Abstract
Recent biochemical and behavioral data implicate reactive oxygen species (ROS) in peripheral and spinal pain mechanisms. However, pain-related functions of ROS in the brain and mechanisms of pain-related ROS activation remain to be determined. Our previous studies showed that the amygdala plays a key role in emotional-affective pain responses and pain modulation. Hyperactivity of amygdala neurons in an animal pain model depends on group I metabotropic glutamate receptors (subtypes mGluR1 and mGluR5), but their signaling pathway remains to be determined. Here we tested the hypothesis that activation of group I mGluRs increases nociceptive processing in amygdala neurons through a mechanism that involves ROS. Extracellular single-unit recordings were made from neurons in the laterocapsular division of the central nucleus of the amygdala (CeLC) in anesthetized adult male rats. Administration of a group I mGluR agonist (DHPG) into the CeLC by microdialysis increased the responses to innocuous and noxious somatosensory (knee joint compression) and visceral (colorectal distention [CRD]) stimuli. A ROS scavenger (PBN) and a superoxide dismutase mimetic (TEMPOL) reversed the facilitatory effects of DHPG. An mGluR5 antagonist (MPEP) also inhibited the effects of DHPG on the responses to innocuous and noxious somatosensory and visceral stimuli, whereas an mGluR1 antagonist (LY367385) decreased only the responses to visceral stimulation. The results show for the first time that ROS mediate group I mGluR-induced facilitation of nociceptive processing in amygdala neurons. The antagonist data may suggest differential contributions of subtypes mGluR1 and mGluR5 to the processing of somatosensory and visceral nociceptive information in the amygdala.
INTRODUCTION
Cytotoxicity and oxidative stress through reactive oxygen species (ROS) formation play a critical role in apoptosis, stroke pathology, spinal cord injury, neurodegenerative disorders, and aging (Chinopoulos and Adam-Vizi 2006; Maher and Schubert 2000). However, ROS, such as superoxide and hydrogen peroxide, also serve as important signaling molecules in physiological plasticity and may be required for normal cognitive functions (Hu et al. 2006; Kishida and Klann 2007; Klann 1998). A novel concept views ROS as a major factor in persistent pain (Chung 2004).
Peripheral and spinal ROS, such as superoxide and hydrogen peroxide, have been implicated in inflammatory and neuropathic pain (Gao et al. 2007; Keeble et al. 2009; Kim et al. 2004, 2009; Schwartz et al. 2008, 2009; Wang et al. 2004). Peripheral or spinal administration of exogenous ROS has pronociceptive effects (Keeble et al. 2009; Schwartz et al. 2008). Production of endogenous ROS is increased in injured peripheral nerve and inflamed tissue (Keeble et al. 2009; Twining et al. 2004) and in the spinal cord in models of neuropathic and inflammatory pain (Kim et al. 2004; Schwartz et al. 2008, 2009; Wang et al. 2004). In the spinal cord, the source of pain-related increase in ROS is superoxide generated from mitochondrial oxidative phosphorylation in dorsal horn neurons, including spinothalamic tract cells (Schwartz et al. 2008, 2009). Inhibition of endogenous ROS production with peripherally (Keeble et al. 2009; Twining et al. 2004) or spinally (Gao et al. 2007; Kim et al. 2004, 2009; Schwartz et al. 2008, 2009; Wang et al. 2004) administered ROS scavengers is antinociceptive in models of inflammatory and neuropathic pain.
Although behavioral and neurochemical data implicate ROS in pain-related central sensitization, direct electrophysiological evidence for the involvement of ROS other than nitric oxide is sparse. Responses of spinal dorsal horn neurons were inhibited by systemic applications of the antioxidant vitamin E in neuropathic animals (Kim et al. 2006) and by systemically administered ROS scavengers in the capsaicin pain model (Lee et al. 2007). C-fiber–induced long-term potentiation (LTP) in the dorsal horn of spinal cord slices was inhibited by ROS scavengers (Lee et al. 2010). Largely unknown is the role of ROS in pain processing in the brain and the mechanisms by which nociceptive signals activate ROS functions. The present study tested the hypothesis that ROS, such as superoxide and hydrogen peroxide, modulate the processing of nociceptive information in the amygdala and are downstream effectors of group I metabotropic glutamate receptors (mGluRs). The rationale is as follows.
The amygdala, as part of the limbic system, plays a critical role in the emotional response to pain and in pain modulation (Carrasquillo and Gereau 2007; Fields 2004; Gauriau and Bernard 2004; Heinricher and McGaraughty 1999; Ikeda et al. 2007; Neugebauer et al. 2004, 2009; Pedersen et al. 2007; Rhudy and Meagher 2001). The amygdala consists of several functionally distinct nuclei, including the lateral (LA), basolateral (BLA), and central (CeA) nuclei (Sah et al. 2003). The amygdala receives nociceptive information through anatomically and functionally distinct lines of input (Braz et al. 2005; Gauriau and Bernard 2004; Neugebauer et al. 2004, 2009). Purely nociceptive information reaches the laterocapsular division of the CeA (CeLC) directly from the spinal cord and brain stem (parabrachial area), thus bypassing the thalamus (Cliffer et al. 1991; Gauriau and Bernard 2004; Spike et al. 2003). Polymodal sensory, including nociceptive, inputs from thalamus (posterior areas) and cortex (insula and association cortices) target the LA, the initial site of sensory convergence and integration in the amygdala (Maren 2005; Pape and Paré 2010; Phelps and Ledoux 2005; Sah et al. 2003). Associative processing in the LA–BLA network is believed to attach emotional significance to sensory information and play an important role in fear and anxiety (Maren 2005; Paré et al. 2004; Phelps and Ledoux 2005; Sah et al. 2003). Highly processed affect-related information is then transmitted to the CeA, which can modulate pain behavior through projections to descending pain control centers in the brain stem (Fields 2004; Heinricher and McGaraughty 1999; Mason 2005; Neugebauer et al. 2004; Tracey and Mantyh 2007).
Central sensitization and synaptic plasticity have been shown in the laterocapsular division of the CeA (CeLC) in models of arthritic pain (Bird et al. 2005; Fu and Neugebauer 2008; Han et al. 2005; Ji and Neugebauer 2007, 2009; Li and Neugebauer 2004a,b; Neugebauer and Li 2003; Neugebauer et al. 2003), visceral pain (Han and Neugebauer 2004), and neuropathic pain (Ikeda et al. 2007). Pharmacologic inhibition of increased amygdala activity decreases nocifensive and affective pain responses (Carrasquillo and Gereau 2007, 2008; Fu and Neugebauer 2008; Fu et al. 2008; Han and Neugebauer 2005; Han et al. 2005; Ji et al. 2007; Palazzo et al. 2008; Pedersen et al. 2007).
Analysis of pain-related activity changes in the amygdala has focused mainly on somatosensory inputs from the skin or deep tissue such as joints (Neugebauer et al. 2009). Little is known about processing of visceronociceptive information in the amygdala. Neuroimaging studies reported activation or deactivation in the amygdala following rectal distention in patients with irritable bowel syndrome (Bonaz et al. 2002; Mayer et al. 2005; Naliboff et al. 2003; Wilder-Smith et al. 2004). Amygdala activation was detected in healthy human volunteers in response to painful gastric stimulation (Lu et al. 2004), in normal rats during colorectal distention (CRD) (Johnson et al. 2010; Lazovic et al. 2005), and in rats with pancreatitis (Westlund et al. 2009). Visceral pain-related neurochemical changes were found in the central nucleus of the amygdala (CeA). These included increased c-fos mRNA (Nakagawa et al. 2003) and c-fos protein expression (Lazovic et al. 2005; Monnikes et al. 2003; Stam et al. 2002; Traub et al. 1996) following CRD and increased corticotropin-releasing factor (CRF) mRNA in a colitis pain model (Greenwood-Van Meerveld et al. 2006). Behavioral data also implicate the CeA in visceral pain. CeA lesions inhibited negative affective behavior in a visceral pain model (Tanimoto et al. 2003). Corticosterone delivery to the CeA increased anxiety and visceromotor responses (Greenwood-Van Meerveld et al. 2001; Myers and Greenwood-Van Meerveld 2007; Myers et al. 2007; Qin et al. 2003a,b,c) and sensitized viscerosensitive spinal neurons (Qin et al. 2003a,b,c). An electrophysiological analysis of visceronociceptive processing in amygdala neurons, however, remains to be done.
Pain-related hyperactivity of amygdala neurons depends on group I metabotropic glutamate receptors (mGluRs) in a model of arthritis pain (Li and Neugebauer 2004b; Neugebauer et al. 2003). The role of mGluRs in visceronociceptive processing in the amygdala is not known. Group I mGluRs typically couple to protein kinase C (PKC) activation, although PKC does not play a role in amygdala pain mechanisms (Bird et al. 2005; Fu et al. 2008; Han et al. 2010; Ji and Neugebauer 2008). Some evidence links group I mGluRs to ROS production (Battaglia et al. 2002). This study addressed the hypothesis that ROS other than nitric oxide (NO) contribute to group I mGluR actions on nociceptive amygdala neurons. We measured the responses of CeLC neurons to somatosensory (joint compression) and visceral (CRD) stimuli and evaluated their modulation by group I mGluRs and ROS.
METHODS
Adult male Sprague–Dawley rats (250–350 g) were housed in a temperature-controlled room and maintained on a 12-h day/night cycle. Water and food were available without restriction. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and conform to the guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health.
Animal preparation and anesthesia
As described in detail previously (Ji and Neugebauer 2007, 2009; Li and Neugebauer 2004b; Neugebauer and Li 2003), the animals were anesthetized with pentobarbital sodium (induction, 50 mg · kg−1, administered intraperitoneally; maintenance, 15 mg · kg−1 · h−1, administered intravenously [iv]), paralyzed with pancuronium (induction: 0.3–0.5 mg, iv; maintenance: 0.3 mg · h−1, iv), and artificially ventilated (3–3.5 ml; 55–65 strokes/min). Depth of anesthesia was assessed by testing the corneal blink, hindpaw withdrawal and tail-pinch reflexes and by continuously monitoring the end-tidal CO2 levels (kept at 4.0 ± 0.2%), heart rate, electrocardiogram (ECG), and breathing patterns. Core body temperature was maintained at 37°C by means of a homeothermic blanket system. The animal was mounted in a stereotaxic frame and a craniotomy was performed at the sutura frontoparietalis level to allow the insertion of the recording electrode and microdialysis probe (for drug application).
Electrophysiological recording
As described previously (Ji and Neugebauer 2007, 2009; Li and Neugebauer 2004b; Neugebauer and Li 2003), extracellular single-unit recordings were made from neurons in the CeLC with glass insulated carbon filament electrodes (4–6 MΩ) using the following stereotaxic coordinates (Paxinos and Watson 1998): 2.1–2.8 mm caudal to bregma; 3.8–4.5 mm lateral to midline; depth 7–9 mm. Recordings were made in the right hemisphere to allow comparison with our previous studies. The recorded signals were amplified and displayed on analog and digital storage oscilloscopes. Signals were also fed into a window discriminator whose output was processed by an interface (CED 1401 Plus; Cambridge Electronic Design [CED], Cambridge, UK) connected to a Pentium 4 PC. Spike2 software (version 4; CED) was used to create peristimulus rate histograms on-line and to store and analyze digital records of single-unit activity off-line.
Identification of amygdala neurons
This study focused on the processing of visceronociceptive information in amygdala neurons, which had not been addressed in previous studies. While the recording electrode was slowly advanced through the CeLC, an individual CeLC neuron was identified by its background activity and responses to brief search stimuli, which included compression of skin folds and deep tissue (joint and muscles) and visceral stimuli (colorectal distention; see Colorectal distention and knee joint compression). Spike size and configuration were continuously monitored on the storage oscilloscope and with the use of Spike2 software. Spikes were detected and recorded by the waveform signal that crossed a trigger level and matched a preset shape or template that was created for the individual neuron at the beginning of the recording period. Included in this study were only those neurons whose spike configuration remained constant (matching the template) and could be clearly discriminated from activity in the background throughout the experiment, indicating that the activity of one and the same one neuron was measured.
Colorectal distention and knee joint compression
For the analysis of drug effects, we focused on the responses to colorectal distention (CRD, to study processing of a visceral input) and knee joint compression (to allow comparison with our previous studies). For CRD, an inflatable latex balloon (5 cm) connected to a sphygmomanometer was inserted into the distal colon. Innocuous (20 mmHg), moderate (40 mmHg), and noxious (60 and 80 mmHg) intraluminal pressures (15 s each) were achieved by inflating the balloon (see Al-Chaer et al. 1996). Brief (15 s) mechanical stimuli were applied to the knee joint by means of a forceps equipped with a force transducer whose calibrated output was amplified, digitized, and recorded on a Pentium PC for on- and off-line analysis. The knee joint was compressed with innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) intensities. Stimulus intensities of <500 g/30 mm2 applied to the knee and other deep tissue are considered innocuous because they do not evoke hindlimb withdrawal reflexes in awake rats and are not felt to be painful when tested on the experimenters. Stimulus intensities of >1,500 g/30 mm2 are noxious because they evoke hindlimb withdrawal reflexes and vocalizations in awake rats and are distinctly painful when applied to the experimenters (Fu and Neugebauer 2008; Han and Neugebauer 2005; Han et al. 2005; Ji et al. 2007). If present, background activity before stimulation was subtracted from the total response during stimulation to calculate the net response evoked by a particular stimulus.
Experimental protocol
Receptive fields in the deep tissue and skin and responsiveness to CRD were determined first. In each experiment one CeLC neuron that responded to CRD and knee joint stimulation was selected for pharmacologic studies to allow the comparison of visceral and somatosensory nociceptive processing. Background activity and evoked responses were measured repeatedly before and during drug administration into the CeLC (see the following section). The sequence of CRD and knee joint compression was alternated between trials to avoid possibly confounding effects of the preceding stimulus. In addition, if a stimulus produced sustained activity, the interstimulus interval was increased to allow activity to return to prestimulus control levels. Background activity was measured before CRD and before knee joint stimulation. The respective values were subtracted from the total activity during stimulation to calculate the net evoked activity (see previous section). Test stimuli were applied at intervals of 5–10 min before and during drug application. The number of stimulations was kept at a minimum to avoid any “sensitization” that might be produced by repeated stimulation.
Drugs and drug administration by microdialysis
The following drugs were tested: phenyl-N-t-butyl nitrone (PBN, ROS scavenger); 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL, superoxide dismutase mimetic); (S)-3,5-dihydroxyphenylglycine (DHPG, group I mGluR agonist); (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385, mGluR1 antagonist); 2-methyl-6-(phenylethynyl)pyridine (MPEP, mGluR5 antagonist). PBN and TEMPOL were purchased from Sigma (St. Louis, MO); DHPG, MPEP, and LY367385 were purchased from Tocris Cookson (Ellisville, MO). Drugs were dissolved in artificial cerebrospinal fluid (ASCF) on the day of the experiment at a concentration 100-fold that predicted to be needed based on in vitro data (for ROS, see Hu et al. 2006; Klann 1998; Lee et al. 2010; for mGluRs, see Lesage 2004; Neugebauer et al. 2003; Pernia-Andrade et al. 2009) because of the concentration gradient across the dialysis membrane. The appropriateness of the factor 100 for microdialysis concentrations of mGluR agonists and antagonists was validated in our previous in vivo studies that used microdialysis to administer these agents into the amygdala (Han and Neugebauer 2005; Li and Neugebauer 2004b). Drugs were administered into the CeLC by microdialysis at a rate of 5 μl/min.
Several hours before the start of the electrophysiological recordings a microdialysis probe (CMA11; CMA/Microdialysis; membrane diameter: 250 μm; membrane length: 1 mm) was positioned stereotaxically in the CeLC, using the following coordinates (Paxinos and Watson 1998): 2.0 mm caudal to bregma; 4.0 mm lateral to midline; depth of tip 9.0 mm (Han et al. 2005; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b). Using PE-50 tubing, the microdialysis probe was connected to an infusion pump (Harvard) and perfused with ACSF containing (in mM): NaCl 125.0, KCl 2.6, NaH2PO4 2.5, CaCl2 1.3, MgCl2 0.9, NaHCO3 21.0, and glucose 3.5; oxygenated and equilibrated to pH = 7.4. Before the recordings, ACSF was pumped through the fiber for >1 h to establish equilibrium in the tissue. ACSF was present throughout the experiment and also served as a vehicle control.
Histology
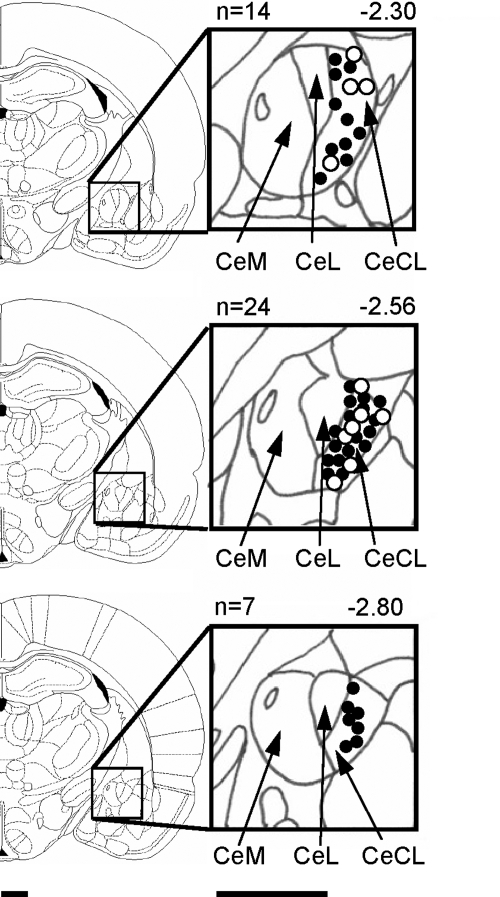
At the end of each experiment the recording site in the CeLC was marked by injecting DC (250 μA for 3 min) through the carbon filament recording electrode. The brain was removed and submerged in 10% formalin and potassium ferrocyanide. Tissues were stored in 20% sucrose before they were frozen-sectioned at 50 μm. Sections were stained with Neutral Red, mounted on gel-coated slides, and coverslipped. The boundaries of the different amygdala nuclei were easily identified under the microscope. Lesion/recording sites were verified histologically and plotted on standard diagrams adapted from Paxinos and Watson (1998) (see Fig. 1).
Fig. 1.
Histologically verified recording sites of 45 neurons in the laterocapsular division of the central nucleus of the amygdala (CeLC) recorded in 45 rats. Filled circles refer to CeLC neurons that were tested in pharmacologic studies (n = 34). Open circles show additional neurons (n = 11) that were characterized without pharmacologic tests. Boundaries of different amygdala nuclei are easily identified under the microscope. Diagrams (adapted from Paxinos and Watson 1998) show coronal brain sections at different levels posterior to bregma (−2.30 to −2.80). Next to each diagram are shown in detail the central nucleus and its medial (CeM), lateral (CeL), and laterocapsular (CeLC) subdivisions. Calibration bars are 1 mm.
Data analysis
Extracellularly recorded single-unit action potentials were analyzed off-line from peristimulus rate histograms using Spike2 software (version 4; CED). Responses to mechanical stimuli were measured and expressed as spikes/s (Hz). Background activity was subtracted from the total activity during the stimulus to obtain the “net” stimulus-evoked activity. For multiple comparisons, one-way ANOVA was used with appropriate posttests as indicated in the text and figure legends (Dunnett's multiple comparison test to compare all sets of data to a control value; Tukey's multiple comparison test to compare all pairs of data; Prism 3.0, GraphPad Software). Statistical analysis was performed on the raw data (firing rate measured as spikes/s). All averaged values are given as the mean ± SE. Statistical significance was accepted at the level P < 0.05.
RESULTS
Extracellular recordings from CeLC neurons were made in 45 rats. Receptive fields in somatic tissues (joints, muscles, and skin) and visceral input from the colon were assessed in a total of 102 CeLC neurons. Recording sites were verified histologically for only the last neuron recorded in each animal (n = 45 neurons; Fig. 1) because the electric lesion was made at the end of each experiment to avoid confounding effects of tissue damage. Additional neurons were recorded either in the same or a nearby (<1 mm) electrode track. Drug studies (see following text) were performed on only one neuron per animal and recording sites of these neurons were verified (n = 34 neurons; Fig. 1).
Visceronociceptive neurons in the CeLC
Ninety-nine of 102 neurons (97%) responded to compression of the knee joint with excitation (91 neurons) or inhibition (8) and had bilateral symmetrical receptive fields in the deep tissue of the entire body (59 neurons) or only in the hindlimbs (40). These neurons also had cutaneous receptive fields mainly on the trunk and plantar surfaces. We did not detect any neurons that responded only to cutaneous stimuli. CeLC neurons responded more strongly to noxious (1,500 g/30 mm2) than to innocuous (500 g/30 mm2) stimuli (tissue compression) and were therefore classified as multireceptive neurons as in our previous studies (Ji and Neugebauer 2007, 2009; Li and Neugebauer 2004b; Neugebauer and Li 2003). Multireceptive CeLC neurons are believed to integrate nociceptive and affective information (Neugebauer et al. 2004, 2009).
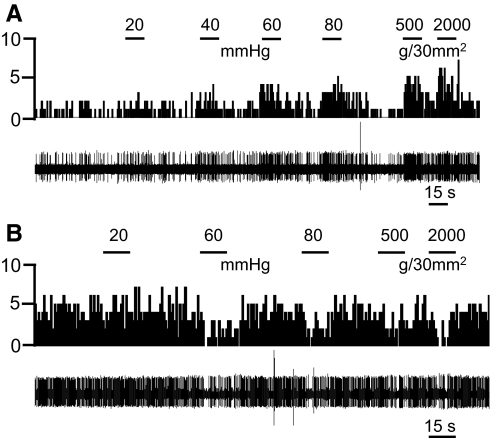
Sixty-three of 102 neurons (62%) responded to CRD with excitation (55 neurons) or inhibition (8). These neurons were classified as visceronociceptive neurons because of their graded responses to stimuli of increasing intensities (20, 40, 60, and 80 mmHg). CRD of 20 mmHg is considered innocuous, whereas 60 and 80 mmHg are noxious stimuli (Al-Chaer et al. 1996). Sixty visceronociceptive neurons also responded to somatosensory stimuli, whereas 3 responded only to CRD. Figure 2 shows individual examples of visceronociceptive CeLC neurons that were excited (Fig. 2A) or inhibited (Fig. 2B) by CRD and knee joint compression.
Fig. 2.
Visceronociceptive CeLC neurons. Background and evoked activity recorded in 2 individual CeLC neurons that were excited (A) or inhibited (B) by colorectal distention (CRD) and knee joint compression. Peristimulus time histograms (PSTHs; bin width, 1 s) show number of action potentials (spikes)/s before and during CRD of increasing intensities (20, 40, 60, and 80 mmHg) and compression of the knee joint with innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) intensities (see methods). Stimulus duration was 15 s. Original recordings of action potentials are shown below the PSTH.
For pharmacologic studies 34 neurons were selected that showed an excitatory response both to CRD and to knee joint stimulation to allow the comparison of visceral and somatosensory processing. Somatosensory receptive fields of these neurons were bilaterally symmetrical on the entire body (25 neurons) or only in the hindlimbs (9).
Facilitatory effects of group I mGluR activation
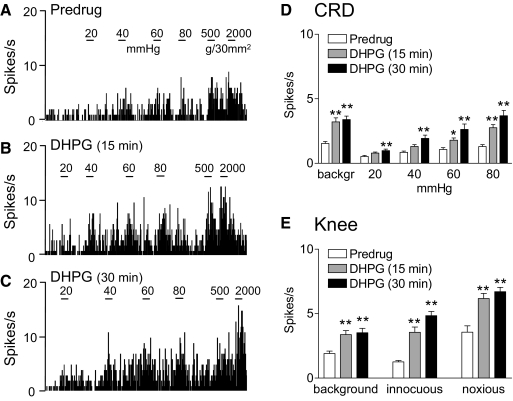
A selective group I mGluR agonist DHPG (100 μM, concentration in microdialysis probe corresponding to a tissue concentration of ∼1 μM; see methods) administered into the CeLC increased the responses of CeLC neurons (n = 6) to both visceral (CRD) and somatosensory (knee joint compression) stimuli. Prolonged application of DHPG for 30 min did not result in desensitization but continued facilitation. Figure 3, A–C shows an individual example. The significant facilitatory effects of DHPG in the sample of neurons are summarized in Fig. 3, D and E (P < 0.05–0.01, Dunnett's multiple comparison tests). Repeating the series of CRDs and knee joint compression three times at 5 min intervals before drug application did not produce sensitization.
Fig. 3.
Group I metabotropic glutamate receptor (mGluR) agonist-induced facilitation. A–C: extracellular single-unit recordings of background activity and evoked responses of a CeLC neuron before drug application (predrug, A) and during administration of (S)-3,5-dihydroxyphenylglycine (DHPG, 100 μM, concentration in microdialysis probe) into the CeLC for 15 min (B) and 30 min (C). A: the neuron showed graded responses to CRD of increasing intraluminal pressure (20, 40, 60, and 80 mmHg) and knee joint compression with innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) intensities. B and C: DHPG increased responses to CRD and knee joint compression. The effects did not show desensitization but persisted during continued drug application. PSTHs (bin width, 1 s) show spikes/s. Horizontal lines indicate stimulus duration (15 s). D and E: summary data. Bar histograms (mean ± SE) show background activity and responses evoked by CRD (D) and by knee joint compression (E) averaged across the sample of neurons (n = 6). *P < 0.05, **P < 0.01 (compared with predrug control; Dunnett's multiple comparison test).
Contribution of mGluR5
Coadministration of an mGluR5 antagonist (MPEP, 100 μM, concentration in microdialysis probe; 15 min) into the CeLC inhibited the facilitatory effects of DHPG on CRD and knee joint compression. Figure 4, A–C shows an individual example. The inhibitory effects of MPEP on DHPG-induced facilitation of responses to CRD (Fig. 4D) and knee joint compression (Fig. 4E) were significant for the sample of neurons (n = 6; P < 0.05–0.001, Tukey's multiple comparison tests). As in our previous study (Li and Neugebauer 2004b) MPEP at a concentration of 100 μM (concentration in microdialysis probe) had no effect on baseline responses to innocuous stimuli, but reduced the responses to noxious stimuli by about 15% (n = 3 neurons).
Fig. 4.
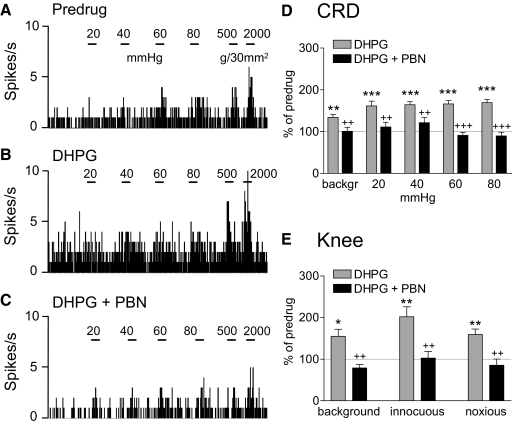
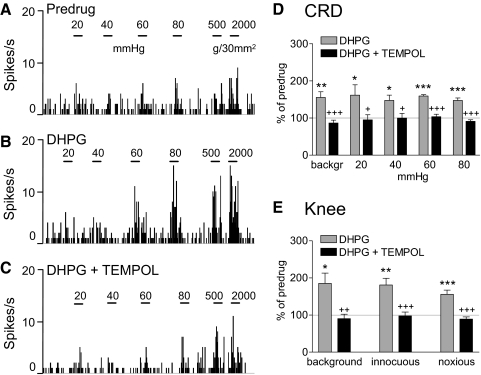
mGluR5 antagonist (2-methyl-6-(phenylethynyl)pyridine [MPEP]) inhibits DHPG-induced facilitation. A–C: extracellular single-unit recordings of background activity and evoked responses of an individual CeLC neuron. A: responses to CRD of increasing intraluminal pressure (20, 40, 60, and 80 mmHg) and to knee joint compression with innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) intensities before drug application (predrug). B: administration of DHPG (100 μM, concentration in microdialysis probe; 15 min) into the CeLC increased the neuron's responses. C: MPEP (100 μM; 15 min) coapplied with DHPG inhibited the facilitatory effects. PSTHs (bin width, 1 s) show spikes/s. Horizontal lines indicate stimulus duration (15 s). D and E: summary data for the sample of neurons (n = 6). Bar histograms (mean ± SE) show background activity and responses evoked by CRD (D) and by knee joint compression (E) normalized to predrug control (set to 100%). *P < 0.05, **P < 0.01 (compared with predrug control); +P < 0.05, ++P < 0.01, +++P < 0.001 (compared with DHPG; Tukey's multiple comparison test).
Contribution of mGluR1
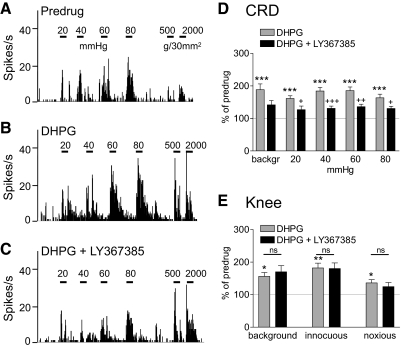
Coadministration of an mGluR1 antagonist (LY367385, 1 mM, concentration in microdialysis probe; 15 min) into the CeLC inhibited the facilitatory effects of DHPG on visceral but not somatosensory responses. Figure 5, A–C shows an individual example. The inhibitory effects of LY367385 on DHPG-induced facilitation of responses to CRD (Fig. 5D), but not to knee joint compression (Fig. 5E), were significant for the sample of neurons (n = 6; P < 0.05–0.001, Tukey's multiple comparison tests). LY367385 (1 mM) had no effect on baseline responses to innocuous or noxious stimuli (n = 6 neurons), consistent with the results of our previous study (Li and Neugebauer 2004b) using a different mGluR1 antagonist (CPCCOEt).
Fig. 5.
mGluR1 antagonist [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385)] inhibits DHPG-induced facilitation of responses to CRD but not knee joint compression. A–C: background activity and evoked responses of an individual CeLC neuron before drug application (predrug, A), during administration of DHPG (100 μM, concentration in microdialysis probe; 15 min) into the CeLC (B), and during coapplication of DHPG and LY367385 (1 mM; 15 min; C). Same display as in Fig. 4. LY367385 inhibited the facilitatory effects of DHPG on responses to CRD but not knee joint stimulation. D and E: summary data for the sample of neurons (n = 6). Bar histograms (mean ± SE) show background activity and responses evoked by CRD (D) and by knee joint compression (E) normalized to predrug control (set to 100%). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with predrug control); +P < 0.05, ++P < 0.01, +++P < 0.001 (compared with DHPG); ns, not significant (compared with DHPG; Tukey's multiple comparison test).
Contribution of ROS
Coadministration of a ROS scavenger (PBN, 100 mM, concentration in microdialysis probe, 15 min; Fig. 6) or superoxide dismutase mimetic (TEMPOL, 100 mM, concentration in microdialysis probe, 15 min; Fig. 7) into the CeLC inhibited the facilitatory effects of DHPG on visceral and somatosensory responses. Figures 6, A–C and 7, A–C show the effects of PBN and TEMPOL, respectively, in individual neurons. In the sample of neurons, inhibition of DHPG-induced facilitation by PBN (Fig. 6D, CRD, n = 6 neurons; Fig. 6E, knee joint compression, n = 5) and TEMPOL (Fig. 7D, CRD, n = 5 neurons; Fig. 7E, knee joint compression, n = 5) was significant (P < 0.05–0.001, Tukey's multiple comparison tests). TEMPOL had no significant effect on its own (n = 3 neurons). The data suggest that ROS, particularly superoxide, are involved in the facilitatory effects of group I mGluR activation.
Fig. 6.
ROS scavenger phenyl-N-t-butyl nitrone (PBN) inhibits DHPG-induced facilitation. A–C: background activity and evoked responses of an individual CeLC neuron before drug application (predrug, A), during administration of DHPG (100 μM, concentration in microdialysis probe; 15 min) into the CeLC (B), and during coapplication of DHPG and PBN (100 mM; 15 min; C). Same display as in Fig. 4. PBN inhibited the facilitatory effects of DHPG on responses to CRD and knee joint stimulation. D and E: bar histograms (mean ± SE) show background activity and responses evoked by CRD (n = 6 neurons; D) and by knee joint compression (n = 5 neurons; E) normalized to predrug control (set to 100%). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with predrug control); ++P < 0.01, +++P < 0.001 (compared with DHPG; Tukey's multiple comparison test).
Fig. 7.
Superoxide dismutase mimetic (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl [TEMPOL]) inhibits DHPG-induced facilitation. A–C: background activity and evoked responses of an individual CeLC neuron before drug application (predrug, A), during administration of DHPG (100 μM, concentration in microdialysis probe; 15 min) into the CeLC (B), and during coapplication of DHPG and TEMPOL (100 mM; 15 min; C). Same display as that in Fig. 4. TEMPOL inhibited the facilitatory effects of DHPG on responses to CRD and knee joint stimulation. D and E: summary data for the sample of neurons (n = 5). Bar histograms (mean ± SE) show background activity and responses evoked by CRD (D) and by knee joint compression (E) normalized to predrug control (set to 100%). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with predrug control); +P < 0.05, ++P < 0.01, +++P < 0.001 (compared with DHPG; Tukey's multiple comparison test).
DISCUSSION
This study addressed two novel and important issues: visceronociceptive processing in the amygdala (CeLC) and the role of ROS other than NO as signaling molecules downstream of group I mGluRs. Key findings are as follows. 1) The majority of CeLC neurons process both visceral (CRD) and somatosensory (compression of deep and cutaneous tissue) nociceptive information. 2) Both visceral processing and somatosensory processing in the CeLC are modulated differently by mGluR subtypes: mGluR1 mediates facilitation of visceral but not somatosensory responses, whereas mGluR5 modulates both. 3) ROS scavengers inhibit facilitatory effects of group I mGluRs on both visceral and somatosensory processing. This pattern of effects suggests a closer association of ROS, particularly superoxide, with mGluR5 than with mGluR1. Superoxide is implicated because TEMPOL acts as a superoxide dismutase (SOD2) mimetic that converts superoxide to hydrogen peroxide (Bindokas et al. 1996; Chinopoulos and Adam-Vizi 2006; Kishida and Klann 2007; Maher and Schubert 2000).
Biologically important neuronal ROS include superoxide and hydrogen peroxide. Although pathophysiological levels of ROS are important factors in neurotoxicity and apoptosis, at physiological levels ROS act as signaling molecules that are required for synaptic plasticity and normal cognitive functions such as learning and memory (Kishida and Klann 2007). Increasing evidence suggests a role of ROS in peripheral and spinal cord pain mechanisms (Chung 2004; Keeble et al. 2009; Schwartz et al. 2009). Peripherally injected hydrogen peroxide induced hyperalgesia and hydrogen peroxide levels increased in inflamed tissue (Keeble et al. 2009). It was speculated that superoxide might be more important centrally than peripherally (Keeble et al. 2009). Increased spinal levels of SOD2, which removes superoxide, and mitochondrial ROS were measured in the capsaicin pain model (Schwartz et al. 2008, 2009). Intrathecal ROS scavengers or SOD2 mimetics decreased capsaicin-induced secondary hyperalgesia (Lee et al. 2007; Schwartz et al. 2008, 2009) and hyperalgesia in a neuropathic pain model (Kim et al. 2009). Hyperalgesia induced by a superoxide generator was not blocked by inhibiting NO production, suggesting independent signaling pathways for superoxide and NO (Kim et al. 2009).
Little is known about the contribution of superoxide or hydrogen peroxide to pain-related neuronal activity. Systemic application of a ROS scavenger (PBN) or an SOD2 mimetic (TEMPOL) decreased capsaicin-induced central sensitization of spinal dorsal horn neurons (Lee et al. 2007). Vitamin E, an antioxidant, inhibited the responses of spinal dorsal horn neurons in neuropathic animals (Kim et al. 2006). A ROS donor produced long-lasting facilitation of Aβ-fiber–evoked synaptic responses of dorsal horn neurons in spinal cord slices (Lee et al. 2010). Conversely, PBN and TEMPOL impaired the induction and maintenance of C-fiber–induced LTP of Aβ-fiber–evoked synaptic responses in the dorsal horn of spinal cord slices (Lee et al. 2010).
The role of ROS in the processing of pain-related information in the brain is largely unknown. Our study shows that ROS are critically important for the facilitation of nociceptive processing by group I mGluRs in amygdala neurons. The inhibitory effect of an SOD2 mimetic (TEMPOL) is consistent with the involvement of superoxide. The pharmacologic properties of ROS scavengers used in this study deserve some consideration. PBN is the prototype of “spin-trapping” nitrones that react with free radicals to form stable nitroxide products (Kotake 1999). PBN (100 μM to 1 mM) can inhibit the formation of different types of ROS, such as superoxide, hydrogen peroxide, hydroxyl radical, and peroxynitrates (Kotake 1999). The stable and nontoxic nitroxide TEMPOL (10 μM to 10 mM) is a potent superoxide dismutase mimetic that converts superoxide radicals to hydrogen peroxide, which is further metabolized into molecular oxygen and water (Tal 1996). TEMPOL has been used to determine the role of superoxide in neuroplasticity (Lee et al. 2010; Schwartz et al. 2008, 2009) and to distinguish superoxide signaling from NO signaling (Kim et al. 2009). Therefore we interpret our data to possibly suggest a predominant involvement of superoxide in group I mGluR signaling in the CeLC. This is consistent with circumstantial evidence that superoxide is necessary for LTP, whereas other forms of ROS, such as hydrogen peroxide, may have a negative impact (Knapp and Klann 2002).
Mechanisms of pain-related ROS activation remain to be determined, but our study suggests that ROS are downstream of group I mGluRs, particularly mGluR5, because the effect of ROS scavengers showed a pattern similar to that of an mGluR5, but not mGluR1, antagonist. Glutamate receptor agonists, including N-methyl-d-aspartate (NMDA), increased superoxide production in cultured hippocampal neurons but not in glia (Bindokas et al. 1996). Increased superoxide production required calcium entry and the mitochondrial electron transport chain (Bindokas et al. 1996; Duan et al. 2007; Dugan et al. 1995). Another source of ROS, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, has been implicated in NMDA-dependent hippocampal LTP and memory formation (Kishida and Klann 2007; but see Bindokas et al. 1996), both of which require ROS (Huddleston et al. 2008; Klann 1998; Serrano and Klann 2004). NMDA receptor-dependent ROS production has also been suggested as an important spinal cord pain mechanism (Gao et al. 2007; Lee et al. 2010). On the other hand, increased phosphorylation of NR1 subunits in models of neuropathic or capsaicin-induced pain was reduced by an ROS scavenger, suggesting that NMDA receptors can be downstream targets of ROS (Gao et al. 2007).
A critical issue with NMDA-induced ROS generation is that in addition to its physiological role in plasticity it is an important mechanism of well-documented glutamate-induced excitotoxicity and neuronal damage that result from the irreversible rise in calcium (delayed calcium deregulation) and ROS release from impaired mitochondria (Chinopoulos and Adam-Vizi 2006; Duan et al. 2007). Therefore we focused on another glutamate receptor as a mechanism of pain-related ROS activation, group I mGluRs. Group I mGluRs couple through Gq proteins to phospholipase C activation, resulting in the formation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (Lesage 2004; Neugebauer 2007). IP3 calcium releases receptors on the endoplasmatic reticulum and DAG-sensitive mitochondrial channels provide a balanced way to regulate cytosolic calcium levels, mitochondrial calcium accumulation, and ROS production (Bianchi et al. 2004; Chinopoulos and Adam-Vizi 2006). DAG is also a native PKC activator and PKC-dependent activation of NADPH oxidase serves as an important source of neuronal ROS (Fontayne et al. 2002). The data suggest that group I mGluRs are well positioned to regulate ROS signaling.
Some evidence links group I mGluRs to ROS. Antagonists for mGluR5 (MPEP or SIB-1893), but not mGluR1 (CPCCOEt), inhibited increased release of ROS in the striatum in an in vivo model of methamphetamine-induced neurotoxicity (Battaglia et al. 2002). In contrast, mGluR5 activation had neuroprotective effects in activated microglial cell cultures by inhibiting NADPH oxidase activity and ROS generation (Loane et al. 2009). The role of group I mGluR subtypes in the regulation of ROS under different conditions, in different systems and cell types, and through different signaling pathways remains to be determined. Our previous studies showed that pain-related synaptic plasticity and central sensitization in the CeLC requires up-regulation and endogenous activation of mGluR1 and mGluR5. Antagonists for mGluR1 (CPCCOEt) and mGluR5 (MPEP) inhibited enhanced synaptic transmission (Neugebauer et al. 2003) and responsiveness of CeLC neurons to somatosensory stimuli (Li and Neugebauer 2004b) in a model of arthritis pain. Under normal conditions, DHPG increased responses to knee joint stimulation and this effect was mimicked by an mGluR5 agonist (CHPG), which is consistent with a predominant role of neuronal mGluR5 under normal conditions (Li and Neugebauer 2004b). The role of group I mGluRs in visceral processing and the underlying signaling mechanisms were not determined.
Results of the present study suggest a differential contribution of mGluR1 and mGluR5 to somatosensory (mGluR5 only) and visceral (mGluR1 and mGluR5) nociceptive processing in the CeLC. Evidence for differential roles of group I mGluR subtypes in various conditions has been reported before; they may involve differences in desensitization factors, signaling mechanisms, anatomical location, or expression pattern (Lea and Faden 2006). The fact that the mGluR1 antagonist affected DHPG-induced facilitation of visceral but not somatosensory processing in neurons that received both types of input may suggest a closer association of mGluR1 with visceral inputs, perhaps through a presynaptic mechanism. This issue remains to be resolved at the synaptic level.
The differential pattern of antagonist effects argues against nonselective drug actions. LY367385 antagonizes mGluR1 in the low micromolar range and does not interact with other mGluR subtypes ≤100 μM (Kingston et al. 2002). Concentration of LY367385 in the microdialysis fiber was 100 μM, confirming the appropriateness of the factor 100 to estimate tissue concentration (see methods). The concentration of MPEP of 100 μM in the microdialysis fiber that we used was previously shown to be selective for mGluR5 compared with mGluR1 (Battaglia et al. 2002). MPEP can have off-site effects on mGluR1 and NMDA receptor subunits at concentrations of >10 μM and >18 μM, respectively (Cosford et al. 2003; Loane et al. 2009). However, differential effects of MPEP and mGluR1 antagonists in this study and in our previous studies (Han and Neugebauer 2005; Li and Neugebauer 2004b; Neugebauer et al. 2003) as well as in the literature (Battaglia et al. 2002) argue against off-site effects as confounding factors. Allosteric potentiation of mGluR4 function is another potential off-site action of MPEP, but significant effects were observed only with higher concentrations (50 or 100 μM) (Mathiesen et al. 2003). Allosteric potentiation of mGluR4 by MPEP also required the presence of an mGluR4 agonist (Mathiesen et al. 2003). In the present study, amygdala neurons were stimulated with DHPG, which does not directly interact with mGluR4. If DHPG-induced (indirect) activation of mGluR4 occurred in our study, potentiating actions of MPEP on mGluR4 would be expected to enhance rather than antagonize the effects of DHPG. mGluR4 is not present in the CeA but in inhibitory neurons of the intercalated cell mass (Ohishi et al. 1995). Therefore allosteric potentiation of mGluR4 function, which typically is inhibitory (Neugebauer 2008), would result in disinhibition of CeA neurons based on the intrinsic amygdala circuitry (Sah et al. 2003). However, the effects of MPEP on CeA neurons in our study were inhibitory rather than facilitatory.
The analysis of visceronociceptive processing in amygdala neurons is another novelty of this study. More than half of the somatosensory responsive neurons also responded to CRD. It is conceivable that the proportion of visceronociceptive amygdala neurons is even larger because this study focused on input only from the colon. An important issue is the sampling technique for identifying visceronociceptive neurons. We monitored spontaneous activity and used brief stimulation of somatic tissue and, more sparingly, brief innocuous stimuli to moderate visceral stimuli (CRD). Every neuron with spontaneous activity or a receptive field in somatic tissues was also tested for its response to CRD. Although it is possible that we overlooked visceronociceptive neurons without spontaneous activity and somatic receptive fields, this population would be relatively small because previous studies showed that only about 20% of CeLC neurons did not respond to any somatosensory stimuli (Neugebauer and Li 2002, 2003). We did not use electrical stimulation in the parabrachial area (PB) as a search stimulus because this would have biased the sample toward neurons with PB input.
It is not known how the amygdala receives visceronociceptive input. Visceronociceptive neurons have been described in the PB that is part of the spino-parabrachio-amygdala pain pathway (Bernard et al. 1994). CRD induced Fos expression in spinal neurons that project to the parabrachial area (Traub and Murphy 2002). A majority of PB neurons (66%) responded to visceral stimuli (intraperitoneal bradykinin and/or CRD) and almost all of them also had cutaneous receptive fields (Bernard et al. 1994). These numbers match the findings of the present study, in which 62% of CeLC neurons responded to CRD and 98% also to somatosensory stimuli. Therefore the PB could serve as the major source of visceronociceptive input to the CeLC. However, the proportion of visceronociceptive CeLC neurons that were excited by CRD (87%) was larger than that of PB neurons (53%; Bernard et al. 1994).
Another source of visceral input could be the thalamus, which receives visceronociceptive information via the spinothalamic tract and the postsynaptic dorsal column pathway (Al-Chaer and Traub 2002; Ness 2000; Palecek et al. 2003; Willis et al. 1999). Polymodal, including nociceptive, information from midline and posterior thalamic nuclei reaches the lateral–basolateral amygdala circuitry, where associative processing generates highly processed information with affective content that is transmitted to the CeLC and other areas of the CeA (Neugebauer et al. 2004, 2009). The thalamic contribution to visceronociceptive processing in the amygdala is somewhat difficult to determine because surprisingly few studies have addressed viscerosomatic convergence at the single-cell level and reported varying degrees of convergence: relatively few neurons with convergent visceronociceptive and somatosensory inputs in the ventroposterior lateral nucleus (VPL) (Zhang et al. 2002) and the lateral part of the ventromedial thalamus (Monconduit et al. 2003); intralaminar neurons have been observed with viscerosomatic convergence (69%), but few of them had responses to CRD (Berkley et al. 1995); visceronociceptive neurons with somatosensory receptive fields (74–77%) in the ventrobasal complex (Berkley et al. 1993; Chandler et al. 1992); a large number of neurons with convergent inputs in the VPL (Al-Chaer et al. 1996); and a cluster of exclusively visceronociceptive cells in the central lateral nucleus of the intralaminar thalamus (Ren et al. 2009). The lines of visceral input to the different nuclei of the amygdala remain to be determined. Differential behavioral effects of lesions of different nuclei (Tanimoto et al. 2003) and patterns of c-fos mRNA expression (Nakagawa et al. 2003) related to somatic versus visceral pain suggest a more important role of the central than the basolateral nucleus of the amygdala in visceral processing, which would be consistent with a predominant role of the spinoparabrachial input.
In conclusion, this study showed a substantial convergence of somatosensory and visceral nociceptive input in amygdala (CeLC) neurons and identified ROS as downstream signaling molecules of group I mGluRs that can modulate the processing of somatosensory and visceronociceptive information. Enhanced responsiveness of amygdala neurons under conditions of increased group I mGluR–ROS function could be a mechanism of pain in the absence of tissue injury such as in irritable bowel syndrome. The effector mechanisms of ROS remain to be determined but may include oxidative modification or phosphorylation of targets such as protein kinases (Kishida and Klann 2007; Knapp and Klann 2002) that are important for pain mechanisms in the amygdala (Carrasquillo and Gereau 2007; Fu et al. 2008) or calcium release receptors of the ryanodine type that link ROS to extracellular signal-regulated kinase activation in hippocampal synaptic plasticity (Kemmerling et al. 2006). Inhibition of phosphatases such as calcineurin has also been suggested as a mechanism by which ROS contribute to LTP (Kishida and Klann 2007; Knapp and Klann 2002). Removing ROS after it forms following activation of group I mGluRs may be a useful strategy to inhibit amygdala hyperactivity that has been associated with pain behaviors and pain-related anxiety.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-38261 and NS-11255.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. William D. Willis Jr. for critical reading of and helpful comments on this manuscript and Drs. Kyungsoon Chung and Jin-Mo Chung for valuable advice on the use of reactive oxygen species scavengers.
REFERENCES
- Al-Chaer et al., 1996.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 76: 2661–2674, 1996 [DOI] [PubMed] [Google Scholar]
- Al-Chaer and Traub, 2002.Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain 96: 221–225, 2002 [DOI] [PubMed] [Google Scholar]
- Battaglia et al., 2002.Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci 22: 2135–2141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley et al., 1995.Berkley KJ, Benoist JM, Gautron M, Guilbaud G. Responses of neurons in the caudal intralaminar thalamic complex of the rat to stimulation of the uterus, vagina, cervix, colon and skin. Brain Res 695: 92–95, 1995 [DOI] [PubMed] [Google Scholar]
- Berkley et al., 1993.Berkley KJ, Guilbaud G, Benoist JM, Gautron M. Responses of neurons in and near the thalamic ventrobasal complex of the rat to stimulation of uterus, cervix, vagina, colon, and skin. J Neurophysiol 69: 557–568, 1993 [DOI] [PubMed] [Google Scholar]
- Bernard et al., 1994.Bernard J-F, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol 71: 1646–1660, 1994 [DOI] [PubMed] [Google Scholar]
- Bianchi et al., 2004.Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta 1742: 119–131, 2004 [DOI] [PubMed] [Google Scholar]
- Bindokas et al., 1996.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16: 1324–1336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird et al., 2005.Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol 564: 907–921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz et al., 2002.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol 97: 654–661, 2002 [DOI] [PubMed] [Google Scholar]
- Braz et al., 2005.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47: 787–793, 2005 [DOI] [PubMed] [Google Scholar]
- Carrasquillo and Gereau, 2007.Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci 27: 1543–1551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo and Gereau, 2008.Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 4: Article 24 (1–8), 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler et al., 1992.Chandler MJ, Hobbs SF, Fu QG, Kenshalo DR, Jr, Blair RW, Foreman RD. Responses of neurons in ventroposterolateral nucleus of primate thalamus to urinary bladder distension. Brain Res 571: 26–34, 1992 [DOI] [PubMed] [Google Scholar]
- Chinopoulos and Adam-Vizi, 2006.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J 273: 433–450, 2006 [DOI] [PubMed] [Google Scholar]
- Chung, 2004.Chung JM. The role of reactive oxygen species (ROS) in persistent pain. Mol Interv 4: 248–250, 2004 [DOI] [PubMed] [Google Scholar]
- Cliffer et al., 1991.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 11: 852–868, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford et al., 2003.Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem 46: 204–206, 2003 [DOI] [PubMed] [Google Scholar]
- Duan et al., 2007.Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol 585: 741–758, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan et al., 1995.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci 15: 6377–6388, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, 2004.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 5: 565–575, 2004 [DOI] [PubMed] [Google Scholar]
- Fontayne et al., 2002.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750, 2002 [DOI] [PubMed] [Google Scholar]
- Fu et al., 2008.Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain 4: 26–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu and Neugebauer, 2008.Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28: 3861–3876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al., 2007.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 131: 262–271, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau and Bernard, 2004.Gauriau C, Bernard J-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468: 24–56, 2004 [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld et al., 2001.Greenwood-Van Meerveld B, Gibson M, Gunder W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 893: 135–142, 2001 [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld et al., 2006.Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA. Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res 1071: 91–96, 2006 [DOI] [PubMed] [Google Scholar]
- Han et al., 2010.Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 6: 10–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al., 2005.Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci 25: 10717–10728, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han and Neugebauer, 2004.Han JS, Neugebauer V. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci Lett 361: 254–257, 2004 [DOI] [PubMed] [Google Scholar]
- Han and Neugebauer, 2005.Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- Heinricher and McGaraughty, 1999.Heinricher MM, McGaraughty S. Pain-modulating neurons and behavioral state. In: Handbook of Behavioral State Control, edited by Lydic R, Baghdoyan HA. New York: CRC Press, 1999, p. 487–503 [Google Scholar]
- Hu et al., 2006.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci 26: 3933–3941, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston et al., 2008.Huddleston AT, Tang W, Takeshima H, Hamilton SL, Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol 99: 1565–1571, 2008 [DOI] [PubMed] [Google Scholar]
- Ikeda et al., 2007.Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127: 161–172, 2007 [DOI] [PubMed] [Google Scholar]
- Ji et al., 2007.Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 3: 13–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji and Neugebauer, 2007.Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol 97: 3893–3904, 2007 [DOI] [PubMed] [Google Scholar]
- Ji and Neugebauer, 2008.Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol 99: 1201–1212, 2008 [DOI] [PubMed] [Google Scholar]
- Ji and Neugebauer, 2009.Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 102: 2253–2264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 2010.Johnson AC, Myers B, Lazovic J, Towner R, Greenwood-Van MB. Brain activation in response to visceral stimulation in rats with amygdala implants of corticosterone: an fMRI study. PLoS One 5: e8573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble et al., 2009.Keeble JE, Bodkin JV, Liang L, Wodarski R, Davies M, Fernandes ES, de Faria Coelho C, Russell F, Graepel R, Muscara MN, Malcangio M, Brain SD. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain 141: 135–142, 2009 [DOI] [PubMed] [Google Scholar]
- Kemmerling et al., 2006.Kemmerling U, Munoz P, Muller M, Sanchez G, Aylwin ML, Klann E, Carrasco MA, Hidalgo C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium 41: 491–502, 2006 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2006.Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 122: 53–62, 2006 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2004.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111: 116–124, 2004 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2009.Kim HY, Wang J, Lu Y, Chung JM, Chung K. Superoxide signaling in pain is independent of nitric oxide signaling. Neuroreport 20: 1424–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston et al., 2002.Kingston AE, Griffey K, Johnson MP, Chamberlain MJ, Kelly G, Tomlinson R, Wright RA, Johnson BG, Schoepp DD, Harris JR, Clark BP, Baker RS, Tizzano JT. Inhibition of group I metabotropic glutamate receptor responses in vivo in rats by a new generation of carboxyphenylglycine-like amino acid antagonists. Neurosci Lett 330: 127–130, 2002 [DOI] [PubMed] [Google Scholar]
- Kishida and Klann, 2007.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal 9: 233–244, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann, 1998.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol 80: 452–457, 1998 [DOI] [PubMed] [Google Scholar]
- Knapp and Klann, 2002.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res 70: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- Kotake, 1999.Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid Redox Signal 1: 481–499, 1999 [DOI] [PubMed] [Google Scholar]
- Lazovic et al., 2005.Lazovic J, Wrzos HF, Yang QX, Collins CM, Smith MB, Norgren R, Matyas K, Ouyang A. Regional activation in the rat brain during visceral stimulation detected by c-fos expression and fMRI. Neurogastroenterol Motil 17: 548–556, 2005 [DOI] [PubMed] [Google Scholar]
- Lea and Faden, 2006.Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev 12: 149–166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2007.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 133: 9–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2010.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol 103: 382–391, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage, 2004.Lesage ASJ. Role of group I metabotropic glutamate receptors mGlu1 and mGlu5 in nociceptive signalling. Curr Neuropharmacol 2: 363–393, 2004 [Google Scholar]
- Li and Neugebauer, 2004a.Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110: 112–122, 2004a [DOI] [PubMed] [Google Scholar]
- Li and Neugebauer, 2004b.Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91: 13–24, 2004b [DOI] [PubMed] [Google Scholar]
- Loane et al., 2009.Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284: 15629–15639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al., 2004.Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil 16: 575–587, 2004 [DOI] [PubMed] [Google Scholar]
- Maher and Schubert, 2000.Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci 57: 1287–1305, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, 2005.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron 47: 783–786, 2005 [DOI] [PubMed] [Google Scholar]
- Mason, 2005.Mason P. Deconstructing endogenous pain modulations. J Neurophysiol 94: 1659–1663, 2005 [DOI] [PubMed] [Google Scholar]
- Mathiesen et al., 2003.Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol 138: 1026–1030, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer et al., 2005.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115: 398–409, 2005 [DOI] [PubMed] [Google Scholar]
- Monconduit et al., 2003.Monconduit L, Bourgeais L, Bernard JF, Villanueva L. Convergence of cutaneous, muscular and visceral noxious inputs onto ventromedial thalamic neurons in the rat. Pain 103: 83–91, 2003 [DOI] [PubMed] [Google Scholar]
- Monnikes et al., 2003.Monnikes H, Ruter J, Konig M, Grote C, Kobelt P, Klapp BF, Arnold R, Wiedenmann B, Tebbe JJ. Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res 966: 253–264, 2003 [DOI] [PubMed] [Google Scholar]
- Myers et al., 2007.Myers B, Dittmeyer K, Greenwood-Van Meerveld B. Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behav Brain Res 181: 163–167, 2007 [DOI] [PubMed] [Google Scholar]
- Myers and Greenwood-Van Meerveld, 2007.Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol 292: G1622–G1629, 2007 [DOI] [PubMed] [Google Scholar]
- Nakagawa et al., 2003.Nakagawa T, Katsuya A, Tanimoto S, Yamamoto J, Yamauchi Y, Minami M, Satoh M. Differential patterns of c-fos mRNA expression in the amygdaloid nuclei induced by chemical somatic and visceral noxious stimuli in rats. Neurosci Lett 344: 197–200, 2003 [DOI] [PubMed] [Google Scholar]
- Naliboff et al., 2003.Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 124: 1738–1747, 2003 [DOI] [PubMed] [Google Scholar]
- Ness, 2000.Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain 87: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- Neugebauer, 2007.Neugebauer V. Glutamate receptor ligands. Handb Exp Pharmacol 177: 217–249, 2007 [DOI] [PubMed] [Google Scholar]
- Neugebauer, 2008.Neugebauer V. Group III metabotropic glutamate receptors (mGlu4, mGlu6, mGlu7, and mGlu8). In: The Glutamate Receptors, edited by Gereau RW, Swanson GT. Totowa, NJ: Humana Press, 2008, p. 489–508 [Google Scholar]
- Neugebauer et al., 2009.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 60: 226–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer and Li, 2002.Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 87: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- Neugebauer and Li, 2003.Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 89: 716–727, 2003 [DOI] [PubMed] [Google Scholar]
- Neugebauer et al., 2003.Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23: 52–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer et al., 2004.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 10: 221–234, 2004 [DOI] [PubMed] [Google Scholar]
- Ohishi et al., 1995.Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol 360: 555–570, 1995 [DOI] [PubMed] [Google Scholar]
- Palazzo et al., 2008.Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology 55: 537–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek et al., 2003.Palecek J, Paleckova V, Willis WD. Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli. Pain 104: 249–257, 2003 [DOI] [PubMed] [Google Scholar]
- Pape and Paré, 2010.Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré et al. 2004.Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson, 1998.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates New York: Academic Press, 1998 [Google Scholar]
- Pedersen et al., 2007.Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 127: 17–26, 2007 [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade et al., 2009.Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325: 760–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps and Ledoux, 2005.Phelps EA, Ledoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187, 2005 [DOI] [PubMed] [Google Scholar]
- Qin et al., 2003b.Qin C, Greenwood-Van Meerveld B, Foreman RD. Visceromotor and spinal neuronal responses to colorectal distension in rats with aldosterone onto the amygdala. J Neurophysiol 90: 2–11, 2003b [DOI] [PubMed] [Google Scholar]
- Qin et al., 2003c.Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 90: 2180–2189, 2003c [DOI] [PubMed] [Google Scholar]
- Qin et al., 2003a.Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 89: 1343–1352, 2003a [DOI] [PubMed] [Google Scholar]
- Ren et al., 2009.Ren Y, Zhang L, Lu Y, Yang H, Westlund KN. Central lateral thalamic neurons receive noxious visceral mechanical and chemical input in rats. J Neurophysiol 102: 244–258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy and Meagher, 2001.Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry 14: 241–245, 2001 [Google Scholar]
- Sah et al., 2003.Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Schwartz et al., 2009.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci 29: 159–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz et al., 2008.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 138: 514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano and Klann, 2004.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3: 431–443, 2004 [DOI] [PubMed] [Google Scholar]
- Spike et al., 2003.Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci 18: 2433–2448, 2003 [DOI] [PubMed] [Google Scholar]
- Stam et al., 2002.Stam R, Ekkelenkamp K, Frankhuijzen AC, Bruijnzeel AW, Akkermans LM, Wiegant VM. Long-lasting changes in central nervous system responsivity to colonic distention after stress in rats. Gastroenterology 123: 1216–1225, 2002 [DOI] [PubMed] [Google Scholar]
- Tal, 1996.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport 7: 1382–1384, 1996 [DOI] [PubMed] [Google Scholar]
- Tanimoto et al., 2003.Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci 18: 2343–2350, 2003 [DOI] [PubMed] [Google Scholar]
- Tracey and Mantyh, 2007.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 55: 377–391, 2007 [DOI] [PubMed] [Google Scholar]
- Traub and Murphy, 2002.Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain 95: 93–102, 2002 [DOI] [PubMed] [Google Scholar]
- Traub et al., 1996.Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett 215: 165–168, 1996 [DOI] [PubMed] [Google Scholar]
- Twining et al., 2004.Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain 110: 299–309, 2004 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2004.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309: 869–878, 2004 [DOI] [PubMed] [Google Scholar]
- Westlund et al., 2009.Westlund KN, Vera-Portocarrero LP, Zhang L, Wei J, Quast MJ, Cleeland CS. fMRI of supraspinal areas after morphine and one week pancreatic inflammation in rats. NeuroImage 44: 23–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith et al., 2004.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53: 1595–1601, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis et al., 1999.Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA 96: 7675–7679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2002.Zhang HQ, Al-Chaer ED, Willis WD. Effect of tactile inputs on thalamic responses to noxious colorectal distension in rat. J Neurophysiol 88: 1185–1196, 2002 [DOI] [PubMed] [Google Scholar]