm.
Lesion characterization.
Based on identification of the most distal tract created by the implanted cannulas, the goats were divided into four anatomical groups, with four goats in the LPBN, MPBN, and KFN groups and three goats in the rostral pontine tegmental nuclei (RPTN) group. Since the injection tubes were inserted only to the tip of the cannula, we reasoned that the major effect of IA injections was on neurons immediately distal to the cannula tip. We recognize that there may have been diffusion in all directions. We point out that in the MPBN group, the physical lesion caused by the cannula as it penetrated through the LPBN would destroy dendritic connections within the LPBN, and in the KFN group, physical destruction occurred in both the LPBN and MPBN. Thus the deficit of neurons as shown in Table 1 would be a result of both the physical and chemical lesion. Accordingly, the acute and chronic effects of the IA injections could be cumulative and not due solely to neurons distal to the cannula tract. Nevertheless, the effects of injections are presented as being associated with specific subnuclei even though we recognize that this might be an oversimplification.
Table 1.
Number of neurons and intact area of RPTN, LPBN, MPBN, and KFN at cannula implantation sites in goats
| Neurons |
Area |
|||||
|---|---|---|---|---|---|---|
| Cannula Implantation Site | LPBN | MPBN | KFN | LPBN | MPBN | KFN |
| RPTN | 100 ± 22 | 104 ± 17 | 102 ± 16 | 99 ± 8 | 102 ± 4 | 106 ± 6 |
| LPBN | 73 ± 12 | 88 ± 15 | 92 ± 9 | 74* ± 8 | 91 ± 2 | 88 ± 5 |
| MPBN | 61 ± 10 | 62 ± 11 | 80 ± 14 | 63* ± 11 | 75* ± 9 | 96 ± 6 |
| KFN | 52* ± 12 | 51* ± 16 | 51* ± 20 | 62* ± 10 | 63* ± 17 | 59* ± 21 |
Values are the number of neurons and intact area (expressed as a percentage in that in control goats) in the rostral pontine tegmental nuclei (RPTN), lateral (LPBN) and medial parabrachial nuclei (MPBN), and Kölliker-Fuse nucleus (KFN) in goats with implanted cannulas. Significant differences (
P < 0.05) between lesioned and control goats are indicated, but because of variations among both lesioned and control goats, several of the deficits in the LPBN, MPBN, and KFN groups were not significantly different from controls.
In the LPBN and MPBN groups, the number of neurons and intact area were significantly (P < 0.05) decreased in the rostral portions of the subnuclei (Fig. 4). In the KFN group, the number of neurons and intact area were significantly (P < 0.05) reduced throughout the PRG (Fig. 4). In the MPBN and KFN groups, there were also deficits in the LPBN and in the LPBN and MPBN, respectively (Table 1). For all three subnuclei, the total number of neurons and intact area in the lesioned goats were less than in the control goats, but the decreases did not reach statistical significance (P > 0.05) at some sites due to variation among control and lesioned goats (Table 1).
Effects of cannula implantation.
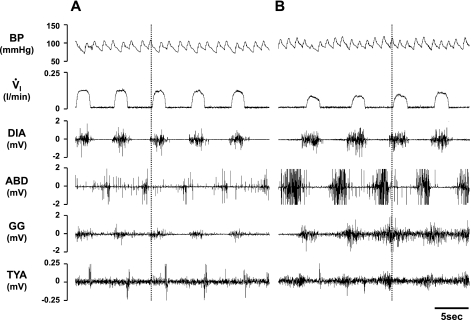
On regaining consciousness from the cannula implantation surgery, several goats exhibited a “pursed-lip” breathing pattern. The magnitude of this phenomenon between goats ranged from mild to robust, with the most robust goats having cannulas implanted in either the MPBN or KFN. In most goats, this pattern disappeared a few hours to a few days after surgery, but in one goat it persisted until injection of IA several weeks after surgery. During studies on this goat before the IA injection, most recordings were typical of goats in that DIA and GG muscle activity corresponded to inspiratory flow, there was a brief burst of TYA muscle activity in the immediate postinspiratory period, and there was ABD muscle activity in the late expiratory period (Fig. 5A). However, during periods of pursed-lip breathing easily identified visually, there was increased phasic activity of the TYA muscle simultaneous with increased activity of the ABD muscle (Fig. 5B). In addition, there was also increased activity of both the DIA and GG muscles concomitant with a reduction in inspiratory flow compared with the normal breathing period (Fig. 5).
Fig. 5.
Periods of pursed-lipped breathing were associated with altered airway and pump muscle activity (B) compared with normal breathing (A). Note that in purse-lipped compared with normal breathing, there was high phasic abdominal (ABD) and thyroid arytenoid (TYA) muscle activity in late expiration, as well as high diaphragm (DIA) and genioglossus (GG) muscle activity, which appeared insufficient to maintain normal inspiratory flow (V̇i).
In the days following cannula implantation surgery, goats hyperventilated (data not shown), which has been previously observed with implantation into the medulla (44). However, by 2 wk after surgery, blood gases were back to presurgery levels and were within normal limits for goats (Fig. 6). In addition, respiratory muscle activity was unchanged pre- vs. postsurgery, except during the pursed-lip breathing pattern. However, V̇i during eupnea was decreased (P < 0.05) compared with preimplantation by an average of 1 l/min (Table 2). This decrease in V̇i was due to a 0.06 l/breath decrease (P < 0.05) in Vt (Table 2) and was secondary to a decrease (P < 0.05) in metabolic rate (data not shown). Eupneic breathing pattern was normal both during the day and at night before injections of IA.
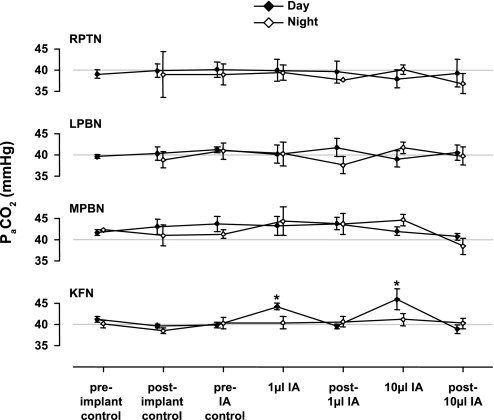
Fig. 6.
Both 1- and 10-μl bilateral injections of IA into the KFN resulted in arterial hypercapnia (PaCO2; hypoventilation) (*P < 0.05). Values shown are peak hypercapnia during the 5 h after the injections. IA injections into the RPTN, LPBN, and MPBN did not significantly (P > 0.05) affect PaCO2. In addition, PaCO2 was unchanged from the preimplant control at other times over the course of the experimental design.
Table 2.
| V̇i, l/min |
f, Breaths/min |
Vt, l/breath |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RPTN | LPBN | MPBN | KFN | RPTN | LPBN | MPBN | KFN | RPTN | LPBN | MPBN | KFN | |
| Preimplant | 7.60 ± 0.98 | 8.59 ± 1.25 | 6.27 ± 0.43 | 7.79 ± 0.78 | 20.68 ± 2.83 | 16.56 ± 0.60 | 16.47 ± 0.90 | 17.84 ± 1.16 | 0.40 ± 0.02 | 0.53 ± 0.07 | 0.40 ± 0.04 | 0.46 ± 0.04 |
| Postimplant | 6.47* ± 0.67 | 6.76* ± 1.18 | 6.39 ± 0.60 | 6.77* ± 0.30 | 19.46 ± 2.06 | 19.77 ± 1.60 | 18.63 ± 1.46 | 17.93 ± 1.06 | 0.38* ± 0.03 | 0.38* ± 0.07 | 0.35* ± 0.02 | 0.40* ± 0.02 |
| Post-Atr MD | 6.48* ± 0.23 | 6.67* ± 0.30 | 6.31 ± 0.58 | 6.85* ± 0.36 | 21.34 ± 1.98 | 17.85 ± 1.72 | 19.11 ± 0.98 | 16.16 ± 1.23 | 0.36* ± 0.02 | 0.42* ± 0.04 | 0.34* ± 0.02 | 0.47 ± 0.04 |
| Post-IA inj | 6.89* ± 0.51 | 6.26* ± 0.71 | 6.14 ± 0.59 | 6.44* ± 0.46 | 22.77 ± 2.41 | 15.90 ± 2.31 | 16.66 ± 1.05 | 15.48 ± 1.10 | 0.38 ± 0.02 | 0.43 ± 0.04 | 0.38 ± 0.02 | 0.45 ± 0.03 |
Values are pulmonary ventilation (V̇i), breathing frequency (f), and tidal volume (Vt) before (preimplant) and after (postimplant) cannula implantation surgery, after atropine microdialysis (Atr MD) studies, and after ibotenic acid injection studies (IA inj) (see Fig. 1 for reference).
P < 0.05, significantly different from control.
Acute daytime effects of IA injection (0–5 h).
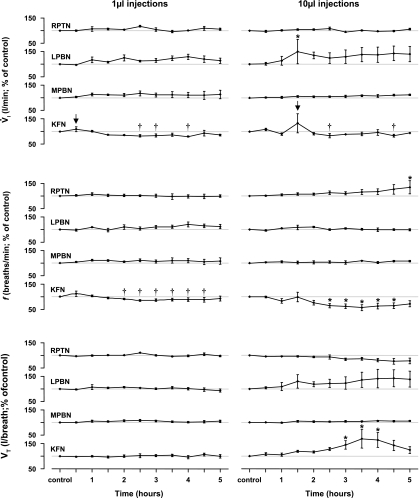
The acute effects of IA injections on breathing varied considerably depending on the anatomical site of the cannula placement. The 10-μl injection of IA into the RPTN increased (P < 0.05) f up to 34% above control levels (Fig. 7). The 10-μl injection of IA into the LPBN increased (P < 0.05) V̇i up to 50% above control (Fig. 7). There were no significant (P > 0.05) acute effects of injecting IA into the MPBN on V̇i, f, or Vt (Fig. 7). However, injections of IA into the KFN had a biphasic effect on breathing (Figs. 7–11). The initial response was an approximate 30-min hyperpnea (Figs. 8B and 9B), which was followed by a prolonged (>3 h) hypopnea (Figs. 8C, 9C, 10, and 11) and hypoventilation (Fig. 6). The hypopnea was due to changes in f; for example, there were 26% (P < 0.05) and 37% decreases (P < 0.05) in f following 1- and 10-μl injections, respectively, whereas Vt changed in the opposite direction (Fig. 7). Correspondingly, Ti and Te were elevated (P < 0.05) during the secondary hypopneic response to IA injection in KFN-injected animals (data not shown). After bilateral injection of IA in the KFN, apneas or paired pattern breathing (Fig. 10) and apneusis-like breathing patterns (Figs. 11–13) were observed during the secondary hypopneic phase. During apneusis-like patterns, there was prolonged activity of the DIA muscle and minimal activity of the ABD muscle for up to 30 s during which inspiratory airflow was negligible. The sustained postinspiratory muscle activity is shown in Figs. 12 and 13 by periods after the injections when the offset time of diaphragm activity after cessation of inspiratory flow was prolonged. In addition, there were also periods after the injections when the onset time of diaphragm activity differed from control (Figs. 12 and 13). Similar observations of abdominal and airway muscle activity also indicated that after the 10-μl injection of IA, there were changes in the period of activity during a respiratory cycle (Figs. 8–11). There were no episodes of prolonged absence of diaphragm activity or terminal apneas as observed in previous studies when IA was injected into the pre-Bötzinger complex (pre-BötzC) (44).
Fig. 7.
Bilateral injections of IA into the rostral pons of awake goats had heterogeneous, site-specific effects; bilateral injection of 1 or 10 μl of IA into the KFN had a biphasic response. First, pulmonary ventilation (V̇i) increased transiently (arrows), and second, V̇i was attenuated over several hours compared with this initial increase (†P < 0.05 compared with value at arrow). V̇i decreased due to a 37% decrease (*P < 0.05) in breathing frequency (f), despite an increase in tidal volume (Vt) (*P < 0.05). Bilateral injection of 10 μl of IA into the LPBN induced a persistent increase (*P < 0.05) in V̇i due to an increase in Vt, whereas injections into RPTN induced a delayed increase (*P < 0.05) in f. Bilateral injection of IA into the MPBN had no significant effect on breathing.
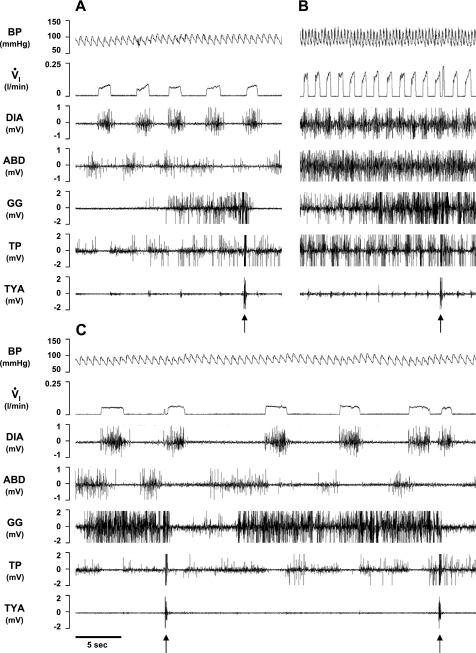
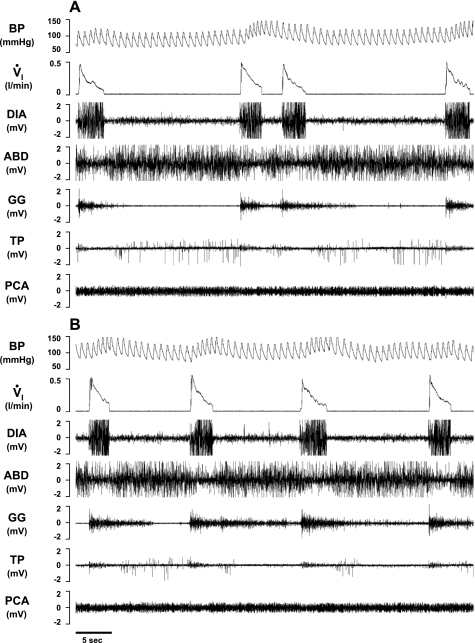
Fig. 8.
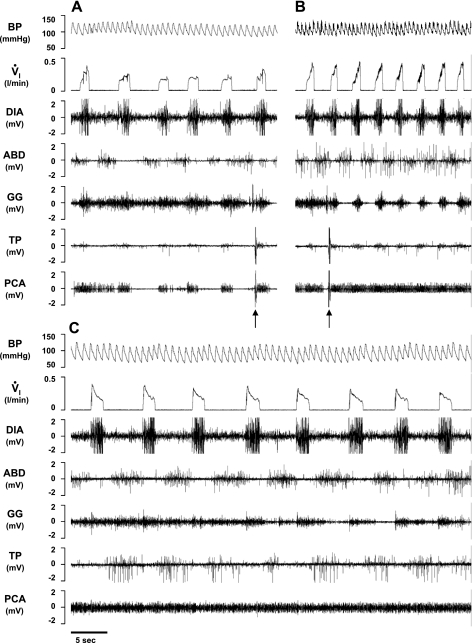
In a KFN-lesioned goat, bilateral injection of IA (10 μl) during the day caused a biphasic response in V̇i, blood pressure (BP), and heart rate compared with control conditions (A), with a transient (<20 min) hyperpnea (B) immediately after the injection, followed by a prolonged (>3 h) hypopnea (C). Arrows denote typical swallow patterns as identified by the abrupt, maximal contraction of the airway thyropharyngeus (TP) and TYA muscles. Electromyograms (EMG) are shown for the DIA, ABD, GG, TP, and TYA muscles. The post mortem histology of this goat's PRG is illustrated in Fig. 3.
Fig. 9.
In a second KFN-lesioned goat, bilateral injection of IA (10 μl) during the day also caused a biphasic response in V̇i, BP, and heart rate compared with control conditions (A), with a transient (<20 min) hyperpnea (B) immediately after the injection, followed by a prolonged (>4 h) hypopnea (C). Arrows denote typical swallow patterns. Note in C the decrementing airflow pattern, decrementing GG muscle activity pattern, and increased ABD and TP activity that begin immediately after inspiration. PCA, posterior cricoarytenoid muscle activity.
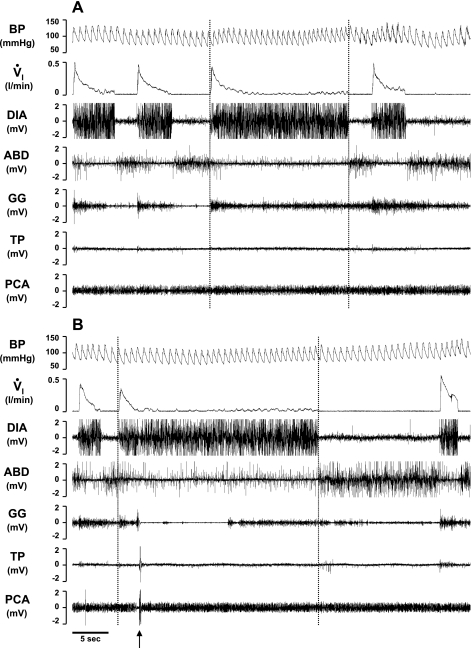
Fig. 10.
In the second KFN-lesioned goat, prolonged expiratory time (central apnea) and/or a paired breathing pattern (A and B) occurred during the secondary hypopneic response to bilateral injection (10 μl) of IA. Note the decrementing airflow pattern, decrementing GG muscle activity, high level of ABD activity, and the change in BP during each inspiration except the second of a doublet inspiration.
Fig. 11.
In the second KFN-lesioned goat, apneusis-like breathing patterns (A and B) occurred during the secondary hypopneic response to bilateral injection (10 μl) of IA. During apneusis-like patterns (record between vertical lines), note the sustained DIA activity, which was terminated during phasic contraction of the ABD muscle. An upward arrow denotes a typical swallow pattern.
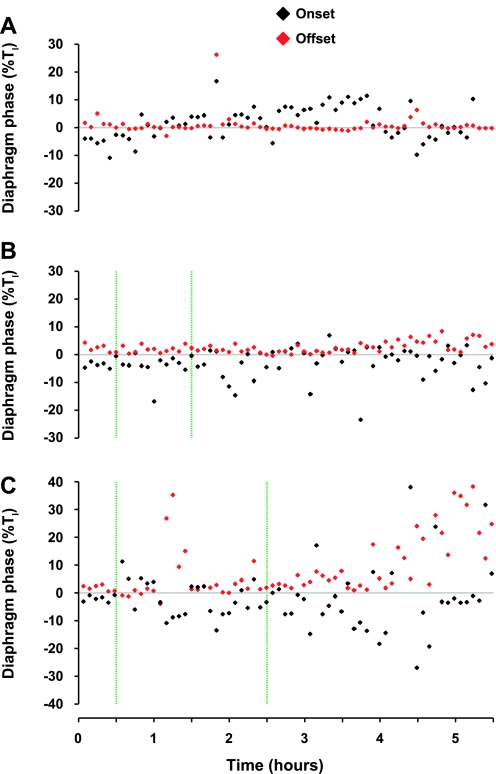
Fig. 12.
In the second KFN-lesioned goat, bilateral injection (ipsi- and contralateral injections made separately) of 10 μl of IA (vertical green lines) increased postinspiratory activity of the diaphragm, particularly 4–5 h after the 10-μl injections (C). Data in A and B were obtained during the control study and after injection of 1 μl of IA, respectively. Black symbols represent the delay in onset of airflow after the initiation of diaphragm activity, and the red symbols represent the delay in offset of diaphragm activity after cessation of inspiratory airflow. The delays in onset and offset are expressed as a percentage of inspiratory airflow time (Ti).
Fig. 13.
Similar to data shown in Fig. 12, in the other 3 KFN-lesioned goats (A–C), bilateral injections (ipsi- and contralateral injections made separately) of IA (vertical green lines) increased postinspiratory activity of the diaphragm during different periods after the injections. See Fig. 12 legend for further explanations.
After 1- and 10-μl injections into the KFN, PaCO2 was elevated (P < 0.05) during the secondary hypopnea by 4.4 and 5.7 Torr, respectively (Fig. 6). PaO2 and pHa were within normal levels and were unaffected by IA injection in these goats (data not shown). Additionally in these animals, compared with control levels, IA injection increased (P < 0.05) the CV of f by 39%. The CVs of the other ventilatory variables were not significantly altered after the IA injections.
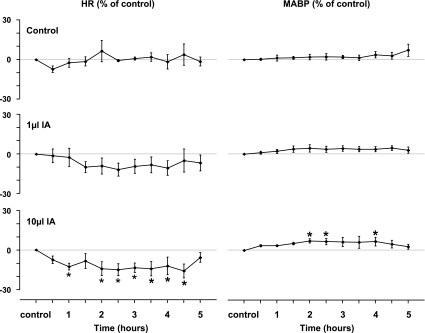
Heart rate after the 10-μl IA injections into the KFN closely followed the changes in V̇i. As shown in Figs. 8B and 9B, the initial effect was increased heart rate, which persisted for less than 30 min, but thereafter for most of the 5-h study, the heart rate was significantly (P < 0.05) below control level (Fig. 14). Also, during periods of gasplike breathing, most gasps were associated with circulatory “respiratory wave” patterns (Figs. 10 and 11), indicating respiratory modulation of the heart rate (sinus arrhythmia) and modulation of pressure (Meyer or Traube-Hering). Mean arterial BP was significantly (P < 0.05) increased for portions of the 5 h after the 10-μl IA injections (Fig. 14). The cardiovascular variables were not significantly (P > 0.05) affected by the 1-μl injection into the KFN (Fig. 14) or by any injection into the other pontine nuclei (data not shown).
Fig. 14.
Heart rate (HR) and mean arterial blood pressure (MABP) were significantly (*P < 0.05) increased and decreased, respectively, during portions of the 5-h period after bilateral 10-μl injections of IA into the KFN. Note the nonsignificant decrease in HR after the 1-μl injection. Neither HR nor MABP changed after injections into other rostral pontine areas.
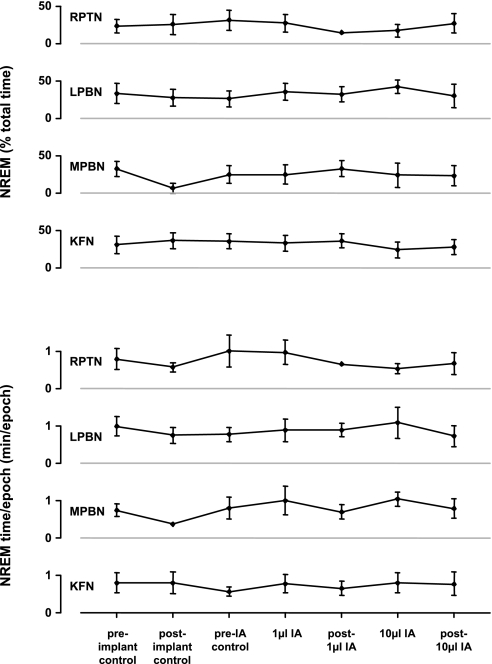
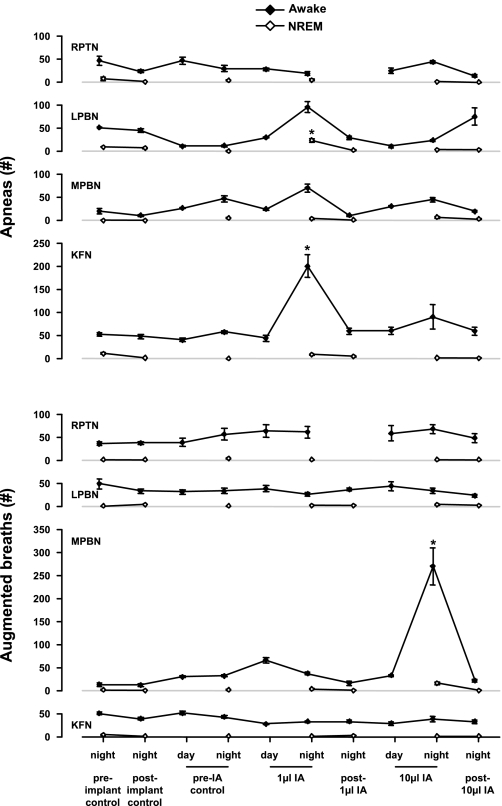
Acute nighttime effects of IA injection (10–15 h).
During the night, 10–15 h after IA injection, levels of NREM (Fig. 15) and REM sleep (data not shown) did not differ from control levels, nor did the average amount of time per NREM sleep epoch. However, the number of apneas (as defined in methods) was elevated (P < 0.05) on the night of 1-μl IA injections into the LPBN (P < 0.05), MPBN, and KFN (Fig. 16). The number of augmented breaths (as defined in methods) was elevated (P < 0.05) on the night of 10-μl IA injections into the MPBN during wakefulness (Fig. 16). Notably, in the RPTN-injected animals, the number of apneas and augmented breaths remained relatively constant throughout the protocol (Fig. 16). Ventilatory parameters were normal and comparable to pre-IA injection night studies (data not shown).
Fig. 15.
Over the course of the experimental protocol, the percentage of the night spent in non-rapid eye movement (NREM) sleep and the duration of sleep epochs did not change significantly in the RPTN, LPBN, MPBN, or KFN.
Fig. 16.
On the night of 1-μl injections of IA, the number of apneas was increased during wakefulness in KFN-lesioned goats (P < 0.05) and during NREM sleep in LPBN-lesioned goats (P < 0.001). Note that the number of apneas was nonsignificantly elevated in LPBN- and MPBN-lesioned goats during wakefulness but was not elevated whatsoever in animals with IA injections into RPTN. On the night of 10-μl injections of IA, the number of augmented breaths was increased during wakefulness in MPBN animals (P < 0.001). Asterisks denote significant differences from preimplant control values.
Chronic effects of IA injection (>22 h).
On the day following IA injections, all ventilatory variables, including muscle activity of the diaphragm, abdominal, and genioglossus, were unchanged (P > 0.05) from preinjection controls (data not shown). In addition, PaCO2 (Fig. 6), PaO2 (data not shown), pHa (data not shown), the amount of NREM (Fig. 15) and REM sleep (data not shown), the amount of time per NREM sleep epoch (Fig. 15), and the number of apneas and augmented breaths (Fig. 16) were also unchanged (P > 0.05) from preinjection controls. In all cannula-implanted goats, injection of IA did not appear to chronically affect eupneic breathing.
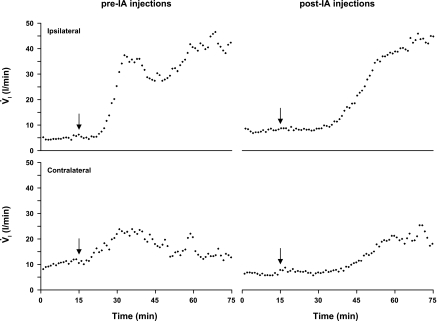
Acute effects of NMDA injection.
Figure 17 shows data for a KFN animal in which unilateral injections (1 μl, 100 mM) of NMDA were made ipsi- and contralaterally the week before the first IA injection and the week after the last IA injection (same goat as for data in Fig. 8). Compared with pre-IA NMDA injections, the magnitude of the hyperpnea was relatively preserved in post-IA NMDA injections, whereas the time course was somewhat delayed (Fig. 17). The same pattern of response to NMDA injection was also seen in the KFN animal from Fig. 9 (data not shown). Injection of NMDA into RPTN, LPBN, and MPBN animals did not have any consistent significant effect (data not shown).
Fig. 17.
In a KFN-lesioned goat, unilateral injections of N-methyl-d-aspartate (NMDA) were made ipsi- and contralaterally the week before the first IA injection and the week after the last IA injection. The arrows indicate the time of the NMDA injection (1 μl, 100 mM). Compared with pre-IA NMDA injections, the magnitude of the hyperpnea was relatively preserved in post-IA NMDA injections, whereas the time course was somewhat delayed. V̇i is expressed in 1-min bins.
DISCUSSION
Summary.
The major findings of this study were the site-specific effects in response to IA injections and the time-dependent plasticity in recovery of the eupneic breathing rhythm and pattern. Daytime IA injections acutely increased f in RPTN animals, increased V̇i in LPBN animals, had no effect in MPBN animals, and had a biphasic V̇i effect in KFN animals. Furthermore, in KFN animals apneas and apneusis-like breathing patterns were observed. Our hypothesis regarding site- and state-specific effects was partially validated in that effects were heterogeneous across cannula implantation groups and there were state-dependent effects on the frequency of apneas and augmented breaths. Our last hypothesis regarding plasticity was validated in that eupneic respiratory rhythm and pattern was restored within 22 h after IA injection.
Limitations.
A limitation of this study is that we are unable to unequivocally define the anatomic regions affected by IA injection. The grouping of the goats was based on the histological localization of the distal end of the tract created by implantation of the cannulas. The extent of the lesions was based on quantitative histological analysis, including absence and disruption of tissue and presence and absence of living and dead neurons. Exactly how far and to what extent the IA diffused was dependent on rate of diffusion, metabolism, and clearance. Although the effects were most consistent when IA was injected into the KFN, we cannot rule out diffusion to more than one subnucleus. In addition, the effects of IA may be due in part to the cumulative effect of destruction of dendritic connections when the cannula was inserted through the LPBN and MPBN (35). Nevertheless, there were clear-cut site-specific effects implicating a primary site for the effect of injections.
A second limitation is that only part of each nucleus was perturbed. The perturbations in the KFN were clearly sufficient to affect the eupneic breathing pattern and therefore are the major finding of this study. The relatively small lesions in the LPBN and MPBN preclude definitive conclusions about whether these subnuclei contribute to the eupneic breathing pattern.
Effects of cannula implantation.
The observation of pursed-lip breathing was an unexpected consequence of the cannula implantation surgery. Pursed-lip breathing is often observed in human chronic obstructive pulmonary disease (COPD) patients (9, 36), because it slows the rate of breathing, keeps the airways open longer, and makes each breath more efficient. It is unclear whether the pursed-lip breathing in goats served the same purpose as in COPD humans. Most of the animals exhibiting robust pursed-lip breathing had cannulas implanted into the MPBN or KFN, which physically lesioned portions of the PRG. Except in one animal, this behavior subsided within 2 wk postsurgery. However, in one animal (MPBN implanted), only injection of IA weeks postsurgery eliminated this behavior. It is possible that this breathing pattern was compensatory for presumed increased airway resistance caused by the abnormal activity of the laryngeal contractor muscle in late expiration (Fig. 5). Alternatively, postinspiratory resistance may have been reduced by the damage to the PRG, and the pursed-lip breathing might be compensatory for this decreased airway resistance during expiration.
Biphasic ventilatory response in KFN animals after IA injection.
There are two possible explanations for the biphasic ventilatory response to IA injections into the KFN. On one hand, both effects might be on the same population of neurons, since IA initially stimulates the neurons inducing the hyperpnea, and subsequently the neurotoxic effect of the IA destroys neurons, resulting in the hypopnea. On the other hand, in a diffusion-dependent manner, it is conceivable IA initially stimulated excitatory neurons proximal to the cannula and subsequently stimulated inhibitory neurons distal to the cannula. For several reasons, we believe the second explanation is more probable. First, the time course of the hyperpneic response was very short. In other studies in the medulla (16, 44) and cerebellum (26), IA injection elicited prolonged tachypnea and hyperpnea, and thus the first scenario seems unlikely.
Second, when the response of the small (1 μl) IA injection volume was compared with that of the subsequent large (10 μl) IA injection volume, there was an hour delay in both the initial hyperpnea and subsequent hypopnea, respectively, with the second injection. The majority of neuronal death caused by the small injection was likely proximal to the cannula, and thus the large injection took longer to diffuse to more distal, dense groups of neurons in the same population. Direct histological examination of this possibility was not possible given animals were only euthanized subsequent to all injections. However, we believe the brief time frame between hyperpnea and hypopnea was not sufficiently long enough for the neurotoxic properties of IA to take effect.
Third, in support of the second option, there also was a time delay in the response to the same volume of NMDA, compared with before and after IA injection studies (Fig. 17). Given the magnitude of the hyperpnea in response to NMDA was equivalent before and after IA injection, it is likely that the same population of neurons was being acted on in both cases, but in the latter injection, time for diffusion to more distal neurons within this population was necessary and resulted in a shifted response. Important to the previous two explanations is the assumption that a sufficient majority of neurons remained subsequent to the small IA injections, on which the large IA injections could act.
Last, if the first scenario were true, then we should have observed the same biphasic response in all animals. On the contrary, there was a delayed f increase in RPTN goats, a prolonged V̇i increase in LPBN animals, and no response in MPBN animals (Fig. 7). Therefore, we conclude that the biphasic V̇i response of IA injection in KFN animals is due to the heterogeneic topography of the PRG. Moreover, responses among implantation groups were heterogeneic, providing supplementary evidence to PRG heterogeneic topography.
Site-specific acute effects of glutamate agonists.
There are several lines of evidence supporting site-specific effects of glutamate agonist injections into the PRG. First, we found injection of IA into the KFN elicited a biphasic ventilatory response. This finding supports site specificity in two respects: 1) the fact that the response was biphasic, implicating two sites of differentially acting neuronal populations, and 2) the fact that it only occurred after injection into KFN animals. Second, a biphasic response did not occur in previous studies of medullary (16, 44) and cerebellar (26) areas, in which IA injections produced a single, distinct response. Third, in KFN animals there was a delayed response in the 10-μl IA injection compared with the 1-μl IA injection and a delayed response in the post-IA NMDA injection compared with the pre-IA NMDA injection. These delays are indicative of the increased time necessary for diffusion of IA to neurons more distal from the tip of the cannula. Finally, other laboratories have reported similar rostral pontine site-specific effects, including Chamberlin and Saper (5), who showed that glutamate injections into the dorsolateral pons in rat elicited hyperpnea and hypopnea, depending on the site and timing (relative to the respiratory cycle) of the injection.
Other groups have found apneas and prolonged inspiratory and expiratory times in response to PRG perturbation/lesioning, albeit in reduced preparations (7, 13, 21, 40, 43). Chamberlin and Saper (6) reported apnea after glutamate microstimulation of the area just ventral to the KFN, known as the intertrigeminal region (ITR). It is possible that in our study IA diffused into this area and elicited apnea. Radulovacki et al. (27) reported in anesthetized, freely breathing rats that the role of the ITR in the control of breathing is to attenuate vagal reflex apneas and dampen respiratory instabilities. Our data agree with both these findings, since IA injection in KFN goats generated both apneas and apneuses and increased variability in f. In contrast, Saponjic et al. (32) reported in anesthetized, spontaneously breathing rats that perturbations of the pedunculopontine tegmental nucleus caused intermittent apneas and increased variability of Te and total breathing duration. We did not observe either of these findings in our RPTN animals, although f was increased after IA injection. Altogether, these data show several examples of site-specific effects on breathing of perturbations in the rostral pons.
State-specific effects.
Similar to observations during the day, an increase in the number of apneas and augmented breaths was observed on the night following IA injections (Fig. 16) during both wakefulness and NREM sleep and was dependent on the site of the cannula. Although IA injection disrupted eupneic breathing at night, it did not affect the amount of NREM or REM sleep or the duration of sleep epochs (Fig. 15). Given there were no significant differences between awake and NREM sleep in the effects of IA injection on nighttime ventilatory parameters, we conclude there were no state-dependent effects. In previous studies in our laboratory when injecting IA into the pre-BötzC, we observed central apneas and increased breath-to-breath variation in breathing during nighttime wakefulness (16). Accordingly, we found the absence of state-dependent ventilatory effects in the present study curious, since the protocols for injection, study, and analysis were the same as in the medullary studies.
Role of the pons in respiratory pattern generation.
As summarized in the Introduction, for years there has been controversy regarding pontine contributions to respiratory rhythm and pattern generation. These views are based primarily on studies in anesthetized, decerebrate, or in vitro preparations. The data of the present study in awake and sleeping goats support a current concept that the KFN, with few exceptions (42), contributes to the generation of the three-phase eupneic pattern. Recent support for this concept were findings of Abdala et al. (1) that sequential transections through the pons eliminated phase 2 of the normal, eupneic, inspiration, postinspiration, and active expiration respiratory pattern (1). Specific to the KFN, Dutschmann and Herbert (11) found that glutamate-induced excitation of the KFN caused tonic excitation of postinspiratory (PI) motor activity or sustained laryngeal constriction in the PI period. Subsequently, isoguvacine inhibition of KFN neurons abolished PI motor activity and laryngeal constriction while prolonging phrenic nerve activity characteristic of apneustic breathing. As shown in Figs. 11 and 12 for one goat and in Fig. 13 in the other three of the four awake, KFN-lesioned goats, we found periods of prolonged PI diaphragm activity after bilateral injection of 10 μl of IA into the KFN. Moreover, as shown in Fig. 5, the pursed-lip breathing observed in KFN- and MPBN-lesioned goats was associated with altered pump and airway muscle activation pattern during the respiratory cycle. These findings support the concept that the KFN in particular is a major contributor to generation of the three-phase respiratory muscle activation pattern. Since our lesions of the LPBN and MPBN were relatively small, we cannot exclude the potential significance of these sites to respiratory pattern generation.
Cardiorespiratory coupling.
The injections of 10 μl of IA into the KFN initially increased V̇i, heart rate, and mean arterial BP (Figs. 8B and 9B), but thereafter for nearly 5 h, V̇i and heart rate were below control values (Figs. 7 and 15). This close coupling, illustrated by cardiovascular “respiratory waves,” was also observed with nearly each breath (Figs. 10 and 11) during periods of gasplike breathing and prolonged Te. It thus appears the injections did not disrupt cardiorespiratory coupling, which has been shown to be mediated by PRG subnuclei (10).
Chronic effects of PRG lesions.
In our studies, mechanical lesion by cannula implantation or chemical lesion by IA injection into the pons acutely disrupted eupneic respiration, but breathing soon thereafter returned to normal. We have observed similar time-dependent plasticity in previous studies lesioning the pre-BötzC in goats (16). The caveat in the present study was that we were unable to lesion the entire PRG in any one animal, compared with near total destruction of the pre-BötzC by IA injection in previous studies (16). Accordingly, we could not conclude whether recovery of eupneic respiration after pontine lesions was attributable to remaining PRG neurons, to respiratory neurons from another site(s), or to a combination of the two. St. John et al. (39) also found recovery or plasticity after lesions in the MPBN, where initially MPBN lesions increased Ti and reduced f, but after 3 mo Ti, while animals were awake and under anesthesia, did not differ between MPBN-lesioned and control cats (39). The exact mechanism of plasticity remains to be determined, but it could involve a reconfiguration of the pontomedullary network such as suggested by a recent computational modeling study (31).
GRANTS
This work was supported by the Department of Veterans Affairs and National Heart, Lung, and Blood Institute Grants HL25739 and HL007852.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We especially thank Greg McQuestion and Heather Vernon for assistance in software development.
REFERENCES
- 1. Abdala A, Rybak I, Smith J, Zoccal D, Machado B, St. John W, Paton J. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol 168: 19– 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdala A, Rybak I, Smith J, Paton J. Adominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539– 3559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellingham M, Funk G. Cholinergic modulation of respiratory brain-stem neurons and its function in sleep-wake state determination. Clin Exp Pharmacol Physiol 27: 132– 137, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bolser D, Poliacek I, Jakus J, Fuller D, Davenport P. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol 152: 255– 265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamberlin N, Saper C. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 14: 6500– 6510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chamberlin N, Saper C. A brainstem network mediating apneic reflexes in the rat. J Neurosci 18: 6048– 6056, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen M. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol 217: 133– 158, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cotran Kumar V R, Collins T. Pathologic Basis of Disease ( 6th ed.). Philadelphia, PA: Sanders, 1999, p. 1295 [Google Scholar]
- 9. Dechman G, Wilson C. Evidence underlying breathing retraining in people with stable chronic obstructive pulmonary disease. Phys Ther 84: 1189– 1197, 2004 [PubMed] [Google Scholar]
- 10. Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol 168: 76– 85, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071– 1084, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Feldman JL. Neurophysiology of breathing in mammals. In: Handbook of Physiology. The Nervous System. Intrinsic Regulatory Systems of the Brain. Bethesda, MD: Am. Physiol. Soc., 1986, vol. IV, sect. 1, p. 463– 524 [Google Scholar]
- 13. Fung M, St. John W. Electrical stimulation of pneumotaxic center: activation of fibers and neurons. Respir Physiol 96: 71– 82, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Gilbert K, Lydic R. Pontine cholinergic reticular mechanisms cause state-dependent changes in the discharge of parabrachial neurons. Am J Physiol Regul Integr Comp Physiol 266: R136– R150, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Janczewski W, Feldman J. Novel data supporting the two respiratory rhythm oscillator hypothesis. Focus on “Respiration-related rhythmic activity in the rostral medulla of newborn rats”. J Neurophysiol 96: 1– 2, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Krause K, Forster HV, Kiner T, Davis S, Bonis J, Qian B, Pan L. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Bötzinger complex and the surrounding region. J Appl Physiol 106: 605– 619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee L, Friedman D, Lydic R. Respiratory nuclei share synaptic connectivity with pontine reticular regions regulating REM sleep. Am J Physiol Lung Cell Mol Physiol 268: L251– L262, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Lieske S, Thoby-Brisson M, Telgkamp P, Ramirez J. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3: 600– 607, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153– 160, 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lumsden T. Observations on the respiratory centres. J Physiol 57: 354– 367, 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lumsden T. The regulation of respiration. Part I. J Physiol 58: 81– 91, 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lumsden T. The regulation of respiration. Part II: Normal type. J Physiol 58: 111– 126, 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mallios V, Lydic R, Baghdoyan H. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol Lung Cell Mol Physiol 268: L941– L949, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Marckwald MZ. Die Atembewegungen und deren Innervation beim Kaninchen. Z Biol 23: 149– 283, 1887 [Google Scholar]
- 25. Marckwald M. Bedeutung des Mettelhirns für die Athmung. Z Biol 26: 259– 289, 1890 [Google Scholar]
- 26. Martino P, Davis S, Opansky C, Krause K, Bonis J, Pan L, Qian B, Forster HV. The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respir Physiol Neurobiol 157: 242– 251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radulovacki M, Pavlovic S, Saponjic J, Carley D. Intertrigeminal region attenuates reflex apnea and stabilizes respiratory pattern in rats. Brain Res 975: 66– 72, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Ramirez J, Zuperku E, Alheid G, Lieske S, Ptak K, McCrimmon D. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol 131: 43– 56, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Richter D, Spyer K. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464– 472, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Rubin J, Shevtsova N, Ermentrout G, Smith J, Rybak I. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol 101: 2146– 2165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rybak I, O'Connor R, Ross A, Shetsova N, Nuding S, Segers L, Shannon R, Dick T, Dunin-Barkowski W, Orem J, Solomon I, Morris K, Lindsey B. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770– 1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saponjic J, Radulovacki M, Carley D. Respiratory pattern modulation by the pedunculopontine tegmental nucleus. Respir Physiol Neurobiol 138: 223– 237, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Smith J, Ellenberger H, Ballanyi K, Richter D, Feldman J. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726– 729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JC, Abdala A, Rybak I, Paton J. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577– 2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song S, Yu Y, Poon C. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci 26: 300– 310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lip breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest 128: 640– 650, 2005 [DOI] [PubMed] [Google Scholar]
- 37. St. John W. Medullary regions for neurogenesis of gasping: noued vital or noeuds vitals? J Appl Physiol 81: 1865– 1877, 1996 [DOI] [PubMed] [Google Scholar]
- 38. St. John W, Bledsoe T. Genesis of rhythmic respiratory activity in pons independent of medulla. J Appl Physiol 59: 684– 690, 1985 [DOI] [PubMed] [Google Scholar]
- 39. St. John W, Glasser R, King R. Rhythmic respiration in awake vagotomized cats with chronic pneumotaxic area lesions. Respir Physiol 15: 233– 244, 1972 [DOI] [PubMed] [Google Scholar]
- 40. St. John W, Zhou D. Rostral pontile mechanisms regulate durations of expiratory phases. J Appl Physiol 71: 2133– 2137, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11: 538– 540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Euler Marttila I C, Remmers J, Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand 96: 324– 337, 1976 [DOI] [PubMed] [Google Scholar]
- 43. Wang W, Fung M, St. John W. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol 74: 2801– 2811, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Wenninger J, Pan L, Klum L, Leekley T, Bastastic J, Hodges M, Feroah T, Davis S, Foster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629– 1636, 2004 [DOI] [PubMed] [Google Scholar]