Abstract
Vasoactive intestinal peptide (VIP) is implicated in cutaneous active vasodilation in humans. VIP and the closely related pituitary adenylate cyclase activating peptide (PACAP) act through several receptor types: VIP through VPAC1 and VPAC2 receptors and PACAP through VPAC1, VPAC2, and PAC1 receptors. We examined participation of VPAC2 and/or PAC1 receptors in cutaneous vasodilation during heat stress by testing the effects of their specific blockade with PACAP6–38. PACAP6–38 dissolved in Ringer's was administered by intradermal microdialysis at one forearm site while a control site received Ringer's solution. Skin blood flow was monitored by laser-Doppler flowmetry (LDF). Blood pressure was monitored noninvasively and cutaneous vascular conductance (CVC) calculated. A 5- to 10-min baseline period was followed by ∼70 min of PACAP6–38 (100 μM) perfusion at one site in normothermia and a 3-min period of body cooling. Whole body heating was then performed to engage cutaneous active vasodilation and was maintained until CVC had plateaued at an elevated level at all sites for 5–10 min. Finally, 58 mM sodium nitroprusside was perfused through both microdialysis sites to effect maximal vasodilation. No CVC differences were found between control and PACAP6–38-treated sites during normothermia (19 ± 3%max untreated vs. 20 ± 3%max, PACAP6–38 treated; P > 0.05 between sites) or cold stress (11 ± 2%max untreated vs. 10 ± 2%max, PACAP6–38 treated, P > 0.05 between sites). PACAP6–38 attenuated the increase in CVC during whole body heating when compared with untreated sites (59 ± 3%max untreated vs. 46 ± 3%max, PACAP6–38 treated, P < 0.05). We conclude that VPAC2 and/or PAC1 receptor activation is involved in cutaneous active vasodilation in humans.
Keywords: laser-Doppler flowmetry, VPAC2, PAC1, thermoregulation, microdialysis
the cutaneous vasculature is a major effector of human thermoregulatory reflex responses. During periods of cold stress, constriction of skin blood vessels with consequent reductions in skin perfusion facilitates maintenance of thermal homeostasis by reducing loss of heat to the environment. In periods of heat stress, thermal homeostasis is maintained by dilation of the cutaneous vasculature to increase heat loss to the environment by delivering excess heat to the body surface where it can be removed in conjunction with evaporative cooling through sweat production.
In nonglabrous (hairy) skin, blood flow (SkBF) changes during thermoregulatory reflexes are effected by two branches of the sympathetic nervous system. During cold stress, cutaneous vasoconstriction is mediated by increased tone in vasoconstrictor nerves that release norepinephrine and NPY to effect reductions in SkBF (17, 41). During heat stress, cutaneous vasodilation is mediated by increased activity in the cutaneous active vasodilator system. This system involves release of acetylcholine (ACh) as well as one or more cotransmitters from cutaneous cholinergic sympathetic nerves (24). According to present understanding, the release of ACh and one or more cotransmitters (probably neuropeptides, but at present of uncertain identity) from cholinergic nerves increases SkBF during heat stress to cause cutaneous vasodilation to facilitate heat loss in combination with sweat production (23). This cutaneous active vasodilator system is critical to human tolerance of endogenous and endogenous heat stress as heat-related illnesses including heat exhaustion and heat stroke occur when it fails (21, 23, 33). This system can cause SkBF to increase to as much as 8 l/min or 60% of cardiac output at the peak of heat stress (34). While the vasodilator potential and overall physiological import of this system is well understood, the nature of the neurotransmitters involved remains unclear.
Neuropeptides of the secretin/glucagon/VIP/pituitary adenylate cyclase activating peptide (PACAP) family have been implicated in cotransmission in the cutaneous active vasodilator system (2, 18) albeit not without controversy (49, 51). The members of this neuropeptide superfamily that have been identified in human skin are the cotranslated and coexpressed peptides, VIP and peptide histidine methionine (PHM), along with the related peptide, PACAP. VIP and PHM are structurally related peptides that are encoded by the same gene and are cosynthesized from the same precursor and therefore co-occur in essentially equal amounts in neurons (9, 45). PACAP has a 68% homology with VIP (9, 19, 29, 32, 37, 40). PACAP occurs in two forms: PACAP38 and a truncated derivative, PACAP27. PACAP38 has been found in cutaneous nerves in association with both sweat glands and blood vessels while PACAP27 appears to be absent from skin (29, 40). VIP/PHM and PACAP can be colocalized with ACh and all cause vasodilation with PACAP being the most potent and PHM the least potent (32); thus any or all of these peptides may be considered as candidate neurotransmitters for the cholinergic cutaneous active vasodilator system (30, 47, 48).
Members of the secretin/glucagon/VIP/PACAP peptide family mediate their effects through G protein-coupled receptors. Since initially identified, several nomenclatures for these receptors have been used, i.e., VIP1, VIP2, PACAP1, etc.; however, a specific nomenclature has now been established with VPAC1, VPAC2, and PAC1 as accepted receptor terminology (15). VPAC1 and VPAC2 receptors have essentially equivalent high affinities for both VIP and PACAP38. PAC1 receptors have high affinity for PACAP38 and bind VIP with much lower affinity (0.01%). PHM has little if any affinity for VPAC1 receptors and binds to VPAC2 receptors with a much lower affinity than does either VIP or PACAP (25–27, 32, 45). VPAC1, VPAC2, and PAC1 receptors all have been found in human nonglabrous skin: VPAC2 and PAC1 receptors appear to be the predominant types while the number of VPAC1 receptors was reported to be sparse (28).
Evidence implicating the secretin/glucagon/VIP/PACAP peptide family in cutaneous active vasodilation in humans is mixed. Bennett et al. (2) found that VIP10–28, a truncated derivative of VIP produced by mast cells with VPAC1 and inconsistent VPAC2 receptor antagonist properties (5, 42), significantly attenuated the increase in cutaneous vascular conductance (CVC) relative to internal temperature during whole body heating (2). These results suggested an important mechanistic role for VPAC1 and/or VPAC2 receptors and VIP in cutaneous active vasodilation; however, contrary evidence also exists. For example, patients with cystic fibrosis have sparse but not zero levels of VIP in nonglabrous skin, yet these patients appear to have normal cutaneous active vasodilator responses to heat stress (22, 37, 50). In addition, Wilkins et al. (49, 51) found that a higher concentration of VIP10–28 than used by Bennett at al (2) augmented cutaneous active vasodilation in heat stress. To add further uncertainty, the antagonist properties of VIP10–28 on VPAC2 receptors appear to be variable (13, 46). The realization that PACAP coexists with VIP in skin neurons compounds the complexity of defining roles for these neuropeptides and their receptors in cutaneous active vasodilation (10).
The foregoing summary of in vivo evidence for and against a mechanistic role for neuropeptides of the secretin/glucagon/VIP/PACAP peptide family in cutaneous active vasodilation outlines why such a role remains under debate. We sought to clarify this issue by examining the effects of an alternative to the VPAC receptor antagonist, VIP10–28, on the vasodilation induced by whole body heating to test whether members of this superfamily of peptides participate in cutaneous active vasodilation. We hypothesized that the two more common receptors for these peptides in skin, specifically VPAC2 and PAC1 receptors rather than VPAC1 type that is relatively sparse in skin (28), are activated to increase SkBF during heat stress in humans. We tested our hypothesis by examining the effect of PACAP6–38, a truncated version of PACAP38 with recognized antagonist properties for VPAC2 and PAC1 receptors, on responses in SkBF during thermoregulatory reflexes in humans.
METHODS
Antagonism of VPAC2 and PAC1 receptors was achieved by administration of PACAP6–38, a VPAC2 and PAC1 receptor selective antagonist that is without activity at VPAC1 receptors (27). Drug delivery was achieved by intradermal microdialysis to permit direct, local administration of PACAP6–38 into the interstitial space of a small area of skin. This approach permitted simultaneous local monitoring of SkBF from an untreated control site and an adjacent locally treated site within each subject without confounding systemic effects. SkBF was monitored by laser-Doppler flowmetry (LDF, MoorLab, Moor Instruments, Devon, UK) from the same small volume of skin (∼1 mm3) over each microdialysis probe. LDF measurements are specific to skin and are uninfluenced by blood flow in the underlying skeletal muscle (36).
Preliminary experiments were performed to determine the appropriate PACAP6–38 concentration to achieve an optimal VPAC2/PAC1 receptor blockade that would consistently antagonize the receptors without the potentially confounding effects of altering basal SkBF (51). In the first of these studies, we used intradermal microdialysis and tested the effects of 50, 100, and 200 μM PACAP6–38 concentrations on basal SkBF. These four studies showed that 50 μM and 100 μM PACAP6–38 did not alter basal SkBF but that 200 μM PACAP6–38 increased SkBF to 32 ± 3% of maximal CVC. Maximal absolute CVC levels as achieved with 58 mM sodium nitroprusside (SNP) were not altered by 50, 100, or 200 μM PACAP6–38. Based on these preliminary studies, we chose to further evaluate the antagonist properties of 100 μM PACAP6–38 for use in our whole body heat stress protocol.
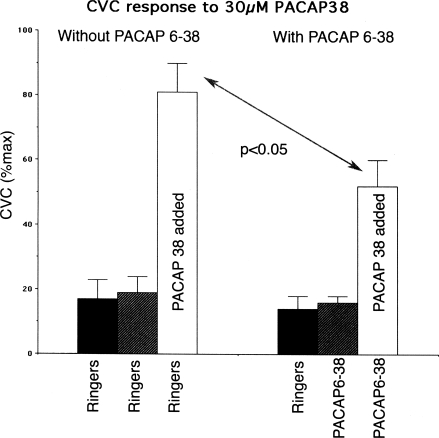
As there is no specific VPAC2/PAC1 agonist available (25–27), we chose to examine the ability of PACAP6–38 to antagonize the vasodilation produced by 30 μM PACAP38, an agonist at VPAC1, VPAC2, and PAC1 receptors. This concentration of PACAP38 was found to increase cutaneous vascular conductance (CVC) to 89 ± 7% of maximal levels. VIP was not chosen because this peptide is only a weak agonist at PAC1 receptors compared with PACAP38 (25–27). As illustrated in Fig. 1, 100 μM PACAP6–38 consistently attenuated the vasodilation induced by 30 μM PACAP38 (P < 0.05). Based on our preliminary studies, we chose 100 μM PACAP6–38 for use in our whole body heat stress protocol.
Fig. 1.
Summary of attenuating effect of 100 μM PACAP6–38 on the vasodilation induced by 30 μM PACAP38 in 4 subjects. PACAP6–38 did not alter basal cutaneous vascular conductance (CVC) levels (P > 0.05). PACAP38 increased CVC significantly from basal levels in the absence and presence of PACAP6–38 (P < 0.05); however, the vasodilation induced by PACAP38 was significantly reduced by PACAP6–38 (P < 0.05).
Nine healthy subjects (4 men and 5 women) participated in the main portion of this study. The subjects' average age (± SE) was 34 ± 4 yr, average weight was 62 ± 2 kg, and average height was 169 ± 2 cm. All subjects were in good health as documented by medical history and physical examination and were taking no medications, and all were nonsmokers. All subjects gave their informed consent to participate in this institutionally approved study. There was no caffeine intake on the day of the study. The menstrual phase of the female subjects at the time of the study was not controlled for.
Microdialysis probes were made in our laboratory from polyimide tubing and a 1-cm length of capillary microdialysis membrane (200-μm diameter, molecular mass cutoff 20 kDa) reinforced by a 51-μm-diameter coated stainless steel wire placed in the lumen of the membrane and tubing. Upon arrival in the laboratory, each subject had two probes placed at separate sites on the ventral aspect of one forearm. Ice was used at each skin site to achieve temporary local anesthesia before microdialysis probe placement (16). For probe placement, a 25-gauge needle was inserted through the dermis using sterile technique. Entry and exit points were approximately 2.5–3 cm apart. A microdialysis probe was subsequently threaded through the lumen of the needle, which was withdrawn, leaving the probe in place with the microdialysis membrane entirely within the dermis. After probe insertion and before additional instrumentation, subjects waited 2 h or longer for resolution of insertion trauma (1).
To induce thermoregulatory reflexes, subjects wore a tube-lined suit to control skin temperature (Tsk) by perfusion with water to control Tsk of different temperatures (35). For normothermia, the suit was perfused with water at approximately 33–34°C. The suit was perfused with 16–17°C water to decrease Tsk and induce cold stress and 48°C water to raise Tsk to 38–39°C during heating periods. Over the suit, subjects wore a water-impermeable plastic garment to insulate them from the environment and prevent sweat evaporation. The suit and garment covered the entire body with the exception of the head, hands, feet, and the forearm from which measurements were made.
Internal temperature was monitored with a thermocouple held in the sublingual sulcus (Tor). Tsk was recorded as the weighted electrical average from six thermocouples taped on the skin surface (20, 44). Pulse rate (PR) and mean arterial pressure (MAP) were recorded continuously from a finger (Finapres BP Monitor, Ohmeda, Madison, WI).
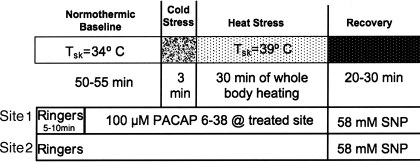
For the study, subjects were placed in the supine position and instrumented. Tsk was maintained at 34°C. Data collection began with a 5- to 10-min baseline control period during which the microdialysis probes were perfused with Ringer's solution only. Perfusion was done at a rate of 3 μl/min using a microinfusion pump. After this control period, the perfusate at one site was changed to 100 μM PACAP6–38 in Ringer's. Perfusion was maintained with Ringer's only at the second site. Perfusion was maintained with these solutions for 40–45 min under normothermic conditions to allow PACAP6–38 to reach a steady state in the intradermal space. Following this normothermic period, Tsk was decreased to induce cold stress for 3 min. Tsk was then raised to 38–39°C and maintained at that level for 35–50 min to induce heat stress and thereby stimulate the active vasodilator system. After heat stress, subjects were cooled and returned to a normothermic Tsk of 34°C. All microdialysis sites were then perfused with 58 mM SNP to effect maximal vasodilation at each site. CVC values were normalized to these maximal levels for data analysis. The protocol is illustrated in Fig. 2.
Fig. 2.
Whole body heat stress protocol. This protocol was designed to examine the effects of antagonism of VPAC2 and PAC2 receptors for VIP and pituitary adenylate cyclase activating peptide (PACAP) on the cutaneous vasodilation induced by whole body heat stress. Two intradermal microdialysis sites were used to perfuse intradermal microdialysis fibers with Ringer's solution alone at an untreated control site and with 100 μM PACAP6–38, a VPAC2/PAC1 receptor antagonist. Maximal cutaneous vasodilation was achieved at the end of the study by perfusion with 58 mM sodium nitroprusside (SNP) at both sites. The perfusion rate at all microdialysis sites was 3 μl/min. Tsk, skin temperature.
Data are presented as means ± SE. CVC values were indexed as LDF/MAP and were normalized to their respective maxima as elicited by 58 mM SNP to facilitate comparisons among sites both within and among subjects after verifying that there were no statistically significant differences among the absolute LDF values achieved with SNP perfusion (P > 0.05 between sites). The CVC responses at the two microdialysis sites were analyzed by comparing the Tor thresholds at which CVC began to increase during whole body heating. The internal temperature threshold for the onset of vasodilation for each site was defined as the Tor that prevailed when a sustained increase in CVC began during whole body heating. Tor thresholds were chosen from graphs of CVC vs. Tor by an investigator who was blinded as to the conditions, subjects, and antagonist treatment. These thresholds were compared by ANOVA for repeated measures. Normothermic baseline CVC values, CVC levels during the final minute of drug infusion in normothermia, CVC during the final minute of cold stress, and CVC from the final 3 min of heat stress were also compared among sites by ANOVA, followed by Student-Newman-Keuls test. MAP, pulse rate (PR), and Tor changes from normothermia to the peak of heat stress were compared by paired t-tests. The level of statistical significance was defined as P < 0.05.
RESULTS
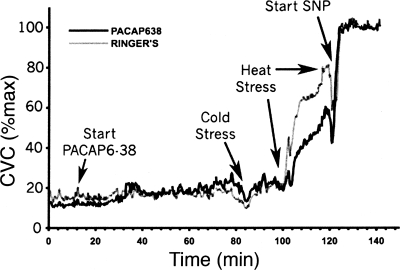
An example of the responses in skin blood flow to the body heating protocol is illustrated in Fig. 3. In normothermia, during perfusion with Ringer's at both microdialysis sites, CVC values did not differ between sites (18 ± 2 vs. 17 ± 2%max, P > 0.05). Changing the microdialysis perfusate to 100 μM PACAP6–38 at one site did not alter CVC (P > 0.05 between sites). In the last 3 min of normothermia, CVC at sites perfused with 100 μM PACAP6–38 averaged 19 ± 3%max while at control sites CVC averaged 20 ± 3%max (P > 0.05 between sites).
Fig. 3.
CVC response to the body heating protocol in 1 subject. Perfusion with 100 μM PACAP6–38 Ringer's began 10 min into the study. Periods of whole body cooling (CS) and heating are indicated. Perfusion with 58 mM SNP began at 120 min. The site receiving PACAP6–38 showed less vasodilation during heat stress than did the control site.
During cold stress, CVC was reduced at both sites (P < 0.05 cold stress vs. normothermia). CVC values fell to 11 ± 2%max CVC at sites treated with PACAP6–38 and to 10 ± 2%max CVC at control sites. These responses did not differ between sites (P > 0.05).
In normothermia, MAP averaged 77 ± 3 mmHg and averaged 71 ± 4 mmHg at the peak of heat stress (P > 0.05, normothermia vs. heat stress). PR averaged 62 ± 2 beats/min in normothermia and increased to 84 ± 5 beats/min at the peak of heat stress (P < 0.01). Tor averaged 36.34 ± 0.09°C in normothermia and increased to 37.11 ± 0.10°C at peak heat stress (P < 0.01).
During whole body heating, CVC at the sites perfused with Ringer's began to rise when Tor reached values of 36.62 ± 0.09°C and 36.59 ± 0.19°C for sites perfused with PACAP6–38. There was no statistically significant difference between the threshold values at the two sites (P > 0.05).
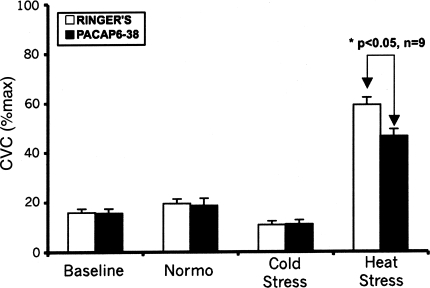
At the peak of heat stress, CVC at sites perfused with Ringer's increased to 59 ± 3%max CVC (P < 0.05 vs. normothermia). CVC at sites perfused with PACAP6–38 increased to 46 ± 3%max CVC (P < 0.05 vs. normothermia), a response that was significantly attenuated compared with that at the Ringer's sites (P < 0.05). Averaged CVC responses for the protocol are summarized in Fig. 4.
Fig. 4.
Summary of CVC responses. Under normothermic conditions during perfusion of both microdialysis sites with Ringer's solution, CVC values, expressed as a percentage of their respective maxima, did not differ significantly (P > 0.05 between sites). In normothermia, changing the perfusion of one site to 100 μM PACAP6–38 in Ringer's did not produce any significant difference with CVC at sites perfused with Ringer's alone (P > 0.05). Cold stress significantly reduced CVC at both sites compared with normothermia (Normo) (P < 0.05). These responses did not differ between sites (P > 0.05). During heat stress, CVC increased at both microdialysis sites compared with normothermia (P < 0.05); however, this increase was significantly attenuated at sites perfused with 100 μM PACAP6–38 (P < 0.05, PACAP6–38 vs. Ringer's).
Following heat stress, maximal CVC in response to 58 mM SNP infusion did not differ between sites that were untreated and those that received PACAP6–38 (P > 0.05).
DISCUSSION
The main finding of this study is that antagonism of VPAC2 and PAC1 receptors in forearm skin significantly attenuates the increases in SkBF during thermoregulatory reflex responses to whole body heat stress in humans. This finding demonstrates that activation of one or both of these receptors by members of the secretin/glucagon/VIP superfamily of peptides mediates a significant part of thermoregulatory reflex cutaneous active vasodilation in nonglabrous skin.
In contrast to attenuating the vasodilation caused by whole body heat stress, we found that VPAC2/PAC1 receptor antagonism did not alter reflex vasoconstriction caused by whole body cooling. This result shows that these receptors and hence members of the VIP/PACAP peptide family are not involved in effecting cutaneous vasoconstriction during cold stress. In addition, that vasoconstriction at untreated control sites did not differ from that observed at VPAC2/PAC1 blocked sites indicates that PACAP6–38 did not alter the responsiveness of the vascular smooth muscle itself, precluding a nonspecific attenuated vascular responsiveness as an explanation of the attenuating effects of PACAP6–38 on SkBF increases during heat stress.
The foregoing conclusions are based on our demonstration that PACAP6–38 attenuated the vasodilator response evoked by administration of the agonist PACAP38 significantly. We were, however, unable to block completely the vasodilation induced by exogenous PACAP38 with PACAP6–38. While we may have been able to achieve greater blockade with higher concentrations of PACAP6–38 we found higher concentrations induced a vasodilation, perhaps due to activating effects of higher concentrations or inflammatory mechanisms such as mast cell destabilization (14, 32). The failure to achieve complete blockade could be a consequence of limiting the concentration of PACAP6–38 to 100 μM to avoid any increase in basal SkBF. We limited our concentration of PACAP6–38 to 100 μM to avoid potentially confounding effects of pharmacological increases in baseline as identified by Wilkins et al. (51). This allowed us to be certain that cutaneous active vasodilation was actually attenuated by PACAP6–38 rather than an artifact of baseline elevation. We therefore chose a PACAP6–38 concentration of 100 μM as an optimal one that would allow us to define whether VPAC2/PAC1 receptor activation was involved in the mechanism of cutaneous active vasodilation. We reasoned that although this choice may have precluded estimation of the absolute VPAC2/PAC1 contribution to cutaneous active vasodilation because of uncertainty about the extent of receptor blockade, an attenuation of cutaneous active vasodilation without potentially confounding baseline effects would best prove or refute our hypothesis of VPAC2/PAC1 receptor involvement.
Another factor that could have contributed to our apparent inability to abolish the vasodilation induced by PACAP38 may be because it is an agonist for VPAC1 as well as VPAC2 and PAC1 receptors. PACAP6–38 antagonizes VPAC2 and PAC1 receptors, but not VPAC1 receptors. While VPAC1 receptors are reported to be few in number in human nonglabrous skin, they are not totally absent (28); thus it is possible that exogenous PACAP38 induced vasodilation via VPAC1 mechanisms unaffected by PACAP6–38. Because of the foregoing, we cannot exclude a role for VPAC1 receptors in active vasodilation during whole body heat stress.
A prior study from our laboratory showed that VIP10–28, an antagonist of VPAC1, and inconsistently of VPAC2 receptors (13, 46), attenuated the rise of SkBF during heat stress (2). While this study supported a role for activation of these receptors in the mechanism of cutaneous active vasodilation, subsequent work by Wilkins et al. (49, 51) suggested that VIP10–28 had some characteristics of a receptor agonist. VIP10–28 is a truncated version of VIP that is produced in vivo by lymphocytes and has a relatively low affinity for VPAC1 and VPAC2 receptors (42). These attributes are problematic in interpreting in vivo effects of this agent; however, the findings of the present study are consistent with the work by Bennett et al. (2).
The present demonstration that VPAC2/PAC2 receptor activation is involved in the mechanism of cutaneous active vasodilation indicates that release of neuropeptides belonging to the VIP/PACAP peptide family is involved in the process. Within this peptide family, both VIP and PACAP38 are found in skin, while the truncated version of PACAP38, PACAP27, is not (40, 47, 48). Both VIP and PACAP38 are potent vasodilators in skin (10, 47, 48). PHM, another member of the VIP/PACAP peptide family, has also been found in human skin colocalized in equal amounts with VIP (9, 19). No specific receptor has been found for PHM in mammals (45). PHM has a much lower binding affinity than VIP and PACAP for VPAC1 and VPAC2 receptors, and especially PAC1 receptors (26) and has a potency several orders of magnitude lower than those of VIP or PACAP (10). Since VIP and PHM co-occur and are coreleased, any contribution of PHM to cutaneous vasodilation would be relatively smaller than that of VIP (10, 25–27).
Cutaneous immunohistochemical studies of VIP-containing neurons have revealed abundant VIP-containing fibers that innervate cutaneous arterioles and sweat glands (8, 11, 12). PACAP38 has also been found in skin nerves innervating arterioles and sweat glands (40). Both VIP and PACAP38 are found colocalized with ACh in cutaneous cholinergic nerves, a type known to be intimately involved in cutaneous active vasodilation (10, 24, 47). They are also found in cutaneous afferent nerves that may be involved in the process (10, 32, 52, 53). These findings show that both VIP and PACAP38 are found in microanatomic locations consistent with a mechanistic role in cutaneous active vasodilation as demonstrated by the attenuating effects of PACAP6–38 on the process.
As previously mentioned, VPAC1, VPAC2, and PAC1 receptors are all found in human nonglabrous skin. Of these, VPAC1 receptors appear to be quite limited in number in skin in general and in cutaneous arterioles in particular (28). In contrast, VPAC2 receptors have been found to be abundant in skin, although mRNA for VPAC2 receptors appears to be absent from dermal vascular smooth muscle cells (11, 12). VPAC2 receptors are also abundantly expressed in sweat glands (11, 12). PAC1 receptors are also found in human nonglabrous skin; however, their precise location has not been elucidated (40). These findings show that the receptors for both VIP and PACAP38 are also found in microanatomic locations, consistent with a mechanistic role in cutaneous active vasodilation.
How VIP and/or PACAP38 activation of the VPAC/PAC1 receptors effects cutaneous vasodilation is unknown, however, given that activation of these receptors by physiological concentrations of VIP and/or PACAP activates adenylyl cyclase in general. On sweat glands in particular, cAMP seems likely to be involved as a second messenger (6, 7, 43). Less likely is the release of histamine. Although exogenous VIP and PACAP38 have been found to induce histamine release from mast cells, such release occurs only at high (micromolar) concentrations of agonist by non-receptor-mediated, direct interaction with G proteins (14, 29, 49). Nitric oxide production may also be involved (49).
Finally, the antagonism by 100 μM PACAP6–38 accounted for ∼30% of the increase in CVC from normothermia to the peak of heating. The source of the remaining 70% of the response is not clear. As previously discussed, the remainder could be due to incomplete blockade of the VPAC2/PAC1 receptors or to activation by the native agonist of unblocked VPAC1 receptors. It might also be ascribed to other transmitter-receptor groups, for example ACh (24, 31, 38, 39), or neurokinins (52, 53). Further research with combinations of more specific antagonists will be required to distinguish among these possibilities.
In summary, antagonism of VPAC2/PAC1 receptors for VIP, PACAP38, and related members of their neuropeptide family attenuates SkBF increases induced by whole body heat stress. This shows that one or more of these neuropeptides and their cognate receptors are involved in increasing SkBF during whole body heat stress. Overall, these data strongly support a role for VPAC2/PAC1 receptor activation in cutaneous active vasodilation and therefore suggest that members of the secretin/glucagon/VIP/PACAP superfamily of neuropeptides are also involved.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-065599.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the authors.
Footnotes
- CVC
- Cutaneous vascular conductance
- LDF
- Laser-Doppler flowmetry
- MAP
- Mean arterial pressure
- PACAP
- Pituitary adenylate cyclase activating peptide
- PACAP6–38
- VPAC2 and PAC1 receptor antagonist
- PAC1
- PACAP Receptor
- PHM
- Peptide histidine methionine
- PR
- Pulse rate
- SkBF
- Skin blood flow
- SNP
- Sodium nitroprusside
- Tloc
- Local skin temperature
- Tor
- Oral temperature
- Tsk
- Skin temperature
- VPAC1
- VIP and PACAP receptor, type 1
- VPAC2
- VIP and PACAP receptor, type 2
- VIP
- Vasoactive intestinal peptide
- VIP10–28
- VPAC1 and VPAC2 receptor antagonist
REFERENCES
- 1. Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser-Doppler perfusion imaging. J Invest Dermatol 102: 808– 811, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg J DL. Evidence for a role for vasoactive intestinal peptide in active vasodilation in the cutaneous vasculature in humans. J Physiol 552: 223– 232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537– 552, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 290: H1601– H1609, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 56: 249– 290, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Dickson L, Aramori I, McMulloch J, Sharkey J, Finlayson K. A systematic comparison of intracellular cyclic AMP and calcium signaling highlights complexities in human VPAC/PAC receptor pharmacology. Neuropharmacology 51: 1086– 1098, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Dickson L, Aramori I, Sharkey J, Finlayson K. VIP and PACAP receptor pharmacology. Ann NY Acad Sci 1070: 239– 242, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Donadio V, Nolano M, Provitera V, Stancanelli A, Lullo F, Liguori R, Santoro L. Skin sympathetic adrenergic innervation: an immunoflourescence confocal study. Ann Neurol 59: 376– 381, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Eedy DJ, Shaw C, Armstrong EP, Johnston CF, Buchanan KD. Vasoactive intestinal peptide (VIP) and peptide histidine methionine in human eccrine sweat glands: demonstration of innervation, specific binding sites and presence in secretions. Br J Dermatol 123: 65– 76, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Fahrenkrug J, Hannibal J. Neurotransmitters co-existing with VIP or PACAP. Peptides 25: 393– 401, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Fischer TC, Dinh QT, Peiser C, Loser A, Groneberg DA. Simultaneous detection of receptor mRNA and ligand protein in human skin tissues. J Cutan Pathol 29: 65– 71, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fischer TC, Hartmenn P, Loser C, Springer J, Peiser C, Dinh T, Fischer A, Groneberg DA. Abundant expression of vasoactive intestinal polypeptide receptor VPAC2 mRNA in human skin. J Invest Dermatol 117: 754– 756, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides 18: 1555– 1560, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Groneberg DA, Welker P, Fischer TC, Dinh QT, Grutzkau A, Peiser C, Wahn U, Henz BM, Fischer A. Downregulation of vasoactive intestinal polypeptide expression in atopic dermatitis. J Allergy Clin Immunol 111: 1099– 1105, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Wasachek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50: 265– 270, 1998 [PMC free article] [PubMed] [Google Scholar]
- 16. Hodges GJ, Chu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol 106: 1112– 1118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodges GJ, Jackson DN, Mattar L, Johnson JM, Shoemaker JK. Neuropeptide Y and neurovascular control in skeletal muscle and skin. Am J Physiol Regul Integr Comp Physiol 297: R546– R555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hökfelt TM, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature 284: 515– 521, 1980 [DOI] [PubMed] [Google Scholar]
- 19. Johansson O. Evidence for PHI-immunoreactive nerve fibers in the human skin: coexistence with VIP? Med Biol 64: 67– 73, 1986 [PubMed] [Google Scholar]
- 20. Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814– 818, 1981. [DOI] [PubMed] [Google Scholar]
- 21. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. I, chapt. 11, p. 215– 243 [Google Scholar]
- 22. Kellogg DJ, Hodges GJ, Orozco CR, Phillips TM, Zhao JL, Johnson JM. Cholinergic mechanisms of cutaneous active vasodilation during heat stress in cystic fibrosis. J Appl Physiol 103: 963– 968, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol 100: 1709– 1718, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Kellogg DL, Jr, Pérgola PE, Kosiba WA, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res 77: 1222– 1228, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept 108: 165– 173, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels 8: 137– 153, 2002 [PubMed] [Google Scholar]
- 27. Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation, and pharmacology. Peptides 28: 1631– 1639, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lundeberg L, Nordlind K. Vasoactive intestinal polypeptide in allergic contact dermatitis: an immunohistochemical and radioimmunoassay study. Arch Dermatol Res 291: 201– 206, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Odum L, Petersen LJ, Skov PS, Ebskov LB. Pituitary adenylate cyclase activating polypeptide (PACAP) is localized in dermal neurons and causes histamine release from skin mast cells. Inflamm Res 47: 488– 492, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Rambotti MG, Simonetti S, Spreca A. PACAP activated adenylate cyclase in human sweat glands. An ultracytochemical study. Eur J Histochem 46: 223– 228, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J Physiol 136: 489– 497, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roosterman D, George T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroendocrine organ. Physiol Rev 86: 1309– 1379, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Rowell LB. Cardiovascular adjustments to thermal stress. In: Handbook of Physiology. The Cardiovascular System. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 27, p. 967– 1024 [Google Scholar]
- 34. Rowell LB. Human Circulation: Regulation during Physical Stress. New York: Oxford Univ; Press, 1986 [Google Scholar]
- 35. Rowell LB, Murray JA, Brengelmann GL, Kraning KK II. Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circ Res 24: 711– 724, 1969 [DOI] [PubMed] [Google Scholar]
- 36. Saumet JL, Kellogg DL, Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478– 481, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Savage MV, Brengelmann GL, Buchan AMJ, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol 69: 2149– 2154, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Shastry S, Minson CT, Wilson SA, Dietz NK, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467– 472, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 93: 1947– 1951, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Steinhoff M, McGregor GP, Radleff-Schlimme A, Steinhoff A, Jarry H, Schmidt WE. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept 80: 49– 55, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Stephens DP, Saad A, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1401– H1409, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Summers MA, O'Doriso MS, Cox MO, Lara-Marquez M, Goetzl EJ. A lymphocyte-generated fragment of vasoactive intestinal peptide with VPAC1 agonist activity and VPAC2 antagonist effects. J Pharmacol Exp Ther 306: 638– 645, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Tainio H. Cytochemical localization of VIP-stimulated adenylate cyclase activity in human sweat glands. Br J Dermatol 116: 323– 328, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586– 1592, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Tse DLY, Pang RTK, Wong AOM, Chan SM, Vaurdy H, Chow BKC. Identification of a potential receptor for both peptide histidine isoleucine and peptide histidine valine. Endocrinology 142: 1327– 1336, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Usdin TB, Bonner TI, Mezey E. Two receptors for Vasoactive Intestinal Polypeptide with similar specificity and complementary distributions. Endocrinology 135: 2662– 2680, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Wallengren J. Vasoactive peptides in the skin. J Invest Derm Symp Proc 2: 49– 55, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Warren JB, Cockcroft JR, Larkin SW, Kajekar R, Macrae A, Ghatei MA, Bloom SR. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator in humans. J Cardiovasc Pharmacol 20: 83– 87, 1992 [PubMed] [Google Scholar]
- 49. Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 97: 1291– 1298, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Wilkins BW, Martin EA, Roberts SK, Joyner MJ. Preserved reflex vasodilation in cystic fibrosis does not include an enhanced nitric oxide-dependent mechanism. J Appl Physiol 102: 2301– 2306, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. The vasoactive intestinal peptide fragment VIP10–28 and active vasodilation in human skin. J Appl Physiol 99: 2284– 2301, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilation in humans. J Physiol 577: 1043– 1051, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in humans. J Physiol 568: 1047– 1056, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoshi-Ichiro K, Lee K, Mack GW. Active cutaneous vasodilation in resting humans during mild heat stress. J Appl Physiol 98: 829– 837, 2005. [DOI] [PubMed] [Google Scholar]