Abstract
A consistent organizational feature of auditory cortex is a clustered representation of binaural properties. Here we address two questions. What is the intrinsic organization of binaural clusters and to what extent does intracortical processing contribute to binaural representation. We address these issues in the auditory cortex of the pallid bat. The pallid bat listens to prey-generated noise transients to localize and hunt terrestrial prey. As in other species studied, binaural clusters are present in the auditory cortex of the pallid bat. One cluster contains neurons that require binaural stimulation to be maximally excited, and are commonly termed predominantly binaural (PB) neurons. These neurons do not respond to monaural stimulation of either ear but show a peaked sensitivity to interaural intensity differences (IID) centered near 0 dB IID. We show that the peak IID varies systematically within this cluster. The peak IID is also correlated with the best frequency (BF) of neurons within this cluster. In addition, the IID selectivity of PB neurons is shaped by intracortical GABAergic input. Iontophoresis of GABAA receptor antagonists on PB neurons converts a majority of them to binaurally inhibited (EI) neurons that respond best to sounds favoring the contralateral ear. These data indicate that the cortex does not simply inherit binaural properties from lower levels but instead sharpens them locally through intracortical inhibition. The IID selectivity of the PB cluster indicates that the pallid bat cortex contains an increased representation of the frontal space that may underlie increased localization accuracy in this region.
INTRODUCTION
Lesion and inactivation studies show that the auditory cortex is necessary for sound localization (Casseday and Diamond 1977; Heffner 1997; Jenkins and Merzenich 1984; Malhotra et al. 2004; Whitfield et al. 1972). This report addresses two related issues regarding how the auditory cortex supports sound localization: the nature of the underlying functional organization and the extent to which this organization is shaped within the auditory cortex.
A consistent observation in auditory cortex is that neurons are clustered according to their binaural properties (Imig and Adrian 1977; Kelly and Sally 1988; Middlebrooks et al. 1980; Reale and Kettner 1986; Recanzone et al. 1999; Rutkowski et al. 2000; Velanovsky et al. 2003). This is also true of the pallid bat, the subject of this study. Unlike most bats, the pallid bat listens for prey-generated noise transients to localize prey with an accuracy ∼2° (Barber et al. 2003; Fuzessery et al. 1993). Echolocation is reserved for general orientation and obstacle avoidance. Its auditory cortex contains a region tuned between 5 and 35 kHz with most neurons selective for noise transients (Razak and Fuzessery 2002). It presumably serves the pallid bat's accurate passive sound localization of prey. This region contains two clusters of neurons with different forms of interaural intensity difference (IID) sensitivity. One contains binaurally inhibited (EI) neurons. The second cluster contains predominantly binaural (PB) neurons. PB neurons have mixed facilitatory and inhibitory interactions that create peaked IID functions (Fuzessery et al. 1990; Semple and Kitzes 1993a,b; Wise and Irvine 1984). These neurons respond weakly or not at all to monaural stimulation of either ear but respond robustly when binaurally stimulated at appropriate IIDs. The first goal of the present study was to map IID sensitivity within the PB cluster. We show that IID sensitivity shifts systematically with frequency tuning within the cluster.
The second goal of this study was to determine if intracortical inhibition plays a role in shaping binaural selectivity of PB neurons. PB type binaural selectivity is seen in both the inferior colliculus (IC) and the cortex suggesting that cortical selectivity may be inherited (Irvine and Gago 1990; Irvine et al. 1996; Lohuis and Fuzessery 2000; Phillips and Irvine 1981; Semple and Kitzes 1993a,b). Anatomical data show considerable convergence and divergence in thalamocortical connections (McMullen and de Venecia 1993; Middlebrooks and Zook 1983). Miller et al. (2001) showed that convergence underlies thalamocortical transformation of spectral and temporal properties, one consequence being that inhibitory receptive field properties are transmitted with less fidelity than excitatory properties. In the pallid bat cortex, GABA is involved in shaping FM sweep direction and rate selectivity even though similar response selectivity is seen in the inferior colliculus (Razak and Fuzessery 2009). Therefore although PB type selectivity is present in both the cortex and the IC, it remains to be tested whether intracortical GABA plays a role in refining PB selectivity locally.
Additional evidence suggests that cortical processing is important in shaping binaural selectivity. There is a greater diversity of binaural properties in the cortex compared with subcortical structures (Irvine and Gago 1990; Irvine et al. 1996; Phillips and Irvine 1981; Semple and Kitzes 1993a,b). Azimuthal sensitivity is narrower in auditory cortex than in the thalamus, suggesting that mechanisms intrinsic to cortex may sharpen spatial selectivity (Barone et al. 1996). In both the cat and the pallid bat, there is a higher percentage of PB neurons in the cortex than in the IC (Irvine and Gago 1990; Irvine et al. 1996; Lohuis and Fuzessery 2000; Phillips and Irvine 1981; Semple and Kitzes 1993a,b), suggesting that additional PB neurons may be synthesized within the auditory cortex. We tested this hypothesis by iontophoretically applying antagonists of GABAA receptors on PB neurons. We show that binaural facilitation in the majority of cortical PB neurons is eliminated by GABAA receptor antagonists. This suggests that the cortex shapes binaural sensitivity locally through intracortical inhibition.
METHODS
Pallid bats were collected in Arizona and New Mexico and held in captivity in a 16 × 21 ft room. The room was maintained on a reversed 12:12 h light cycle. All procedures followed the animal welfare guidelines required by the National Institutes of Health and the Institutional Animal Care and Use Committee.
Surgical procedures
Recordings were obtained from bats that were anesthetized with methoxyflurane (Metofane) inhalation followed by an intraperitoneal injection of pentobarbital sodium (30 μg/g body wt) and acepromazine (2 μg/g body wt). To expose the auditory cortex, the head was held in a bite bar, a midline incision was made in the scalp, and the muscles over the dorsal surface of the skull were reflected to the sides. The front of the skull was scraped clean and a layer of glass microbeads applied, followed by a layer of dental cement. The bat was then placed in a Plexiglas restraining device. A cylindrical aluminum head pin was inserted through a cross bar over the bat's head and cemented to the previously prepared region of the skull. This pin served to hold the bat's head secure during the recording session. The location of the auditory cortex was determined relative to the rostrocaudal extent of the midsagittal sinus, the distance laterally from the midsagittal sinus, and the location of a prominent lateral blood vessel that travels parallel to the midsagittal sinus. The size of the exposure was usually <2 mm2. Exposed muscle was covered with petroleum jelly (Vaseline) and exposed brain surface was covered with paraffin oil to prevent desiccation.
Recording procedures
Experiments were conducted in a heated (85–90°F), sound-proofed chamber lined with anechoic foam. Bats were kept anesthetized throughout the course of the experiments with additional pentobarbital sodium (one-third of presurgical dose) injections. Stimuli were generated using Modular Instruments and Tucker Davis Technologies digital hardware and custom-written software (Fuzessery et al. 1991). The waveforms were amplified with a stereo amplifier and presented as closed-field stimuli through Infinity emit-K ribbon tweeters fitted with funnels that were inserted into the pinnae and sealed in place with petroleum jelly. This procedure attenuated speaker intensity level at the opposite ear by ≥30 dB and permitted presentation of the ±20 dB IIDs that the bat normally experiences (Fuzessery 1996) without significant acoustic interaction between speaker outputs. The speaker-funnel frequency response curve showed a gradual increase of ∼5 dB from 8 to 40 kHz as measured with a Bruel and Kjaer 1/8-in microphone placed at the tip of the funnel. The two speakers used for dichotic studies did not differ significantly in their frequency response.
For mapping experiments, extracellular single-unit recordings were obtained using glass electrodes (1 M NaCl, 2–10 MΩ impedance) at depths between 200 and 600 μm. Penetrations were made orthogonal to the surface of the cortex. Action potentials were acquired and stored with the use of a Modular Instruments high-speed clock controlled by custom software. Responses were quantified as the total number of spikes elicited over 20 stimulus presentations.
Data acquisition
Pure tones (5–75 kHz, 5 ms duration, 1 ms rise/fall times, 1 Hz repetition rate) were used to determine best frequency (BF) and frequency tuning to ensure that recordings were made in the region selective for noise. We have termed this region the low-frequency region (LFR) in previous papers to distinguish it from the region tuned between 30 and 70 kHz and selective for echolocation calls (Razak et al. 2007). BF was defined as the frequency that elicited action potentials to at least five successive stimulus repetitions at the lowest intensity. The intensity was then increased in 5 or 10 dB steps to record tuning characteristics. For neurons that did not respond to monaural tones, BF was determined by presenting binaural tones. The IID at which peak response occurred with noise (see following text) was used to determine BF with binaural tones. Monaural responses to contralateral (CL) and ipsilateral (IL) ear stimulation were determined by presenting broadband noise with intensities between 5 and 60 dB above threshold. The binaural response type was also determined with broadband noise as the stimulus. Noise was presented at least two different CL intensities, from 10 to 30 dB above threshold, while the intensity at the IL ear was varied from 25 dB below to 25 dB above CL intensity in 5 dB steps. Neurons were considered EI if the response to binaural stimulation at any IID within the test range was ≤50% of the maximal response. A neuron was considered PB if the response to binaural stimulation was at least twice the response to monaural CL stimulation and the response subsequently decreased with increasing IL intensity. The example neuron shown in Fig. 1A was typical of most PB neurons in the pallid bat cortex in that it hardly responded to monaural CL ear stimulation. Monaural IL stimulation did not elicit responses (not shown). The neuron responded robustly to binaural input (Fig. 1B). IID selectivity of PB neurons was quantified using two measures. The peak IID was measured as the geometric center of the range of IIDs eliciting >80% of maximum response (e.g., Fig. 1B). The PB neuron shown in Fig. 1B exhibited a peak at +1.5 dB. The bandwidth (BW) of IID selectivity of PB neurons was the difference between the maximum and minimum IIDs that produced >50% of maximum response (Fig. 1B). The BW of the example neuron was 14 dB. As IID selectivity was measured at more than one CL intensity, the peak IID and BW were measured at each CL intensity and averaged.
Fig. 1.
Measurement of peak interaural intensity differences (IIDs) and bandwidth of predominantly binaural (PB) neurons. A: monaural [contralateral (CL) ear] rate-level function of a PB neuron. B: IID selectivity was measured by keeping the intensity at the CL ear fixed and increasing the ipsilateral (IL) intensity from 20 dB below to 20 dB above the CL intensity in 5 dB steps. The peak IID was the geometric center of the range of IIDs that produced 80% of maximum response (short dash horizontal line). In the example shown, IIDs between −3 and +5 dB caused a response >80% of maximum. The peak IID of this neuron was +1.5 dB (solid arrow). The solid horizontal line indicates the maximum response. Bandwidth (BW) of IID selectivity was the difference between the maximum and minimum IIDs that produced a response >50% of maximum. The BW of the neuron shown was 14 dB (−6 to +8 dB, long dash arrows). The long dash horizontal line indicates 50% of maximum response.
Iontophoresis methods
Iontophoresis of bicuculline (BIC) or gabazine (GBZN) was accomplished using a three-barrel glass pipette. The tip of the electrode was broken to an outer diameter of 5–10 μm. One barrel was used for recording action potentials (1 M NaCl, 2–6 MΩ impedance). The second barrel was used for injection of bicuculline methiodide (BIC, Sigma, St. Louis, MO) or gabazine (GBZN, Sigma). BIC (10 mM concentration, pH 3.0 with 0.1 N HCl) and GBZN (5 mM, pH 3.0 with 0.1 N HCl) were prepared fresh in isotonic saline. Iontophoretic currents were presented with a BH-2 Neurophore System (Medical Systems). In all experiments with BIC, recordings were commenced at 10 min after turning on the injection current. For experiments with GBZN, the waiting time was 15 min as initial studies showed stable effects after 10 min. The third barrel was used as the balance electrode (filled with 1 M NaCl).
A retaining current between −15 and −25 nA was applied during the search phase and the predrug (control) recording phase. Ejection currents between 5 and 40 nA were used to eject BIC and GBZN. In most cases, a low ejection current was followed by at least one higher current to ensure that the neuron was capable of firing additional spikes and saturation was not an issue. Both BIC and GBZN were used in this study because of previous reports suggesting that at high ejection currents, BIC may affect both GABAA receptors and potassium channels (Johansson et al. 2001; Kurt et al. 2006). GBZN may be more specific for GABAA receptors. At the low ejection currents used here (<40 nA), GBZN and BIC have similar effects in the pallid bat auditory cortex (Razak and Fuzessery 2009). After recording the effect of the receptor antagonists on response properties, recovery data were obtained at 5 min intervals after turning off the injection current. Neurons typically recovered from BIC effects in 10–15 min. Recovery was typically longer following GBZN injections, and a waiting time of 20 min was used. Control data were also obtained by injecting current (up to ≥2 times the magnitude of the injection current used) through the balance barrel to ensure that the current by itself did not cause changes in spike rate.
RESULTS
Peak IIDs and bandwidths of PB neurons
IID selectivity was examined in 120 PB neurons within the LFR in 16 pallid bats, using broadband noise as a stimulus. Stimulus choice was based on previous selectivity studies (Razak and Fuzessery 2002) and the putative involvement of the LFR in the localization of prey-generated noise. The IID selectivity of these neurons was determined at two or more CL intensities. A typical example is shown in Fig. 2A. The peak IID and BW of this neuron were 0 and 13 dB, respectively. There was little change in the peak IID and BW of this neuron as CL intensity was increased. Across the population, peak IIDs ranged from −10 to +10 dB, with the majority of neurons showing peaks at IIDs between −4 to +6 dB (Fig. 2B). This suggests that most neurons in the PB cluster represent sound locations near the midline. The BW ranged from 7 to 30 dB across the population, with the majority of neurons exhibiting values between 10 and 20 dB (Fig. 2C).
Fig. 2.
Properties of the population of PB neurons. A: the responses of a PB neurons to binaural stimulation of a range of intensity levels, showing little change in peak IIDs or BWs. B: the majority of neurons exhibited peak IID values between −4 and +6 dB. C: the BW of selectivity ranged between 7 and 30 dB with the majority of neurons exhibiting values between 10 and 16 dB. D: peak IIDs and bandwidth of selectivity were stable across absolute intensity (see text for details).
The ratio of changes in peak IID or BW relative to changes in CL intensity level (termed IID/CL ratio) was measured to test the stability of IID preference or BW over different CL intensities. This is the same measure used by Irvine and Gago (1990) to quantify stability of binaural properties across absolute intensity. For example, if the peak IIDs of a neuron were 0, 0, and 3 dB at CL intensities of 35, 40, and 50 dB, respectively, then the IID/CL ratio for peak IID is 0.2 [i.e., (3–0)/(50–35) = 3/15 = 0.2]. Similarly if the BWs were 11, 13, and 15 dB at 35, 40, and 45 dB, respectively, then the IID/CL ratio for BW is 0.4. The distribution of the IID/CL ratio for peak IID (Fig. 2D) shows that ∼70% of the PB neurons had a ratio <0.2, and ∼90% of the neurons had a ratio <0.4. Similarly, the distribution of IID/CL ratio for BW shows that ∼70% of the neurons had a ratio <0.4. These results suggest that the peak IID and BW of the majority of PB neurons remain relatively stable with changes in absolute intensity level.
Peak IIDs vary systematically within the PB cluster
The PB cluster was mapped using single unit recordings in four bats to determine the intrinsic organization of the PB cluster. In all four cortices, peak IIDs changed systematically from positive to negative IID values in a caudorostral direction (Figs. 3 and 4). Figure 3 shows IID sensitivity plots recorded from the LFR of a pallid bat auditory cortex. The number above each IID plot corresponds to the recording location in the map shown in the middle of the figure. The two most medial neurons in the map were binaurally inhibited (shown as EI in the map). The most caudolateral neuron was monaural (EO – showed no change in response with increasing IL intensities). All other neurons exhibited PB sensitivity. The peak IID of each PB neuron is shown in parentheses in the map, and by the arrow in each IID plot. Peak IIDs changed systematically from +8 dB caudally to −6 dB rostrally. The BF of each recording site is also noted in the IID plots. Because both BF and peak IID change in a rostrocaudal direction, there was a correlation between these two properties. Neurons with lower BFs had more positive IID peaks.
Fig. 3.
Peak IIDs are arranged systematically in a PB cluster. IID sensitivity of single neurons was recorded at several sites within the low-frequency region (LFR, experiment CTX24). The recording sites are indicated by numbers in the map shown in the middle of this figure. The number on top of each IID plot identifies the location of the recorded neuron within this map. The numbers within parentheses in the map, and arrows in each IID plot, correspond to the peak IID value recorded at each site. The peak IIDs change systematically from CL preferring to IL preferring in the caudorostral direction. Sites marked as binaurally inhibited (EI) were binaurally inhibited, and the site marked EO was monaural.
Fig. 4.
Peak IIDs are arranged systematically in PB clusters. A, C, and E: BF map within PB clusters in 3 different bats. B, D, and F: the corresponding peak IID map. Sites marked as EI were binaurally inhibited. Left: - - -, isofrequency contours; right: - - -, different ranges of peak IIDs. Broadband noise was used as stimulus to map peak IID. G: correlation between BF and peak IID from all the neurons recorded in this study.
The actual range of BF and peak IIDs within each PB cluster varied across individuals. Figure 4, left, shows changes in BF within the PB clusters of the other three cortices, and the right column shows the corresponding peak IID maps. In the example shown in Fig. 4A, BF changed from 8 to 26 kHz in a caudolateral to rostromedial direction. The peak IIDs of these PB neurons changed systematically from +5 dB caudally to −8 dB rostrally. The other examples in Fig. 4 also show the relationship between BF and IID peak and serve to illustrate the individual variability in the range of peak IIDs and in the overlap between the tonotopic map and peak IID range. The correlation between BF and IID peak was significant (Fig. 4G). Together, these data show that the arrangement of peak IIDs within the PB cluster is systematic and correlated with tonotopy.
Comparison of IID selectivity in response to noise and BF tones
IID selectivity in response to broadband noise and BF tones was compared in 37 PB neurons. Almost all (33/37) PB neurons were more sharply tuned for IID in response to noise than BF tones. The examples in Fig. 5 illustrate the range of differences observed in neural responses to binaural noise and tones. The neuron shown in Fig. 5A was sharply tuned for IID when presented with noise. The peak IID was near 0 dB, and the 50% BW was 10 dB. In response to binaural BF tone (16 kHz), the BW increased to 26 dB (Fig. 5B). The peak IID remained centered near 0 dB, indicating that the increase in BW for tones was due to a nearly symmetrical expansion of the IID function. The neuron shown in Fig. 5, C and D, also showed an increase in BW from 21 dB in response to noise to 31 dB in response to BF tone (21 kHz). In this neuron, the peak IID changed from 0 dB for noise to 7 dB for tones, indicating that the neuron now responded better to IIDs favoring the CL ear.
Fig. 5.
PB neurons were more sharply tuned for IID in response to noise than to BF tones. Left: IID selectivity for broadband noise. Right: IID selectivity for BF tones. A and B: the 50% BW of this neuron was broader for noise than BF tone, but the center of the peak IIDs (↓) was similar for the 2 stimuli. C and D: this neuron's IID selectivity was broader for tones, and the peak IID shifted to more positive IIDs. E and F: this neuron was PB for noise. When presented with the BF tone, however, the response was not inhibited for increasing IL intensities. G and H: this neuron was PB in response to noise and EI in response to BF tones.
Nearly 30% (11/37) of PB neurons exhibited either a different binaural selectivity or poor responses to tones compared with noise. For example, the neuron shown in Fig. 5E was sharply tuned for IID in response to noise. However, when tested with a BF tone, the neuron now responded to IIDs favoring the IL ear (Fig. 5F). The neuron shown in Fig. 5, G and H, showed the opposite change. It had a peaked PB function for noise but was no longer inhibited at IIDs favoring the CL ear. Overall, eight neurons that were PB for noise either exhibited EI type responses favoring the CL (4/8) or IL ears (4/8). Three neurons that responded robustly to noise, responded poorly (<20% of maximum noise response) to tones. Thus more than a quarter of neurons in the PB cluster of the noise-selective region did not exhibit a peaked PB response when tested with BF tones. Across the population of PB neurons that showed a peaked IID response for both noise and tones (26/37), BW was significantly higher for tones than noise (noise: 16.9 ± 1.1 dB, BF tone: 26.5 ± 1.1 dB, paired t-test, P < 0.0001, Fig. 6A). Most (19/26) of these neurons showed at least a 3 dB difference in the peak IID for noise and tones (Fig. 6B). No trend was observed in terms of the direction of shift of peak IIDs for noise and tones. Taken together, these data show that the PB neurons integrate across multiple frequencies to generate sharper IID selectivity for broadband stimuli.
Fig. 6.
Population summary of IID selectivity for noise and tones. A: the 50% BW was higher for tones than for noise in all except 1 neuron (paired t-test, P < 0.0001). The thick horizontal lines show the average 50% BW for noise and tones. B: most neurons exhibited different peak IID values for noise and tones.
The difference in binaural sensitivity for noise and tones suggests that the systematic map of IID sensitivity in the PB cluster may not be clearly evident if probed with BF tones. To test this, we mapped one cortex based on single unit recordings in response to both binaural noise and BF tones (Fig. 7). When mapped with noise as the stimulus, peak IIDs varied systematically from +8 dB caudally to −5 dB rostrally. When the IID sensitivity of the same neurons was mapped with the BF tone, there was a general increase from positive to negative values in a caudorostral direction. However, the map is disrupted by the presence of neurons with weak response (WR) to tones and neurons with different binaural sensitivity to tones compared with noise. Even when only the neurons with PB response to tones are considered, the map is less systematic. For instance, a neuron with a negative peak IID (−1 dB) occurs among neurons with positive values. Another neuron with 0 dB peak IID occurs among neurons with more positive peak IIDs. These data suggest that neurons in PB clusters integrate binaural cues across frequency to sharpen IID sensitivity to noise.
Fig. 7.
IID sensitivity of PB clusters is more systematic when mapped with noise compared with tones. A: BF map in a PB cluster. B: when mapped with noise as stimulus, IID sensitivity varied systematically from −5 dB rostrally to +8 dB caudally. - - -, different ranges of IID sensitivity. C: when the IID sensitivity of the same neurons was mapped with the BF tone, there is a general increase from positive to negative values in a caudorostral direction. However, the map is disrupted by the presence of neurons with weak response (WR) to tones and neurons with different binaural sensitivity to tones (EI) compared with noise. Even when only the neurons with PB response to tones are considered, the map is less systematic.
GABA shapes binaural sensitivity of PB neurons
The role of GABA in shaping cortical binaural sensitivity was studied in 22 neurons within the PB cluster. During the application of BIC or GBZN, 17/22 (78%) PB neurons were strongly excited by monaural stimulation, resulting in a loss of their peaked IID sensitivity. A typical example is shown in Fig. 8. This neuron responded poorly to monaural CL stimulation in the control condition (Fig. 8A, CTRL). The neuron responded robustly to binaural stimulation with a peak IID centered at 0 dB (Fig. 8B). When BIC was injected, the neuron responded to monaural CL stimulation, with response magnitude increasing with current strength (Fig. 8A). Consistent with an enhanced monaural response to CL stimulation, the neuron responded well to binaural stimuli that favored the CL ear (Fig. 8C). That is, binaural selectivity changed from a PB to an EI type response. The neuron continued to be inhibited for binaural stimuli that favored the IL ear in the presence of BIC. These observations were consistent at the three different CL intensities tested. Figure 8A shows that the neuron was capable of firing additional spikes when the injection current was increased. This suggests that the loss of the peak was not due to a saturation effect. The neuron's dependence on binaural input for strong responses was restored a few minutes after the BIC current was turned off (Fig. 8D).
Fig. 8.
Bicuculline converts PB type IID sensitivity to EI type sensitivity. A: monaural rate-level functions. In the predrug control (CTRL) condition, the neuron responded poorly to CL ear stimulation. In the presence of bicuculline (BIC, BIC15), injected with a current of 15 nA, the neuron responded to monaural CL stimulation. The response increased for a larger injection current (BIC20). 10 min after the injection current was turned off (REC), the response of the neuron was similar to control. B: in the control condition, the neuron responded to binaural stimuli with a peak IID around 0 dB. IID sensitivity was tested at 3 different CL intensities. C: binaural stimulation in the presence of BIC (15 nA) resulted in an EI type function, wherein the neuron responded to a broad range of IIDs favoring the CL ear and was inhibited when the intensity at the IL ear was increased. D: the neuron recovered control-like binaural sensitivity 10 min after the injection current was turned off.
To quantify the effects of the antagonists on binaural responses of PB neurons, two trough/peak ratios were calculated. The first, termed contralateral trough/peak ratio is the ratio between response at a CL ear favoring IID (+20 dB IID) and the response at the best IID. The second, termed ipsilateral trough/peak ratio is the ratio between response at an IL ear favoring IID (−20 dB IID) and the response at the best IID. The best IID is defined in the control (predrug) condition when neurons exhibit PB type selectivity. During iontophoresis when most neurons show EI type selectivity, best IID refers to the IID at which the neuron's response peaked in the control condition. These ratios provide a measure of the peak height by comparing response at best IID with response to IIDs at which the neuron hardly responds (±20 dB IID). A smaller ratio indicates a larger difference between response at the reference IID and response at best IID. These ratios were calculated before and during antagonist iontophoresis. For the example shown in Fig. 8, the CL trough/peak ratio changed from 0.2 in the control condition to 0.9 during BIC application. This shows that in the control condition, the response to the best IID was approximately fivefold larger than the response to +20 dB IID. When BIC was applied, the response to a CL favoring IID was almost as high as the response to the IID to which the neuron responded best in the control condition. The IL trough/peak ratio hardly changed (0–0.07), indicating that the neuron continued to be strongly suppressed at IL favoring IIDs during iontophoresis.
Both GBZN and BIC produced similar effects in the LFR as has been previously shown in the echolocation call selective region of the pallid bat cortex (Razak and Fuzessery 2009). BIC increased responses to monaural stimulation in 13/16 neurons, whereas GBZN had similar effects in 4/6 neurons. The binaural response of the neuron shown in Fig. 9 was converted from PB to EI type when GBZN was applied. The neuron hardly responded to monaural stimulation of either ear in the control condition but showed robust responses to CL ear stimulation in the presence of GBZN (Fig. 9A). There was no response to the IL ear stimulation. Its binaural response was strong and peaked at an IID centered at 0 dB (Fig. 9B). In the presence of GBZN, the neuron responded well to binaural stimulation favoring the CL ear and continued to be suppressed when the IL intensity was increased, resulting in EI type sensitivity (Fig. 9B). In this neuron, the CL trough/peak ratio changed from 0.1 in the control condition to 1.1 during GBZN application, indicating a strong response to CL IIDs. The IL trough/peak ratio changed from 0.1 to 0.4, suggesting a decrease in the inhibition driven by the IL ear.
Fig. 9.
Gabazine (GBZN) converts PB type IID sensitivity to EI type sensitivity. A: monaural rate-level functions in response to either the CL or the IL ear. B: IID selectivity before and during ejection of GBZN (10 nA).
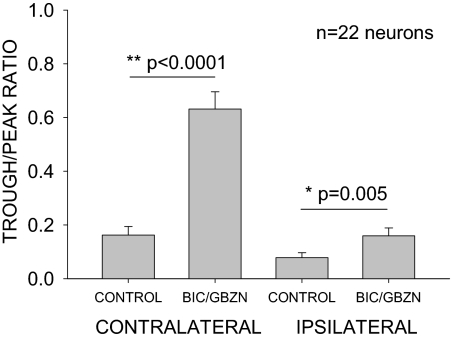
Across the population of PB neurons the main effect of BIC/GBZN was to reduce or eliminate the peak mainly by increasing responses to the IIDs favoring the CL ear. Figure 10 summarizes the change in the CL and IL trough/peak ratios following antagonist application. The CL trough/peak ratio is significantly larger (paired t-test, P < 0.0001) in the drug condition compared with control showing that GABA signaling is important in shaping the peaked response of PB neurons in the cortex. The IL trough/peak ratio was also significantly (paired t-test, P = 0.005) different between the control and drug conditions. However, the mean ratio was still <0.2 in both conditions indicating that the response to IL favoring IID was strongly suppressed even in the presence of GABA antagonists. Taken together, these data suggest that cortical GABAA receptors play a critical role in shaping the binaural sensitivity of PB neurons with the main effect being a suppression of responses to monaural CL input or to binaural input favoring the CL ear. Thus cortical GABA sculpts out a peaked binaural response from EI type inputs.
Fig. 10.
Summary of effects of GABAA receptor antagonists on facilitation in PB neurons. Two trough/peak ratios were calculated. The 1st, termed contralateral trough/peak ratio, is the ratio between response at a CL ear favoring IID (+20 dB IID) and the response at the best IID. The second, termed ipsilateral trough/peak ratio is the ratio between response at an IL ear favoring IID (−20 dB IID) and the response at the best IID. These ratios were calculated before and during antagonist iontophoresis. A smaller ratio indicates a larger difference between response at the reference IID and response at best IID. The contralateral trough/peak ratio was significantly larger (paired t-test, P < 0.0001) in the drug condition compared with control showing that GABA signaling is important in shaping binaural facilitation in the cortex. The ipsilateral trough/peak ratio was also significantly (paired t-test, P = 0.005) different between the control and drug conditions. However, the mean ratio was still <0.2 in both conditions indicating that the response to IL favoring IID was strongly inhibited even in the presence of GABA antagonists.
Binaural facilitation was not affected by GABAA receptor antagonists in 5/22 PB neurons. Figure 11 shows an example in which GBZN enhanced the response magnitude nearly fourfold, but the peaked IID function remained. The CL trough/peak ratio changed from 0.15 to 0.17 with GBZN. The IL trough/peak ratio changed from 0.05 to 0.38, suggesting a decrease in IL driven inhibition relative to control. The best IID also was unchanged. In these neurons, peaked IID functions are either inherited from subcortical structures or depend on non-GABAA receptor mediated mechanisms.
Fig. 11.
GBZN does not change facilitation despite a four-fold increase in response magnitude. A: the response to monaural CL stimulation before and during GBZN application. B: the binaural response.
DISCUSSION
There are two novel findings in this study regarding cortical binaural processing. First, the peak IID shifts systematically within the PB cluster and is correlated with BF. The peak IIDs and BWs of PB neurons were relatively stable across absolute intensities, suggesting that they provide reliable spatial information in a changing acoustic environment. Second, cortical GABA shapes the peak IID in the majority of PB neurons. The auditory cortex uses local inhibition to create peaked IID functions and to shape the intrinsic organization of the PB cluster.
PB cluster: increased representation of azimuths near the midline
PB neurons have been reported in the inferior and superior colliculi as well as the auditory cortex (Hirsch et al. 1985; Kitzes et al. 1980; Semple and Kitzes 1993a,b; Wise and Irvine 1984). As observed in the present study, these neurons typically respond best to IIDs near 0 dB. In a direct test of the azimuthal sensitivity of binaurally facilitated neurons in the IC, Fuzessery et al. (1990) used a sequential closed-field/free-field paradigm to show that neurons with PB type selectivity responded best to sounds from near the midline. Likewise, in auditory cortex, Samson et al. (1994) reported neurons that responded best to midline locations but lost responses when either ear was occluded. While the IID sensitivity of these neurons was not tested in this study, the monaural occlusion results suggest that these were PB cells.
The PB cluster in the pallid bat cortex is likely to focus on azimuth locations close to the midsagittal plane based on the monaural and binaural cues created by the bat's ears (Fuzessery 1996). BFs in the PB cluster were between 8 and 39 kHz. The distribution of maximum IIDs in the pallid bat's frontal sound field is highly frequency dependent (Fuzessery 1996) over the range of BFs in the PB cluster. Therefore while the majority of neurons show >50% maximum firing within ±10o of the midsagittal plane, their azimuthal sensitivity will vary considerably with BF. From 5 to 30 kHz, the directionality of the ears sharpen considerably, and both the areas of maximum sensitivity and peak IIDs are reduced in size and move toward the midline. Assuming bilateral symmetry in the binaural properties of the PB clusters, neurons tuned to ∼10 kHz can be expected to be most sensitive to sound locations of ±40° azimuth, whereas those tuned around 30 kHz will have a more restricted sensitivity for sound locations of ±10° azimuth. Thus the PB clusters may be a specialization to increase representation of space near the midline and subserve the remarkably accurate passive sound localization ability of the pallid bat (Barber et al. 2003).
The presence of a systematic IID map in the PB cluster does not necessarily predict a point-to-point map of space as seen in the midbrain of the barn owl. First, the PB cluster focuses on a narrow range of space in front of the pallid bat. Second, the 50% BW of most PB neurons is broad (between 12 and 15 dB). As sound frequency increases, the range of azimuth locations that produce this range of IIDs decreases. This predicts that azimuth receptive fields will become narrower with increasing BF. Thus as the sound moves from ∼30o CL or IL space toward the midline, the extent of activity will spread from mostly the low BF neurons to include neurons with systematically higher BF with most of the PB cluster activated when the sound is at midline. One can imagine this as a “bull's eye” with neurons with the highest BFs having spatial receptive fields restricted to the center of the target. This suggests that the relationship among IID sensitivity, frequency tuning, and ear directionality results in a substrate within the PB cluster that represents sounds near the midline based on systematic changes in the extent of activity.
We have previously suggested that the EI cluster also encodes sound locations based on the extent of activation model (Irvine and Gago 1990; Wenstrup et al. 1988; Wise and Irvine 1984). Neurons in the EI cluster are arranged according to the IIDs at which they are inhibited, which suggests a systematic shift in areas of excitation as a sound moves along the azimuth (Razak and Fuzessery 2002). Thus the two binaural clusters may use similar substrates to represent overlapping regions of space with the EI cluster serving a larger region including midline and the PB cluster focusing on the midline. The two clusters may also have complementary sound localization functions with the EI clusters lateralizing a sound source, resulting in a head orientation that places the sound within the focus of the PB cluster.
Comparison of responses to noise and tones
The sharper IID tuning and more systematic topography observed when broadband noise was used as a stimulus compared with a BF tone indicates that PB neurons integrate binaural information over a broad frequency range, which is consistent with the sound localization behavior of the pallid bat in that it relies on prey-generated noise transients (Bell 1982; Fuzessery et al. 1993). Perhaps in a manner similar to neurons in the optic tectum of the barn owl (Brainard et al. 1992), the main priority of the PB cluster is to resolve space, not frequency. This is consistent with both neurophysiological (Clarey et al. 1995; Recanzone et al. 2000) and behavioral data (Butler 1986) showing sharper azimuth localization for broad- versus narrowband sounds.
Role of GABA in shaping cortical binaural properties
Our results with GABAA receptor antagonists show intracortical inhibition shapes the peaked IID sensitivity of PB neurons. Specifically, it is responsible for the inhibition at IIDs favoring the CL ear. In contrast, inhibition at IL IIDs is less affected, suggesting a subcortical origin. A model for how inhibition shapes binaural facilitation in PB neurons was proposed by Park and Pollak (1993) and is applicable in the pallid bat cortex. This model requires two EI neurons converging on the PB neuron (Fig. 12). One EI neuron is excitatory, and the other EI neuron is GABAergic and provides inhibition. Another requirement of the model is that the GABAergic EI neuron be inhibited at more positive (CL) IIDs than the excitatory EI neuron (Fig. 12). When binaural stimulation favors the CL ear, the inhibition provided by the GABAergic EI neuron reduces the response of the PB neuron. When the IID is near 0 dB, the GABAergic EI neuron is inhibited, allowing the excitatory EI neuron to drive the PB neuron. For IIDs that favor the IL ear, both EI neurons are inhibited, and the PB cell is not activated. The response peak therefore occurs near 0 dB IID.
Fig. 12.
Neural interactions that may generate a PB neuron and explain the effects of GABAA receptor antagonists observed in this study (modified from Park and Pollak 1993). See text for details.
One likely scenario for the inputs to PB neurons is that the inhibitory EI neuron is located in the cortex, while the excitatory EI input is a thalamocortical projection (Fig. 12). Application of GABAA receptor antagonists blocks the actions of GABA released from the inhibitory EI neuron located in the cortex. This causes a relative increase in response to stimuli favoring the CL ear. The systematic shift in the peak IID within the PB cluster will occur if the properties of the two EI inputs change systematically across the cortical surface. The BW of PB neurons is controlled by the difference between the IIDs at which the two EI neurons are maximally inhibited.
It must be noted that these results were obtained from pentobarbital anesthetized cortex. Pentobarbital may increase apparent inhibition and sharpen selectivity. However, IID sensitivity varied as a function of stimulus, so the effect was not overwhelming. Also the effect of disinhibition was more than just an increase in response magnitude. It specifically involved loss of inhibition evoked by sound at one ear. This suggests that while the system may have been depressed by pentobarbital, the underlying inhibitory mechanisms could nonetheless be revealed.
Hierarchical processing in the auditory system
Why shape PB sensitivity de novo when such selectivity is already present in the IC? There is considerable convergence and divergence in the thalamocortical pathway that may reduce selectivity in the cortex (Middlebrooks and Zook 1983). In addition, inhibitory transmission may be more subject to degradation than excitatory transmission. In a study of thalamocortical transference of receptive fields, Miller et al. (2001) showed that while thalamic excitatory fields are largely unchanged at the cortical level, inhibitory components are added there. Previous studies support the idea that the cortex uses local inhibitory processes to shape response selectivity. Whole cell recording also shows that intracortical inhibition shapes the spectrotemporal properties of cortical neurons (Kaur et al. 2004; Wehr and Zador 2003). Zhang et al. (2003) reported that inhibitory currents recorded in cortical neurons enhance selectivity for FM sweep direction by differentially suppressing responses to the nonpreferred direction. The blockade of GABAergic receptors has revealed that the response patterns, frequency tuning, and FM sweep selectivity of cortical neurons are shaped by intracortical inhibition (Chen and Jen 2000; Kurt et al. 2006; Razak and Fuzessery 2009; Schulze and Langner 1999; Wang et al. 2000, 2002). Thus even if a similar response property is present in both subcortical and cortical neurons, it cannot be assumed that the cortex simply inherits these properties. If cortical processing refines binaural selectivity, one prediction is that there will be layer-specific differences in the PB cluster (Atencio et al. 2009). This will be tested in future studies.
Origin of cortical binaural clusters
Middlebrooks and Zook (1983) showed that binaural clusters occur in the cortex through systematic connections with thalamic loci with similar binaural properties (Fig. 13, left). Cortical bands of EI and EE (binaurally excited) neurons receive inputs from segregated thalamic loci that presumably have similar binaural properties, forming functionally distinct thalamocortical subunits. Our data suggest a second mechanism (Fig. 13, right). PB neurons are present in a single cluster in the pallid bat LFR. The finding that disinhibition transforms most PB neurons to EI neurons suggests that intracortical inhibition may play a role in creating this cluster of PB neurons from a larger cluster of EI neurons.
Fig. 13.
Mechanisms through which 2 clusters of binaural properties can arise in the auditory cortex. Left: inheritance of different types of binaural properties through systematic thalamocortical connections. Right: inheritance of 1 type of binaural input and conversion of this input at some locations into another type using local GABA. This creates 2 different binaural clusters.
GRANTS
This research was supported by National Institute of Deafness and Other Communication Disorders Grant DC-05202.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank D. Richardson for participating in preliminary stages of this study, T. Zumsteg for comments on the manuscript, and G. McLellan for programming the software required for this study.
Present address of K. A. Razak: Dept. of Psychology, University of California, 900 University Ave, Riverside, CA 92521.
REFERENCES
- Atencio et al. 2009.Atencio CA, Sharpee TO, Schreiner CE. Hierarchical computation in the canonical auditory cortical circuit. Proc Natl Acad Sci USA 106: 21894–21899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber et al. 2003.Barber JR, Razak KA, Fuzessery ZM. Can two streams of auditory information be processed simultaneously? Evidence from the gleaning bat Antrozous pallidus. J Comp Physiol [A] 189: 843–855, 2003 [DOI] [PubMed] [Google Scholar]
- Barone et al. 1996.Barone P, Clarey JC, Irons WA, Imig TJ. Cortical synthesis of azimuth-sensitive single-unit responses with nonmonotonic level tuning: a thalamocortical comparison in the cat. J Neurophysiol 75: 1206–1220, 1996 [DOI] [PubMed] [Google Scholar]
- Bell 1982.Bell GP. Behavioral and ecological aspects of gleaning by the desert insectivorous bat, Antrozous pallidus (Chiroptera: Vespertilionidae). Behav Ecol Sociobiol 10: 217–223, 1982 [Google Scholar]
- Brainard et al. 1992.Brainard MS, Knudsen EI, Esterly SD. Neural derivation of sound source location: resolution of spatial ambiguities in binaural cues. J Acoust Soc Am 91: 1015–1027, 1992 [DOI] [PubMed] [Google Scholar]
- Butler 1986.Butler RA. The bandwidth effect on monaural and binaural localization. Hear Res 21: 67–73, 1986 [DOI] [PubMed] [Google Scholar]
- Casseday and Diamond 1977.Casseday JH, Diamond IT. Symmetrical lateralization of function in the auditory system of the cat: effects of unilateral ablation of cortex. Ann NY Acad Sci 299: 255–263, 1977 [DOI] [PubMed] [Google Scholar]
- Chen and Jen 2000.Chen QC, Jen PH-S. Bicuculline application affects discharge patterns rate-intensity functions and frequency tuning characteristics of bat auditory cortical neurons. Hear Res 150: 161–174, 2000 [DOI] [PubMed] [Google Scholar]
- Clarey et al. 1995.Clarey JC, Barone PW, Irons A, Samson FK, Imig TJ. Comparison of noise and tone azimuth tuning of neurons in cat primary auditory cortex and medial geniculate body. J Neurophysiol 74: 961–980, 1995 [DOI] [PubMed] [Google Scholar]
- Furukawa et al. 2000.Furukawa S, Xu L, Middlebrooks JC. Coding of sound source location by ensembles of cortical neurons. J Neurosci 20: 1216–1228, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery 1996.Fuzessery ZM. Monaural and binaural spectral cues created by the external ears of the pallid bat. Hear Res 95: 1–17, 1996 [DOI] [PubMed] [Google Scholar]
- Fuzessery et al. 1993.Fuzessery ZM, Buttenhoff P, Andrews B, Kennedy JM. Passive sound localization of prey by the pallid bat (Antrozous p. pallidus). J Comp Physiol [A] 171: 767–777, 1993 [DOI] [PubMed] [Google Scholar]
- Fuzessery et al. 1991.Fuzessery ZM, Gumtow RG, Lane R. A microcomputer-controlled system for use in auditory physiology. J Neurosci Methods 36: 45–52, 1991 [DOI] [PubMed] [Google Scholar]
- Fuzessery et al. 1990.Fuzessery ZM, Wenstrup JJ, Pollak GD. Determinants of horizontal sound location selectivity of binaurally excited neurons in an isofrequency region of the mustache bat inferior colliculus. J Neurophysiol 63: 1128–1147, 1990 [DOI] [PubMed] [Google Scholar]
- Heffner 1997.Heffner HE. The role of macaque auditory cortex in sound localization. Acta Otolaryngol 532: 22–27, 1997 [DOI] [PubMed] [Google Scholar]
- Hirsch et al. 1985.Hirsch JA, Chan JCK, Yin TCT. Responses of neurons in the cat's superior colliculus to acoustic stimuli. I. Monaural and binaural response properties. J Neurophysiol 53: 726–745, 1985 [DOI] [PubMed] [Google Scholar]
- Imig and Adrian 1977.Imig TJ, Adrian HO. Binaural columns in the primary field (A1) of cat auditory cortex. Brain Res 138: 241–257, 1977 [DOI] [PubMed] [Google Scholar]
- Irvine and Gago 1990.Irvine DRF, Gago G. Binaural interaction in high-frequency neurons in inferior colliculus of the cat: effects of variations in sound pressure level on sensitivity to interaural intensity differences. J Neurophysiol 63: 570–591, 1990 [DOI] [PubMed] [Google Scholar]
- Irvine et al. 1996.Irvine DRF, Rajan R, Aitkin LM. Sensitivity to interaural intensity differences of neurons in primary auditory cortex of cat. I. Types of sensitivity and effects of variations in sound pressure level. J Neurophysiol 75: 75–96, 1996 [DOI] [PubMed] [Google Scholar]
- Jenkins and Merzenich 1984.Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol 52: 819–847, 1984 [DOI] [PubMed] [Google Scholar]
- Johansson et al. 2001.Johansson S, Druzin M, Haage D, Wang M-D.The functional role of a bicuculline-sensitive Ca2+-activated K+ current in rat medial preoptic neurons. J Physiol 532: 625–635, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur et al. 2004.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol 91: 2551–2567, 2004 [DOI] [PubMed] [Google Scholar]
- Kelly and Sally 1988.Kelly JB, Sally SL. Organization of auditory cortex in the albino rat: binaural response properties. J Neurophysiol 59: 1756–1769, 1988 [DOI] [PubMed] [Google Scholar]
- Kitzes et al. 1980.Kitzes LM, Wrege KS, Cassady JM. Patterns of responses of cortical cells to binaural stimulation. J Comp Neurol 192: 455–472, 1980 [DOI] [PubMed] [Google Scholar]
- Kurt et al. 2006.Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res 212: 224–235, 2006 [DOI] [PubMed] [Google Scholar]
- Lohuis and Fuzessery 2000.Lohuis TD, Fuzessery ZM. Neuronal sensitivity to interaural time differences in the sound envelope in the auditory cortex of the pallid bat. Hear Res 143: 43–57, 2000 [DOI] [PubMed] [Google Scholar]
- Malhotra et al. 2004.Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol 92: 1625–1643, 2004 [DOI] [PubMed] [Google Scholar]
- McMullen and de Venecia 1993.McMullen NT, de Venecia RK. Thalamocortical patches in auditory neocortex. Brain Res 620: 317–322, 1993 [DOI] [PubMed] [Google Scholar]
- Middlebrooks et al. 1980.Middlebrooks JC, Dykes RW, Merzenich MM. Binaural response-specific bands in primary auditory cortex (AI) of the cat: topographical organization orthogonal to isofrequency contours. Brain Res 181: 31–48, 1980 [DOI] [PubMed] [Google Scholar]
- Middlebrooks and Knudsen 1984.Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat's superior colliculus. J Neurosci 4: 2621–2634, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks et al. 1998.Middlebrooks JC, Xu L, Eddins AC, Green DM. Codes fror sound-source location in nontonotopic auditory cortex. J Neurophysiol 80: 863–881, 1998 [DOI] [PubMed] [Google Scholar]
- Middlebrooks and Zook 1983.Middlebrooks JC, Zook JM. Intrinsic organization of the cat's medial geniculate body identified by projections to binaural response-specific bands in the primary auditory cortex. J Neurosci 3: 203–224, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. 2001.Miller LM, Escabi MA, Read HL, Schreiner CE. Functional convergence of response properties in the auditory thalamocortical system. Neuron 32: 151–160, 2001 [DOI] [PubMed] [Google Scholar]
- Nakamoto et al. 2004.Nakamoto KT, Zhang J, Kitzes LM. Response patterns along an isofrequency contour in cat primary auditory cortex (AI) to stimuli varying in average and interaural levels. J Neurophysiol 91: 118–135, 2004 [DOI] [PubMed] [Google Scholar]
- Park and Pollak 1993.Park TJ, Pollak GD. GABA shapes sensitivity to interaural intensity disparities in the mustache bat's inferior colliculus: implications for encoding sound location. J Neurosci 13: 2050–2067, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips and Irvine 1981.Phillips DP, Irvine DRF. Responses of single neurons in physiologically defined area A1 of cat cerebral cortex: sensitivity to interaural intensity differences. Hear Res 5: 299–307, 1981 [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2002.Razak KA, Fuzessery ZM. Functional organization of the pallid bat auditory cortex: Emphasis on binaural organization. J Neurophysiol 87: 72–86, 2002 [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2009.Razak KA, Fuzessery ZM. GABA shapes selectivity for the rate and direction of frequency modulated sweeps in the auditory cortex. J Neurophysiol 102: 1366–1378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak et al. 2007.Razak KA, Shen W, Zumsteg T, Fuzessery ZM. Parallel thalamocortical pathways for echolocation and passive sound localization in a gleaning bat, Antrozous pallidus. J Comp Neurol 500: 322–338, 2007 [DOI] [PubMed] [Google Scholar]
- Reale and Kettner 1986.Reale RA, Kettner RE. Topography of binaural organization in primary auditory cortex of the cat: effects of changing interaural intensity. J Neurophysiol 56: 663–682, 1986 [DOI] [PubMed] [Google Scholar]
- Recanzone et al. 2000.Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol 83: 2723–2739, 2000 [DOI] [PubMed] [Google Scholar]
- Recanzone et al. 1999.Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol 415: 460–481, 1999 [DOI] [PubMed] [Google Scholar]
- Rutkowski et al. 2000.Rutkowski RG, Wallace MN, Shackleton TM, Palmer AR. Organisation of binaural interactions in the primary and dorsocaudal fields of the guinea pig auditory cortex. Hear Res 145: 177–189, 2000 [DOI] [PubMed] [Google Scholar]
- Samson et al. 1994.Samson FK, Barone P, Clarey JC, Imig TJ. Effect of ear plugging on single-unit azimuth sensitivity in cat primary auditory cortex. II. Azimuth tuning dependent upon binaural stimulation. J Neurophysiol 71: 2194–2216, 1994 [DOI] [PubMed] [Google Scholar]
- Schulze and Langner 1999.Schulze H, Langner G. Auditory cortical responses to amplitude modulations with spectra above frequency receptive fields: evidence for wide spectral integration. J Comp Physiol [A] 185: 493–508, 1999 [DOI] [PubMed] [Google Scholar]
- Semple and Kitzes 1993a.Semple MN, Kitzes LM. Binaural processing of sound pressure level in the cat primary auditory cortex: evidence for a representation based on absolute levels rather than interaural level differences. J Neurophysiol 69: 449–461, 1993a [DOI] [PubMed] [Google Scholar]
- Semple and Kitzes 1993b.Semple MN, Kitzes LM. Focal selectivity for binaural sound pressure level in cat primary auditory cortex: two-way intensity network tuning. J Neurophysiol 69: 462–473, 1993b [DOI] [PubMed] [Google Scholar]
- Velanovsky et al. 2003.Velanovsky DS, Cetas JS, Price RO, Sinex DG, McMullen NT. Functional subregions in the primary auditory cortex defined by thalamocortical terminal arbors: an electrophysiological and anterograde labeling study. J Neurosci 23: 308–316, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. 2000.Wang J, Caspary D, Salvi RJ. GABAA antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport 11: 1137–1140, 2000 [DOI] [PubMed] [Google Scholar]
- Wang et al. 2002.Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res 944: 219–231, 2002 [DOI] [PubMed] [Google Scholar]
- Wehr and Zador 2003.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Wenstrup et al. 1988.Wenstrup JJ, Fuzessery ZM, Pollak GD. Binaural neurons in the mustache bat's inferior colliculus. II. Determinants of spatial response among 60-kHz EI units. J Neurophysiol 60: 1384–1404, 1988 [DOI] [PubMed] [Google Scholar]
- Whitfield et al. 1972.Whitfield IC, Cranford J, Ravizza R, Diamond IT. Effects of unilateral ablation of auditory cortex in cat on complex sound localization. J Neurophysiol 35: 718–731, 1972 [DOI] [PubMed] [Google Scholar]
- Wise and Irvine 1984.Wise LZ, Irvine DRF. Interaural intensity difference sensitivity based on facilitatory binaural interactions in cat superior colliculus. Hear Res 16: 181–187, 1984 [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2003.Zhang LI, Tan AYY, Schreiner CE, Merzenick MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424: 201–205, 2003 [DOI] [PubMed] [Google Scholar]