Abstract
Appropriate interlimb coordination of the lower extremities is particularly important for a variety of functional human motor behaviors such as jumping, kicking a ball, or simply walking. Specific interlimb coordination patterns may be especially impaired after a lesion to the motor system such as stroke, yet this has not been thoroughly examined to date. The purpose of this study was to investigate the motor deficits in individuals with chronic stroke and hemiparesis when performing unilateral versus bilateral inphase versus bilateral antiphase voluntary cyclic ankle movements. We recorded ankle angular trajectories and muscle activity from the dorsiflexors and plantarflexors and compared these between subjects with stroke and a group of healthy age-matched control subjects. Results showed clear abnormalities in both the kinematics and EMG of the stroke subjects, with significant movement degradation during the antiphase task compared with either the unilateral or the inphase task. The abnormalities included prolonged cycle durations, reduced ankle excursions, decreased agonist EMG bursts, and reduced EMG modulation across movement phases. By comparison, the control group showed nearly identical performance across all task conditions. These findings suggest that stroke involving the corticospinal system projection to the leg specifically impairs one or more components of the neural circuitry involved in lower extremity interlimb coordination. The express susceptibility of the antiphase pattern to exaggerated motor deficits could contribute to functional deficits in a number of antiphase leg movement tasks, including walking.
INTRODUCTION
Many daily activities require precise patterns of interlimb coordination. An inphase pattern is one in which the limbs perform the same movement with homologous muscles on opposite sides working simultaneously, such as reaching to grasp and lift a box or jumping. An antiphase pattern involves homologous muscles on opposite sides working reciprocally rather than simultaneously, for example, during swimming freestyle. For other tasks, the limbs may perform two completely dissimilar actions to achieve a goal, such as opening a jar or playing the violin. In humans, the most commonly executed mode of interlimb coordination between the legs is the rhythmic antiphase pattern of flexion and extension that occurs during walking.
These bilateral movements have specific constraints (Kelso et al. 1979; Swinnen 2002; Walter et al. 2001). For example, when trying to draw a circle with one hand and a square with the other, the two tasks interfere with one another such that both are disrupted, and the resulting shapes reflect something intermediate between circle and square. Another constraint is the well-documented predisposition for an inphase pattern (Byblow et al. 1994; Kelso 1984). Specifically, repetitive cyclic limb flexion and extension movements more closely follow the correct phasing and have less timing variability when performed in the inphase pattern compared with antiphase (Kelso and Jeka 1992). Additionally, when the cycle frequency is increased, antiphase patterns often convert to inphase patterns, but the reverse is not observed. This preference for inphase patterns has been reported in both the upper (Byblow et al. 1994; Kelso 1984) and lower (Baldissera et al. 1991; Kelso and Jeka 1992) extremities in healthy individuals.
After unilateral stroke, interlimb coordination is often impaired (Dietz and Berger 1984; Roerdink et al. 2007; Wu et al. 2009), whether caused by the primary brain lesion itself and/or resulting adaptive changes (Crone et al. 2003; Lamy et al. 2009; Liepert et al. 2000a; Murase et al. 2004) in other connected central structures. Some studies have identified modest improvements in paretic arm movements when they are performed simultaneously with the nonparetic arm (i.e., inphase) compared with performed with the paretic arm alone (Cunningham et al. 2002; Harris-Love et al. 2005; Rose and Winstein 2005). In contrast, others have shown no improvement (Steenbergen et al. 2000) or even marked degradation (Lewis and Byblow 2004) of paretic arm movements during bilateral compared with unilateral tasks. The presence of increased mirroring (the involuntary mirror symmetric movement of a limb induced by effortful movement of the opposite limb) in individuals with stroke suggests that there is some bias toward the inphase pattern that is either evoked or inadequately suppressed after unilateral brain lesion (Mayston et al. 1999; Nelles et al. 1998).
Stroke-related interlimb coordination deficits of voluntary leg movements have not been studied as commonly. However, it has been shown that paretic motor deficits during unilateral pedaling are worsened during antiphase pedaling (Kautz and Patten 2005; Kautz et al. 2006). To our knowledge, no study has directly compared inphase versus antiphase cyclic leg movements in persons with poststroke hemiparesis. If lower extremity behavior is similar to the upper extremity and if stroke does not alter the apparent preference for inphase patterns seen in healthy subjects, we could expect that voluntary bilateral leg movements would be performed better in the inphase pattern than the antiphase pattern. On the other hand, important structural and functional differences between arm and leg must be considered. After stroke, brain stem and spinal circuits are disinhibited (Crone et al. 2003; Delwaide and Oliver 1988; Lamy et al. 2009; Masakado et al. 2005). With the lumbosacral spinal cord containing robust circuitry controlling antiphase patterned human locomotion, it is possible that bilateral leg movements poststroke may be predisposed toward the antiphase pattern over the inphase pattern. If correct, stroke may result in a preference for the antiphase pattern of leg movements, even during behaviors that are not related to locomotion, e.g., a sitting voluntary cyclic ankle movement task.
The purpose of this study was to compare motor behavioral deficits in individuals with chronic stroke and hemiparesis performing a voluntary, single-joint cyclic ankle movement task in either a unilateral, bilateral inphase or bilateral antiphase pattern. We assessed kinematics and muscle activity in a group of subjects with stroke and hemiparesis and a comparison group of healthy controls to determine the effects of these three different patterns of nonparetic leg movement on the motor behavior of the paretic leg. In accordance with the findings from lower extremity pedaling studies (Kautz and Patten 2005; Kautz et al. 2006), we hypothesized that individuals with stroke would have greater motor deficits during antiphase ankle movements than unilateral movements.
METHODS
Subjects
Eighteen adults, 8 individuals with chronic poststroke hemiparesis (4 females; 60.4 ± 11.1 yr, mean age ± 1 SD) and 10 age-matched healthy adults (6 females; 60.2 ± 8.9 yr) participated in the study. The healthy controls had no known acute or ongoing orthopaedic or neurological deficits, no pain, and no other medical diagnoses that would limit best performance in the study tasks. For the stroke group, inclusion criteria were 1) a history of a single unilateral ischemic stroke occurring ≥12 months prior, 2) clinical evidence of involvement of the corticospinal system projection to the leg, measured by the presence of at least mild paresis, incoordination and/or hyperreflexia in the contralesional lower extremity, 3) able to achieve 15° or more of passive dorsiflexion-plantarflexion range of motion at the paretic ankle, 4) able to achieve passive dorsiflexion to at least to 5° less than the neutral position at the paretic ankle, and 5) have some visible active dorsiflexion and plantarflexion against-gravity movement at the paretic ankle. Exclusion criteria were 1) any clinical or radiological evidence of brain lesion to the nonstroke hemisphere or to the cerebellum, 2) history of traumatic brain injury, 3) history of any other significant neurological condition affecting sensorimotor function, 4) score of 21 or lower on the Mini Mental State Examination (Folstein et al. 1975), 5) peripheral neuropathy, 6) acute pain, or 7) any restrictions in active or passive movement of the nonparetic leg. All subjects gave informed consent before participation and the University of Maryland School of Medicine Institutional Review Board approved the study.
For the subjects with hemiparesis, stroke was confirmed by MRI or CT scan. Before testing, these subjects also underwent a thorough neurological examination to quantify sensorimotor impairments. Ankle movement was categorized grossly as either “good” (can achieve nearly full active range of motion against a moderate resistance), “partial” (active motion to ∼25–75% of available range), or “minimal” (active motion to <25% of available range). Reflexes were measured via tendon tap at the Achilles tendon and quantified numerically as 0 (absent), 1 (diminished), 2 (normal), 2+ (slightly brisk), 3 (brisk and excessive), or 4 (sustained clonus) (Dyrek 1994). Light touch sensation was classified based on the ability to detect Semmes-Weinstein monofilaments (Weinstein 1993) and categorized as “intact” (able to detect monofilament sizes ≤3.61 on the great toe and ≤4.31 on the lower leg), “impaired” [able to detect sizes ≤4.56 (toe) and ≤6.65 (leg)], or “absent” [unable to detect sizes >4.56 (toe) or >6.65 (leg)]. Proprioception was classified based on the ability to identify the correct direction of passive movement: intact (successful on 6 of 6 trials at the great toe and ankle), impaired (successful on 4 or 5 of 6 trials), or absent (successful on ≤3 trials). The degree of visuospatial neglect was assessed with performance on the BIT star cancellation test (Wilson et al. 1987): correctly crossing out ≥51 of 54 stars was considered having no deficits (Bailey et al. 2004). The overall level of leg impairment was assessed by the lower extremity Fugl-Meyer assessment, which uses a 0–34 point scale, with 34 indicating the best possible performance (Fugl-Meyer et al. 1975). The sensorimotor status for all stroke subjects is provided in Table 1.
Table 1.
Stroke subject characteristics
| Motion/strength |
Somatosensation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | Sex | Dur, mo | Lesion | DF | PF | Refl PF | Cutan | Proprio | LE FMA | Antiphase score | |
| S1 | 59.4 | F | 66 | L subc | Partial | Good | 2+ | Intact | Intact | 27 | 2 |
| S2 | 62.9 | F | 51 | R subc | Partial | Partial | 2+ | Impaired | Intact | 21 | 1 |
| S3 | 37.2 | M | 69 | L cort | Partial | Partial | 3 | Impaired | Impaired | 22 | 2 |
| S4 | 71.9 | M | 73 | L cort | Partial | Partial | 2+ | Impaired | Impaired | 18 | 1 |
| S5 | 53.5 | F | 91 | R subc | Partial | Partial | NT | Impaired | Intact | 24 | 1 |
| S6 | 69.7 | M | 250 | L cort | Partial | Partial | 2+ | Impaired | Impaired | NT | 3 |
| S7 | 61.0 | F | 139 | R subc | Partial | Good | 2 | Absent | Impaired | 26 | 3 |
| S8 | 67.4 | M | 128 | L cort | Partial | Partial | 4 | Absent | Absent | 16 | 0 |
| 60.4 ± 11.1 | 108.4 ± 65.0 | 22.0 ± 4.0 | |||||||||
Leftmost column represents the stroke subject number. Lesions were grossly categorized as either cortical (cort) or subcortical (subc), depending on whether the sensorimotor cortex was damaged. Antiphase scores indicate the number of trials (of 3) in which the subject correctly executed the antiphase pattern. Cutan, cutaneous; DF, dorsiflexion; Dur, duration (since stroke); F, female; L, left; M, male; mo, months; NT, not tested; PF, plantarflexion; Proprio, proprioception; R, right; Refl, deep tendon reflex; yr, years; LE FMA, lower extremity Fugl-Meyer Assessment. Bottom row, group means ± 1 SD.
Paradigm
Subjects sat on a custom chair in the long-sitting position (lower legs extended out in front and resting on a support unit) such that the trunk was semireclined, hips were flexed to ∼60–80°, and knees were fully extended and ankles were just off the edge of the support surface. A Velcro strap was secured around the thighs and lower legs to help maintain knee extension. In this position, to achieve full ankle dorsiflexion or plantarflexion required an active concentric contraction of the ankle dorsiflexor or plantarflexor muscles, respectively.
We compared voluntary ankle movements between individuals with poststroke hemiparesis and healthy controls during three movement pattern conditions: unilateral, bilateral inphase, and bilateral antiphase. In each case, the task was to maximally dorsiflex and plantarflex the ankle cyclically and continuously for the duration of a trial. Each trial lasted 10 s. An auditory metronome sounding at 1.33 Hz was used as a cue to pace the cycles. Subjects were instructed to bend the ankle back and forth to the beat so as to reach the end position of each movement at the exact time of each beat (i.e., to achieve an ankle cycle frequency of 0.67 Hz). Thus six to seven full cycles of ankle movement would be completed in each trial if the subject kept to the beat. This cycle frequency was selected because it produced a moderate speed of ankle movement that was effortful but could be achieved by all subjects in the stroke group while also not considered overly slow to most subjects in the control group. Subjects were often reminded to make sure they moved the ankle all the way through its full range with each movement, to stay with the beat, and to move only the requested ankle. In the unilateral condition, subjects moved one ankle only while keeping the other ankle and all other limb segments at rest. In the inphase condition, subjects moved both ankles together always in the same direction so that homologous muscles on both sides were activated simultaneously. In the antiphase condition, subjects moved both ankles together but always in opposite directions so that homologous muscles on both sides were activated sequentially and not simultaneously. During the bilateral conditions, subjects were given the additional instruction to “make sure you maintain the correct [exactly same or exactly opposite] timing across the two sides.” During the unilateral condition, subjects were reminded to keep the nonmoving ankle relaxed.
A brief practice session was completed first to familiarize subjects with all three conditions. A total of nine test trials were collected: three in each movement condition. The order of conditions was counterbalanced across subjects to minimize possible effects of practice or fatigue. In addition, a 1-min rest break was inserted between conditions for all subjects.
Data collection
Three-dimensional leg position data were collected using the Optotrak System (Northern Digital, Waterloo, Ontario, Canada). Six infrared-emitting markers were placed on the fifth metatarsal heads, the lateral malleoli, and the lateral knee joint spaces, bilaterally. Four pairs of bipolar surface electrodes (Motion Lab Systems, Baton Rouge, LA) were placed over the tibialis anterior (TA) and medial gastrocnemius (MG) muscle bellies bilaterally to record muscle activation patterns. Skin was prepped by light abrasion with an alcohol pad before electrode application. A ground electrode was placed on the left patella. EMG signals were amplified with a gain of ×20 at the electrode site and on-line band-pass filtered at 20–500 Hz. Kinematic data were collected at 100 Hz. EMG were recorded at 1,000 Hz and time-synchronized with the kinematics.
Data analysis
All analyses were conducted using custom Matlab (MathWorks, Natick, MA) software. Off-line, kinematic data were low-pass filtered at 10 Hz. Ankle joint angular excursions were derived from the three-dimensional position data. EMG signals were demeaned, notch filtered to eliminate noise at 60 Hz, and rectified for further analysis.
For each trial, a cycle was defined as the time from peak ankle plantarflexion to the next peak ankle plantarflexion. We identified four movement phases within each cycle: initial dorsiflexion (phase I), late dorsiflexion (phase II), initial plantarflexion (phase III), and late plantarflexion (phase IV). The dorsiflexion phase was the time interval from peak ankle plantarflexion to peak ankle dorsiflexion; the plantarflexion phase was the time interval from peak dorsiflexion to peak plantarflexion. Within the dorsiflexion and plantarflexion phases, initial and late components of each were further defined as the times before and after, respectively, the ankle reached its midpoint angle between consecutive peak angles.
Because of the difficulty in identifying clear bursts of muscle activity in individuals with hemiparesis, EMG signals were instead analyzed over each of the four movement phases determined by kinematic events. We calculated the integrated EMG (IEMG) over the duration of each of the four movement phases and expressed each as a percentage of the total IEMG in a cycle by dividing the IEMG for each movement phase by the total IEMG for that cycle (Brown et al. 1997). To quantify the ability to modulate levels of muscle activity across the four phases of the ankle cycle movement, we also generated IEMG phase difference measures. IEMG phase differences were calculated as the difference between IEMG percentages across each set of successive movement phases. For example, modulation of the TA muscle from phase I to phase II was measured as the TA %IEMG during phase II minus the TA %IEMG during phase I and so on. Thus positive phase difference values would indicate IEMG of a muscle increasing from one phase to the next; negative values would indicate IEMG decreasing between phases. Greater absolute values represent greater modulation between phases; values approaching zero represent little modulation between phases.
Our prediction was that the stroke group would show differences in performance across the three movement conditions. One way in which the difficulty of a specific condition might become apparent is if a subject fails to perform that condition as instructed and instead reverts to a movement pattern that more closely resembles another test condition. Therefore before we proceeded with our main analyses comparing performance across movement conditions, we performed preliminary analyses to quantify the degree to which each movement was performed successfully. We defined successful performance as no movement on the “resting” side during the unilateral condition and the correct phasing relationship during the bilateral inphase and antiphase conditions.
Preliminary analysis: movement condition success
To determine whether the unilateral condition was really performed unilaterally, we assessed ankle angular excursions and the magnitude of TA and MG EMG on the instructed relaxed leg (the noninstructed side) relative to these values during an active voluntary movement. Thus a score of 0% would indicate no activity of the noninstructed leg, whereas a score of 100% would indicate the noninstructed leg was as active as would be expected if it were moving voluntarily alone. We also compared IEMG phase difference measures on the noninstructed side to see whether any muscle activity we detected appeared more static, as might be used to hold the ankle still, or appeared more modulatory, as it would be during the voluntary cyclic task. For the bilateral conditions, we assessed the phase relationship between the two ankle angles using a cross-correlation analysis. Lag times at the peak of the cross-correlation function over the cycle duration were expressed numerically ranging between 0 and 1. A lag time of 0 (or 1) would indicate a perfect inphase pattern and 0.5 would indicate a perfect antiphase pattern. We considered a trial to have the correct phase relationship if the lag time ranged between 0.0 and 0.15 or 0.85 and 1 for the instructed inphase movements and between 0.35 and 0.65 for the instructed antiphase movements. We assessed these lag times for both inphase and antiphase trials and quantified the frequency with which noninstructed movements were performed for all trials from both groups.
Main analysis: movement condition performance
Here, we compared the total ankle angle cycle duration, the duration of each phase of the cycle, and the total angular excursion at the ankle joint across the three conditions. We also compared the percentage IEMG over the duration of each of the four movement phases and the IEMG phase differences.
These variables were averaged over all available cycles for each trial and all trials for each condition and each subject and averaged over all subjects to create group means. We compared the left leg of control subjects to the paretic leg of the subjects with stroke. Statistical comparisons were made using SAS/STAT software (SAS, Cary, NC). A two-way (group × condition) mixed-model ANOVA with repeated measures on one factor (condition) was used. When the ANOVA was significant, post hoc analyses were performed using Tukey's honest significant difference test. In all cases, the level for statistical significance was set at P < 0.05.
RESULTS
Movement condition success
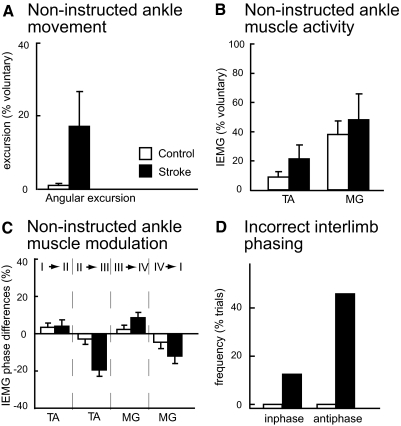
Subjects in the stroke group sometimes had difficulty in performing the instructed task. For example, several individuals were unable to keep the nonparetic leg relaxed and unmoving during the unilateral condition. In addition, some subjects performed a movement more closely resembling the inphase condition when attempting to perform the antiphase condition or vice versa. Figure 1 shows the capability of stroke and control group subjects to complete the cyclic movements according to instructions. For the unilateral movement condition, the magnitude of ankle movements (Fig. 1A) and muscle activity (Fig. 1B) in the noninstructed leg (i.e., the nonparetic leg for stroke subjects and the right leg for healthy controls) is expressed as a percent of the ankle excursion (or muscle activity) from that leg during active voluntary unilateral ankle cycle on that side. In general, the stroke group had greater ankle excursions and higher levels of muscle activity on the noninstructed side but not significantly so (Fig. 1A; 1-way ANOVA group effect, P = 0.08; Fig. 1B; group effects, P = 0.21 and P = 0.60 for TA and MG, respectively). Modulation of the muscle activity from the noninstructed leg was also generally increased in the stroke group compared with controls. In particular, TA muscle modulation was significantly greater between movement phases II and III (Fig. 1C; group effect, P = 0.002). None of the other phase difference modulation measures showed a significant difference between groups (phase I to II, P = 0.88; phase III to IV, P = 0.14; phase IV to I, P = 0.16). Figure 1D shows the frequency of trials in which an incorrect phasing pattern was observed during inphase and antiphase conditions. Control subjects always maintained the correct interlimb phasing for both inphase and antiphase trials (i.e., 0% of instructed inphase and antiphase trials incorrectly), whereas stroke subjects did not always maintain the correct interlimb phase relationship (χ2; group effects, P = 0.063 and P < 0.001 for inphase and antiphase conditions, respectively).
Fig. 1.
A: average ankle excursions on the noninstructed side during the unilateral condition, expressed as a percent of the excursion during an active voluntary ankle cycle movement on that side. B: average integrated EMG (IEMG) activity from the tibialis anterior (TA) and medial gastrocnemius (MG) muscles on the noninstructed side during the unilateral condition, expressed as a percent of the total muscle activity produced from each muscle, respectively, during an active voluntary ankle cycle movement on that side. C: average IEMG modulation (phase differences) in the noninstructed ankle muscles during the unilateral condition. D: frequency of inappropriate interlimb phase relationships observed during inphase or antiphase conditions, expressed as a percent of the total number of trials. See results for the description of the criteria for determining whether each trial was correctly or incorrectly phased. Error bars, ±SE.
The inability on the part of some stroke subjects to perform all task conditions as instructed could have confounded the comparison of results between conditions. To test for this, we compared the dataset containing all trials of the stroke group to the dataset containing only those trials in which the correct movement condition (inphase vs. antiphase) was performed. For every variable of interest described in the main results below, we found no difference between these two datasets (all comparisons, P ≥ 0.45). Therefore we elected to report performance measures on the dataset containing all trials and all individuals. This approach would maintain the maximal sample size and would not eliminate any data based on relatively arbitrary criteria for successful task performance.
Movement condition performance
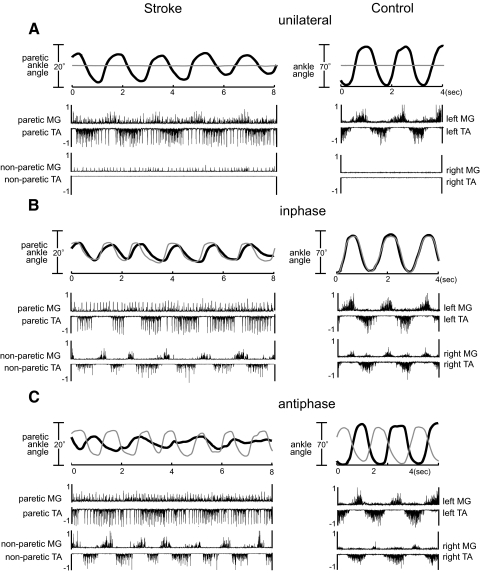
Individual traces of ankle angular trajectories and muscle activation are depicted in Fig. 2 for all three conditions from a typical subject with chronic stroke and hemiparesis and an age-matched control. As expected, the subject with stroke showed smaller ankle motions and abnormal muscle activation patterns on the paretic side compared with the control subject. When comparing across conditions, it is also clear that the paretic ankle excursion was reduced during the inphase condition compared with unilateral and further reduced during the antiphase condition compared with inphase. In contrast, the control subject showed a similar magnitude of ankle angular excursion across all conditions. The stroke subject also showed impaired interlimb coordination characterized by increased inconsistency in the angular trajectories and phasing abnormalities during the bilateral conditions but particularly during the antiphase movement (Fig. 2, B and C, left). In addition, both paretic muscles of the stroke subject showed a lack of modulation across cycle phases.
Fig. 2.
Individual traces of ankle angular trajectories (top) and muscle activity (bottom) plotted vs. time during typical trials from the (A) unilateral, (B) inphase, and (C) antiphase conditions for a subject in the stroke group (left) and an age-matched control subject (right). On the angle traces, thick, black lines represent the leg of interest (paretic leg of stroke group; left leg of control group); thin gray lines represent the opposite leg. Upward deflections represent movement in the plantarflexor direction; downward deflections represent the dorsiflexor direction. Note the scaling changes between stroke and control subjects' ankle excursions. Muscle activity is normalized to the average peak during a series of maximal voluntary isometric contractions. TA traces are inverted to facilitate viewing. TA, tibialis anterior; MG, medial gastrocnemius.
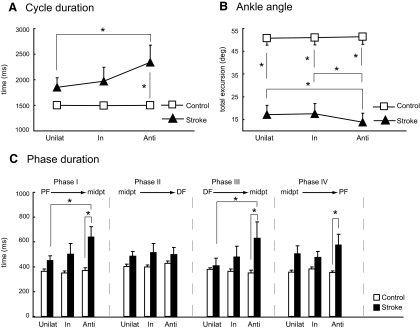
Average kinematic data for both groups is shown in Fig. 3. Overall, the stroke group had longer cycle durations across all conditions compared with the control group (group effect, P = 0.02). There was also a significant group × condition interaction (P = 0.018): cycle duration incrementally increased across conditions (unilateral vs. inphase vs. antiphase) in the stroke group, whereas the control group showed no such change across conditions (Fig. 3A). Cycle duration in the stroke group was significantly longer during the antiphase condition compared with the unilateral condition (post hoc, P = 0.006) and compared with the control group antiphase condition (post hoc, P = 0.016). We also assessed separately each of the four movement phases of the full cycle to determine whether any specific phase(s) were responsible for the total cycle duration differences. In general, most movement phases showed a similar trend to that of the total cycle duration (Fig. 3C). Phases I, III, and IV all showed a significant group × condition interaction effect (P = 0.02, P = 0.004, and P = 0.03, respectively), with the stroke group antiphase condition consistently having the longest duration (phase I: post hoc, stroke antiphase vs. unilateral P = 0.004, antiphase stroke vs. control P = 0.004; phase III: post hoc, stroke antiphase vs. unilateral P = 0.003, antiphase stroke vs. control P = 0.04; phase IV: post hoc, antiphase stroke vs. control P = 0.02). Interestingly, the pattern was most evident in phases I and III and not present at all in phase II. Phases I and III represent initial dorsiflexion and initial plantarflexion, respectively, immediately after a reversal in movement direction.
Fig. 3.
Average group kinematics. Average (A) total cycle durations, (B) ankle angular excursions, and (C) cycle phase durations across all conditions for stroke and control groups. Unilat, unilateral condition; In, inphase condition; Anti, antiphase condition; PF, peak plantarflexion; DF, peak dorsiflexion; midpt, midpoint range of ankle motion. Error bars, ±SE. Asterisks indicate specific significant differences from the post hoc comparisons of interaction effects.
Ankle angular excursions during cycles (Fig. 3B) showed significant group (P < 0.001) and interaction effects (P = 0.016) but no condition effect (P = 0.09). Not surprisingly, the stroke group consistently produced smaller ankle excursions at each condition compared with the control group (post hoc, all P < 0.001). Notably, the antiphase condition resulted in the smallest ankle excursion in the stroke group, which was significantly smaller than either the inphase or unilateral conditions (post hoc, both P = 0.047). In contrast, the control group showed no difference in ankle excursions across any condition.
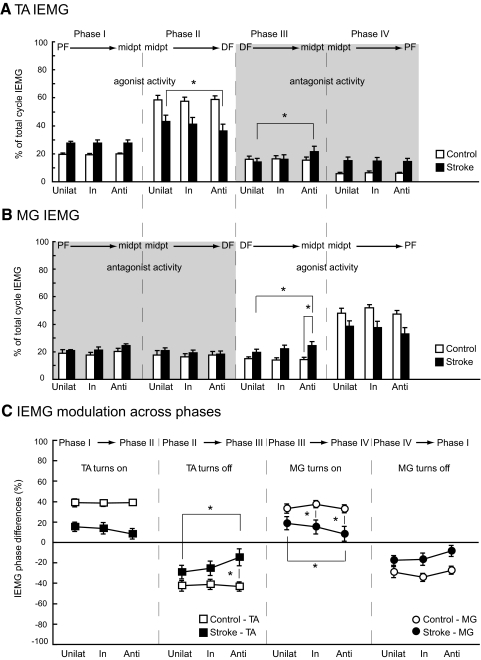
Average IEMGs are depicted in Fig. 4, A and B, for the four phases of ankle cycles. Recall that phases I and II correspond to early and late dorsiflexion periods and phases III and IV correspond to early and late plantarflexion. In both groups, the maximal IEMG activity was generally produced during phase II for the TA muscle and phase IV for the MG. However, stroke subjects did not produce as much agonist muscle activity during these phases as control subjects (TA phase II group effect, P = 0.002; MG phase IV group effect, P = 0.02). In addition, stroke subjects produced a greater percentage of the total IEMG of the TA muscle during phase IV, the period of late plantarflexion, when the TA muscle is expected to be relatively inactive (TA phase IV group effect, P = 0.001). Average IEMG were significantly higher for the stroke group TA during phase I and MG during phase III (group effects, P < 0.001 and P < 0.01, respectively).
Fig. 4.
Average group EMG. Average (A) TA and (B) MG IEMG for all cycle phases, across all conditions for stroke and control groups. IEMG magnitudes are a percentage of the total cycle IEMG for each muscle. C: IEMG modulation values from phase to phase, across all conditions for stroke and control groups. Unilat, unilateral condition; In, inphase condition; Anti, antiphase condition; PF, peak plantarflexion; DF, peak dorsiflexion; midpt, midpoint range of ankle motion; TA, tibialis anterior; MG, medial gastrocnemius. Error bars, ±SE. Asterisks indicate specific significant differences from the post hoc comparisons of interaction effects.
In addition to group differences, we also found that stroke subjects exhibited the greatest abnormalities of IEMG magnitudes during the antiphase condition (significant group × condition interaction effects). In the TA muscle (Fig. 4A), this was evident during phases II (borderline significance; P = 0.05) and III (P = 0.02), or late dorsiflexion and early plantarflexion. In the stroke group, TA IEMG during phase II was significantly lower during the antiphase condition compared with unilateral (post hoc, P = 0.047), whereas during phase III, TA IEMG was significantly greater during the antiphase condition compared with unilateral (post hoc, P = 0.01). Note that decreased TA (agonist) activity during phase II and increased TA (antagonist) activity during phase III would both be expected to slow or limit the intended movement direction (dorsiflexion during phase II; plantarflexion during phase III). For the MG muscle (Fig. 4B), there was also a group × condition interaction during phase III (P = 0.02). Here, stroke subjects produced a significantly greater percentage of IEMG during the antiphase condition compared with either the control group antiphase condition (post hoc, P = 0.02) or the stroke group unilateral condition (post hoc, P = 0.01).
Figure 4C shows the average IEMG modulation measures for both groups at each key phase transition. Overall, individuals with stroke and hemiparesis showed reduced modulation levels in the ankle muscles across all four phase comparisons compared with the control group (group effect, P < 0.05 for all). Importantly, it can also be seen that the modulation in the individuals with stroke appeared to degrade when comparing unilateral to inphase to antiphase, with the antiphase condition consistently showing the least modulation. Note (Fig. 4, A and B) that the primary modulation of IEMG activity levels between phases I and II occurs in the TA muscle, because the TA becomes dramatically more active to move the ankle into full dorsiflexion. Between phases II and III, the greatest modulation again occurs in the TA muscle with a large drop in activity to change the movement direction from dorsiflexion to plantarflexion. On the other hand, as the movement continues from phase III to phase IV, and again from phase IV to the next cycle phase I, the greatest change in IEMG occurs in the MG. Thus we focused our attention specifically on the modulation of the TA muscle between phases I and II and between II and III and the modulation of the MG muscle between phases III and IV and between IV and I (Fig. 4C).
Statistically, there was a group × condition interaction in TA modulation (P = 0.02) from phases II to III, in which the ankle reversed movement direction from dorsiflexion to plantarflexion (TA decreases activity). The TA in stroke subjects showed less modulation during the antiphase condition compared with unilateral (post hoc, P = 0.01) and compared with the control group antiphase condition (post hoc, P = 0.049). The MG modulation values from phases III to IV also showed an interaction effect (P = 0.04). Again, the individuals with poststroke hemiparesis had less modulation, this time when the MG should have increased its activity to move the ankle from initial to full plantarflexion. The post hoc analysis showed significantly less modulation of the MG muscle in the stroke group in the antiphase condition compared with the unilateral condition (P = 0.02) and in both the inphase and antiphase conditions compared with same conditions in the control group (both P < 0.05).
DISCUSSION
In this study, we compared deficits in cyclic paretic ankle movements across three different interlimb coordination patterns in individuals with chronic stroke. Kinematic and EMG findings were consistent: paretic ankle movements were the least impaired when performed unilaterally, only moderately more impaired when performed in an inphase bilateral pattern, but significantly more impaired during an antiphase bilateral pattern. Specifically, ankle movements were slower and had smaller amplitudes, and TA and MG muscles showed reduced modulation over the four ankle cycle phases during the antiphase pattern. In contrast, healthy controls exhibited nearly identical performance across all three conditions. To our knowledge, this is the first study to compare inphase versus antiphase lower extremity movements in the chronic poststroke population. Because of the paradigm we used (i.e., a simple, single-joint movement tested in supported sitting), it is unlikely that these results are attributable to mechanical coupling changes, postural deficits, or paresis. Rather, we think the deficits shown here indicate specific impairment of central control mechanism(s) for interlimb coordination during voluntary control of ankle movements.
Although control subjects maintained the requested speed and full ankle excursion across conditions, cycle durations were significantly increased in the stroke group and were the greatest during antiphase movements. Even with the increased time taken to complete each cycle, ankle excursions in the stroke group showed the same pattern of impairments: ankle movements were the smallest during antiphase movements and the largest during unilateral movements. The substantial increase in total movement duration in the antiphase condition is attributed primarily to slowness during the initial phases of each movement direction (phases I and III; Fig. 3C), suggesting that the stroke group had more difficulty in quickly reversing movement directions and/or during the initial portions of each movement direction rather than during the terminal portions of each movement direction. This is consistent with other studies in persons with chronic stroke showing prolonged times to peak velocity during discrete reaching (Harris-Love et al. 2005) and prolonged reversal durations during continuous aiming (Winstein and Pohl 1995). With regard to the EMG findings, the stroke group showed reduced levels of agonist muscle activation during the transition phases of each movement direction (phases II and IV, respectively; Fig. 4, A and B) compared with controls. Other studies of cyclic leg movements have shown a similar impaired phasing of paretic leg muscle activity poststroke, leading to abnormal movement transitions (Kautz and Brown 1998; Schindler-Ivens et al. 2004). This study extends previous work by showing the magnitude of the reduced agonist activity and the degree of impaired phase modulation are dependent on activity of the other leg: deficits are most impaired during antiphase patterned movements, slightly better during inphase patterned movements, and the least impaired during unilateral movements.
Overall, what conclusions can be drawn regarding the effect of nonparetic sensorimotor activity, and particularly the interlimb coordination mode, on the motor behavior of the paretic ankle? Our results suggest clearly that activity of the nonparetic ankle influences the motor output of the paretic leg but only in the antiphase pattern and not in the inphase pattern. Here we have reported only the effects on the paretic side, but our anecdotal evidence suggests the nonparetic leg is similarly affected by paretic side sensorimotor activity. The general idea that activity on one side of the body can affect the contralateral side is well accepted, having been shown in the upper and lower extremities, in single-joint as well as whole limb movements, and in healthy and neurologically impaired individuals (Baldissera et al. 1991; Byblow et al. 1994; Dietz et al. 1994; Ferris et al. 2004; Harris-Love et al. 2005; Kautz et al. 2002; Kelso 1984; Kelso and Jeka 1992; Ting et al. 1998). However, the effects of contralateral activity, including whether it assists or hinders motor performance, depend on a number of factors, including the specific task and the degree of effort required. For example, in the current paradigm, healthy subjects showed no effects of contralateral activity or interlimb coordination mode whatsoever. This may be because the task was relatively simple and slow for these subjects. We also observed no significant differences between the unilateral and bilateral inphase conditions in the stroke group, even though it was considerably more effortful for these individuals. Previous studies have also shown that pacing frequency affects interlimb coordination in healthy adults (Baldissera et al. 1991; Kelso 1984; Kelso and Jeka 1992). It is possible that we may have observed different performance patterns across conditions if we had allowed subjects to use their preferred frequency instead of a fixed frequency. Average performance on all of the principle variables of interest (both kinematics and EMG) indicated either a slight but nonsignificant degradation or no difference at all between inphase and unilateral performance. Therefore at a minimum, we can conclude that the inphase condition offers no immediate improvement of stroke subjects' ankle motor performance. This result is in contrast to other studies that have reported a performance advantage associated with bilateral arm movements after stroke (Cunningham et al. 2002; Harris-Love et al. 2005; Rose and Winstein 2005) when performed in a bilateral mode compared with unilateral mode. These studies and ours report single-session performance changes only and cannot be taken as evidence for or against specific training interventions. In fact, several bimanual upper extremity training protocols have been initiated for recovery of arm movements after stroke (Luft et al. 2004; Mudie and Matyas 1996, 2000; Whitall et al. 2000).
Few studies have compared lower extremity coordination patterns after stroke, and those that have largely focused on comparing EMG patterns in unilateral versus antiphase activities. During reclined pedaling, EMG magnitudes of some of the large, biarticular leg muscles are increased when performed bilaterally (antiphase cycling) compared with unilaterally (Kautz and Patten 2005). Similarly, adults with chronic complete spinal cord injury show enhanced reflex responses (Onushko and Schmit 2007) or locomotor-like EMG patterns (Kawashima et al. 2005) in the legs during passive bilateral hip movements compared with unilateral. Generally, these results have been interpreted as evidence that existing spinal circuits controlling the predominant antiphase pattern for locomotion are either selectively recruited or disinhibited. Interestingly, however, the additional muscle activation associated with antiphase pedaling does not translate to a behavioral improvement. Instead, the movement itself actually degrades, by producing significantly more negative work, or greater forces opposing the pedal upstroke (Kautz and Brown 1998; Kautz and Patten 2005). In this study, in contrast to the inphase condition, moving the nonparetic ankle in an antiphase mode strongly degraded paretic ankle movements compared with unilateral performance. For the EMG in particular, we saw a consistent decrease in EMG amplitudes (although this was not significant) of agonist muscle activity (TA, phase II; MG, phase IV) and EMG phase modulation across the three conditions, with the most abnormal performance occurring during the antiphase condition. The tasks used in these other studies differ substantially from ours, most notably in terms of the additional components of body weight loading, involvement of multisegmental coordination, and hip movement. Therefore these tasks might be considered to more closely resemble human locomotion than our task, which is an isolated, voluntary, and distal single-joint movement. However, it is interesting that our paradigm and these others show similar findings: subjects with chronic stroke and hemiparesis do not improve paretic limb control from any enhanced muscle activity but rather experience temporary performance decrements, indicating that these EMG changes, whether increases or decreases, are maladaptive.
The current paradigm does not allow us to determine specific mechanism(s) responsible for the exacerbation of deficits during antiphase movements in persons with chronic stroke, but other evidence points to several possibilities. For instance, antiphase patterns may be more impacted by impaired spinal inhibitory mechanisms, which have been well described in chronic stroke (Crone et al. 2000, 2003; Delwaide and Oliver 1988; Lamy et al. 2009; Mazevet et al. 2003). Antiphase deficits might also be attributed to abnormal interhemispheric interactions between motor cortices. Previous upper extremity studies have shown that, after stroke, the inhibition from lesioned to nonlesioned hemisphere becomes weaker (Butefisch et al. 2008; Murase et al. 2004). Because antiphase motor patterns probably require strong amounts of interhemispheric inhibition in both directions to suppress an apparent inclination toward bilateral activation of homologous muscles on both sides, this could contribute to functional performance deficits during intended antiphase movements. Changes in inhibitory mechanisms within a hemisphere could also play a role, such as reduced intracortical inhibition in the lesioned (Liepert et al. 2000b) and the nonlesioned hemisphere (Liepert et al. 2000a; Shimizu et al. 2002) after stroke. Recent evidence suggests that healthy adults produce less intracortical inhibition during synchronous (inphase) bimanual wrist movements compared with asynchronous wrist movements (in this case, interlimb phase difference of 60°; Stinear and Byblow 2002). If inphase movements involve less cortical inhibition, the disinhibited state of one or both cortices after stroke may therefore be better capable of executing inphase patterns compared with antiphase. In summary, the interlimb coordination deficits observed in this study could reflect altered control mechanisms at any number of sites. Also note that, because very little is known about cortical interactions controlling lower extremity movements, we base our comments primarily on evidence from upper extremity research, which ultimately may not hold true for leg movements.
Conclusions
We showed that individuals with poststroke hemiparesis have greater motor deficits during voluntary antiphase ankle movements compared with either unilateral or inphase movements, whereas healthy subjects perform all patterns equally well. Thus stroke results in impairments of one or more components of the neural circuitry involved in lower extremity interlimb coordination. The specific vulnerability of the antiphase pattern to exaggerated motor deficits could contribute to functional deficits in a number of leg movement tasks that are reliant on this coordination mode, including walking. Future studies should address the specific neural mechanisms associated with this form of poststroke lower extremity interlimb coordination deficit.
GRANTS
This study was supported by National Institutes of Health Grants K01 HD-050369 and M01 RR016500 to the University of Maryland General Clinical Research Center and the University of Maryland School of Medicine Office for Research and Graduate Studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank S. Hartman for assistance with data collection and L. Cohen and J. Whitall with helpful discussion about this project.
REFERENCES
- Bailey et al., 2004.Bailey MJ, Riddoch MJ, Crome P. Test-retest stability of three tests for unilateral visual neglect in patients with stroke: Star Cancellation, Line Bisection, and the Baking Tray Task. Neuropsychol Rehabil 14: 403–419, 2004 [Google Scholar]
- Baldissera et al., 1991.Baldissera F, Cavallari P, Marini G, Tassone G. Differential control of in-phase and anti-phase coupling of rhythmic movements of ipsilateral hand and foot. Exp Brain Res 83: 375–380, 1991 [DOI] [PubMed] [Google Scholar]
- Brown et al., 1997.Brown DA, Kautz SA, Dairaghi CA. Muscle activity adapts to anti-gravity posture during pedalling in persons with post-stroke hemiplegia. Brain 120: 825–837, 1997 [DOI] [PubMed] [Google Scholar]
- Butefisch et al., 2008.Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inibhition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008 [DOI] [PubMed] [Google Scholar]
- Byblow et al., 1994.Byblow WD, Carson RG, Goodman D. Expressions of asymmetries and anchoring in bimanual coordination. Hum Mov Sci 13: 3–28, 1994 [Google Scholar]
- Crone et al., 2003.Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003 [DOI] [PubMed] [Google Scholar]
- Crone et al., 2000.Crone C, Johnsen LL, Nielsen J. Reciprocal inhibition in hemiplegic patients–a longitudinal study. Suppl Clin Neurophysiol 53: 187–191, 2000 [DOI] [PubMed] [Google Scholar]
- Cunningham et al., 2002.Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychol (Amst) 110: 321–337, 2002 [DOI] [PubMed] [Google Scholar]
- Delwaide and Oliver, 1988.Delwaide PJ, Oliver E. Short-latency autogenic inhibition (IB inhibition) in human spasticity. J Neurol Neurosurg Psychiatry 51: 1546–1550, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz and Berger, 1984.Dietz V, Berger W. Interlimb coordination of posture in patients with spastic paresis. Impaired function of spinal reflexes. Brain 107: 965–978, 1984 [DOI] [PubMed] [Google Scholar]
- Dietz et al., 1994.Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994 [DOI] [PubMed] [Google Scholar]
- Dyrek, 1994.Dyrek DA. Assessment and treatment planning strategies for musculoskeletal deficits. In: Physical Rehabilitation: Assessment and Treatment, edited by O'Sullivan SB, Schmitz TJ. Philadelphia, PA: FA Davis, 1994, p. 61 [Google Scholar]
- Ferris et al., 2004.Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord 42: 14–23, 2004 [DOI] [PubMed] [Google Scholar]
- Folstein et al., 1975.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975 [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer et al., 1975.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Harris-Love et al., 2005.Harris-Love ML, McCombe Waller S, Whitall J. Exploiting interlimb coupling to improve paretic arm reaching performance in people with chronic stroke. Arch Phys Med Rehabil 86: 2131–2137, 2005 [DOI] [PubMed] [Google Scholar]
- Kautz and Brown, 1998.Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain 121: 515–526, 1998 [DOI] [PubMed] [Google Scholar]
- Kautz et al., 2002.Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Mutability of bifunctional thigh muscle activity in pedaling due to contralateral leg force generation. J Neurophysiol 88: 1308–1317, 2002 [DOI] [PubMed] [Google Scholar]
- Kautz and Patten, 2005.Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol 93: 2460–2473, 2005 [DOI] [PubMed] [Google Scholar]
- Kautz et al., 2006.Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol 95: 3154–3163, 2006 [DOI] [PubMed] [Google Scholar]
- Kawashima et al., 2005.Kawashima N, Nozaki D, Abe MO, Akai M, Nakazawa K. Alternate leg movement amplifies locomotor-like muscle activity in spinal cord injuried persons. J Neurophysiol 93: 777–785, 2005 [DOI] [PubMed] [Google Scholar]
- Kelso, 1984.Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol 246: R1000–R1004, 1984 [DOI] [PubMed] [Google Scholar]
- Kelso and Jeka, 1992.Kelso JA, Jeka JJ. Symmetry breaking dynamics of human multilimb coordination. J Exp Psychol Hum Percept Perform 18: 645–668, 1992 [DOI] [PubMed] [Google Scholar]
- Kelso et al., 1979.Kelso JA, Southard DL, Goodman D. On the nature of human interlimb coordination. Science 203: 1029–1031, 1979 [DOI] [PubMed] [Google Scholar]
- Lamy et al., 2009.Lamy JC, Wargon I, Mazevet D, Ghanim Z, Pradat-Diehl P, Katz R. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 132: 734–748, 2009 [DOI] [PubMed] [Google Scholar]
- Lewis and Byblow, 2004.Lewis GN, Byblow WD. Bimanual coordination dynamics in poststroke hemiparetics. J Mot Behav 36: 174–188, 2004 [DOI] [PubMed] [Google Scholar]
- Liepert et al., 2000a.Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 23: 1761–1763, 2000a [DOI] [PubMed] [Google Scholar]
- Liepert et al., 2000b.Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol 111: 671–676, 2000b [DOI] [PubMed] [Google Scholar]
- Luft et al., 2004.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. J Am Med Assoc 292: 1853–1861, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masakado et al., 2005.Masakado Y, Kagamihara Y, Takahashi O, Akaboshi K, Muraoka Y, Ushiba J. Post-activation depression of the soleus H-reflex in stroke patients. Electromyogr Clin Neurophysiol 45: 115–122, 2005 [PubMed] [Google Scholar]
- Mayston et al., 1999.Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol 45: 583–594, 1999 [DOI] [PubMed] [Google Scholar]
- Mazevet et al., 2003.Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain 126: 988–1000, 2003 [DOI] [PubMed] [Google Scholar]
- Mudie and Matyas, 1996.Mudie MH, Matyas TA. Upper extremity retraining following stroke: effects of bilateral practice. J Neuro Rehab 10: 167–184, 1996 [Google Scholar]
- Mudie and Matyas, 2000.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil 22: 23–37, 2000 [DOI] [PubMed] [Google Scholar]
- Murase et al., 2004.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004 [DOI] [PubMed] [Google Scholar]
- Nelles et al., 1998.Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke 29: 1182–1187, 1998 [DOI] [PubMed] [Google Scholar]
- Onushko and Schmit, 2007.Onushko T, Schmit BD. Reflex response to imposed bilateral hip oscillations in human spinal cord injury. J Neurophysiol 98: 1849–1861, 2007 [DOI] [PubMed] [Google Scholar]
- Roerdink et al., 2007.Roerdink M, Lamoth CJ, Kwakkel G, van Wieringen PC, Beek PJ. Gait coordination after stroke: benefits of acoustically paced treadmill walking. Phys Ther 87: 1009–1022, 2007 [DOI] [PubMed] [Google Scholar]
- Rose and Winstein, 2005.Rose DK, Winstein CJ. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil 19: 452–462, 2005 [DOI] [PubMed] [Google Scholar]
- Shimizu et al., 2002.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125: 1896–1907, 2002 [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens et al., 2004.Schindler-Ivens S, Brown DA, Brooke JD. Direction-dependent phasing of locomotor muscle activity is altered post-stroke. J Neurophysiol 92: 2207–2216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen et al., 2000.Steenbergen B, Van Thiel E, Hulstijn W, Meulenbroek RGJ. The coordination of reaching and grasping in spastic hemiparesis. Hum Mov Sci 19: 75–105, 2000 [Google Scholar]
- Stinear and Byblow, 2002.Stinear JW, Byblow WD. Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. J Physiol 543: 307–316, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen, 2002.Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3: 348–359, 2002 [DOI] [PubMed] [Google Scholar]
- Ting et al., 1998.Ting LH, Raasch CC, Brown DA, Kautz SA, Zajac FE. Sensorimotor state of the contralateral leg affects ipsilateral muscle coordination of pedaling. J Neurophysiol 80: 1341–1351, 1998 [DOI] [PubMed] [Google Scholar]
- Walter et al., 2001.Walter CB, Swinnen SP, Dounskaia N, Van Langendonk H. Systematic error in the organization of physical action. Cogn Sci 25: 393–422, 2001 [Google Scholar]
- Weinstein, 1993.Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. J Hand Ther 6: 11–22, 1993 [PubMed] [Google Scholar]
- Whitall et al., 2000.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 31: 2390–2395, 2000 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 1987.Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil 68: 98–102, 1987 [PubMed] [Google Scholar]
- Winstein and Pohl, 1995.Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res 105: 163–174, 1995 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2009.Wu CY, Chou SH, Chen CL, Kuo MY, Lu TW, Fu YC. Kinematic analysis of a functional and sequential bimanual task in patients with left hemiparesis: intra-limb and interlimb coordination. Disabil Rehabil 31: 958–966, 2009 [DOI] [PubMed] [Google Scholar]