Abstract
Simultaneous behavior and multielectrode neural recordings in freely behaving rodents holds great promise to study the neural bases of behavior and disease models in combination with genetic manipulations. Here, we introduce the use of three-axis accelerometers to characterize the behavior of rats and mice during chronic neural recordings. These sensors were small and light enough to be worn by rodents and were used to record three-axis acceleration during freely moving behavior. A two-layer neural network-based pattern recognition algorithm was developed to extract the natural behavior of mice from the acceleration data. Successful recognition of resting, eating, grooming, and rearing are shown using this approach. The inertial sensors were combined with continuous 24-h recordings of neural data from the striatum of mice to characterize variations in neural activity with circadian cycles and to study the neural correlates of spontaneous action initiation. Finally, accelerometers were used to study the performance of rodents in traditional operant conditioning, where they were used to extract the reaction time of rodents. Thus the addition of accelerometer recordings of rodents to chronic multielectrode neural recordings provides great value for a number of neuroscience applications.
INTRODUCTION
One of the most important challenges in neuroscience is to understand how behavior emerges from the activity of the nervous system. Implantable microelectrode arrays are extensively used in systems neuroscience research to study the neural correlates of behavior in awake behaving animals, with a growing focus on using rodents to study complex behavior. Neural data acquisition in freely roaming rodents is interesting for a number of studies including sensory processing (Krupa et al. 2004), changes in neural processing with behavioral state (Shin and Chapin 1990), decision-making (Uchida and Mainen 2003), and disease models like Parkinson's disease (van der Meer and Redish 2009). However, most current experiments require the animal to be performing a particular task and use external sensors like levers and nose pokes to identify behaviors of interest in the animal. As a result, there are limited data characterizing neural correlates of behavior in freely roaming rodents. Continuous, long-duration neural recordings from multiple electrodes along with a measure of animal behavior might allow novel paradigms studying sequences of natural behaviors, circadian transitions between behavioral states and neural correlates of intertrial behavioral changes. Here, we introduce the use of inertial sensors to characterize the behavior of rats and mice during chronic neural recordings.
Many systems have been proposed for the recognition of behavioral states in rodents. One commonly used technique is video surveillance, which has the important advantage of being nonintrusive. Video tracking systems (Belongie et al. 2005; Twining et al. 2001) have been used to characterize animal behavior with some success. However, the quantity of video data necessary for continuous recording experiments spanning several days makes video unattractive for these purposes. Furthermore, these algorithms typically do not detect subtle movements or the precise instant of movement initiation. Some other approaches developed include the use of piezo or pressure sensors on the floor of the cage (May 2003) and the use of continuous-wave Doppler radar (CWDR) signals to discriminate animal behaviors (Austin and Rose 1997). Both technologies need to be further developed to achieve the desired temporal precision and fare poorly in the presence of multiple animals in a cage.
Recent advances in micro-electro-mechanical systems (MEMS) technologies have enabled the development of packaged low-power accelerometers and other inertial sensors. These accelerometers provide three-dimensional acceleration data, which can be used as an excellent measure of the movement of the animal. Moreover, they are small and light enough (e.g., ADXL330 from Analog Devices is 4 × 4 mm and weighs <0.1 g) to enable small boards and connectors to be worn by rats and mice. Researchers have attempted analysis of animal behavior using inertial sensors in the past, but these have been restricted to large systems unsuitable for small animals (Martiskainen et al. 2009; Pfau et al. 2005; Robert et al. 2009; Scheibe and Gromann 2006; Yoda et al. 2001). Accelerometers have also been used in conjunction with EEG measurements to track states of vigilance in rodents (Sunderam et al. 2007). Measurement of human motion and classification of human behavior using accelerometers is a widely researched topic (Godfrey et al. 2008).
Recent work (Chestek et al. 2009; Santhanam et al. 2007) has shown that it is possible to acquire neural and accelerometer recordings simultaneously from freely behaving primates. This system was, however, restricted to recording two channels of neural data because of technical limitations. Furthermore, we would like to perform long-term experiments in rodents, namely rats and mice, which allow us to study more disease models, and to combine the recordings with genetic manipulations, which is not easily done in primates. However, this places a much stricter limit on the size and weight of the sensors we can use and impacts the design of the system. We have previously shown (Venkatraman et al. 2007) that small and light wireless accelerometers can be used to collect information on the movement of freely behaving rats.
Here we show that behavior of the freely roaming animal can be extracted from acceleration data using a simple neural network algorithm. We also show that inertial sensors can be used with continuous 24-h recordings of neural data from the striatum of mice to study neural correlates of movement and initiation of specific natural actions. Finally, we show that such accelerometers are also useful in studying the performance of rodents in traditional operant conditioning paradigms, where they can be used to extract the reaction time of rodents.
METHODS
Electrophysiology
This study was a collaboration between University of California at Berkeley and the National Institutes of Health. Adult female Sprague-Dawley rats were used in this study at UC Berkeley, and adult male and female mice (C57Bl6/J) were used at National Institutes of Health. All animal procedures were approved by ACUC committees at UC Berkeley and at National Institutes of Health and were consistent with National Institutes of Health and USDA regulations.
Neural signals were recorded from the somatosensory and motor cortices of adult female Sprague-Dawley rats (200–300 g in weight) with implanted tungsten (35 μm diam) microwire electrode arrays (Venkatraman and Carmena 2009). Neural signals in mice were recorded bilaterally from the dorsal striatum (Costa et al. 2006).
Accelerometer
A miniature custom-printed circuit board was designed to hold the ADXL330 accelerometer (Analog Devices, Norwood, MA; sensitivity of 300 mV/g, where g = acceleration caused by gravity, using power supply of +3 V, dynamic range ±3g). This board also contains a 3 V voltage regulator (MAX6030, Maxim, Sunnyvale, CA), three 0.1 μF surface mount capacitors, and an 18-pin Omnetics connector (Minneapolis, MN) typically used to connect neural recording arrays to amplifier headstages (Fig. 1A). This system is small and light enough (9 × 9 mm in size, 350 mg in weight) to allow a mouse to carry multichannel neural recording headstages (Plexon, Dallas, TX), as well as the accelerometer board (Fig. 1B). The accelerometer board was attached to neural headstages to accurately measure acceleration of the animal. Cables with 34-gauge wires attached to a multichannel commutator (HSC/16o50, Plexon) were used to allow the animal free movement in the cage. This is a cable typically used for neural recording headstages and was used to allow easy adoption by laboratories with rodent electrophysiology setups. The acceleration data were sampled at 1,000 samples/s and recorded along with simultaneous recording of neural activity from implanted multielectrode arrays.
Fig. 1.
Accelerometer tested on behaving mice and rats. A: the 9 × 9 mm accelerometer board. The net weight without connector is 350 mg. B: simultaneous recording of neural activity (32 channels) and acceleration signals in a freely behaving mouse. C: acceleration data recorded from the mouse that was initially moving around its cage and later became immobile (fell asleep).
Figure 1C shows three-axis acceleration data collected from a mouse freely moving in its home cage using this system. The accelerometer measures both dynamic acceleration (Mizell 2003) related to movement of the animal and gravitational acceleration (1g). During this recording, the mouse was initially moving around the cage and stopped moving (fell asleep by human observer classification), as is clearly seen from the acceleration data. Thus the acceleration data can easily be used to provide a quantitative measure of activity of the animal and to monitor sleep patterns.
Neural network-based behavior recognition
A neural network-based pattern recognition algorithm was used to classify behaviors of mice. A human observer manually recorded rearing, eating, resting, and grooming to train the algorithm. Rearing was characterized as when the animal stood on its hind legs, raising its forelimbs and head, eating when the animal ate a small piece of food, resting when the animal stood still, and grooming when the animal used its forearms to clean its face and whiskers in a repetitive motion. Supplemental Fig. S11 shows three-axis acceleration as recorded from a mouse along with manual recording of its behaviors during one 30-min session. There exist some periods where the mouse was performing other behaviors (like walking) that were not classified as one of our four behaviors. Continuous sessions (including such unclassified behaviors) were used for all data analyses.
Data analysis was performed using Matlab (Mathworks, Natick, MA). Preprocessing of acceleration data involved decimation to 20 samples/s. To recognize patterns of behavior from the acceleration data, we needed a supervised learning algorithm. Neural networks have been extensively used for pattern recognition in time series data (Dorffner 1996); hence, we chose a two-layer neural network with five hidden units for this purpose. Each unit performs the computation
where yi is the output of the unit, xj are the inputs to the unit, Wij are the weights assigned to individual inputs, and σ is a nonlinear (sigmoidal) function. A different algorithm was used for each of the four behaviors.
The neural network is provided with information regarding the frequency content of the acceleration data by feeding it data from the current time instant and six previous time instants (each separated by 50 ms). One of the recorded behaviors served as the desired output for the neural network during the training period. The network was trained using a standard back-propagation of errors algorithm. The output of the neural network predicts whether the animal was engaged in that particular behavior for every time instant. Because we know that behaviors do not switch rapidly on the order of 50 ms, we low-pass filter this prediction and look for sustained periods where the neural network predicts the particular behavior. Therefore postprocessing involved low-pass filtering and thresholding the neural network output. This results in a binary output that classifies whether the animal is engaged in the particular behavior for every time instant.
In certain behaviors like rearing, which are well defined, precise timing of behavioral pattern changes can be easily identified. The output of the neural network is low-pass filtered at 1 Hz so it does provide resolution on the order of 1 s as is evident from Supplemental Fig. S2A. However, other behaviors such as eating or grooming are difficult to identify based on the acceleration data. Moreover, such behaviors tend to occur in long bouts (>5 s). Therefore the algorithm output is typically noisy during state transition periods (Supplemental Fig. 2B).
Other possible algorithms for such pattern recognition problems include independent component analysis (ICA), support vector machines (SVM), and the K nearest neighbor algorithm. It is still an open question as to which algorithm is optimally suited to analyzing data from such inertial sensors (Bao 2003).
RESULTS
Behavior recognition
The acceleration data from mice can be used to extract periods of relevant behaviors using pattern recognition algorithms. The behaviors that we decided to categorize were rearing, eating, resting, and grooming. Grooming is a behavior of particular interest because it is an innate sequence of stereotyped behaviors (Kalueff et al. 2007; Spruijt et al. 1992; Suckow et al. 2006) used by rodents to keep themselves clean and is observed less frequently when rodents are unhealthy.
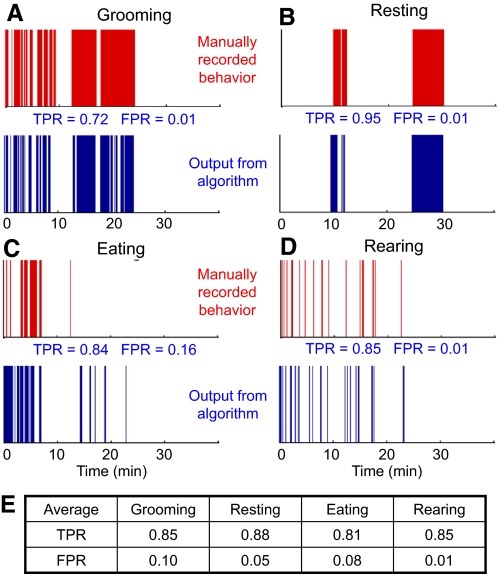
The neural network was trained on 10 min of data from one session and used to test the rest of the session and other sessions from the same animal. These results are shown in Fig. 2, A–D. We tested the performance of the algorithm on six sessions recorded on three mice (2 sessions per mouse), each 30 min long, where the behavior of each mouse was manually recorded by a human observer. The true positive rate (TPR) and false positive rate (FPR) compared with the human observer for the shown session, as well as the average TPR and FPR across all six sessions for each behavior, are shown in Fig. 2. All reported results exclude the training set used for training the algorithm.
Fig. 2.
Recognition of behavioral states in a mouse using a neural network-based algorithm. The data used for this analysis is shown in Supplemental Fig S1. Traces in red show manually recorded behaviors and traces in blue show the output of the algorithm with quantification of true positive rate (TPR) and false positive rate (FPR). A: grooming. B: resting. C: eating. D: rearing. E: average performance of the algorithm over 6 recording sessions in 3 mice.
In earlier work (Venkatraman et al. 2007), we mounted the accelerometer on a jacket worn by the rat and discussed how the performance of the algorithm is degraded if trained on 1 day of data and tested on another day's data because of changes in the orientation of the sensor across days. This problem was resolved in this study by using a different attachment technique for the accelerometer, where it is directly attached to the neural recording headstage. This ensured that the accelerometer was mounted in an identical fashion every day. Using this new procedure, we were able to accurately (compared with manual scoring) perform behavior recognition using the same algorithm across days (Supplemental Fig. S3).
Neural correlates of behavior
NEURAL AND ACCELERATION RECORDINGS IN FREELY BEHAVING RODENTS.
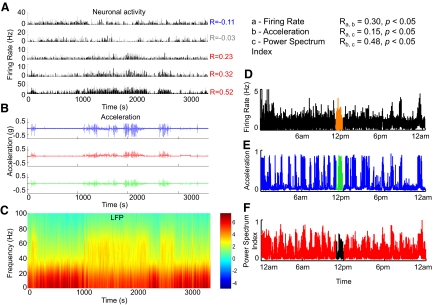
Simultaneous chronic neural recordings and acceleration measurements (Supplemental Fig. S4) allow us to study neural correlates of movement. In mice, neural signals were recorded bilaterally from the striatum, as in Costa et al. (2006). Figure 3 shows histograms of action potentials of single units (Fig. 3A; bin = 1 s) and local field potential (LFP; Fig. 3C) recorded from the dorsal striatum of a mouse, with simultaneously recorded acceleration data in X-, Y-, and Z-directions (Fig. 3B). During this session, it was visually observed that periods of immobility were interrupted by several periods when the mouse was moving, and this was clearly captured in the acceleration data: 77.9 ± 2.9 and 17.5 ± 2.9% of the striatal neurons (data from 9 mice) showed positive and negative modulation with movement (Fig. 3A; correlation indicated by R, P < 0.001), similar to previous observations using EMG and infrared beam recordings (Costa et al. 2006). We quantified movement activity as the sum of the squares of the acceleration in the X-, Y-, and Z-directions and binned both movement activity and neural firing rate at 1 Hz.
Fig. 3.
Neural activity and acceleration signals recorded in the same mouse. A: firing rates of 5 example neurons recorded in the dorsal striatum (the time bin used is 1 s). The activity of different neurons shows strong positive (e.g., the 3 neurons from bottom) or negative (neuron in the top panel) modulation of firing rate during movement. Some neurons seem to be less modulated, e.g., the neuron shown in the 2nd panel from the top. B: simultaneously recorded acceleration signals along 3 axes (X, Y, Z). C: power spectrum of the simultaneously recorded local field potential. D: Twenty-four-hour recording showing simultaneous recording of single units, acceleration data (E), and power spectrum index of local field potential (LFP) (F). The power spectrum index reflects the relative power of theta and gamma oscillations over beta and delta. Clear correlations are seen between all 3 measures.
We also found an increase in the relative power of gamma frequency oscillations (Fig. 3C) in relation to lower-frequency oscillations during movement indicated by accelerometer signals (Fig. 3B). Thus accelerometer recordings provide a convenient measure of global activity of the animal comparable to invasive surface EMG recordings (Supplemental Fig. S5), which have traditionally been used in such applications (Costa et al. 2006).
LONG-TERM RECORDINGS OF NEURAL AND BEHAVIORAL ACTIVITY DURING CIRCADIAN CYCLES.
Precise long-term monitoring of neural activity and behavior is critically important for studying wake-sleep and circadian cycles. Therefore we recorded the neural activity and accelerometer signals simultaneously and continuously in mice for multiple periods of 24 h (up to 1 wk). To analyze the changes in the relative power of different frequency oscillations across different movement states, we calculated a power spectrum index as the average power at (theta × gamma)/(delta × beta) [i.e., (4.5–9 × 30–55 Hz)/(1.5–4 × 11–30 Hz); Costa et al. 2006)]. Figure 3D shows an example of a striatal neuron's activity, the simultaneously recorded accelerometer signal (Fig. 3E), and the power spectrum index (Fig. 3F) during a 24-h period. Significant correlations are seen between all three measurements as quantified in Fig. 3. These data illustrate the possibility of using accelerometers to study the neural correlates of wake-sleep or circadian cycles in mice, and they may provide additional information for state classification methods that are traditionally based on power spectrum analysis of LFPs and behavioral observations (Dzirasa et al. 2006; Gervasoni et al. 2004).
Neural correlates of action initiation in mouse striatum
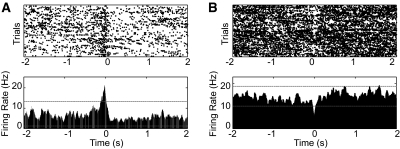
The striatum is one of the main areas involved in action selection and initiation and is implicated in diseases like Parkinson's disease, where voluntary movement initiation and execution are compromised. We analyzed the simultaneously recorded accelerometer data and striatal neuronal activity to look for periods of action initiation throughout the 24-h recording in freely behaving mice. Figure 4 shows two example neurons in dorsal striatum correlated with the initiation of a particular action. Time 0 indicates the moment of initiation of a particular movement, which we calculated as the time that the acceleration change exceeds 0.2g in the X-negative direction. The first neuron (Fig. 4A), a putative medium spiny neuron (Burkhardt et al. 2009), increased its firing phasically during the initiation of this particular action over 24-h periods, whereas the second neuron (Fig. 4B) decreased its firing during the initiation of the action. We recorded a total of 156 striatal neurons from nine mice and found that 27.6 ± 4.4% of the neurons show modulation of firing rate during movement, with 15.6 ± 3.0% showing a positive modulation and 12.0 ± 3.3% showing a negative modulation, which is well above chance. Similar to Belova et al. (2007), criterion for an excitatory response was met if ≥20 consecutive bins (bin size, 20 ms) exceeded 99% of the baseline activity and for an inhibitory response if ≥20 consecutive bins contained fewer spikes than 95% of baseline bins. In the future, this technique can be used to characterize the identity of these striatal medium spiny neurons (e.g., striatonigral or striatopallidal) during spontaneous movement initiation and termination and to study the cellular and circuit mechanism of action initiation in models of motor disorders (Costa et al. 2006; Lu et al. 2009).
Fig. 4.
Neural correlates of the initiation of a particular spontaneous action in mouse striatum. Time 0 indicates the moment of action initiation, which was defined as the time that the acceleration change exceeded 0.2g in the X-negative direction. Top: trial-to-trial plot where each dot indicates an action potential. Bottom: perievent time histogram (PETH) of the activity of striatal neurons. Examples of positive and negative rate modulation neurons are shown in A and B, respectively. The dashed lines in each figure indicate the significance threshold for positive and negative rate modulation.
Reaction times in rodents
Another potential application of this technology is to measure the reaction times of rodents being trained on a task. Operant conditioning of rodents is typically performed in a test chamber with the animal performing tasks such as a forelimb reach for food, lever pressing, or activating an infrared sensor within a nose poke. The current methods of quantifying behavior have some inherent limitations because they tend to record only part of the behavior of the animal. For example, it has been shown that reach-related activity in shoulder muscles and shoulder movement can precede attainment of the goal of the movement (attaining food) by 400 ms or more (Hyland and Reynolds 1993). Such analysis is of particular importance for studies of motor areas in the brain, if the goal is to assign neural activity to the premotor and motor phases of the task. Researchers typically solve this problem by designing more complex tasks, or by using other techniques, such as subcutaneous EMG recordings (Hyland and Reynolds 1993) or frame-by-frame video monitoring to accurately measure the behavior of the animal. Accelerometer recordings provide a convenient substitute for these techniques.
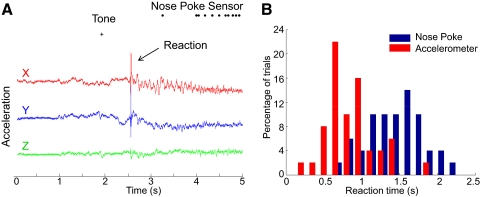
Figure 5A shows an example recording of three axes of acceleration from a rat while it performs a go/no-go task in response to a tone. The rat is trained to react to a tone and poke its nose in a nose poke within 3 s after the tone. The reaction time of the rat can be measured using the time the rat enters the nose poke, which provides an overestimate of the reaction time. A more accurate estimate is obtained using the accelerometer data, which shows the instant when the rat starts moving after hearing the tone. However, it may not be possible to identify the moment of movement initiation in all trials because the rat may have been moving before the tone. We calculated the standard deviation of the acceleration data for 1 s before the tone (σ) and identified movement initiation as the first time instant after the tone when the acceleration was >3σ. This metric agreed well with manual estimates of movement initiation on trials like Fig. 5A. During a 1-h behavioral session, we found that we could identify movement initiation and thus reaction time in 85% of the trials using this technique. The reaction time (tacc) thus obtained from the acceleration data shows a smaller mean and smaller standard deviation variance than the reaction time (tnose) obtained from the nose poke sensor (tacc = 0.86 ± 0.32 s, tnose = 1.56 ± 0.36 s; Fig. 5B). This experiment suggests that accelerometer recordings can also provide additional information of rat behavior in conventional operant conditioning paradigms.
Fig. 5.
Recording of acceleration data while a rat performs a go/no-go task based on a tone. A: example trial shows the ability to extract the animal's reaction time from the accelerometer data. Traditionally, the only information available is the time a sensor on the nose-poke is triggered, but the accelerometer trace provides a significantly more accurate measure of reaction time. B: a histogram of the reaction time calculated using the accelerometer and nose poke. The mean and variance of the reaction time calculated from the accelerometer (red) is less than time taken to enter the nose poke (blue).
DISCUSSION
The measurement of three-axis acceleration in conjunction with chronic neural recordings in rodents presents an excellent technique to study the neural correlates of behavior in freely moving animals. As shown in this study, this technique can be used to study the neural correlates of action initiation, the neural correlates of wake-sleep or circadian cycles, the recognition of different behavioral states, and the measurement of reaction times in operant conditioning paradigms. Considering the numerous applications, low cost, size, and weight of these sensors, we believe that acceleration recordings will become an integral part of chronic neural recording studies in rodents.
Several disorders affecting nigrostriatal circuits in mammals, like Parkinson's and Huntington's diseases, affect the generation of voluntary movements (Lang and Lozano 1998; Walker 2007). The continuous measurement of global movement with high temporal resolution in rodent models can be of great importance for studying the neural mechanisms of action generation and for better studying the mechanisms underlying movement dysfunction in disorders like Parkinson's and Huntington's diseases. This is especially true considering the growing development of chronic models for these diseases (Lu et al. 2009), where continuous monitoring of movement of the animals for long periods of time may be desirable. Moreover, the fact that this technique can be used in mice implies that it can be used in genetically modified models to study motor function with a temporal resolution previously only achieved with EMG recordings in mice (Costa et al. 2006).
We verified that accelerometers may be very useful in determining overall movement of an animal, and we observed similar (or higher) correlations between accelerometer and neural activity and surface EMG and neural activity (Supplemental Fig. S5). Accelerometers have the obvious advantage of being noninvasive. However, EMG signals do provide great advantages in studies focusing on particular movements where recording the activity of specific muscles is valuable.
Video tracking with a light-emitting diode (LED) mounted on a headstage provides a convenient form of rodent tracking for some applications. This technique has the important advantage that it provides the location of the animal, which might be essential for some studies. On the other hand, the accelerometer provides behavioral information at a much lower data rate, which might be advantageous for long-duration studies. Moreover, accelerometers avoid some commonly encountered problems with video tracking such as occlusion. Although the choice of technology for behavior monitoring will finally be decided by the needs of the scientific study, we believe that accelerometers can provide easy access to behavioral information in many cases with small capital investment and minor changes to current experimental setups.
Numerous groups are working on wireless neural interfaces that transmit multiple channels of neural data over a radio link. Early prototypes are commercially available (Triangle Biosystems, Durham, NC) and can wirelessly transmit 16–64 channels of neural data. Academic research has focused on fully integrated solutions that can amplify and wirelessly transmit ≤100 channels of digitized neural data (Harrison et al. 2007; Sarpeshkar et al. 2007). These systems open the possibility for long-term “untethered” neural recordings from rodents for multiple days. The wireless accelerometer that we developed earlier (Venkatraman et al. 2007), or recent systems with similar functionality (Kusserow et al. 2009), could be used in conjunction with such wireless neural interfaces to provide truly untethered neural and behavior recordings in rodents. We believe that such systems will enable new experimental paradigms, with continuous, long-duration neural and behavioral recordings from multiple electrodes on freely moving animals. These paradigms will allow us to study varied topics such as long-term stability of neural recordings, sequences of natural or operant behaviors, and circadian transitions between different behavioral states.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Austin and Rose, 1997.Austin K, Rose G. Automated behavior recognition using continuous-wave Doppler radar and neural networks. Proc IEEE Eng Med Biol Soc 4: 1458–1461, 1997 [Google Scholar]
- Bao, 2003.Bao L. Physical Activity Recognition from Acceleration Data under Semi-Naturalistic Conditions. Cambridge, MA: MIT Press, 2003 [Google Scholar]
- Belongie et al., 2005.Belongie S, Branson K, Dollár P, Rabaud V. Monitoring animal behavior in the smart vivarium. In: Measuring Behavior. Wageningen, The Netherlands, 2005, p. 70–72 [Google Scholar]
- Belova et al., 2007.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron 55: 970–984, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht et al., 2004.Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427: 704–710, 2004 [DOI] [PubMed] [Google Scholar]
- Burkhardt et al., 2009.Burkhardt JM, Jin X, Costa RM. Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum. Front Integr Neurosci 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestek et al., 2009.Chestek C, Gilja V, Nuyujukian P, Kier RJ, Solzbacher E, Ryu SI, Harrison RR, Shenoy KV. HermesC: low-power wireless neural recording system for freely moving primates. IEEE Trans Neural Systems Rehabil Eng 17: 330–338, 2009 [DOI] [PubMed] [Google Scholar]
- Costa et al., 2006.Costa RM, Lin S, Sotnikova T, Cyr M, Gainetdinov R, Caron M, Nicolelis M. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52: 359–369, 2006 [DOI] [PubMed] [Google Scholar]
- Dorffner, 1996.Dorffner G. Neural networks for time series processing. Neural Netw World 6: 447–468, 1996 [Google Scholar]
- Dzirasa et al., 2006.Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL. Dopaminergic control of sleep-wake states. J Neurosci 26: 10577–10589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni et al., 2004.Gervasoni D, Lin S, Ribeiro S, Soares ES, Pantoja J, Nicolelis MAL. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci 24: 11137–11147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey et al., 2008.Godfrey A, Conway R, Meagher D, Laighin G. Direct measurement of human movement by accelerometry. Med Eng Phys 30: 1364–1386, 2008 [DOI] [PubMed] [Google Scholar]
- Harrison et al., 2007.Harrison RR, Watkins PT, Kier RJ, Lovejoy RO, Black DJ, Greger B, Solzbacher F. A low-power integrated circuit for a wireless 100-electrode neural recording system. IEEE J Solid-State Circuits 42: 123–133, 2007 [Google Scholar]
- Hyland and Reynolds, 1993.Hyland BJ, Reynolds JN. Pattern of activity in muscles of shoulder and elbow during forelimb reaching in the rat. Hum Mov Sci 12: 51–69, 1993 [Google Scholar]
- Kalueff et al., 2007.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protocols 2: 2538–2544, 2007 [DOI] [PubMed] [Google Scholar]
- Krupa et al., 2004.Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MAL. Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304: 1989–1992, 2004 [DOI] [PubMed] [Google Scholar]
- Kusserow et al., 2009.Kusserow M, Amft O, Tröster G. BodyANT: miniature wireless sensors for naturalistic monitoring of daily activity. In Fourth International Conference on Body Area Networks2009 [Google Scholar]
- Lang and Lozano, 1998.Lang AE, Lozano AM. Parkinson's Disease- second of two parts. N Engl J Med 339: 1130–1143, 1998 [DOI] [PubMed] [Google Scholar]
- Lu et al., 2009.Lu X-H, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, Sulzer D, Jackson GR, Maidment NT, Chesselet MF, Yang XW. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant {alpha}-synuclein. J Neurosci 29: 1962–1976, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon et al., 2006.Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau J, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci 26: 12587–12595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiskainen et al., 2009.Martiskainen P, Jarvinen M, Skon J, Tiirikainen J, Kolehmainen M, Mononen J. Cow behaviour pattern recognition using a three-dimensional accelerometer and support vector machines. Appl Anim Behav Sci 119: 32–38, 2009 [Google Scholar]
- May, 2003.May EL. Application of a piezoelectric sensor for measuring shivering in a small marsupial. J Therm Biol 28: 469–475, 2003 [Google Scholar]
- Mizell, 2003.Mizell D. Using gravity to estimate accelerometer orientation. In Proceedings of the 7th IEEE International Symposium on Wearable Computers.IEEE Computer Society, 2003, p. 252 [Google Scholar]
- Neafsery et al., 1986.Neafsery E, Bold E, Haas G, Hurley-Guis K, Quirk G, Sievert C, Terreberry R. The organization of the rat motor cortex: a microstimulation mapping study. Brain 396: 77–96, 1986 [DOI] [PubMed] [Google Scholar]
- Pfau et al., 2005.Pfau T, Witte TH, Wilson AM. A method for deriving displacement data during cyclical movement using an inertial sensor. J Exp Biol 208: 2503–2514, 2005 [DOI] [PubMed] [Google Scholar]
- Robert et al., 2009.Robert B, White BJ, Renter DG, Larson RL. Evaluation of three-dimensional accelerometers to monitor and classify behavior patterns in cattle. Comput Electron Agric 67: 80–84, 2009 [Google Scholar]
- Santhanam et al., 2007.Santhanam G, Linderman M, Gilja V, Afshar A, Ryu S, Meng T, Shenoy K. HermesB: a continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng 54: 2037–2050, 2007 [DOI] [PubMed] [Google Scholar]
- Sarpeshkar et al., 2007.Sarpeshkar R, Wattanapanitch W, Rapoport BI, Arfin SK, Baker MW, Mandal S, Fee MS, Musallam S, Andersen RA. Low-power circuits for brain-machine interfaces. In IEEE International Symposium on Circuits and Systems2068–2071, 2007 [DOI] [PubMed] [Google Scholar]
- Scheibe and Gromann, 2006.Scheibe KM, Gromann C. Application testing of a new three-dimensional acceleration measuring system with wireless data transfer (WAS) for behavior analysis. Behav Res Methods 38: 427–433, 2006 [DOI] [PubMed] [Google Scholar]
- Shin and Chapin, 1990.Shin H, Chapin J. Movement induced modulation of afferent transmission to single neurons in the ventroposterior thalamus and somatosensory cortex in rat. Exp Brain Res 81: 515–522, 1990 [DOI] [PubMed] [Google Scholar]
- Spruijt et al., 1992.Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev 72: 825–852, 1992 [DOI] [PubMed] [Google Scholar]
- Suckow et al., 2006.Suckow M, Weisbroth S, Franklin C. The Laboratory Rat. Elsevier, 2006 [Google Scholar]
- Sunderam et al., 2007.Sunderam S, Chernyy N, Peixoto N, Mason JP, Weinstein SL, Schiff SJ, Gluckman BJ. Improved sleep-wake and behavior discrimination using MEMS accelerometers. J Neurosci Methods 163: 373–383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining et al., 2001.Twining CJ, Taylor CJ, Courtney P. Robust tracking and posture description for laboratory rodents using active shape models. Behav Res Methods Instruments Comput 33: 381–391, 2001 [DOI] [PubMed] [Google Scholar]
- Uchida and Mainen, 2003.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003 [DOI] [PubMed] [Google Scholar]
- van der Meer and Redish, 2009.van der Meer AAM, Redish AD. Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front Integr Neurosci 3: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman and Carmena, 2009.Venkatraman S, Carmena JM. Behavioral modulation of stimulus-evoked oscillations in barrel cortex of alert rats. Front Integr Neurosci 3: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman et al., 2007.Venkatraman S, Long J, Pister K, Carmena J. Wireless inertial sensors for monitoring animal behavior. In Proceedings of IEEE Engineering in Medicine and Biology Society378–381, 2007 [DOI] [PubMed] [Google Scholar]
- Walker, 2007.Walker FO. Huntington's disease. Lancet 369: 218–228, 2007 [DOI] [PubMed] [Google Scholar]
- Yoda et al., 2001.Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y. A new technique for monitoring the behaviour of free-ranging Adelie penguins. J Exp Biol 204: 685–690, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.