Abstract
Neurons in several areas of monkey frontal cortex exhibit ordinal position (rank) selectivity during the performance of serial order tasks. It has been unclear whether rank selectivity or the dependence of rank selectivity on task context varies across the areas of frontal cortex. To resolve this issue, we recorded from neurons in the supplementary motor area (SMA), presupplementary motor area (pre-SMA), supplementary eye field (SEF), and dorsolateral prefrontal cortex (dlPFC) as monkeys performed two oculomotor tasks, one requiring the selection of three actions in sequence and the other requiring the selection of three objects in sequence. We found that neurons representing all ranks were present in all areas. Only to a moderate degree did the prevalence and nature of rank selectivity vary from area to area. The two most prominent inter-area differences involved a lower prevalence of rank selectivity in the dlPFC than in the other areas and a higher proportion of neurons preferring late ranks in the SMA and SEF than in the other areas. Neurons in all four areas are rank generalists in the sense of favoring the same rank in both the serial action task and the serial object task.
INTRODUCTION
The performance of serial order tasks is thought to depend critically on cortical areas of the frontal lobe (Ashe et al. 2006; Curran and Keele 1993; Dominey et al. 1998; Hikosaka et al. 1999; Hikosaka et al. 2002; Jueptner et al. 1997; Marshuetz 2005; Rhodes et al. 2004; Tanji 2001). One sign of frontal-lobe involvement is the observation that numerous frontal areas contain rank-selective neurons: neurons the activity of which depends on the stage the monkey has attained in a learned sequence of actions (Akkal et al. 2002; Averbeck et al. 2002, 2003; Clower and Alexander 1998; Funahashi et al. 1997; Inoue and Mikami 2006; Isoda and Tanji 2002, 2003; Lee and Quessy 2003; Lu et al. 2002; Nakajima et al. 2009; Shima and Tanji 2000; Sohn and Lee 2007). Presumably these areas act in concert to mediate serial order behavior with each making a distinctive contribution. However, it is still unclear in what way the contributions of multiple areas differ from each other. This issue remains unresolved because for the most part, studies of rank selectivity in different areas have been carried out under different conditions. Rank selective neurons have been observed in the frontal and supplementary eye fields (FEF and SEF, respectively) in the context of oculomotor sequence tasks (Isoda and Tanji 2002, 2003; Lu et al. 2002), in the presupplementary and supplementary motor areas (pre-SMA and SMA, respectively) in the context of manual sequence tasks (Clower and Alexander 1998; Lee and Quessy 2003; Nakajima et al. 2009; Shima and Tanji 2000; Sohn and Lee 2007; but see also Isoda and Tanji 2004), and in the dorsolateral prefrontal cortex (dlPFC) in the context of sequential tasks requiring button presses (Barone and Joseph 1989), tracing of geometrical shapes (Averbeck et al. 2002, 2003), saccades (Averbeck et al. 2006), and memorization of the order of presentation of a sequence of images (Inoue and Mikami 2006; Ninokura et al. 2003, 2004). In only a few cases were two closely related areas directly compared in the same behavioral context (pre-SMA and SMA: Clower and Alexander 1998; Nakajima et al. 2009; Shima and Tanji 2000; Sohn and Lee 2007; SEF and FEF: Isoda and Tanji 2003). Because of the procedural inconsistencies, it is unclear whether rank-related activity varies systematically across areas.
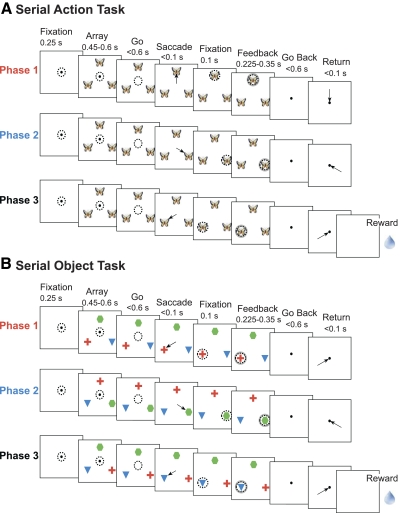
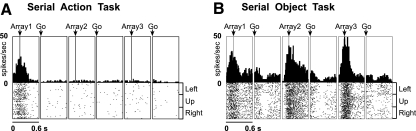
To resolve this issue, we recorded from four frontal areas (SMA, pre-SMA, SEF, and dlPFC) in the same monkeys during performance of the same two tasks: a serial action task and a serial object task (Berdyyeva and Olson 2009). The serial action task required making saccades in a fixed sequence of directions (Fig. 1A). The serial object task required making saccades to a fixed sequence of objects (Fig. 1B). The tasks shared a requirement that the monkeys remain oriented with respect to position in the sequence but differed with respect to the rule governing what should be selected at each stage. This approach allowed us to compare the four areas systematically with regard not only to the presence and nature of rank selectivity but also its tendency to generalize across task contexts.
Fig. 1.
Sequence of events in 2 serial order tasks. Arrows indicate saccades and broken circles indicate direction of gaze. A: a typical trial in the serial action task. The identity of an image presented at 3 locations in the visual field periphery served as a sequence cue (e.g., the picture of a butterfly instructed the monkey to make a saccade upward during the 1st trial phase, rightward during the 2nd phase, and leftward during the 3rd phase). Six different pictures signaled 6 different sequences. B: a typical trial in the serial object task. Monkeys had to make a saccade toward the red cross in the 1st trial phase, to the green hexagon during the 2nd phase, and to the blue triangle during the 3rd phase irrespective of the objects' locations. The object positions on the screen were changed in each trial phase.
METHODS
General methods
SUBJECTS.
We used two adult male rhesus monkeys (Macaca mulatta). Monkey O had been trained previously on a variety of manual and oculomotor tasks. Monkey T was experimentally naïve. Experimental procedures were approved by the Carnegie Mellon University Animal Care and Use Committee and were in compliance with the guidelines set forth in the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
PREPARATORY SURGERY.
At the outset of the training period, each monkey underwent sterile surgery under general anesthesia maintained with isofluorane inhalation. The top of the skull was exposed, bone screws were inserted around the perimeter of the exposed area, a continuous cap of rapidly hardening acrylic was laid down so as to cover the skull and embed the heads of the screws, a head-restraint bar was embedded in the cap, and scleral search coils were implanted on the eyes with the leads directed subcutaneously to plugs on the acrylic cap. Following initial training, recording chambers were implanted into the acrylic. At each selected site, a 2-cm-diam disk of acrylic and skull was removed. A cylindrical recording chamber was cemented into the hole with its base flush to the exposed dural membrane.
Chambers were placed either at a medial location (over SEF, pre-SMA, and SMA), or at a lateral location (over dlPFC). The medial chambers were centered on the midline ∼21 mm anterior to the Horsley–Clarke interaural plane. The lateral chambers were centered approximately at anterior 23 mm and lateral 23 mm in the left hemisphere. Recording was carried out from the medial chamber first and from the lateral chamber second in monkey O. In monkey T, the order was reversed. The reversal of order was intended to minimize the possibility that measured differences between the lateral and medial areas were secondary to differences in the level of practice.
SINGLE UNIT RECORDING.
At the beginning of each day's session, a varnish-coated tungsten microelectrode with an initial impedance of several megohms at 1 kHz (Frederick Haer and Co., Bowdoinham, ME) was advanced vertically through the dura into the underlying cortex using a hydraulic microdrive (Narishige, Tokyo, Japan). The electrode could be placed reproducibly at points forming a square grid with 1 mm spacing (Crist et al. 1988). In the case of monkey O, the action potentials of single neurons were isolated from the multineuronal trace by means of an on-line spike-sorting system using a template-matching algorithm (Signal Processing Systems, Prospect, Australia); the spike-sorting system, on detection of an action potential, generated a pulse the time of which was stored with 1 ms resolution. In case of monkey T, three microelectrodes were inserted simultaneously at different grid locations, and single neurons from each microelectrode were sorted using both on-line and off-line template matching and principal components analysis (Plexon, Dallas, TX).
RECORDING SITES.
After installing a recording chamber, we confirmed the location of the chamber relative to the gross anatomical landmarks by collecting structural magnetic resonance (MR) images. The images were collected by use of a Bruker BioSpin 4.7 T magnet in which the anesthetized monkey was supported by an MR-compatible stereotaxic device. Fiducial marks made visible by means of a contrast agent included the centers of the ear bars and selected locations inside the recording chamber. Frontoparallel 2-mm-thick slices spanning the entire brain were collected. In addition, 2-mm-thick slices were collected in monkey T parallel to the cortical surface underlying the lateral chamber.
Recording sites under the lateral chambers were in dlPFC within and ventral and dorsal to the principal sulcus as revealed by examination of structural MR images (Fig. 2).1 They were located 7–16 mm anterior to the posterior tip of the principal sulcus in monkey O [mean = 10.5 ± 2.7 (SD) mm] and 7–17 mm anterior to the posterior tip of the principal sulcus in monkey T (11.1 ± 2.5 mm). They overlap regions examined in comparable studies from other laboratories (Hasegawa et al. 2004; Shima et al. 2007) but may extend a few mm farther anterior. Recording sites under the medial chambers were assigned to three areas (SEF, SMA, and pre-SMA) according to the following criteria (Matsuzaka et al. 1992; Picard and Strick 1996; Russo and Bruce 1993 2000).
Fig. 2.
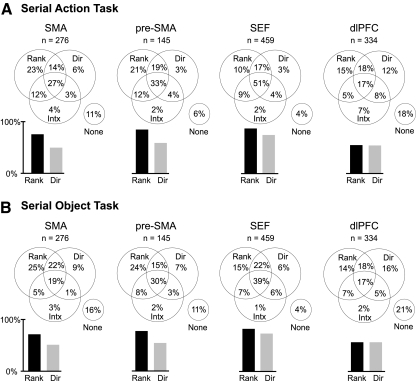
Frequency of significant neuronal effects of rank, direction, and their interaction in each area during performance of the serial action task (A) and serial object task (B). Venn diagrams parcel out all possible combinations of effects. Bar histograms give total percentages of neurons exhibiting main effects of rank and direction regardless of what other effects were present. The presence of a main effect of rank indicated that firing varied significantly as a function of rank with the effect of direction factored out, regardless of what other effects were present. Likewise for a main effect of direction.
Supplementary eye field.
A site was assigned to the SEF if it met the following conditions: it was located 4–8 mm anterior to the genu of the arcuate sulcus and 2–6 mm lateral to the midline as determined by structural MRI; at it or an immediately adjacent site, fixed vector or convergent saccades could be reproducibly elicited with electrical microstimulation at current levels of ≤80 μA (100 ms train of biphasic pulses of 0.2 ms duration at 333 Hz); and neither at this nor at an immediately adjacent site did electrical microstimulation at current levels ≤200 μA elicit orofacial or other bodily movements. These sites were located anteriolateral to the SMA and pre-SMA.
Presupplementary motor area.
A site was assigned to the pre-SMA if it met the following conditions: it was located on the medial wall of the hemisphere 1–6 mm anterior to the genu of the arcuate sulcus as determined by structural MRI; at it or an immediately adjacent site, electrical microstimulation at a current strength 75 −150 μA (100 ms train of biphasic pulses of 0.2 ms duration at 333 Hz) elicited complex multijoint arm movements involving large groups of muscles but did not elicit any bodily or orofacial movements at currents ≤70 μA and did not elicit any eye movements at currents ≤200 μA; and light cutaneous stimulation of the body including the face did not elicit brisk phasic neuronal responses (although visual stimulation such as turning the room lights on and off might). These sites were located anterior to the SMA and posteromedial to the SEF.
Supplementary motor area.
A site was assigned to the SMA if it met the following conditions: it was located 1–6 mm caudal to the genu of the arcuate sulcus either on the medial wall of the hemisphere or on the gyral surface ≤4 mm from the midline as determined by structural MRI; electrical microstimulation at a current strength under 50 μA (50–100 ms train of biphasic pulses of 0.2 ms duration at 333 Hz) elicited simple, brisk body twitching or simple brisk single-joint hind- or forelimb movements involving a small isolated groups of muscles and did not elicit eye movements at currents ≤100 μA; and light cutaneous stimulation of the body elicited brisk phasic neuronal responses but visual stimulation did not. These sites were located posterior to the pre-SMA and posteriomedial to the SEF.
Finding the border between the SMA and pre-SMA.
Beginning at the back of the recording chamber, we stimulated a succession of sites at progressively more anterior levels in 1 mm steps (50–100 ms train of biphasic pulses of 0.2 ms duration at 333 Hz, 50–75 μA). At each site, we noted the type of movement elicited at lowest current strength, for example distal or proximal hind- or forelimb, upper or lower body or orofacial. With anterior progress of the stimulation site, we noted a rough topographical trend from the distal hindlimbs to the orofacial region followed by a topographical reversal to complex, slow, multijoint forelimb movements elicited at relatively high currents. The level of reversal was taken as the border between the SMA and the pre-SMA. This level coincided with the level at which neuronal somatosensory responses gave way to visual responses.
BEHAVIORAL CONTROL AND DATA COLLECTION.
All aspects of behavioral procedure, including presentation of stimuli, monitoring of eye movements, and delivery of reward, were under the control of a computer running Cortex software (NIMH Cortex) in a DOS operating system. Eye position was monitored by means of a scleral search coil system (Riverbend Instruments, Birmingham, AL). The x and y coordinates of eye position were stored at 4-ms intervals. Reward was delivered through a spigot under control of a solenoid valve on successful completion of each trial.
Task design
The monkeys performed two tasks. Each task required generating a series of three saccades to peripheral targets. However, the tasks differed with regard to the rule governing target selection. The rule was spatial in the case of the serial action task and object-based in the case of the serial object task. During recording from each site, the monkey performed both the serial action task and serial object task. The order of the tasks alternated from site to site.
SERIAL ACTION TASK.
Basic design principle.
On any given trial, the same image was placed at all three target locations. The identity of this image indicated in what order saccades must be made to the three locations.
Sequence of events in a trial.
The monkey initiated a trial by acquiring central fixation. Two hundred and fifty milliseconds after attainment of fixation, three identical pictures (∼4.5° across) appeared at locations 11.4° eccentric spaced at equal intervals of 120° around fixation – straight up, down and to the right, and down and to the left. Disappearance of the central fixation spot after a variable interval (450–600 ms) signaled the monkey to make a saccade to the first location. One hundred milliseconds after completion of the saccade, a feedback stimulus appeared: a white annulus centered on the target. After a variable additional interval of eccentric fixation (225–350 ms), all peripheral stimuli vanished and the central fixation spot reappeared, signaling the monkey to execute a saccade back to the center. After 150 ms of central fixation, the array of pictures reappeared and the series of events was repeated with the sole exception that the saccade must be directed to the second location. The third phase of the trial consisted of an equivalent series of events with the third location as the target. The trial terminated with 25 ms of central fixation followed by offset of all stimuli and delivery of reward. In the case of a saccade toward an incorrect location or a fixation break, the trial was aborted.
Number of conditions.
Six different pictures instructed six different movement sequences. These represented all possible sequences in which the three locations could be, once each, successively selected.
Blocking.
To reduce task difficulty, the monkey was allowed to complete a block of four successful trials under a given condition before another condition was imposed. Within a group of six successive blocks, each condition was imposed once.
Number of trials.
The session terminated when the monkey had successfully completed 24 trials under each of the six conditions.
Visually guided trials.
In the event that the monkey selected an incorrect target on three successive trials, visual guidance was provided on the fourth trial by making the image at the location of the current target brighter than the other two. The frequency of trials on which visual guidance was provided, expressed as a percentage of all correct trials, was small (monkey O: SMA, 2.1%; pre-SMA, 2.0%, SEF, 2.7%, dlPFC, 2.5%; monkey T: SMA, 13.2.0%; pre-SMA, 14.2%, SEF, 15.9%, dlPFC, 16.2%). Visually guided trials were excluded from all subsequent analysis.
SERIAL OBJECT TASK.
Basic design principle.
The monkey was required to make saccades to three images (red cross, green hexagon, and blue triangle in succession) on every trial. After each saccade, the images were subject to rearrangement. Thus the monkey could not plan in advance a fixed series of movements.
Sequence of events in a trial.
The sequence and timing of events and the spatial arrangement of stimuli were the same as in the serial action task.
Number of conditions.
The current target (red cross during phase 1, green hexagon during phase 2, and blue triangle during phase 3) could occupy any of three locations. There were 27 conditions representing all possible sequences of target locations. The two nontarget images were assigned randomly to the two nontarget locations at each phase of the trial.
Number of trials.
A session terminated when the monkey had successfully completed four trials under each of the 27 conditions.
Neuronal database
We discontinued recording at a given site, if it appeared that neuronal activity was unmodulated during performance of the first task (which task this was alternated from site to site) as indicated by inspection of raster and histogram displays. In the case of a neuron from which a full data set had been collected, we subsequently discarded the data if it did not fulfill even a generous criterion for task-related activity, namely that during at least one within-trial epoch, the firing rate was significantly different (t-test, P < 0.05) from the firing rate during a pretrial baseline period of 200 ms, terminating with the initial onset of the fixation spot. The within-trial epochs considered in this analysis were 1) peri-array epoch (50 ms before to 450 ms after onset of the array); each peri-array epoch was considered separately for three trial phases and three directions, resulting in nine peri-array epochs for each neuron; and 2) peri-saccade epoch (200 ms before to 300 ms after initiation of saccade); each peri-saccade epoch was considered separately for six saccades (3 to the target and 3 back to the center) and three directions, resulting in 18 peri-saccade epochs for each neuron.
Only neurons recorded in both tasks and exhibiting modulation in at least one of them were included in the database. The number of neurons selected for analysis in each area and in each monkey is provided in Table 1.
Table 1.
Counts of neurons recorded and selected for study in each area
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||
|---|---|---|---|---|---|---|---|---|
| Recorded | Selected | Recorded | Selected | Recorded | Selected | Recorded | Selected | |
| Monkey O | 86 | 85 | 67 | 65 | 117 | 113 | 189 | 185 |
| Monkey T | 195 | 191 | 80 | 80 | 349 | 346 | 158 | 149 |
| Combined | 281 | 276 | 147 | 145 | 466 | 459 | 347 | 334 |
SMA, supplementary motor area; pre-SMA, presupplementary motor area; SEF, supplementary eye field; dlPFC, dorsolateral prefrontal cortex.
Counts of significant neuronal effects
To characterize the dependence of each neuron's firing rate on rank and saccade direction, we carried out two ANOVAs. The first concerned data from the three peri-array epochs (50 ms before to 450 after array onset). The second concerned data from the three peri-saccade epochs (200 ms before to 300 ms after initiation of the centrifugal saccade). In each case, firing rate was the dependent variable and the two factors were rank (1st, 2nd, or 3rd) and saccade direction (left, up, or right). The threshold for significance was taken as P < 0.05.
Histograms of single neuron and population activity
To create a histogram representing the activity of a single neuron or of a population under a selected set of conditions, we parsed the data from each trial into six blocks. These were obtained by crossing three trial phases (1st, 2nd, and 3rd) with two periods of each phase (array and saccade). The array period extended from 150 ms before array onset (when the only stimulus visible on the screen was the fixation spot) to 450 ms after array onset. The saccade period extended from offset of the fixation spot to a point in time 600 ms later and thus encompassed the planning and execution of both the centrifugal and the centripetal saccade. Cutting out and splicing these blocks allowed creating a continuous record of activity during the trial in which the effects of trial-to-trial jitter (arising from random variation of the delay period and from spontaneous variation of the saccadic reaction time) were minimized.
Representing rank selectivity as a continuous variable
For each neuron, we computed the mean firing rate during the peri-saccade epoch (200 ms before to 300 ms after initiation of the centrifugal saccade) of phases 1–3: R1–R3. We removed information about absolute (as distinct from relative) firing rate by normalizing the resulting values to their sum, obtaining a three-component normalized rank vector capturing the neuron's pattern of serial position preference: (R1, R2, R3). We reduced this to two-component vector by projection onto the two-dimensional (2-D) simplex. This involved no loss of information because the components of the normalized vector, summing to one, possessed only 2 df. We oriented the plot so that the vector representing a pure rank 1 neuron (a neuron active only during phase 1) would point straight up (0°) and vectors representing pure rank 2 and rank 3 neurons would point 120 and 240° clockwise, respectively. We counted the vectors in each of 48 bins centered on values ranging in 7.5° steps from 0 to 352.5°. The counts are represented by radial height in the resulting plots.
Cross-task correlation of rank selectivity
We carried out independent correlation analyses on data from the peri-saccade epoch (200 ms before to 300 ms after initiation of the centrifugal saccade) and the peri-array epoch (50 ms before to 450 after array onset). In each case, we first computed for each neuron the mean firing rate during the during phases 1–3 in the serial action task: A1–A3. We then normalized the values to their sum so as to obtain a three-component normalized rank vector: (A1, A2, A3). By an identical procedure, we obtained a three-component normalized rank vector based on activity in the serial object task: (O1, O2, O3). We then computed the coefficient of correlation across neurons between the two rank vectors using the Matlab corrcoef function.
RESULTS
Rank selectivity in the serial action task
To identify neurons that fired differentially as a function of trial phase, we carried out an ANOVA (α = 0.05) with saccade direction and rank as factors and with firing rate during the peri-saccadic epoch (200 ms before to 300 ms after initiation of the centrifugal saccade) as the dependent variable. A majority of neurons in each area exhibited a significant main effect of rank (Fig. 2A; Table 2) indicating that, with saccade direction factored out, they fired significantly more strongly in conjunction with some trial phases than with others. We conclude that rank selectivity was robust in all four areas.
Table 2.
Counts of neurons significantly selective for rank in the serial action task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | Rank | Total | Rank | Total | Rank | Total | Rank | |
| Monkey O | 85 | 81 | 65 | 53 | 113 | 90 | 185 | 91 |
| Monkey T | 191 | 129 | 80 | 70 | 346 | 311 | 149 | 92 |
| Combined | 276 | 210 | 145 | 123 | 459 | 401 | 334 | 183 |
| % of Total | 76 | 85 | 87 | 55 | ||||
To determine whether the differences from area to area in the proportion of rank-selective neurons were significant, we carried out a χ2 test. This revealed that the cross-area variation in the frequency of rank selectivity was highly significant (χ2 test, P < 10−8). Pairwise comparison of areas (χ2 test on 6 pairs, α = 0.05) revealed that a single robust difference accounted for this effect: the frequency of rank selectivity was significantly lower in the dlPFC than in the other areas ( P < 10−6 for the pairs SMA–dlPFC, pre-SMA—dlPFC, and SEF-dlPFC). On independent analysis of each monkey's data, we found that the trends were the same and that they were significant (P < 0.003) with the exception of the SMA–dlPFC comparison in monkey T. We conclude that rank selectivity was less prevalent in the dlPFC than in the other areas.
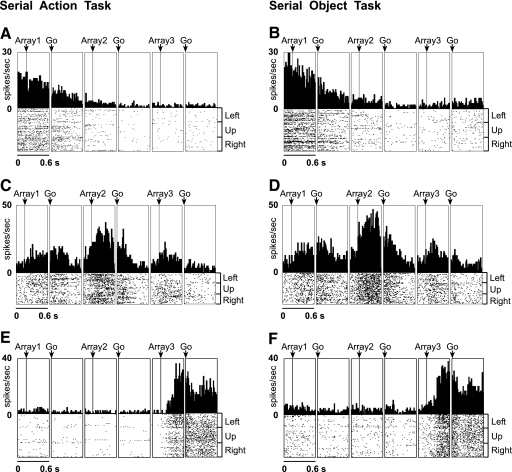
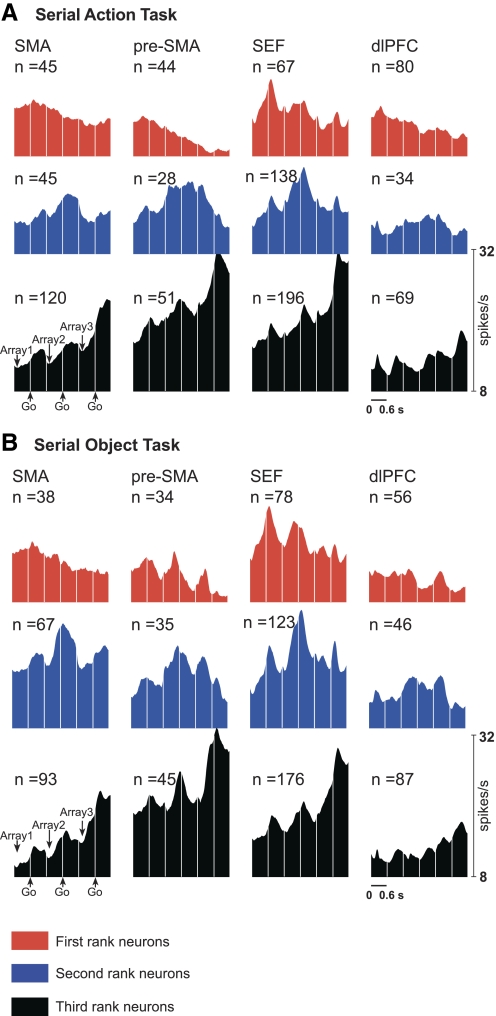
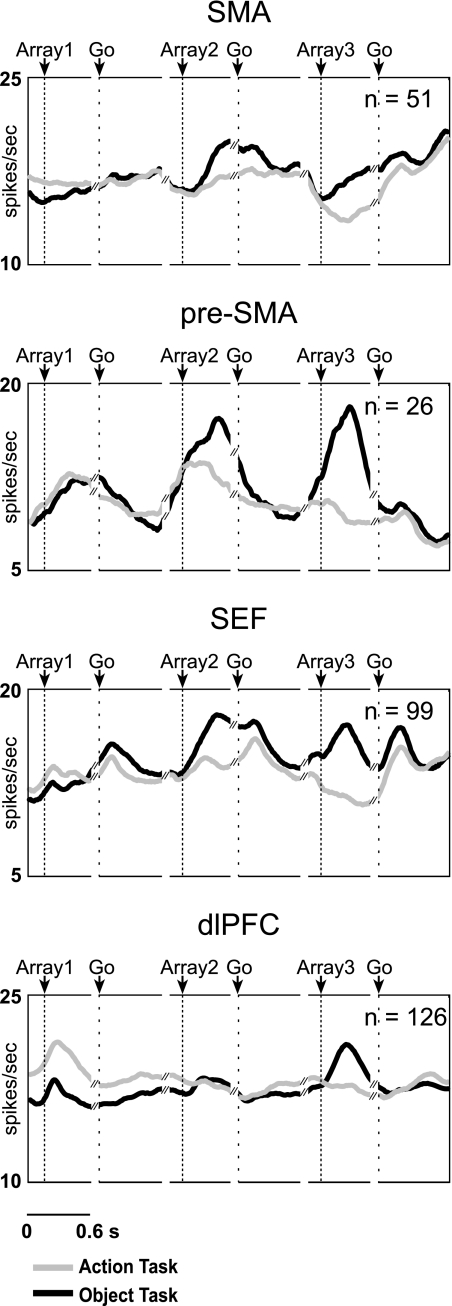
We next asked whether each area contained neurons selective for the first, second, and third rank. Examples of selectivity for each rank are shown in Fig. 3,A, C, and E. The preferred rank of each significantly rank-selective neuron was taken as the phase of the trial during which its firing rate was highest. The distribution of preferred ranks in each area is provided in the bar histograms of Fig. 4A and in Table 3. The timing and strength of activity in each rank-preferring group of neurons can be judged from the population histograms of Fig. 5A. The results indicate that each area contained neurons selective for each rank.
Fig. 3.
Examples of rank-selective neurons. Data in the “array” panels are aligned on array presentation. Data in the “go” panels are aligned on the go signal for the centrifugal saccade (offset of the central fixation spot). Presupplementary motor area (pre-SMA) neuron selective for 1st rank in the serial action task (A) and serial object task (B). Supplementary eye field (SEF) neuron selective for 2nd rank in the serial action task (C) and serial object task (D). SMA neuron selective for 3rd rank in the serial action task (E) and serial object task (F).
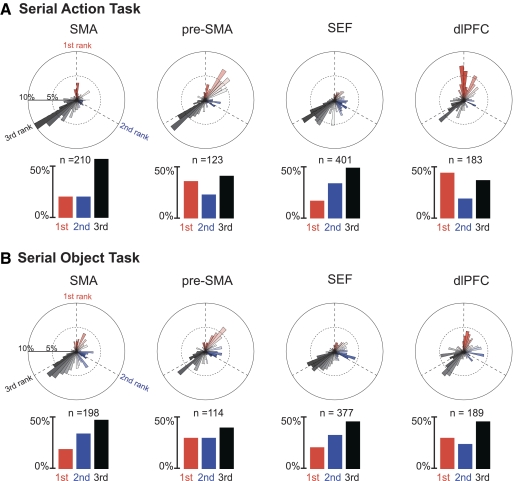
Fig. 4.
Distribution of rank-selective neurons by preferred rank in the serial action task (A) and serial object task (B). Polar plots represent the frequency distribution (radial axis) of neurons according to a continuous measure of preferred rank (theta axis) in which pure categorical rank 1, 2, or 3 selectivity corresponded to 0, 120, and 240° (- - -). Bar histograms represent the frequency distribution of neurons on the basis of which trial phase elicited strongest firing.
Table 3.
Distribution of selective neurons by preferred rank in the serial action task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | |
| Monkey O | 7 | 14 | 60 | 81 | 24 | 18 | 11 | 53 | 20 | 30 | 40 | 90 | 38 | 16 | 37 | 91 |
| Monkey T | 38 | 31 | 60 | 129 | 20 | 10 | 40 | 70 | 47 | 108 | 156 | 311 | 42 | 18 | 32 | 92 |
| Combined | 45 | 45 | 120 | 210 | 44 | 28 | 51 | 123 | 67 | 138 | 196 | 401 | 80 | 34 | 69 | 183 |
| % of Total | ||||||||||||||||
| First | 21 | 36 | 17 | 44 | ||||||||||||
| Second | 21 | 23 | 34 | 19 | ||||||||||||
| Third | 58 | 41 | 49 | 37 | ||||||||||||
Tot *, total number of rank selective neurons in a given area.
Fig. 5.
Mean firing rate as a function of time for SMA, pre-SMA, SEF, and dlPFC neurons classified as selective for the 1st, 2nd, or 3rd rank in the serial action task (A) and serial object task (B). Data in the array panels are aligned on array presentation. Data in the go panels are aligned on the signal for the centrifugal saccade (offset of the central fixation spot).
To determine whether the differences from area to area in the proportion of neurons preferring the first, second, and third rank were significant, we carried out a χ2 test. This revealed that the variation across areas with respect to the relative frequency of neurons preferring the first, second and third rank was highly significant (P < 10−10; χ2 test). Pairwise comparison of areas (χ2 test on 6 pairs, α = 0.05) revealed a single pattern that was both significant and consistent across monkeys. The dlPFC differed from the SMA (P < 10−7 in combined data) and the SEF (P < 10−8 in combined data) in that first-rank neurons were preponderant in the dlPFC, whereas third-rank neurons were preponderant in the two other areas. We conclude that the dlPFC differed from other areas by virtue of containing more neurons with a preference for rank 1.
A rank-selective neuron might exhibit categorical selectivity (firing at a high rate for rank 3 and at a uniformly low rate for ranks 2 and 1) or continuous selectivity (firing at a high rate for rank 3, at an intermediate rate for rank 2, and at a low rate for rank 1). Neurons with categorical selectivity tend to take pride of place in figures, including our own (Fig. 3) but are not necessarily representative of the population as a whole. To determine to what degree rank selectivity was categorical as opposed to continuous, we assigned to each neuron a two-dimensional vector based on its firing rates during phases 1–3 (methods). The distribution of vectors across neurons is summarized in the polar bar histograms of Fig. 4A, where the principal directions representing pure categorical selectivity are straight up, 120° clockwise and 240° clockwise for ranks 1–3, respectively. Bars representing vectors within ±30° of the principal axes for ranks 1–3 are colored red, blue, and black, respectively. If there were no tendency toward categorical selectivity, we would expect the count of vectors within ±30 of the principle axes to equal the count of vectors outside this range. Contrary to this prediction, in the SMA, SEF, and dlPFC, vectors close to the principal axes were more numerous than vectors far from them. The trend was significant in combined data (χ2 test; SMA, P = 0.0001; SEF, P < 10−6; dlPFC, P = 0.0019) and was present in both monkeys. We conclude that there was an overall trend toward categorical rank selectivity.
The tendency toward categorical selectivity might have been present for one rank or for several. To investigate this issue, we repeated the analysis for each 120° domain of the plane centered on a principal axis. We found that, in each area, neurons selective for rank 3 tended to exhibit categorical selectivity. This effect was significant in the combined data (SMA: P < 10−6; pre-SMA: P = 0.0008; SEF: P < 10−8; dlPFC: P = 0.03) and was present in each monkey. Among neurons preferring rank 1, categorical selectivity was significantly preponderant only in the dlPFC (P = 0.0012), where the trend was present in both monkeys. Among neurons preferring rank 2, there was no trend toward categorical selectivity (P = 0.99; pre-SMA: P = 0.85; SEF: P = 0.44; dlPFC: P = 0.39). We conclude that the trend toward categorical rank selectivity arose from the presence in all four areas of neurons that fired markedly more strongly during the third phase of the trial than during the prior two phases and from the presence in the dlPFC alone of neurons that fired markedly more strongly during the first phase of the trial than during the ensuing two phases.
Rank selectivity in the serial object task
To identify neurons that fired differentially as a function of trial phase, we carried out an ANOVA based on activity during the peri-saccadic epoch as described in the preceding section. A majority of neurons in each area exhibited a significant main effect of rank (Fig. 2B; Table 4). We conclude that—as in the serial action task—rank selectivity was robust in all four areas.
Table 4.
Counts of neurons significantly selective for rank in the serial object task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | Rank | Total | Rank | Total | Rank | Total | Rank | |
| Monkey O | 85 | 76 | 65 | 54 | 113 | 91 | 185 | 112 |
| Monkey T | 191 | 122 | 80 | 60 | 346 | 286 | 149 | 77 |
| Combined | 276 | 198 | 145 | 114 | 459 | 377 | 334 | 189 |
| % of Total | 72 | 79 | 87 | 57 | ||||
To determine whether the differences from area to area in the proportion of rank-selective neurons were significant, we carried out a χ2 test. This revealed that the cross-area variation in the frequency of rank selectivity was highly significant (χ2 test, P < 10−8). Pairwise comparison of areas (χ2 test on 6 pairs, α = 0.05) revealed that a single robust difference accounted for this effect. The frequency of rank selectivity was significantly lower in the dlPFC than in the other areas (SMA–dlPFC, P = 0.0002; pre-SMA–dlPFC, P < 10−6; SEF–dlPFC, P < 10−7). On independent analysis of each monkey's data, we found that the trends were the same and that they were significant (P < 0.03 for all comparisons). We conclude that—as in the serial action task—rank selectivity was less prevalent in the dlPFC than in the other areas.
We next asked whether each area contained neurons selective for the first, second, and third rank. Examples of selectivity for each rank are shown in Fig. 3, B, D, and F. The distribution of preferred ranks in each area is provided in the bar histograms of Fig. 4B and in Table 5. The timing and strength of activity in each rank-preferring group of neurons can be judged from the population histograms of Fig. 5B. The results indicate that—as in the serial action task—each area contained neurons selective for each rank.
Table 5.
Distribution of selective neurons by preferred rank in the serial object task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | 1st | 2nd | 3rd | Tot* | |
| Monkey O | 4 | 17 | 55 | 76 | 17 | 25 | 12 | 54 | 12 | 35 | 44 | 91 | 36 | 29 | 47 | 112 |
| Monkey T | 34 | 50 | 38 | 122 | 17 | 10 | 33 | 60 | 66 | 88 | 132 | 286 | 20 | 17 | 40 | 77 |
| Combined | 38 | 67 | 98 | 198 | 34 | 35 | 45 | 114 | 78 | 123 | 176 | 377 | 56 | 46 | 87 | 189 |
| % of Total | ||||||||||||||||
| First | 19 | 30 | 21 | 30 | ||||||||||||
| Second | 34 | 30 | 33 | 24 | ||||||||||||
| Third | 47 | 40 | 46 | 46 | ||||||||||||
Tot *, total number of rank selective neurons in a given area.
To determine whether the differences from area to area in the proportion of neurons preferring the first, second, and third rank were significant, we carried out a χ2 test. The result approached significance (P = 0.052). Pairwise comparison between areas (χ2 test on six pairs, α = 0.05) revealed a significant trend present in both monkeys whereby the dlPFC differed from the SMA (P = 0.010) and SEF (P = 0.011) in containing more rank-one-selective neurons. We conclude that—as in the serial action task—the dlPFC differed from other areas in containing more neurons with a preference for rank 1 but that the strength of this effect was reduced relative to its strength in the serial action task.
To determine whether neurons encoded rank in a categorical or continuous manner, we compared the count of neurons with rank vectors closer than ±30° to a principal rank axis to the count of neurons with rank vectors farther away than ±30° (Fig. 4B). In three areas, neurons with vectors close to a principal axis significantly outnumbered those with vectors farther away (SMA: P = 0.0001; SEF: P = 0.0020; dlPFC: P = 0.0057; pre-SMA: P = 0.70). In each case, the trend was present in both monkeys. We conclude that—as in the serial action task—there was an overall trend toward categorical rank selectivity.
To check whether this effect was present for all or only for some ranks, we repeated the analysis separately for each rank. We found that, in each of the three areas, there was a significant preponderance of categorical selectivity among neurons favoring rank 3 (SMA, P = 0.0004; pre-SMA, P = 0.015; SEF, P < 10−8; dlPFC, P = 0.0006) but not among neurons favoring other ranks. We conclude that—as in the serial action task—the trend toward categorical rank selectivity was present in all four areas during phase 3 but that—unlike in the serial action task—it was not present in the dlPFC during phase 1.
Overall the rate of incidence and the pattern of rank-related activity was closely similar between the two tasks. The single marked exception arose in the dlPFC, where the number of neurons selective for rank 1 was higher and the tendency for rank 1 selectivity to be categorical was greater in the serial action than in the serial object task. The higher prevalence of neurons active during phase 1 may have been related to the greater information processing opportunities present during this phase: in the serial action task, the monkey could plan out the entire sequence of saccades at the beginning of the trial, whereas in the serial object task he could only plan the first saccade.
Cross-task concordance of rank signals
Neurons in a given area might encode rank at an abstract level, signaling rank identically regardless of task context, or, alternatively, might signal rank in a context-specific manner. To distinguish between these possibilities, we analyzed the degree of concordance between rank signals observed during the peri-saccadic epoch in the serial action and serial object tasks. In all areas, neurons exhibiting rank selectivity in one task showed a highly significant tendency to do so in the other (χ2 test; SMA, SEF, and dlPFC: P < 10−8; pre-SMA: P < 10−5). Moreover, among neurons exhibiting rank selectivity in both tasks, cases of cross-task agreement in preferred rank (counted along the diagonal in each 3 × 3 matrix of Table 6) were highly significantly more numerous than expected on the assumption of independence (χ2 test, P < 10−10 in each area). Three examples supporting this point are shown in Fig. 3. We conclude that there was a strong tendency for neurons preferring a given rank in one task to do so in the other.
Table 6.
Distribution of neurons significantly rank selective in both tasks with regard to preferred rank in the action task (Act) and in the object task (Obj)
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Obj | 2nd Obj | 3rd Obj | 1st Obj | 2nd Obj | 3rd Obj | 1st Obj | 2nd Obj | 3rd Obj | 1st Obj | 2nd Obj | 3rd Obj | |
| Monkey O | ||||||||||||
| 1st Act | 3 | 2 | 1 | 16 | 6 | 0 | 8 | 6 | 0 | 17 | 6 | 3 |
| 2nd Act | 0 | 10 | 2 | 0 | 16 | 1 | 1 | 17 | 8 | 0 | 9 | 4 |
| 3rd Act | 0 | 5 | 51 | 0 | 2 | 8 | 1 | 5 | 29 | 7 | 4 | 22 |
| Monkey T | ||||||||||||
| 1st Act | 23 | 4 | 0 | 13 | 1 | 2 | 30 | 6 | 1 | 12 | 6 | 6 |
| 2nd Act | 1 | 18 | 3 | 2 | 6 | 0 | 20 | 68 | 5 | 2 | 4 | 3 |
| 3rd Act | 3 | 14 | 32 | 0 | 2 | 31 | 9 | 12 | 119 | 0 | 1 | 23 |
| Combined | ||||||||||||
| 1st Act | 26 | 6 | 1 | 29 | 7 | 2 | 38 | 12 | 1 | 29 | 12 | 9 |
| 2nd Act | 1 | 28 | 5 | 2 | 22 | 1 | 21 | 85 | 13 | 2 | 13 | 7 |
| 3rd Act | 3 | 19 | 83 | 0 | 4 | 39 | 10 | 17 | 148 | 7 | 5 | 45 |
| Totals | 172 | 106 | 345 | 129 | ||||||||
Although there was a tendency in all areas for rank selectivity to be conserved across tasks, nevertheless it might have been the case that the degree of cross-task concordance varied from area to area. To assess this possibility, we tested whether the proportion of rank-selective neurons preferring the same rank in both tasks varied significantly across the four areas studied. We found that it did (χ2 test, P = 0.009). To identify the source of the effect, we carried out post hoc pairwise comparisons. We found that the dlPFC consistently differed from other areas in containing a lower proportion of neurons that preferred the same rank in both tasks (χ2 test, SMA–dlPFC, P = 0.023; pre-SMA–dlPFC, P = 0.003; SEF–dlPFC, P = 0.017). This trend was present in both monkeys.
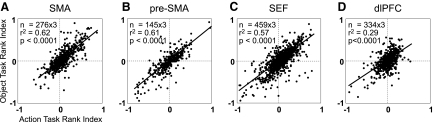
The preceding analysis was based on counts obtained by separating neurons into discrete categories (nonselective and selective with strongest firing for rank 1, 2, or 3). To supplement this approach, we employed an analysis based on a continuous measure. We computed, for each neuron, a three-component normalized rank vector based on firing in the serial action task (A1, A2, A3) and an analogous vector based on firing in the serial object task (O1, O2, O3; methods). Then, for each area, we computed the coefficient of correlation across neurons between the two rank vectors. In each area, the correlation between rank indices in two serial tasks was positive and highly significant: (Table 7; P < 0.0001 for each area). To assess whether areas differed with respect to the degree of correlation between rank vectors observed in the two tasks, we carried out pairwise comparisons of the R values (methods). We found that the dlPFC differed significantly from the other areas in exhibiting a lower degree of concordance (P < 10−6 for all comparisons). The trend was present in both monkeys.
Table 7.
Cross-task correlation between rank indices measured during the peri-saccade epoch and peri-array epoch
| SMA | pre-SMA | SEF | dlPFC | |
|---|---|---|---|---|
| R2 | ||||
| Peri-saccade | 0.62 | 0.61 | 0.57 | 0.29 |
| Peri-array | 0.58 | 0.50 | 0.46 | 0.13 |
All correlations were highly significant: P < 0.0001.
To convey graphically the degree of cross-task correlation in each area, we departed from the use of three-dimensional rank vectors and instead computed three scalar indices dependent on the ability of the neuron to discriminate rank 1 from rank 2, rank 1 from rank 3, and rank 2 from rank 3. For the serial action task, these were (A2 − A1)/(A2 + A1), (A3 − A1)/(A3 + A1) and (A3 − A2)/(A3 + A2), where A1–A3 represent firing rates during trial phases 1–3, respectively. For the serial object task, the corresponding indices were (O2 − O1)/(O2 + O1) and so on mutatis mutandis. We then constructed for each area a scatter plot based on all three indices. The results, shown in Fig. 6, make clear that cross-task concordance was present in all areas but was less in the dlPFC than in the other areas.
Fig. 6.
Correlation between indices of rank selectivity measured in the serial action task (horizontal axis) and serial object task (vertical axis) in each area. Each neuron contributed 3 indices for each task: (F2 − F1)/(F2 + F1), (F3 − F1)/(F + F1) and (F3 − F2)/(F3 + F2), where F1, F2 and F3 represent firing rates during trial phases 1- 3. Graphic presentation required breakdown into three indices. Statistical assessment of the correlation (text) did not.
To say that a neuron's signaling of rank in one task was positively and significantly correlated with its signaling of rank in the other task is not to say that the signals in the two tasks were identical. To determine how often rank-related activity differed significantly between the two tasks, we carried out an ANOVA with rank, saccade direction, and task as factors. The results of this analysis indicate that around half of neurons exhibited a main effect of task, that around half exhibited an interaction between task and rank, and that around a third exhibited an interaction between task and direction (Table 8, A–D). Of particular interest in the present context are the interactions between task and rank. Their frequent occurrence suggests that in a substantial number of neurons the strength of the rank signal differed significantly between the two tasks. However, we are hindered from drawing a firm conclusion on this point by the fact that the two tasks were run consecutively. The properties of a neuron could have changed between sessions 1 and 2.
Table 8.
Counts of neurons with significant effects of rank, direction, and task
| Rank | Dir | Rank* Dir | Task | Rank* Task | Dir* Task | Total | |
|---|---|---|---|---|---|---|---|
| A. SMA | |||||||
| Monkey O | 82 | 58 | 66 | 53 | 52 | 26 | 86 |
| Monkey T | 162 | 103 | 154 | 83 | 48 | 38 | 195 |
| Combined | 244 | 161 | 220 | 136 | 100 | 64 | 281 |
| % of Total | 87 | 57 | 78 | 48 | 36 | 23 | 100 |
| B. Pre-SMA | |||||||
| Monkey O | 57 | 40 | 50 | 27 | 35 | 12 | 67 |
| Monkey T | 74 | 62 | 66 | 54 | 34 | 27 | 80 |
| Combined | 131 | 102 | 116 | 81 | 69 | 39 | 147 |
| % of Total | 89 | 69 | 79 | 55 | 47 | 27 | 100 |
| C. SEF | |||||||
| Monkey O | 98 | 84 | 90 | 64 | 66 | 39 | 117 |
| Monkey T | 314 | 296 | 285 | 268 | 195 | 152 | 349 |
| Combined | 412 | 380 | 375 | 332 | 261 | 191 | 466 |
| % of Total | 88 | 82 | 80 | 71 | 56 | 41 | 100 |
| D. dlPFC | |||||||
| Monkey O | 125 | 118 | 151 | 88 | 74 | 66 | 189 |
| Monkey T | 102 | 95 | 136 | 47 | 39 | 44 | 158 |
| Combined | 227 | 213 | 287 | 135 | 113 | 110 | 347 |
| % of Total | 65 | 61 | 83 | 39 | 33 | 32 | 100 |
Dir, direction.
Saccade direction selectivity
Although the main focus of this paper is on rank selectivity, we will comment on selectivity for saccade direction during the peri-saccadic epoch because it forms a useful benchmark against which to assess selectivity for rank. At least half of the neurons in every area exhibited direction selectivity (Tables 9 and 10), but in no area did the rate of incidence of direction selectivity exceed the rate of incidence of rank selectivity (Tables 2 and 4). We conclude that rank selectivity is at least as robust a trait in all areas as direction selectivity.
Table 9.
Counts of neurons significantly selective for direction in the serial action task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | Direction | Total | Direction | Total | Direction | Total | Direction | |
| Monkey O | 85 | 47 | 65 | 30 | 113 | 68 | 185 | 101 |
| Monkey T | 191 | 91 | 80 | 56 | 346 | 274 | 149 | 81 |
| Combined | 276 | 138 | 145 | 86 | 459 | 342 | 334 | 182 |
| % of Total | 50 | 59 | 75 | 55 | ||||
Table 10.
Counts of neurons significantly selective for direction in the serial object task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | Direction | Total | Direction | Total | Direction | Total | Direction | |
| Monkey O | 85 | 55 | 65 | 27 | 113 | 78 | 185 | 105 |
| Monkey T | 191 | 87 | 80 | 54 | 346 | 258 | 149 | 82 |
| Combined | 276 | 142 | 145 | 81 | 459 | 336 | 334 | 187 |
| % of Total | 52 | 56 | 73 | 56 | ||||
The rate of incidence of direction selectivity varied significantly across areas in both the serial action task (Table 9, χ2 test, P < 10−6) and the serial object task (Table 10, χ2 test, P < 10−8). Pairwise comparisons (χ2 test on 6 pairs of areas) revealed that direction-selective neurons were more numerous in the SEF than in other areas both in the serial action task (SMA–SEF, P < 10−6; pre-SMA–SEF, P = 0.0007; dlPFC–SEF, P < 10−6) and in the serial object task (SMA–SEF, P < 10−6; pre-SMA–SEF, P = 0.0001; dlPFC–SEF, P < 10−6). The trend was present in both monkeys in both tasks. We conclude that the cross-area distribution of rank selectivity (less in the dlPFC than in other areas) was different from the cross-area distribution of direction selectivity (greater in the SEF than in other areas).
The tendency for a neuron to display significant direction selectivity in one task was highly correlated with its tendency to do so in the other task (χ2 test; SMA, SEF, and dlPFC: P < 10−8; pre-SMA: P = 0.0013). To assess whether the preferred direction tended to be conserved across tasks, we computed a three-component normalized direction vector based on firing during the peri-saccade epoch during execution of leftward, upward, and rightward saccades, regardless of trial phase, in the serial action task (AL, AU, AR) and in the serial object task (OL, OU, OR). Then for each area, we computed the coefficient of correlation across neurons between the two vectors. In each area, the correlation was positive and highly significant (SMA: R2 = 0.53; pre-SMA: R2 = 0.59; SEF: R2 = 0.64; dlPFC: R2 = 0.28; P < 10−4 in each area). This degree of cross-task concordance was commensurate with the degree observed for rank selectivity (Table 7).
Timing of direction signals as an index of serial order planning
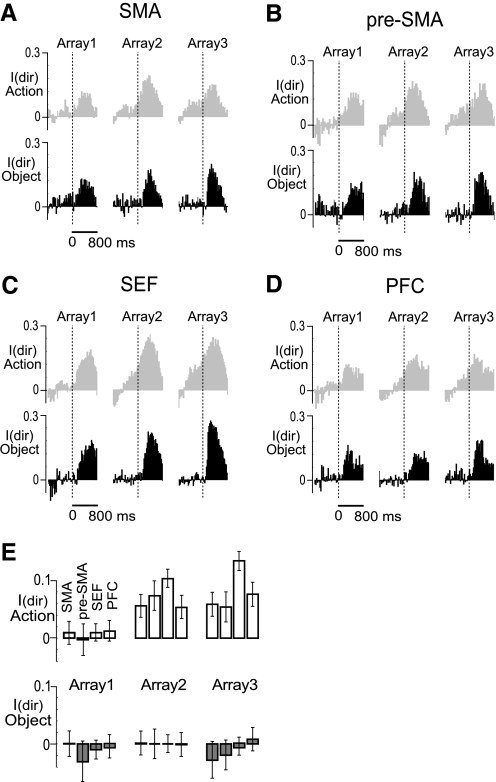
In the serial action task, the required direction of each saccade could in principle be known from the time of onset of the target array presented during phase 1 of the trial. In contrast, in the serial object task, the required direction of each saccade was conveyed by the arrangement of objects in the array presented at the outset of that phase of the trial. Accordingly, we conjectured that neuronal activity reflecting the direction of the impending saccade would develop earlier in the serial action than in the serial object task. To test this idea, we defined as the best and the worst directions for each neuron as those directions that were associated with maximal and minimal firing during the postarray epoch (150–450 ms after array onset) in data pooled across three trial phases. For all neurons in each area, we then computed, for each 25 ms bin in a period from 800 ms before to 800 ms after array onset, an index of directional activity. This was (B − W)/(B + W) where B and W were the firing rates preceding saccades in the best and worst direction.
The results for the serial action task and serial object task are shown in Fig. 7, A–D. In this figure, the time of the array onset is indicated by the dashed vertical line. In the serial object task (black), the directional signal at each phase of the trial became consistently positive (an indication that neurons were signaling the direction of the impending saccade) only after onset of the array. In contrast, in the serial action task (gray), the directional signal became positive well in advance of array onset. To assess the statistical significance of this difference between tasks, we computed the average directional index during an epoch 0–125 ms after onset of the array. The mean and standard error of the direction index in each area for each phase of the trial in each task is shown in Fig. 7E. To compare the indices obtained in the two tasks, we employed a nonpaired one-tailed t-test. In each area and each monkey, the directional index in the action task was consistently higher than in the object task during the second and third trial phases (SMA, 1st phase, P = 0.34, 2nd phase, P = 0.0002, 3rd phase, P < 10−7; pre-SMA, 1st phase, P = 0.13, 2nd phase, P = 0.0004, 3rd phase, P = 0.0006; SEF, 1st phase, P = 0.1, 2nd phase, P < 10−6, 3rd phase, P < 10−7; dlPFC, 1st phase, P = 0.15, 2nd phase, P = 0.0001, 3rd phase, P < 10−5). The results validate the notion that monkeys predictively programmed the second and third saccades in the serial action task but waited for instruction in the serial object task.
Fig. 7.
A–D: population direction index (mean across neurons of the firing rate difference between the best and the worst direction normalized to the sum) in the serial action task (gray) and serial object task (black). Data are aligned on array presentation. The direction index becomes consistently positive earlier in the serial action task than in the serial object task because the monkey has advance knowledge of the direction of the next required saccade. E: mean ± SE of the direction index during the first 125 ms after each array presentation in the serial action task (white bars) and the serial object task (gray bars).
Combination of rank and direction signals in single neurons
In several areas, the number of neurons exhibiting main effects of both rank and direction during the peri-saccadic epoch was greater than expected from independent distribution of the two traits. This trend was significant in the combined data and present in both monkeys in both the serial action task (χ2 test: SMA, P = 0.03; SEF, P < 10−6; dlPFC, P = 0.0011) and the serial object task (SMA, P = 0.0019; dlPFC, P = 0.018). It is as if some neurons were task-related (in which case they tended to possess both attributes) and others were not.
VARIATION ACROSS AREAS IN CO-EXPRESSION OF RANK AND DIRECTION SELECTIVITY.
To determine whether areas differed significantly with respect to the tendency for neurons to exhibit main effects of both rank and direction, we computed for each area the number of co-expressing neurons in excess of the number expected from independent distribution of the two traits. We found that this number, expressed as a fraction of the total neuronal count, was greater in the dlPFC than in other areas both in the serial action and in the serial object task although the only significant comparison was between the dlPFC and the SEF in the latter task (χ2 test: P = 0.005). Perhaps neurons that were task-related and therefore possessed both traits stood out more starkly in the dlPFC because they were less numerous than in other areas.
INTERACTION OF RANK AND DIRECTION SIGNALS.
In around half of all neurons, the influences of rank and direction combined nonlinearly, as reflected by an interaction effect (Fig. 2). The frequency of interaction effects varied significantly from area to area (χ2 test, action: P < 10−8, object: P < 10−7). Pairwise post hoc comparisons revealed that the rate was significantly higher in the SEF than in the pre-SMA and dlPFC in the action task (χ2 test, SEF—pre-SMA, P = 0.0041; SEF–dlPFC, P < 10−5) and significantly higher in the SEF than in the SMA and dlPFC in the object task (SEF–SMA, P < 10−5; SEF–dlPFC, P < 10−6). Thus interaction effects occurred frequently in all areas and were especially frequent in the SEF.
What is the significance of interaction effects with regard to how neurons encoded rank and saccade direction? Examination of data from individual neurons including the one in Fig. 8 indicated one possibility. This neuron preferred ranks 1 and 2 to rank 3 and preferred upward saccades to leftward and rightward saccades. If the influences of rank and direction had combined linearly (as expected if there were no interaction effect), then this neuron would have fired at an intermediate level when the rank was preferred and the direction was not and vice versa. In fact, it fired only if a preferred rank and a preferred direction were both present (and exhibited an interaction effect). We hypothesized on the basis of observations such as this that interaction effects reflected the tendency for neurons to show sharper tuning for a preferred combination of rank and direction than expected from linear summation of the two influences.
Fig. 8.
Data from an SEF neuron exhibiting a main effect of rank, a main effect of direction and a rank × direction interaction effect in both the serial action task (A) and the serial object task (B). The interaction between rank and direction reflects the neuron's responding to only two conjunctions of rank and direction (rank 1 + up and rank 2 + up). Main effects alone cannot give rise to such sharp tuning.
To test this hypothesis, we carried out an analysis of tuning sharpness in two groups of neurons exhibiting main effects of both rank and saccade direction: those in which there was an interaction effect and those in which there was not. The measure of sharpness of tuning of each neuron was derived in the following manner. We defined the best, middle, and worst ranks as those associated with maximal, intermediate, and minimal firing in data pooled across all saccade directions. Likewise, we defined the best, middle, and worst saccade directions as those associated with maximal, intermediate, and minimal firing in data pooled across all ranks. We then computed, for each neuron, the average firing rate under each of nine rank-direction combinations (ranging from “best rank, best direction” to “worst rank, worst direction”). We normalized the nine values to the value associated with best rank, best direction. Then we carried out an ANOVA on the resulting values with neuronal population (2 levels: interaction effect and no interaction effect) and rank-direction combination (8 levels: all combinations except best-best) as factors. This revealed that in the serial action task, tuning was significantly sharper (the mean of the index was lower) among neurons with interaction effects than among those without (Table 11, A and B, P < 10−8) and remained significant in data broken down by area (SMA: P = 0.0151, SEF: P = 0.0029; dlPFC: P < 10−6; with the exception of pre-SMA where general trend did not achieve significance: P = 0.49). The results obtained for the serial object task were generally the same as in the serial action task: tuning was significantly sharper among neurons with interaction effects than among those without (Table 12, A and B, P < 10−8). This effect remained significant in data broken down by area (P < 10−6 except pre-SMA, P = 0.87). We conclude that interaction effects arose in part from a tendency for neurons to fire only when the rank and the saccade direction were both optimal.
Table 11.
Normalized firing rate for each combination of direction and rank (serial action task)
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | |
| A. Neurons with main effects of rank and direction but no interaction effect | ||||||||||||
| Worst rank | 0.52 | 0.59 | 0.71 | 0.49 | 0.50 | 0.63 | 0.46 | 0.54 | 0.66 | 0.54 | 0.62 | 0.74 |
| Middle rank | 0.60 | 0.72 | 0.82 | 0.60 | 0.69 | 0.80 | 0.55 | 0.66 | 0.79 | 0.63 | 0.72 | 0.88 |
| Best rank | 0.79 | 0.87 | 1.0 | 0.78 | 0.86 | 1.0 | 0.74 | 0.87 | 1.0 | 0.77 | 0.85 | 1.0 |
| B. Neurons with main effect of rank and direction and an interaction effect | ||||||||||||
| Worst rank | 0.44 | 0.50 | 0.63 | 0.42 | 0.45 | 0.53 | 0.41 | 0.48 | 0.61 | 0.46 | 0.55 | 0.64 |
| Middle rank | 0.54 | 0.61 | 0.80 | 0.47 | 0.58 | 0.78 | 0.49 | 0.61 | 0.78 | 0.54 | 0.65 | 0.75 |
| Best rank | 0.67 | 0.83 | 1.0 | 0.70 | 0.85 | 1.0 | 0.63 | 0.77 | 1.0 | 0.61 | 0.74 | 1.0 |
Table 12.
Normalized firing rate for each combination of direction and rank (serial object task)
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | |
| A. Neurons with main effect of rank and direction but no interaction effect | ||||||||||||
| Worst Rank | 0.47 | 0.54 | 0.65 | 0.40 | 0.54 | 0.60 | 0.45 | 0.54 | 0.67 | 0.50 | 0.58 | 0.70 |
| Middle Rank | 0.57 | 0.68 | 0.82 | 0.50 | 0.68 | 0.77 | 0.56 | 0.67 | 0.81 | 0.59 | 0.71 | 0.85 |
| Best Rank | 0.75 | 0.85 | 1.0 | 0.74 | 0.87 | 1.0 | 0.72 | 0.86 | 1.0 | 0.74 | 0.84 | 1.0 |
| B. Neurons with main effect of rank and direction and an interaction effect | ||||||||||||
| Worst Rank | 0.37 | 0.42 | 0.55 | 0.46 | 0.50 | 0.62 | 0.39 | 0.46 | 0.58 | 0.39 | 0.48 | 0.57 |
| Middle Rank | 0.45 | 0.56 | 0.76 | 0.56 | 0.65 | 0.78 | 0.46 | 0.56 | 0.78 | 0.49 | 0.56 | 0.79 |
| Best Rank | 0.63 | 0.83 | 1.0 | 0.70 | 0.85 | 1.0 | 0.58 | 0.75 | 1.0 | 0.54 | 0.73 | 1.0 |
Comparison between dorsal and ventral subdivisions of dlPFC
A large majority (84%) of dlPFC neurons characterized in this study was located dorsal to the principal sulcus. The minority located ventral to the sulcus consisted of 37/185 neurons in monkey O and 15/149 neurons in monkey T. The small number of such neurons precluded thorough comparison between dorsal and ventral populations. However, we did compare the populations on one crude measure, namely the outcome of the ANOVA assessing sensitivity to rank and saccade direction (Fig. 9). The distribution of counts across the eight possible categories was significantly different in the serial object task (χ2 test, P = 0.0003) but not in the serial action task (P = 0.25). To identify the source of the difference in the serial object task, we performed post hoc pairwise tests comparing dorsal and ventral dlPFC with regard to the frequency of the eight kinds of effect: no effect, rank alone, direction alone, interaction alone, rank and direction, rank and interaction, direction and interaction, and rank, direction, and interaction. Only one pairwise difference was significant at the Bonferroni-corrected level of 0.05/8 = 0.00625. The fraction of neurons exhibiting two main effects and an interaction effect in the serial object task was significantly greater in the ventral than in the dorsal population (χ2 test with Yates correction, P = 0.0000019). Neurons in this category are the very ones demonstrated above to show sharper tuning for a preferred combination of rank and direction than expected from linear summation of the two influences.
Fig. 9.
Frequency of significant neuronal effects of rank, direction and their interaction in each subdivision of PFC during performance of the serial action task (A) and serial object task (B).
To test the idea that tuning for a particular combination of rank and direction was sharper among ventral than among dorsal neurons in the serial object task, we carried out an analysis of the sharpness of tuning for particular combinations of rank and direction exactly analogous to the one described in the preceding text (combination of rank and direction signals in single neurons). An ANOVA with neuronal population (2 levels: dorsal and ventral) and rank-direction combination (8 levels: all combinations except best-best) as factors revealed that tuning was indeed significantly sharper (the mean of the index was lower) among ventral than among dorsal neurons (Table 13; P = 0.00002).
Table 13.
Normalized firing rate for each combination of direction and rank (based on all neurons exhibiting main effects of both rank and direction and/or an interaction effect)
| Ventral PFC |
Dorsal PFC |
|||||
|---|---|---|---|---|---|---|
| Worst Dir | Middle Dir | Best Dir | Worst Dir | Middle Dir | Best Dir | |
| Worst Rank | 0.42 | 0.47 | 0.59 | 0.48 | 0.58 | 0.69 |
| Middle Rank | 0.52 | 0.61 | 0.82 | 0.59 | 0.67 | 0.85 |
| Best Rank | 0.61 | 0.75 | 1.0 | 0.70 | 0.84 | 1.0 |
Comparison between the peri-array and peri-saccadic epochs
In analyses considered up to this point, neurons were classified as rank and direction selective on the basis of activity during the peri-saccadic epoch. On repeating all of the analyses with attention to the antecedent epoch during which the monkey maintained central fixation (from 50 ms before to 450 ms after onset of the array), we observed identical trends with two notable exceptions.
First, the number of neurons with a significant main effect of direction during this epoch was higher in the serial action task than in the serial object task (Table 14). The trend was present in each monkey in each area and was significant in combined data (χ2 test: SMA, P = 0.0007; pre-SMA, P = 0.012; SEF, P < 10−4; dlPFC, P < 10−5). This observation is in accordance with the observation that firing reflecting the direction of the impending saccade arose earlier in the serial action task than in the serial object task (as described in the preceding text in the section on the timing of direction signals).
Table 14.
Counts of neurons exhibiting significant direction selectivity in the serial action task (Act) and serial object task (Obj) during the peri-array epoch
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Dir Act | Dir Obj | Total | Dir Act | Dir Obj | Total | Dir Act | Dir Obj | Total | Dir Act | Dir Obj | |
| Monkey O | 85 | 44 | 38 | 65 | 26 | 21 | 113 | 65 | 64 | 185 | 85 | 57 |
| Monkey T | 191 | 62 | 30 | 80 | 46 | 29 | 346 | 224 | 163 | 149 | 50 | 23 |
| Combined | 276 | 106 | 68 | 145 | 72 | 50 | 459 | 289 | 227 | 334 | 135 | 80 |
| % of Total | 39 | 25 | 50 | 35 | 63 | 50 | 40 | 24 | ||||
Second, concordance between rank signals observed in the two tasks was lower than during the peri-saccadic epoch (Table 7). This trend was present in each area of each monkey although it attained significance only in the dlPFC (SMA, P = 0.45; pre-SMA, P = 0.052; SEF, P = 0.064, dlPFC P = 0.0087). The reduced level of cross-task preferred-rank concordance during the peri-array epoch might have been due to a random factor (such as greater noise in the neuronal signal) or a systematic factor (such as a tendency for neurons to prefer 1 rank in the serial action task and a different rank in the serial object task). In support of the latter interpretation, we noted that neurons preferring the first rank during the peri-array epoch were more numerous in the serial action task whereas those preferring the third rank during this epoch were more numerous in the serial object task (Table 15).
Table 15.
Counts of neurons by preferred rank during the peri-array epoch in the serial action task and serial object task
| SMA |
Pre-SMA |
SEF |
dlPFC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | Tot | 1st | 2nd | 3rd | Tot | 1st | 2nd | 3rd | Tot | 1st | 2nd | 3rd | Tot | |
| Serial action task | ||||||||||||||||
| Monkey O | 15 | 9 | 51 | 75 | 23 | 14 | 19 | 56 | 31 | 22 | 45 | 98 | 66 | 29 | 29 | 124 |
| Monkey T | 49 | 52 | 26 | 127 | 21 | 29 | 27 | 77 | 53 | 134 | 125 | 312 | 39 | 18 | 36 | 93 |
| Combined | 64 | 61 | 77 | 202 | 44 | 43 | 46 | 133 | 84 | 156 | 170 | 410 | 105 | 47 | 65 | 217 |
| % of Total | ||||||||||||||||
| First | 32 | 33 | 21 | 48 | ||||||||||||
| Second | 30 | 32 | 38 | 22 | ||||||||||||
| Third | 38 | 35 | 41 | 30 | ||||||||||||
| Serial object task | ||||||||||||||||
| Monkey O | 8 | 13 | 57 | 78 | 12 | 16 | 24 | 52 | 14 | 33 | 55 | 102 | 33 | 33 | 54 | 120 |
| Monkey T | 37 | 53 | 22 | 112 | 17 | 20 | 33 | 70 | 47 | 118 | 127 | 292 | 26 | 11 | 52 | 89 |
| Combined | 45 | 66 | 79 | 190 | 29 | 36 | 57 | 122 | 61 | 151 | 182 | 394 | 59 | 44 | 106 | 209 |
| % of Total | ||||||||||||||||
| First | 24 | 24 | 16 | 28 | ||||||||||||
| Second | 35 | 30 | 38 | 21 | ||||||||||||
| Third | 41 | 46 | 46 | 51 | ||||||||||||
To visualize this effect, we considered neurons that tilted from firing during rank 1 in the serial action task to firing during rank 3 in the serial object task as indicated by meeting either of the following criteria: in the serial action task (but not the serial object task), the neuron fired significantly more strongly during trial phase 1 than trial phase 3 (1-tailed t-test, α = 0.05); in the serial object task (but not in the serial action task), the neuron fired significantly more strongly during trial phase 3 than trial phase 1 (1-tailed t-test, α = 0.05). This condition was met by 18% of neurons in the SMA, 18% in the pre-SMA, 22% in the SEF, and 38% in the dlPFC. An example from the dlPFC is shown on Fig. 10. The population activity of all such neurons is displayed in Fig. 11. The fact that firing during trial phases 2 and 3 was greater in the serial object task (black curve) than in the serial action task (gray curve) might be related to the fact that the second and third arrays convey information necessary for selecting the second and third saccades in the serial object task whereas, in the serial action task, they convey no new information. According to this line of interpretation, neurons exhibiting systematic cross-trial discordance in preferred rank during the peri-array period are likely those in which firing is correlated not with rank per se but with processing information from the array.
Fig. 10.
Data from a dlPFC neuron selective for rank 1 in the serial action task but not in the serial object task.
Fig. 11.
Mean firing rate as a function of time of neurons tending to shift from rank 1 preference in the serial action task (gray line) to rank 3 preference in the serial object task (black line).
DISCUSSION
Rank signals are widespread and uniform in frontal cortex
Although there have been numerous prior studies of rank-related activity in the frontal cortex of the monkey, few of these have been designed in such a way as to allow posing the question whether areas differ with respect to the strength or nature of rank-related activity. We have addressed this issue by directly comparing the activity of neurons in the SMA, pre-SMA, SEF, and dlPFC in the same monkeys performing the same serial order tasks. We have found that some aspects of rank selectivity vary across areas. For example, rank selectivity is less common in the dlPFC than in the other areas and neurons selective for late ranks are more common in the SMA and SEF than in the pre-SMA and dlPFC. However, the overarching general finding is that a majority of neurons in each area carries rank signals.
The finding that rank signals are widely distributed in frontal cortex may help to explain the limited nature of the impairments that result from damage confined to one area. The SMA syndrome is characterized by a reduction in spontaneous movement and difficulty in voluntary movement initiation but by little or no impairment of serial order performance (Bannur and Rajshekhar 2000). Thus patients with SMA lesions show an increase in reaction time on a sequential digit task but not much impairment in the production of the correct sequence (Halsband et al. 1993). To the extent that mild deficits of sequence production do occur, these are confined to the limb-movement domain and recover quickly (Bleasel et al. 1996; Chassoux et al. 1999; Hanlon et al. 1995; Krainik et al. 2001; Laplane et al. 1977; Rostomily et al. 1991; Zentner et al. 1996; see also review by Goldberg 1985).
Clinical reports suggest that pre-SMA is important for the learning of new motor sequences but not for the execution of well practiced ones (Deblieck et al. 2003; Fukaya et al. 2003). Insofar as the performance of overlearned sequences is impaired, the impairment appears to involve only initiation of the sequence “chunk” (Kennerley et al. 2004; Muri et al. 1994). The idea that the pre-SMA is important for acquisition of new sequences but not for performance of overlearned sequences is further supported by studies employing transient inactivation in the monkey (Nakamura et al. 1999; Shima and Tanji 1998).
Reversible inactivation of the SEF in monkeys produces a significant but moderate impairment in the performance of a task requiring successive saccades to two targets flashed in a rapid succession (Sommer and Techovnik 1999). Early clinical studies of human patients with the SEF lesions reported moderately poor performance in tasks requiring two or more memory guided saccades (Gaymard et al. 1998; Pierrot-Deseilligny et al. 1995). However, a more recent study of a patient with a rare highly focal lesion of the SEF reported no significant deficit with respect to the order of a sequence of memory-guided saccades (Parton et al. 2007).
Lesions to the dorsolateral prefrontal cortex are well known to affect the ability to plan and organize purposive goal-directed behavior. However, this impairment has rarely been reported to play out in the form of impaired serial order performance. There is no solid indication that inactivation or damage of the dlPFC has deleterious effects specifically on the learning or production of movement sequences (Fuster 2001; Hanna-Pladdy 2007; Miller and Cohen 2001; Shallice and Burgess 1991). Indeed patients with prefrontal lesions can still learn hierarchically structured action sequences in the context of a task requiring associating motor responses with visual cues (Koch et al. 2006).
We conclude that the ability to perform serial order tasks shows graceful degradation with brain damage. This is a classic feature of systems with parallel and distributed representations (McLeod et al. 1998). Graceful degradation in principle could arise from the representation of different performance strategies (for example, chaining and rank-based performance) in different brain areas. However, our finding that rank is widely represented in frontal cortex favors the alternative idea that graceful degradation is the product of multiple areas contributing to rank-based performance.
Comparison to previous studies of individual areas in frontal cortex
RANK SIGNALS IN SMA.
We found that 76% of task related neurons in SMA were influenced by rank of saccade in the oculomotor serial action task. This is a novel finding because rank selectivity in the SMA was previously characterized only in tasks requiring sequential arm movements. In the context of a sequential arm-movement task, 20–70% of task-related SMA neurons have been reported to be to be selective for rank. The large range of the reported values is probably a consequence of differences in experimental procedure. For example, Clower and Alexander (1998) reported that ∼40% of task-related neurons in SMA were rank selective; however, the analysis of rank selectivity was confined to neurons demonstrably selective for the parameters of movements in the sequence. In contrast, Shima and Tanji (2000) reported that 22% of SMA neurons were influenced by rank and not by the parameters of the movements. This proportion matches approximately the proportion we found to be influenced by rank and not saccade direction (23%). Sohn and Lee (2007) reported that ∼60% of SMA neurons were influenced by the ordinal position (as counted from the beginning of the sequence), and ∼70% were influenced by the number of remaining elements in the sequence (the ordinal position as counted from the end of the sequence). These proportions are well matched to the proportion of neurons that exhibited a main effect of rank in our study (76%).
We found that the majority of rank-selective neurons in SMA prefers the third rank (58%, Table 3). The results of some previous studies likewise suggest that the last action in the sequence is overrepresented in the SMA (Clower and Alexander 1998; Sohn and Lee 2007; but see also Shima and Tanji 2000).
RANK SIGNALS IN PRE-SMA.
We found that 85% of task-related neurons in pre-SMA were influenced by rank of saccade in the oculomotor serial action task. This finding is in a good agreement with the results of previous studies of rank selectivity in pre-SMA (Clower and Alexander 1998; Isoda and Tanji 2004; Shima and Tanji 2000; Sohn and Lee 2007).
We found that an approximately equal number of neurons preferred each ordinal position. Although we cannot directly compare the frequencies of neurons preferring each rank to the previous studies because of the differences in the criteria for establishing preferred rank, it appears that a similar trend was observed in an oculomotor sequence task (Isoda and Tanji 2004) but not in tasks involving arm movements, in which the majority of the rank-selective neurons was preferentially active either at the beginning of the sequence (Nakajima et al. 2009; Shima and Tanji 2000; Sohn and Lee 2007) or at its beginning and end (Clower and Alexander 1998).
It is interesting to note that the majority of studies comparing rank selectivity in the pre-SMA and SMA in the context of an arm-movement task found that the number of rank-selective neurons is higher in pre-SMA than in the SMA (Clower and Alexander 1998; Nakajima et al. 2009; Shima and Tanji 2000; Sohn and Lee 2007). Our results reveal a similar trend although the difference did not achieve significance.
RANK SIGNALS IN SEF.
We found that 87% of task-related neurons in SEF were influenced by rank of saccade in the oculomotor serial action task. The occurrence of rank selectivity in the SEF has been documented in previous studies (Berdyyeva and Olson 2009; Isoda and Tanji 2002, 2003; Lu et al. 2002). In one of the first relevant studies, Lu et al. (2002), although their focus was not on rank selectivity, did note that rank selectivity could explain some of their results. Isoda and Tanji (2002, 2003), in a study focused on rank selectivity, found that ∼55% of task-related neurons were influenced by rank; however, as they noted, their criteria for rank selectivity may have led them to underestimate the rate of incidence of the phenomenon (Isoda and Tanji 2002). In addition to the difference in data analysis, the two studies differed in behavioral design. In their task, the sequence was committed to working memory over the course of a few trials. In our task, the association between the cue and the sequence was held in long-term memory.
We have found that rank-selective neurons in the SEF tend to prefer later ranks (17, 34, and 49% of the sample preferred the 1st, 2nd, and 3rd rank, respectively). Previous studies did not report any such overrepresentation of later ranks. The explanation for the discrepancy is not clear.
RANK SIGNALS IN DLPFC.
We found that a comparatively large proportion (55%) of neurons in the dlPFC was rank selective in the context of the serial action task. To the best of our knowledge, no previous study has reported on the rate of incidence of rank selectivity in the dlPFC during sequential action performance although there is some evidence that dlPFC neurons carry rank-related signals (Averbeck et al. 2002; Barone and Joseph 1989; Hasegawa et al. 2004). The limited data suggest that neurons in dlPFC either prefer the first ordinal position (Barone and Joseph 1989) or that the sequential elements occupying the first and the last positions are represented the most strongly by population activity (Averbeck et al. 2002). We too have found that more neurons preferred the first rank (44%) and the third rank (37%) than the second rank (19%).
Neurons in frontal cortex are rank generalists
One of the central goals of our study was to investigate whether the neurons in frontal cortex represent rank in a serial object task as distinct from a task requiring the execution of a series of movements in a memorized sequence. There have been reports to the effect that neurons in dlPFC signal rank during the serial presentation of images during passive fixation (Inoue and Mikami 2006; Ninokura et al. 2003, 2004) and during performance of a three-step self-ordered task (Hasegawa et al. 2004); however, rank selectivity during the selection of objects in a memorized sequence has not previously been studied anywhere in the frontal cortex except SEF (Berdyyeva and Olson 2009). Here we report that a majority of neurons in all four areas that we studied signal rank during performance of the serial object task (72% in SMA, 79% in pre-SMA, 82% in SEF, and 57% in dlPFC). The proportion of rank-selective neurons in the serial object task was similar to that in the serial action task.
Neurons representing serial order at a fully abstract level should signal ordinal position irrespective of the particular task context. It is a question of considerable interest whether the ability of neurons to generalize across task context varies from area to area. To resolve this issue, we compared four frontal cortical areas with respect to the ability to generalize across two serial task contexts. We found that neurons in the SMA, pre-SMA, and SEF and to a lesser degree in dlPFC preserve their rank selectivity across different serial order tasks. The lower incidence of cross-task concordance in the dlPFC could in principle have any of several explanations: rank signals in the dlPFC might be noisier; neurons in the dlPFC might represent different ranks in different task contexts, or neurons in the dlPFC might represent identical ranks in the two tasks but the rank signals might be masked by signals related to other factors that vary between the tasks. Our results tend to support the third explanation as explained in the following section.
Strong firing early in the serial action task and late in the serial object task
We discovered, in all areas, and in particular in the pre-SMA and dlPFC, a population of neurons that fired early in the trial in the serial action task and throughout the trial in the serial object task. On the basis of our standard measure of rank selectivity, these neurons tended to be classified as rank 1 selective in the serial action task and rank 3 selective in the serial object task. Given the systematic nature of the cross-task shift, we speculate that these neurons were not rank selective but rather were sensitive to a factor that was most strongly present at the outset of a trial in the serial action task but was present throughout a trial in the serial object task. This factor might have been the need to attend to the target array to glean from it information about which saccade to execute next. The array in phase 1 of the serial action task conveyed all of the information needed for the planning of all three successive saccades. The monkeys registered this information as evidenced by the fact that neuronal activity representing the directions of the second and third saccades built up before the second and third arrays appeared. In contrast, in the serial object task, the directions of saccade 2 and 3 were not known until arrays 2 and 3 conveyed the information.
In previous studies of rank selectivity, all of them based on the performance of tasks in which the entire sequence of required actions was known at the outset of the trial, there have been indications that rank 1 is overrepresented in the pre-SMA and dlPFC. For example, Shima and Tanji (2000) reported that the majority of the rank-selective neurons in pre-SMA was preferentially active at the beginning of the sequence. Likewise, Clower and Alexander (1998) reported a predominance in the pre-SMA of first and the third rank neurons. Preferential activation of neurons early in a serial order trial has also been observed in the dlPFC (Averbeck et al. 2002). Our findings suggest that the neurons giving rise to an apparent overrepresentation of rank 1 in these areas are not carrying genuine rank signals but rather are sensitive to an extraneous factor present at task onset. In accordance with this view, it has been suggested that the pre-SMA is critically involved in retrieving visuomotor associations (Sakai et al. 1999) and in the initiation of sequences and sequence chunks (Kennerley et al. 2004; Muri et al. 1994). The latter notion has found recent support in a report by Nakajima et al. (2009) showing that the activity of many pre-SMA neurons anticipates the nature of the second movement in a sequence even before the first movement has begun.
Continuous rather than categorical representation of rank
Neurons presented as examples in our paper (Fig. 3) and in reports from other groups tend to be those that exhibit beautiful categorical rank selectivity: they fire at a high rate during one phase of the trial and at a nearly identical low rate during the other phases. It is an interesting question whether neurons of this type are outliers or are genuinely representative of the populations from which they are drawn. To answer this question, we characterized each neuron's pattern of selectivity by an index that varied as a function of the degree to which the representation was categorical. Applying a cut-off such that, by chance alone, 50% of neurons would be classified as carrying a categorical representation and 50% as carrying a noncategorical representation, we found that the proportion carrying a categorical representation was >50% in all areas and that this effect was due primarily to the existence of neurons that fired strongly only during phase 3. It is difficult to compare this outcome to results reported in previous studies because the systems of classification employed in those studies do not lend themselves readily to forming an estimate of the number of neurons representing rank categorically. Clower and Alexander (1998) classified SMA neurons into 12 groups; Isoda and Tanji (2002, 2004 classified pre-SMA and SEF neurons into 7 and 12 groups, respectively. To some degree, individual groups can be described as approximating or not approximating to the categorical ideal. Overall, it appears that pure categorical representations of rank were uncommon and that the strongest tendency toward categorical representation of rank was present, as in our study, among neurons favoring rank 3.
Of course there is no need for rank to be represented in a categorical code. A linear classifier could, without question, identify the current phase of the trial from the activity of neurons carrying noncategorical rank signals in any of the areas that we have studied. However, the existence of neurons in which, for example, the rate of firing increases continuously from the beginning to the end of the trial does raise a question. How do we know that the activity of these neurons encodes the phase of the trial rather than some factor that increases steadily over the course of the trial? Although we have found recently that rank representations in at least one area of frontal cortex—SEF–cannot be explained entirely by factors correlated with rank such as the approach of reward or to the passage of time, it is still an open question whether that holds true in other areas.
GRANTS
This work was supported by National Institutes of Health Grants RO1 EY-018620 and P50 MH-45156. Technical support was from NIH Grants P30 EY-08098 and P41RR-03631.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank K. McCracken for excellent technical assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Akkal et al., 2002.Akkal D, Bioulac B, Audin J, Burbaud P. Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur J Neurosci 15: 887–904, 2002 [DOI] [PubMed] [Google Scholar]
- Ashe et al., 2006.Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol 16: 213–221, 2006 [DOI] [PubMed] [Google Scholar]
- Averbeck et al., 2002.Averbeck BB, Chafee MV, Crowe DA, Georgopoulos AP. Parallel processing of serial movements in prefrontal cortex. Proc Natl Acad Sci USA 99: 13172–13177, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck et al., 2003.Averbeck BB, Crowe DA, Chafee MV, Georgopoulos AP. Neural activity in prefrontal cortex during copying geometrical shapes. II. Decoding shape segments from neural ensembles. Exp Brain Res Exp Hirnforsch 150: 142–153, 2003 [DOI] [PubMed] [Google Scholar]
- Averbeck and Lee, 2007.Averbeck BB, Lee D. Prefrontal neural correlates of memory for sequences. J Neurosci 27: 2204–2211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck et al., 2006.Averbeck BB, Sohn J, Lee D. Activity in prefrontal cortex during dynamic selection of action sequences. Nat Neurosci 9: 276–282, 2006 [DOI] [PubMed] [Google Scholar]
- Bannur and Rajshekhar, 2000.Bannur U, Rajshekhar V. Post operative supplementary motor area syndrome: clinical features and outcome. Br J Neurosurg 14: 204–210, 2000 [DOI] [PubMed] [Google Scholar]
- Barone and Joseph, 1989.Barone P, Joseph J-P. Prefrontal cortex and spatial sequence in macaque monkey. Exp Brain Res Exp Hirnforsch 78: 447–464, 1989 [DOI] [PubMed] [Google Scholar]
- Berdyyeva and Olson, 2009.Berdyyeva T, Olson CR. Monkey supplementary eye field neurons signal the ordinal position of both actions and objects. J Neurosci 29: 591–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasel et al., 1996.Bleasel A, Comair Y, Luders HO. Surgical ablations of the mesial frontal lobe in humans. Adv Neurol 70: 217–235, 1996 [PubMed] [Google Scholar]
- Chassoux et al., 1999.Chassoux F, Devaux B, Landre E, Chodkiewicz JP, Talairach J, Chauvel P. Postoperative motor deficits and recovery after cortical resections. Adv Neurol 81: 189–199, 1999 [PubMed] [Google Scholar]
- Clower and Alexander, 1998.Clower WT, Alexander GE. Movement sequence-related activity reflecting numerical order of components in supplementary and presupplementary motor areas. J Neurophysiol 80: 1562–1566, 1998 [DOI] [PubMed] [Google Scholar]
- Crist et al., 1988.Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988 [DOI] [PubMed] [Google Scholar]
- Curran and Keele, 1993.Curran T, Keele S. Attentional and nonattentional forms of sequence learning. J Exp Psychol Learn Mem Cognit 19: 189–202, 1993 [Google Scholar]
- Deblieck et al., 2003.Deblieck C, Pesenti G, Scifo P, Fazio F, Bricolo E, Lo Russo G, Scialfa G, Cossu M., Bottini G, Paulesu E. Preserved functional competence of perilesional areas in drug-resistant epilepsy with lesion in supplementary motor cortex: fMRI and neuropsychological observations. NeuroImage 20: 2225–2234, 2003 [DOI] [PubMed] [Google Scholar]
- Dominey et al., 1998.Dominey PF, Lelekov T, Ventre-Dominey J, Jeannerod M. Dissociable processes for learning the surface structure and abstract structure of sensorimotor sequences. J Cognit Neurosci 10: 734–751, 1998 [DOI] [PubMed] [Google Scholar]
- Fukaya et al., 2003.Fukaya C, Katayama Y, Kobayashi K, Kasai M, Oshima H, Yamamoto T. Impairment of motor function after frontal lobe resection with preservation of the primary motor cortex. Acta Neurochir Suppl 87: 71–74, 2003 [DOI] [PubMed] [Google Scholar]
- Funahashi et al., 1997.Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behav Brain Res 84: 203–223, 1997 [DOI] [PubMed] [Google Scholar]
- Fuster, 2001.Fuster JM. The prefrontal cortex–an update: time is of the essence. Neuron 30: 319–333, 2001 [DOI] [PubMed] [Google Scholar]
- Gaymard et al., 1998.Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res Exp Hirnforsch 123: 159–163, 1998 [DOI] [PubMed] [Google Scholar]
- Goldberg, 1985.Goldberg G. Supplementary motor area structure and function: Review and hypotheses. Behav Brain Sci 8: 567–616, 1985 [Google Scholar]
- Halsband et al., 1993.Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 116: 243–266, 1993 [DOI] [PubMed] [Google Scholar]
- Hanlon et al., 1995.Hanlon R, Clontz B, Snow D, Thomas M. Treatment of supplementary motor area syndrome. J Neuro Rehab 10: 197–204, 1995 [Google Scholar]
- Hanna-Pladdy, 2007.Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther 31: 119–127, 2007 [DOI] [PubMed] [Google Scholar]
- Hasegawa et al., 2004.Hasegawa RP, Blitz AM, Goldberg ME. Neurons in monkey prefrontal cortex whose activity tracks the progress of a three-step self-ordered task. J Neurophysiol 92: 1524–1535, 2004 [DOI] [PubMed] [Google Scholar]
- Hikosaka et al., 1999.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci 22: 464–471, 1999 [DOI] [PubMed] [Google Scholar]
- Hikosaka et al., 2002.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol 12: 217–222, 2002 [DOI] [PubMed] [Google Scholar]
- Inoue and Mikami, 2006.Inoue M, Mikami A. Prefrontal activity during serial probe reproduction task: encoding, mnemonic, and retrieval processes. J Neurophysiol 95: 1008–1041, 2006 [DOI] [PubMed] [Google Scholar]
- Isoda and Tanji, 2002.Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol 88: 3541–3545, 2002 [DOI] [PubMed] [Google Scholar]
- Isoda and Tanji, 2003.Isoda M, Tanji J. Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J Neurophysiol 90: 3054–3065, 2003 [DOI] [PubMed] [Google Scholar]
- Isoda and Tanji, 2004.Isoda M, Tanji J. Participation of the primate presupplementary motor area in sequencing multiple saccades. J Neurophysiol 92: 653–659, 2004 [DOI] [PubMed] [Google Scholar]
- Jueptner et al., 1997.Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337, 1997 [DOI] [PubMed] [Google Scholar]
- Kennerley et al., 2004.Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol 91: 978–993, 2004 [DOI] [PubMed] [Google Scholar]
- Koch et al., 2006.Koch I, Reverberi C, Rumiati RI. Learning hierarchically structured action sequences is unaffected by prefrontal-cortex lesion. Exp Brain Res Exp Hirnforsch 175: 667–675, 2006 [DOI] [PubMed] [Google Scholar]
- Krainik et al., 2001.Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, Capelle L, Cornu P, Clemenceau S, Sahel M, Valery CA, Boch AL, Mangin JF, Bihan DL, Marsault C. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 57: 871–878, 2001 [DOI] [PubMed] [Google Scholar]
- Laplane et al., 1977.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci 34: 301–314, 1977 [DOI] [PubMed] [Google Scholar]
- Lee and Quessy, 2003.Lee D, Quessy S. Activity in the supplementary motor area related to learning and performance during a sequential visuomotor task. J Neurophysiol 89: 1039–1056, 2003 [DOI] [PubMed] [Google Scholar]
- Lu and Ashe, 2005.Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron 45: 967–973, 2005 [DOI] [PubMed] [Google Scholar]
- Lu et al., 2002.Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron 34: 317–325, 2002 [DOI] [PubMed] [Google Scholar]
- Marcus et al., 2006.Marcus DJ, Karatekin C, Markiewicz S. Oculomotor evidence of sequence learning on the serial reaction time task. Mem Cognit 34: 420–432, 2006 [DOI] [PubMed] [Google Scholar]
- Marshuetz, 2005.Marshuetz C. Order information in working memory: an integrative review of evidence from brain and behavior. Psych Bull 131: 323–339, 2005 [DOI] [PubMed] [Google Scholar]
- Matsuzaka et al., 1992.Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol 68: 653–662, 1992 [DOI] [PubMed] [Google Scholar]
- McLeod et al., 1998.McLeod P, Plunkett K, Rolls ET. Introduction to Connectionist Modelling of Cognitive Processes. Oxford: Oxford., 1998 [Google Scholar]
- Miller and Cohen, 2001.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001 [DOI] [PubMed] [Google Scholar]
- Miyashita et al., 1996.Miyashita K, Rand MK, Miyachi S, Hikosaka O. Anticipatory saccades in sequential procedural learning in monkeys. J Neurophysiol 76: 1361–1366, 1996 [DOI] [PubMed] [Google Scholar]
- Muri et al., 1994.Muri RM, Rosler KM, Hess CW. Influence of transcranial magnetic stimulation on the execution of memorized sequences of saccades in man. Exl Brain Res Exp Hirnforsch 101: 521–524, 1994 [DOI] [PubMed] [Google Scholar]
- Nakajima et al., 2009.Nakajima T, Hosaka R, Mushiake H, Tanji J. Covert representation of second-next movement in the pre-supplementary motor area of monkeys. J Neurophysiol 101: 1883–1889, 2009 [DOI] [PubMed] [Google Scholar]
- Nakamura et al., 1998.Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol 80: 2671–2687, 1998 [DOI] [PubMed] [Google Scholar]
- Nakamura et al., 1999.Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol 82: 1063–1068, 1999 [DOI] [PubMed] [Google Scholar]
- Ninokura et al., 2003.Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol 89: 2868–2873, 2003 [DOI] [PubMed] [Google Scholar]
- Ninokura et al., 2004.Ninokura Y, Mushiake H, Tanji J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J Neurophysiol 91: 555–560, 2004 [DOI] [PubMed] [Google Scholar]
- Parton et al., 2007.Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, Morgan PS, Jackson S, Rees G, Husain M. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia 45: 997–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard and Strick, 1996.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353, 1996 [DOI] [PubMed] [Google Scholar]
- Picard and Strick, 2003.Picard N, Strick PL. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb Cortex 13: 977–986, 2003 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny et al., 1995.Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Ann Neurol 37: 557–567, 1995 [DOI] [PubMed] [Google Scholar]
- Rhodes et al., 2004.Rhodes BJ, Bullock D, Verwey WB, Averbeck BB, Page MP. Learning and production of movement sequences: behavioral, neurophysiological, and modeling perspectives. Hum Move Sci 23: 699–746, 2004 [DOI] [PubMed] [Google Scholar]
- Rostomily et al., 1991.Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg 75: 62–68, 1991 [DOI] [PubMed] [Google Scholar]
- Russo and Bruce, 1993.Russo GS, Bruce CJ. Effect of eye position within the orbit on electrically elicited saccadic eye movements: a comparison of the macaque monkey's frontal and supplementary eye fields. J Neurophysiol 69: 800–818, 1993 [DOI] [PubMed] [Google Scholar]
- Russo and Bruce, 2000.Russo GS, Bruce CJ. Supplementary eye field: representation of saccades and relationship between neural response fields and elicited eye movements. J Neurophysiol 84: 2605–2621, 2000 [DOI] [PubMed] [Google Scholar]
- Sakai et al., 1999.Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci 19: RC1–RC1, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller and Chou, 1998.Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci 1: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- Schiller and Chou, 2000.Schiller PH, Chou I. The effects of anterior arcuate and dorsomedial frontal cortex lesions on visually guided eye movements in the rhesus monkey. I. Single and sequential targets. Vision Res 40: 1609–1626, 2000 [DOI] [PubMed] [Google Scholar]
- Shallice and Burgess, 1991.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain 114: 727–741, 1991 [DOI] [PubMed] [Google Scholar]
- Shima et al., 2007.Shima K, Isoda M, Mushiake H, Tanji J. Categorization of behavioural sequences in the prefrontal cortex. Nature 445: 315–318, 2007 [DOI] [PubMed] [Google Scholar]
- Shima et al., 1996.Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 93: 8694–8698, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima and Tanji, 1998.Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol 80: 3247–3260, 1998 [DOI] [PubMed] [Google Scholar]
- Shima and Tanji, 2000.Shima K, Tanji J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol 84: 2148–2160, 2000 [DOI] [PubMed] [Google Scholar]
- Sohn and Lee, 2007.Sohn J, Lee D. Order-dependent modulation of directional signals in the supplementary and presupplementary motor areas. J Neurosci 27: 13655–13666, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer and Tehovnik, 1999.Sommer MA, Tehovnik EJ. Reversible inactivation of macaque dorsomedial frontal cortex: effects on saccades and fixations. Exp Brain Res Exp Hirnforsch 124: 429–446, 1999 [DOI] [PubMed] [Google Scholar]
- Tanji, 2001.Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci 4: 631–651, 2001 [DOI] [PubMed] [Google Scholar]
- Zentner et al., 1996.Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J. Functional results after resective procedures involving the supplementary motor area. J Neurosurg 85: 542–549, 1996 [DOI] [PubMed] [Google Scholar]