Abstract
In freely moving rats that are actively performing a discrimination task, single-unit responses in primary somatosensory cortex (S1) are strikingly different from responses to comparable tactile stimuli in immobile rats. For example, in the active discrimination context prestimulus response modulations are common, responses are longer in duration and more likely to be inhibited. To determine whether these differences emerge as rats learned a whisker-dependent discrimination task, we recorded single-unit S1 activity while rats learned to discriminate aperture-widths using their whiskers. Even before discrimination training began, S1 responses in freely moving rats showed many of the signatures of active responses, such as increased duration of response and prestimulus response modulations. As rats subsequently learned the discrimination task, single unit responses changed: more cortical units responded to the stimuli, neuronal sensory responses grew in duration, and individual neurons better predicted aperture-width. In summary, the operant behavioral context changes S1 tactile responses even in the absence of tactile discrimination, whereas subsequent width discrimination learning refines the S1 representation of aperture-width.
INTRODUCTION
The rat whisker system has been a productive arena in which to study the relationship between sensory coding and neuronal plasticity. This is partly because of the somatotopic map of the whiskers that is present at multiple levels of the trigeminal system (Fox 2008). This map has facilitated the study of the effects of sensory deprivation (Faggin et al. 1997; Feldman and Brecht 2005; Nicolelis et al. 1993), overstimulation (Welker et al. 1992), and enriched environments (Polley et al. 1999) on sensory processing (for review, see Feldman and Brecht 2005).
The organization of the barrel field has also aided the study of the effects of topographic organization on learning. For instance, successful generalization of the performance of a one-whisker behavioral task depended on the distance between the cortical representation of the original and novel whisker used in the task (Harris et al. 1999).
On the other hand, single-whisker stimulation is likely to be rare in the rat's natural environment, and not all variables are encoded in a topographic fashion. For instance, the representation of radial distance along the whiskers is ethologically important but is not known to be coded topographically (Krupa et al. 2004; Szwed et al. 2006). Rather, information about radial touch location, or aperture width, is distributed across the whisker representation in somatosensory cortex (S1) (Krupa et al. 2004). Therefore studying the S1 representation of aperture width may reveal unique principles of coding multiwhisker stimuli.
In a previous study of aperture-width coding (Krupa et al. 2004), we found that S1 neural responses while rats actively discriminate apertures of different widths (active responses) are quite different from responses to similar stimulation of immobilized rats that are awake or anesthetized in a neutral behavioral context (passive responses). These active responses were generally more diverse, characterized by longer duration (hundreds of milliseconds) responses, a high incidence of response inhibition, and anticipatory firing rate modulations that began before whisker stimulation. In contrast, the passive responses were relatively stereotyped phasic excitations lasting 10–50 ms. Based on this previous work, we hypothesized that top-down modulations of S1 were needed to account for the shaping of active response profiles.
Clearly, there are many differences between the active and passive contexts that could account for the different tactile responses observed in rat S1. Rats engaged in an active discrimination task move voluntarily and receive reward for correct performance. Passively stimulated rats, on the other hand, are immobile and likely less attentive to stimulus values because the stimulus is not associated with any rewards. We do not know if the active response profile in S1, and the associated top-down inputs to S1, depend on voluntary movement during the task, reward and motivational state, or tactile discrimination itself. Nor it is known whether active response profiles of neurons in the rat S1 emerge as the rats learn the task. To address this question we recorded single-neuron responses in layer V of the S1 in rats throughout the time it took these animals to learn an aperture-width discrimination task.
Whereas rats often whisk their vibrissa rhythmically in air or across object surfaces, they typically do not whisk while performing the aperture-width discrimination task, and in fact, can discriminate successfully even when the motor nerves that control whisker movements are cut after they learn the task (Krupa et al. 2001). Nevertheless, it is possible that the rats' behaviors change as they learn the task. To assess this possibility, we analyzed high-resolution digital video of rat behavior before and after they learned the aperture-width discrimination task.
METHODS
Behavioral discrimination task
We trained rats to discriminate between a “wide” and “narrow” aperture using only their large facial whiskers (see Krupa et al. 2001 for detailed description of the discrimination task, training procedures, and behavioral apparatus). Briefly, the discrimination task with well-trained rats proceeded as follows on each trial. At the start of each session, we placed rats in the outer reward chamber (Fig. 1). The first trial of a session began when the computer opened the sliding door between the outer reward chamber and the inner reward chamber. Rats quickly moved toward the center nose poke in the back of the reward chamber. In doing so, rats disrupted an infrared reward in front of the variable-width aperture and, immediately after, their large facial whiskers contacted the aperture. On breaking the infrared photobeam and sampling the aperture, rats backed out into the reward chamber.
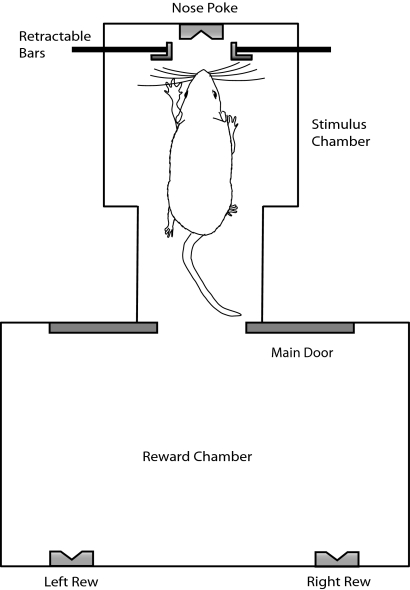
Fig. 1.
Schematic of the aperture-width discrimination task. On each correct trial, the distance between 2 retractable bars is set to a narrow or wide value, the main door opens, the rat enters the stimulus chamber, and samples the aperture with its facial whiskers and retreats to the left reward port if the aperture was narrow or the right reward port if the aperture was wide.
To receive a water reward, rats had to poke their nose into the left nose poke if the aperture was narrow (54 mm) and the right nose poke if the aperture was wide (78 mm). The aperture settings of 54 and 78 mm are relatively easy; rats are capable of significantly finer discriminations (Krupa et al. 2001). Rats received a 50-μl water reward for a correct response; no reward was delivered for an incorrect response. Immediately after rats poked into either the correct or incorrect reward nose poke, the center door between the chambers was closed, and the aperture was randomly reset to either wide (78 mm) or narrow (54 mm). The next trial began 8–30 s later when the center door was again opened. Wide and narrow trials were presented randomly throughout each ∼75-min training session. The average number of trials performed during our recorded behavior sessions was 175. Rats well trained on the discrimination were typically correct on >75% of trials.
Training proceeded in three phases. In phase 1, rats simply learned to receive water from the reward holes in the outer reward chamber. In phase 2, rats learned to enter the discrimination chamber and poke their noses into a hole on the rear wall before retreating to receive reward from alternating holes in the outer reward chamber, regardless of what aperture stimulus was presented. In phase 3, reward was contingent on correct discrimination of aperture-width as described in the previous paragraph. This study examined neural responses in rats from phase 2 (before discrimination training) through phase 3 (during discrimination training).
Rats performed the task in complete darkness to eliminate the use of visual cues. Previous experiments showed that rats use only their large facial whiskers to perform the discrimination and that SI cortex is necessary for accurate discrimination (Krupa et al. 2001). Behavior was monitored using infrared cameras and video recordings to insure that rats were motivated and following relatively stereotypical motor trajectories as observed in previous studies.
Once trained to phase 2 in the task, rats were removed from water restriction for ≥4 days, followed by surgical implantation of arrays of microwire recording electrodes over the whisker barrel region of SI cortex bilaterally (see Surgery). After ≥7 days of postsurgical recovery, rats were returned to water restriction and the behavioral task, and S1 single unit activity was recorded at phase 2 and subsequent stages of learning the discrimination.
Surgery
Details of surgery and recording procedures are as published elsewhere (Wiest et al. 2007). Briefly, surgery was performed under sodium pentobarbital anesthesia to implant rats with pairs of 32-electrode arrays (2 × 16) bilaterally in S1. Electrodes were 37-μm-diam tungsten microwires separated from neighboring wires by 250 μm. We positioned the arrays with a stereotax to record in cortical layer 5 (3.0 mm caudal from bregma, 5.5 mm mediolateral, and 1.5 mm depth from brain surface). Rats were given ≥7 days after surgery to recover.
Electrophysiology
Single-unit (SU) neural activity was recorded through a Multi-neuron Acquisition Processor (MAP; Plexon, Dallas, TX) using electrode arrays built in-house. Data were digitized at 40 kHz, and we initially sorted units on-line during each recording session to define unit waveforms on each channel with spikes (∼2 SD or more) above background noise. These on-line–sorted units may include spikes from multiple neurons. To establish an SU, we further sorted units off-line according to their clustering in the principle component space representing the spike waveforms. Those units that showed a distinct cluster in principle component space from the cluster of noise waveforms and displayed <0.1% of interspike intervals (ISIs) within a refractory period of 1 ms are termed SUs.
Analysis of neuronal data
SU RESPONSE ANALYSIS.
We constructed peristimulus time histograms (PSTHs) for each SU using 50-ms bins, with 0 defined as the time at which the rat's snout broke a beam between the bars of the stimulus aperture. We identified significant firing rate modulations of SUs from PSTHs using a method based on the statistical distribution of cumulative-summed spike counts (Wiest et al. 2005). This approach takes into account the statistics of all the spiking activity up to a given moment, as opposed to considering each bin independently. Additionally, the method requires no smoothing of the PSTH data nor any assumption of a particular parametric form of the spiking statistics. Moreover, the method is able to identify multiple excited and inhibited response modulations within a single PSTH. We used a bootstrapped empirical distribution of prestimulus firing to find the poststimulus bin (if any) at which the cumulative-summed poststimulus spike count was in the 1st or 99th percentile of the baseline distribution (Martinez and Martinez 2002). We recorded this bin as the onset of an excited or inhibited response.
The response offset was defined as the first 0 crossing of the derivative of the cumulative deviation from the baseline spike count, meaning that the cumulative sum was no longer deviating from its expected growth with time. This procedure identified the time at which the PSTH returned to a baseline firing rate. The magnitude of the response was quantified as the number of excess spikes (or, for inhibited responses, the deficit in the number of spikes) compared with the baseline expected number, divided by the number of trials. The duration of a significant response is defined as the number of significantly modulated bins between response onset and offset, multiplied by the bin-width.
APERTURE-WIDTH TUNING CURVES.
To calculate aperture-width tuning curves for each stimulus (wide and narrow), we first determined the mean number of spikes in a 1-s window centered at the time of the aperture stimulus beam break. We include time before the beam break in the response integration window because rats' whiskers sometimes make contact with the aperture stimulus before their nose breaks the beam across the aperture. The value of the tuning curve for aperture-width i is defined as
where ri is the mean response to stimulus i.
We examined whether the aperture-width tuning curves become steeper in the trained animals. For each neuron, we call the stimulus that evoked a larger response the preferred stimulus and the other aperture the nonpreferred stimulus. A neuron's preferred response (PR) is its mean response ri to its preferred stimulus, whereas its nonpreferred response (NR) is its response to its nonpreferred stimulus. The slope of a neuron's tuning curve is directly proportional to the difference between PR and NR, so we use the difference (PR − NR) to examine how receptive field slopes change with learning. Note that, using this measure, the slope of the tuning curves will always be positive, as by definition PR > NR.
χ2 ANALYSIS FOR COMPARING FREQUENCY DISTRIBUTIONS.
We used a χ2 analysis to determine whether latency histograms or distributions of wide- and narrow-preferring tuning curves differed significantly in different conditions. Because histograms in any pair of different conditions could have different total numbers of events (i.e., neural responses, tuning curves), violating the requirements for a χ2 analysis (Zar 1999), we generated count histograms assuming equal totals from normalized frequency distributions. For each pairwise comparison, we conservatively set the total number of responses in each histogram to the smaller of the two totals from that pair. For example, to compare the two-bin histogram h1 = [50 50] containing 100 counts to another, h2 = [22 27], containing 50 counts, we would rescale the first by one half, making h1 = [25 25]. The χ2 analysis would proceed using h2 and the rescaled h1. This is a conservative procedure because scaling to the smaller number of events reduces the statistical power of the test, so that a larger experimental effect is required to reach significance.
SINGLE-TRIAL CLASSIFICATION.
We examined how accurately neuronal firing patterns differentiated between the stimuli using a nonparametric method for statistical pattern recognition called learning vector quantization (LVQ) (Krupa et al. 2004; Laubach et al. 2000). The algorithm classifies single-trial neuronal firing patterns as being evoked by either a wide or narrow aperture. The percentage of correct predictions indicates how well the neuron discriminates the stimuli; technically, it measures the area of overlap of the two distributions of responses to the two stimuli (Thomson and Kristan 2005). We carried out LVQ in Matlab (Mathworks) using the Matlab Neural Network Toolbox in which LVQ is implemented as an artificial neural network (ANN). In the studies described here, we used a modified version of the Matlab algorithm to implement optimized LVQ (OLVQ) (Kohonen 1997; Krupa et al. 2004), which adaptively adjusts the learning rate during training. For further details, see the on-line supplement to Krupa et al. (2004).
We constructed single-trial perievent histograms (100-ms bins) for the epoch starting 1,000 ms before to 1,500 ms after the time when the rats sampled the tactile stimuli. The data sets were divided into training and testing subsets. The training data were used to initialize the OLVQ network by setting the coefficients for each competitive neuron (i.e., unit in the 2nd layer of the network) dedicated to a given type of trial (i.e., wide or narrow trials) equal to the mean neuronal ensemble response for that type of trial (plus a small random noise term). In this study, each network had four competitive neurons (twice the number of stimuli to be classified). We used leave-one-out cross-validation (i.e., iteratively used all but 1 trial as training data and tested performance of the OLVQ network on a single “hold-out” trial) to estimate error rates for each data set. Neuronal discrimination of aperture-width was quantified in terms of percentage of single trials classified correctly compared with expected chance levels of classification of 50% correct.
We used a moving window analysis to assess the time course of predictive accuracy of the wide and narrow apertures. OLVQ was applied sequentially to a 500-ms epoch of neuronal activity that “moved” in 100-ms steps through the 1-s epochs before and after the time of whisker contact. This analysis produces a continuous quantitative readout of the recorded neuron's ability to distinguish between the wide and narrow apertures.
In 3 of 38 sessions, the moving window OLVQ analysis showed better-than-chance predictions based on neural activity >500 ms before the stimulus beam-break. These anomalous results could be caused by fluctuations in the neural data, predictive electrical artifacts during stimulus preparation while the stepper motors moved the apertures to the correct locations in the box, or light leakage into the behavior chamber allowing rats to see the stimulus before whisker contact. Units from these three sessions (1 phase 2 session including 34 units, 2 phase 3 sessions including 49 units) were not included in the results shown in Fig. 6, but including them did not change our basic results.
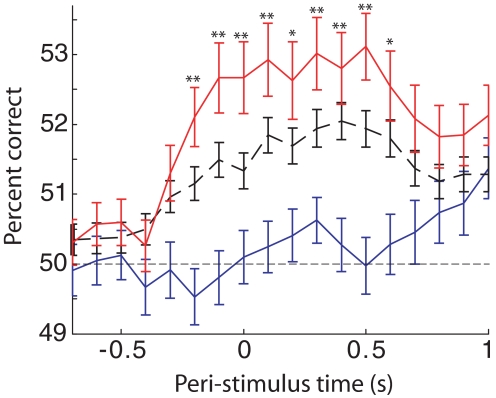
Fig. 6.
S1 single-unit single-trial stimulus predictions improve with learning. The average percent of trials correctly classified as narrow or wide stimulus trials is plotted as a function of time relative to the stimulus beam-break at t = 0, for all prediscrimination units (phase 2, solid blue curve), for all units during discrimination training sessions (phase 3, dashed black curve), and for phase 3 units from sessions with 75% or greater correct behavioral performance (P3 > 75%, solid red curve). The classification algorithm attempts to predict the stimulus on each trial based on each neuron's firing during a 0.5-s window. Performance is plotted at the start of each window, so better than chance performance can begin ≤500 ms before t = 0. Error bars denote SE based on the average over units. Asterisks indicate result of statistical comparison between the population of learning vector quantization (LVQ) results from neurons in P3 > 75% sessions and phase 2 sessions (*P < 0.005, **P < 0.0005; two-sided t-test).
Aperture whisker stimuli delivered to anesthetized rats
To approximate in anesthetized rats the whisker stimulus during the aperture discrimination task, a movable aperture (same dimensions as the aperture in the active discrimination) was swept across the rat's whiskers in a manner that simulated the whisker deflections that occurred during the active discrimination. Specifically, we matched the velocity of the moveable aperture to the speed of the rats' movement during the discrimination task, and pseudorandomly varied the trajectory from trial to trial to reproduce “lateral jitter” observed by video analysis of rats performing the active discrimination. This resulted in whisker deflection dynamics that replicated the whisker deflections that occurred during active discrimination (for details, see Krupa et al. 2004, especially their Fig. 2B and on-line supplement). In anesthetized recording experiments, we repeated each stimulus width 200 times at randomized intertrial intervals between 2 and 4 s. For the PSTHs, we took the reference time (0) as the time at which we sent the signal to the moveable aperture. Rats were anesthetized with ketamine (30 mg/kg) and xylazine (2 mg/kg).
Fig. 2.
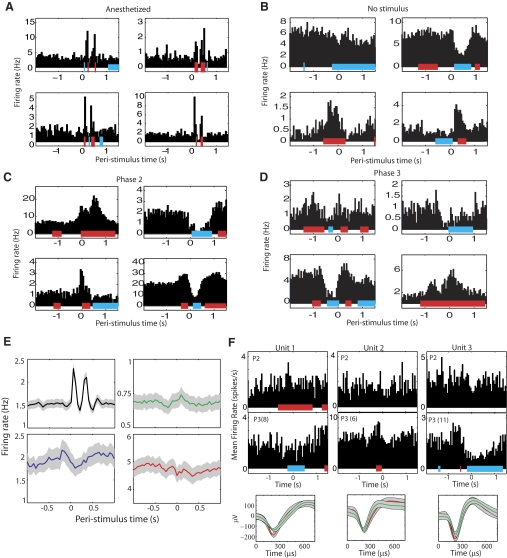
Typical single unit responses. Colored bars beneath each response histogram indicate periods of significantly increased (red) or decreased firing (blue). A: single-unit responses recorded in anesthetized rats in response to moving aperture that reproduces the stimulus in awake behaving rats. The dual peaks reflect onset and offset responses. B: responses recorded before discrimination training, with no aperture stimulus presented (the no stimulus case). Robust, diverse response modulations persist in somatosensory cortex (S1) despite the absence of whisker stimulation around t = 0. C: responses during 2nd phase of training, when a stimulus is present in the box but the rats are not required to discriminate aperture width. D: responses during phase 3 in a rat that successfully performed the aperture-width discrimination task. E: mean poststimulus time histograms (PSTHs) ± SE in anesthetized animals (black curve), in the absence of the aperture stimulus during phase 2 of training (green curve), before discrimination training, phase 2 (blue curve), and during discrimination training, phase 3 (red curve). The relatively flat average during the active task conditions (green, blue, red curves) reflects the diversity of active responses and contrasts with the stereotyped, stimulus-locked phasic passive responses recorded in anesthetized animals (black curve). Note the difference in y-axis limits. F: examples of changes in PSTHs of individual neurons. Top row: single-unit PSTHs from 3 different units recorded during phase 2 of training. Second row: PSTHs from the same units as in the 1st row, with the units recorded during phase 3 [P3(N) indicates the PSTH is from day N of phase 3 training]. Third row: mean ± SE of the action potential for the units in phase 2 (red trace) and phase 3 (green trace). The amplitude of the waveforms is smaller on later days of training, but we were able to trace this change in waveform shape over the course of training the animals.
Video acquisition
We captured image sequences at relatively high spatial resolution (∼150 μm/pixel) at 50 frames/s (SI-1300M-H-CL, Silicon Imaging). We analyzed behavior in four rats without electrophysiological recordings. We placed the camera in the discrimination chamber above the path of the rat's movement. To increase the contrast between the dark whiskers and the background, we inserted a matte white acrylic floor panel into the discrimination chamber. To increase brightness, we placed six infrared LEDs in the ceiling of the chamber (8 in above the floor). We fine-tuned the focus of the camera by hand over several sessions before beginning the actual recording. Individual whiskers were clearly visible on most frames (for an example, see Fig. 7A).
Fig. 7.
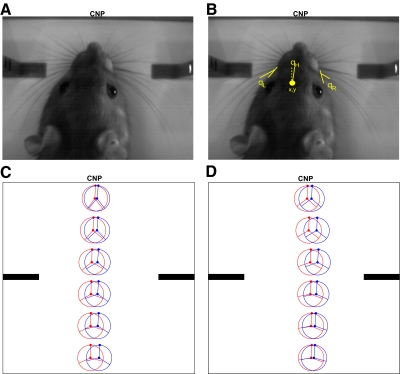
Quantifying rat behavior during learning. A: image from the discrimination chamber as the rat approaches the center nose poke (CNP) at the back of the chamber. B: the same image as in A, with the 5 behavioral variables shown. C and D: the mean behavioral trajectory before (red) and after (blue) learning. The filled circle in the center of the ring is the mean position of the rat's head, the line connecting a circle's center and edge represents the mean orientation of the rat's head, and the left and right lines represent the mean angle of the left and right whiskers, respectively, with respect to the rat's face. C: the mean trajectory for the rat with the most significant change in trajectory over learning (P < 0.001), whereas D shows the trajectories for the rat with the least significant trajectory change (P = 0.05).
Video analysis
REPRESENTING THE BEHAVIOR.
For each video frame, we extracted six variables: the head x-position (x), head y-position (y), left whisker angle with respect to the face (θL), right whisker angle (θR), and head bearing (θH) (for an example, see Fig. 7B). For whisker angles, we calculated the angle between the whiskers in arc 1 (the arc of whiskers just anterior to the Greek whiskers) and the posterior edge of the rat's face. On each trial, we extracted these data from all the frames starting when the rat entered the chamber until its nose entered the central nose poke in the rear of the chamber (between 6 and 27 frames). We also recorded the first frame in which the monitored whiskers contacted the discriminanda.
We extracted the data from the image sequences in a semiautomated way using custom-written Matlab code (Mathworks, Natick, MA).
ANALYSIS OF TRAJECTORIES.
To prepare for analyzing the behavioral trajectory, we first conditioned the data so that each trial could be represented by the same number of data points. In each trial, we binned the y-axis into six bins of 200 pixels (∼30 mm), and for each trial, calculated the mean of the behavioral variables within each bin (Fig. 7C).
We denote the mean behavior in bin i with a behavior vector b(i), where i varies from one to six. Quantitatively, b(i) = [μx(i), μθL(i), μθR(i), μθH(i)], where μZ(i) indicates the mean value of Z within bin i. We exclude the y-variable from b because the y-values were the same on each trial, because that is the dimension along which we binned the data. A trial can be fully represented by concatenating the behavior vectors b(1) through b(6), leaving us with a 1 × 24 element vector associated with each trial. The trials from all sessions were represented with an Nx24-element behavior matrix B for each rat, where N is the number of trials over all sessions (including prelearning and postlearning phase), and each row contains the six behavior vectors from each trial.
In 4% of the trials, there were missing data points. These had various causes, such as the rat moving so quickly in the y-direction that it “skipped” a bin. Hence, before running principal components analysis (PCA), we filled in the missing values using imputation algorithms (Troyanskaya et al. 2001). Using benchmark data without missing values, we found that the k-nearest neighbor (Troyanskaya et al. 2001) imputation algorithm minimized the mean-squared error between the estimates and actual data for all variables except head x-location, for which Bayesian PCA (Oba et al. 2003) provided the best fit. We implemented k-nearest neighbor using the knnimpute function in Matlab's Bioinformatics toolbox (Mathworks).
To reduce the dimensionality of B, we applied PCA to the data using Laplace's method to determine how many components to include (Minka 2000).
TESTS FOR INDEPENDENCE OF BEHAVIORAL TRAJECTORY AND LEARNING PHASE.
To determine whether a rat's behavioral trajectory was independent of learning phase, we used a classifier to predict the phase of learning based on the principal component scores (also known as loadings) extracted from the full behavior matrix B. We used a learning vector quantization (OLVQ)–trained ANN classifier with four hidden units, as described above in the section on single-unit activity classification. Application of the classifier yielded a confusion matrix, in this case a 2 × 2 table with actual and estimated learning phase on its two axes. For instance, the entry in row 1, column 1 included the number of prelearning trials that the classifier predicted were actually prelearning based on the behavioral performance (see Victor and Purpura 1996). We performed a χ2 test on the average confusion matrix after running the OLVQ algorithm on the same data 10 times. The P value yielded is the probability of getting that confusion matrix under the null hypothesis that rat behavior and learning phase are independent (Thomson and Kristan 2005).
ANALYSIS OF BEHAVIOR DURING INITIAL WHISKER CONTACTS.
To determine whether behavior at the time of initial left whisker contact was independent of learning phase, we used methods similar to those for the analysis of the full trajectory. Briefly, we pooled all pre- and postlearning data together into an Nx5 matrix, where each row included the data (x, y, θL, θR, θH) during whisker contact. We used LVQ to predict learning phase from the behavior at the time of whisker contact. We tested the hypothesis that behavior at the time of whisker contact was independent of learning phase as above by applying the χ2 test to the confusion matrix of the classifier. Because in general the behavior during left whisker contact was not the same as that during right whisker contact, we performed this analysis for both right and left whisker contacts.
ANALYSIS OF ANIMAL SPEED.
Binning the behavioral data as described above left out information about how quickly the rat moved through the chamber. Averaging the data within a bin could yield the same b(i) value if the rat was moving quickly through the chamber or if it sat at the same point for many frames. Hence, we also performed the above LVQ analysis on the average speed of the rat between all frames in the analysis.
ANALYSIS OF WHISKER CONTACT DURATION.
We also measured the number of frames the whiskers contacted the discriminanda during each trial. For this analysis, we counted all frames in which any whisker contacted the discriminanda, for both the right and left whiskers. To calculate the total contact duration, we multiplied the number of frames by our sampling period (20 ms), so if the whiskers contacted the discriminanda for five frames, that counted as 100 ms of contact.
RESULTS
We recorded from 907 units in layer 5 of S1 during 38 behavioral sessions in 12 rats at different phases of learning the aperture-width discrimination task. For a description of the task and the different phases of learning, see Behavioral discrimination task and Fig. 1.
Of the 38 recording sessions, 9 took place before discrimination training had begun but after exposure to the aperture stimuli (i.e., during phase 2, methods; n = 216 units). The other 29 recording sessions took place at various stages of discrimination learning (phase 3, n = 691 units). Of the nine sessions during phase 2, four were on the first day of exposure to the aperture-width stimuli (n = 123 units). Typical neural responses and average neuronal responses are shown in Fig. 2. The average number of trials performed in each recording session was 175 ± 81 (SD). The average number of trials performed did not differ significantly in the phase 2 sessions compared with the phase 3 sessions (2-tailed t-test; P = 0.47).
In three additional sessions, we also recorded 352 units in one rat trained through phase 2 but in the absence of the aperture stimulus (the no stimulus case). Also, a separate anesthetized data set includes responses from 513 S1 neurons recorded over five sessions in three anesthetized rats (Aperture whisker stimuli delivered to anesthetized rats).
In what follows, we first describe results from our electrophysiological data and then the results of high-speed videography of a separate group of four rats before and after discrimination training.
Prediscrimination responses
As described in the Introduction, our goal was to characterize the origin of the differences that we previously observed between active and passive responses (Krupa et al. 2004). Before the rats began to discriminate between aperture widths (i.e., during phase 2), S1 responses already qualitatively fit the active response profile rather than the stereotyped phasic excitation characteristic of passive responses.
First, the average duration of responses recorded during phase 2 training was relatively long. Excitatory response durations lasted 430 ± 30 (SE) ms, whereas the same stimuli delivered to the whiskers of awake head-fixed animals evoked mean excitatory response durations of 48.8 ± 5 ms (as reported and described in Krupa et al. 2004). This was a significant difference (P = 3.1 × 10−10; 1-tailed t-test). Inhibitory responses lasted 400 ± 30 ms during phase 2 compared with 12.4 ± 1.8 ms in head-fixed animals (Krupa et al. 2004). This was a significant difference (P = 1 × 10−13; 1-tailed t-test).
Second, the percentage of inhibited responses was large compared with the percentage of inhibited responses in the passive animal (42% inhibited responses in phase 2 animals compared with 7% in the anesthetized animals from Krupa et al. 2004; a significant difference; P < 1 × 10−13; z-test for one proportion; Moore and McCabe 1993). Third, we observed the prestimulus response modulations characteristic of the active responses rather than responses locked to stimulus time, which is always observed in the passive case.
On the first day of exposure to aperture stimuli, excited neural responses were greater in magnitude and duration than in subsequent phase 2 exposure (P = 0.02 and 0.003 for magnitude and duration, respectively; 2-tailed t-test). Table 1 shows the specific values of the variables. This suggests that phase 2 training sessions extinguished the salience of the aperture stimuli until discrimination training began in phase 3.
Table 1.
Summary of S1 single unit response statistics at different phases of discrimination training
| Phase 2 Day 1, 123 Units | Phase 2 All, 216 Units | Phase 3 <75% Correct, 485 Units | Phase 3 >75% Correct, 206 Units | |
|---|---|---|---|---|
| Percent responding | 63 | 53 | 70 | 70 |
| Percent inhibited | 28 | 42 | 43 | 30 |
| Excited response durations, s | 0.58 ± 0.05 | 0.43 ± 0.03 | 0.56 ± 0.03 | 0.72 ± 0.04 |
| Inhibited response durations, s | 0.44 ± 0.07 | 0.40 ± 0.03 | 0.51 ± 0.03 | 0.52 ± 0.05 |
| Percent excited durations > 0.5 s | 37 | 27 | 42 | 49 |
| Percent inhibited durations > 0.5 s | 26 | 23 | 36 | 37 |
| Excited magnitudes, spks/trial | 17 ± 3 | 6.9 ± 0.9 | 27 ± 3 | 23 ± 3 |
| Inhibited magnitudes, spks/trial | 8 ± 2 | 10 ± 2 | 32 ± 5 | 18 ± 4 |
Values are means ± SE. S1, Somatosensory cortex. The overall trend was for response durations and magnitudes to grow over the course of discrimination training. Phase 2 Day 1 refers to the first day of exposure to the aperture stimuli, with no discrimination training. Phase 2 All refers to all sessions before any discrimination training (phase 2), Phase 3 <75% Correct indicates discrimination training sessions when the rat correctly discriminated fewer than 75% of trials (phase 3), and Phase 3 >75% Correct refers to sessions when the rat correctly discriminated at least 75% of trials (phase 3). Percent responding, the percentage of units that significantly modulated their firing rate relative to the time of a beam break between the bars of the aperture stimulus; Percent inhibited, the percentage of inhibited responses, i.e. suppressions of firing; Excited response durations, the average duration of excited responses in seconds; Inhibited response durations, the average duration of inhibited responses in seconds; Percent excited durations > 0.5 s, the percentage of excited responses that lasted longer than 0.5 s; Percent inhibited durations > 0.5 s, the percentage of inhibited responses that lasted longer than 0.5 s; Excited magnitudes, the average magnitude of excited responses in spikes per trial; Inhibited magnitudes, the average magnitude of inhibited responses in spikes per trial.
Surprisingly, similar response modulations occurred in S1 neurons during the execution of the task in the absence of the aperture stimuli (Fig. 2, C and D). In one rat, we recorded data from 352 units when no stimulus was present (the no stimulus case described at the beginning of this section) over three sessions. Even in the absence of whisker stimulation, 50% of units significantly modulated their firing rates around t = 0 defined as a photobeam. This proportion of units is not statistically different from the 53% of units that responded when the aperture stimulus was present but before discrimination training (phase 2; P = 0.66; z-test for 2 proportions) and was less than the 70% of units that responded during discrimination training (phase 3; P = 3.0 × 10−10; z-test for 2 proportions). These results are consonant with a previous observation that, even when rats whiskers were cut, there were still significant response modulations during performance of the aperture-width discrimination task (Krupa et al. 2004).
Change in responses with discrimination training
Task-related response modulations evolved over the course of discrimination learning. As mentioned in the previous section, the percentage of units responding increased from 53 to 70% once discrimination training began (i.e., phase 2 vs. phase 3). This is a significant increase in the proportion of units responding (P < 10−5; z-test for 2 proportions).
RESPONSE DURATIONS.
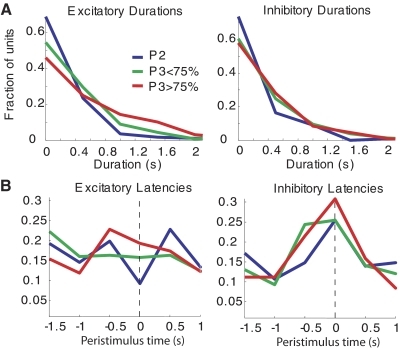
The most salient change in response properties was the general increase in response durations over the course of discrimination training. Figure 3A shows the distribution of response durations during learning for both excited and inhibited responses. The average excited response duration grew from 430 ± 30 ms during phase 2 (P2: before discrimination training) to 560 ± 30 ms at an intermediate stage of discrimination learning (P3 < 75%: phase 3 with <75% correct discriminations) and further to 720 ± 40 ms at a late stage of discrimination learning (P3 > 75%: phase 3 with 75% correct or higher). Each of these changes was significant at a 99% confidence level (unpaired 2-tailed t-test, P < 0.01). The increase in inhibited response durations was less pronounced, but the change from an average duration of 400 ± 30 ms during phase 2 to 510 ± 30 ms during phase 3 was also significant (P < 0.01; t-test).
Fig. 3.
S1 response latency and duration distributions before discrimination training (P2, blue curves), at an intermediate stage of learning (P3 < 75%, green curves), and after reaching a criterion of 75% correctly discriminated trials (P3 > 75%, red curves). A: distribution of response durations for excited (left) and inhibited (right) response modulations. B: distribution of latencies (relative to the aperture beam break at t = 0) of excited (left) and inhibited (right) response modulations.
Interestingly, on the first day of exposure to the aperture stimuli (phase 2 day 1 in Table 1), excited response durations were longer than in the data from subsequent phase 2 sessions. On the first day of phase 2 training with aperture stimuli, excitatory response durations averaged 580 ± 50 ms, which is significantly longer than the durations in the remaining phase 2 sessions (430 ± 30 ms; P < 0.01; t-test).
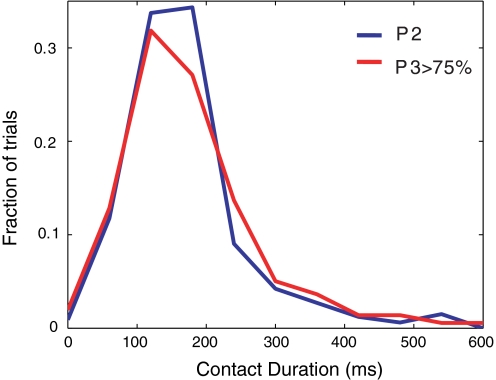
To determine whether longer response durations observed at more advanced stages of training were driven by longer whisker contact times, we directly compared the duration of whisker contacts with the discriminanda before (phase 2: n = 12 sessions) and after learning (phase 3: n = 13 sessions) using video analysis (Fig. 4). We found that contact durations were statistically indistinguishable in the two phases (170.1 ± 4.8-ms contact duration in phase 2 and 175.4 ± 5.1-ms duration in phase 3; P = 0.23; 1-sided t-test of hypothesis that mean contact duration for phase 3 is larger than for phase 2). For further discussion of analysis of video data, see Analysis of behavior.
Fig. 4.
Distributions of whisker contact durations. Comparison of relative frequency of time of contact between whiskers and discriminanda in phase 2 (blue: n = 4 rats; 346 total trials) and phase 3 (red: n = 4 rats; n = 374 total trials) animals. Phase 3 rats were above criterion in performance (i.e., >75% correct).
RESPONSE LATENCIES.
In addition to response durations, response latency distributions also changed with learning. The distributions of excited and inhibited response latencies are shown in Fig. 3B. We used a χ2 statistic to compare latency distributions in different conditions as described in methods. The χ2 analysis shows a significant difference between the prediscrimination P2 and P3 < 75% distributions of excited latencies (degrees of freedom = 5, P = 0.05). The change in the distribution of excited latencies from P3 < 75% to the advanced stage P3 > 75% is also significant (P = 0.01). For the inhibited response latency distributions, the only difference that reaches significance is between the prediscrimination stage P2 and the advanced discrimination stage P3 > 75% (P = 0.03). The quantitative trend for all comparisons is for latencies to shift closer to t = 0 at later stages of training. That is, as rats learn the task, the neuronal response onset times tended to cluster more at the time the rat broke the photobeam on the aperture.
RESPONSE MAGNITUDES.
As expected based on the general increase in response durations with discrimination learning, response magnitudes also grew significantly from phase 2 to phase 3. The average excited response grew significantly from 6.9 ± 0.9 spikes/trial during phase 2 to 27 ± 3 spikes/trial during intermediate phase 3 (t-test, P < 0.01). Similarly, inhibited responses grew significantly from an average of 10 ± 2 fewer spikes/trial to 32 ± 5 fewer spikes per trial (t-test, P < 0.01). On the first day of exposure to the aperture stimuli, inhibited response magnitudes did not differ from the rest of phase 2, but excited response magnitudes were significantly greater (17 ± 3 vs. 6.7 ± 0.9 spikes/trial, P < 0.01; t-test), consistent with the increased excited durations on the first day of exposure to the stimuli, noted above.
In contrast to the general trend toward longer response durations with further discrimination learning during phase 3, excited response magnitudes remained constant or decreased as rats progressed during phase 3 of learning. Excited response magnitudes were not significantly different during P3 < 75% and P3 > 75% (t-test; P > 0.05). Also, inhibited response magnitudes decreased significantly from 32 ± 5 to 18 ± 4 fewer spikes/trial (t-test, P < 0.01).
Changes in aperture-width tuning curves
A simple way for single neurons to encode aperture-width is to fire action potentials at different rates in response to the narrow and wide apertures. Hence, we calculated aperture-width tuning curves for each recorded neuron, normalizing the response to each stimulus to the sum of the responses to both stimuli (see methods). The mean single-unit aperture-width tuning curves for phase 2 (prediscrimination) and phase 3 (discrimination training) are shown in Fig. 5A.
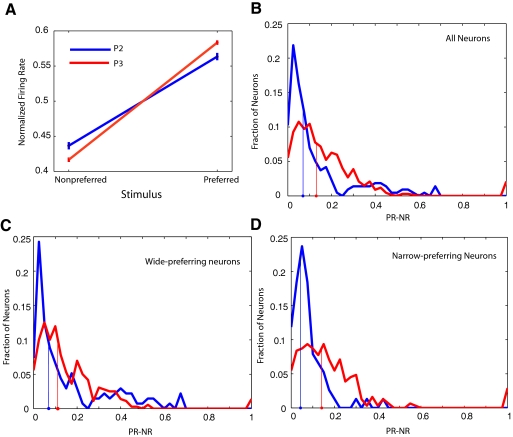
Fig. 5.
S1 single-unit aperture-width tuning curves from behaving rats get steeper with discrimination training. A: average tuning curve ± SE for phase 2 (blue) and phase 3 (red) animals. The difference between the 2 responses is greater in phase 3. B: frequency histogram of tuning-differences for all neurons recorded in phase 2 (blue) and phase 3 (red). The abscissa is proportional to the slope of the tuning curve for aperture-width. The median response-difference over all cells is indicated by the filled circles on the abscissa. C: same histogram as in B, with the analysis restricted to wide-preferring neurons. The median response-difference changes very little between phase 2 and phase 3. D: when the analysis is restricted to narrow-preferring neurons, the change in the slope of the tuning curves is significantly different in phase 2 (blue) vs. phase 3 (red).
As discussed in more detail in methods, we call the stimulus that evokes a larger response the preferred stimulus and the corresponding response the preferred response (PR). Conversely, the stimulus that evokes a smaller response is the nonpreferred stimulus that evokes the nonpreferred response (NR). We use the response-difference (PR − NR) as a convenient measure of the slope of each tuning curve (see methods). This difference varies between 0 (preferred and nonpreferred responses are identical) and 1.0 (the nonpreferred response is 0).
The median response-difference changed from 0.067 in phase 2 to 0.13 in phase 3, a significant difference (P ∼ 10−10; exact median test; Hutson 2007). This indicates that the slope of the S1 tuning curves was significantly different in the two phases of learning. Figure 5B shows the distribution of response-differences in all neurons, with the medians indicated on the abscissa. Note that we examined differences in median values because the distributions were highly skewed.
In contrast to what has been observed in the trigeminal ganglion (Szwed et al. 2006), a significant proportion of S1 neurons preferred the wide stimulus. Specifically, 63% of the neurons recorded during phase 2 tuning responded more to the wider aperture compared with 52% during phase 3. This marked a significant decrease in the fraction of units that prefer the wide aperture (i.e., more wide-preferring neurons; χ2 degrees of freedom = 1, P = 0.002; see methods) with discrimination training.
We repeated our analysis of changes in RF slope with learning, restricting our analysis to the 495 wide-preferring neurons in our dataset. As seen in Fig. 5C, in wide-preferring neurons, the median slope difference was smaller between phase 2 and phase 3 (medians, 0.07 and 0.11, respectively); this difference approached significance (P = 0.03, exact median test) because of our large sample size [note we used an α level of 0.016 based on the Bonferonni correction (Abdi 2007) for 3 comparisons].
When we restricted our analysis to the 386 narrow-preferring neurons in our dataset (Fig. 5D), we observed that the median response-difference increased from 0.06 to 0.14 as rats learned the task, a highly significant change (P ∼ 10−13; exact median test). As seen in Fig. 5D, the distribution of response-differences is clearly shifted to the right for the neurons recorded during phase 3, and it is likely this is the subset of neurons that contributed most to the observed changes in the pooled dataset (Fig. 5B).
Analysis in anesthetized rats
Because the naïve rats (phase 1) in our data set were already trained in a (nondiscriminative) task to obtain water reward, we did not expect their motivational state to differ from the rats trained in the discrimination task. Supporting this, the number of trials that rats performed during a session did not vary significantly before and after training in the aperture-width discrimination task (discussed in results).
However, it is still possible that behavioral or motivational changes during learning contributed to changes in neuronal response properties (see Behavioral analysis). To examine neuronal responses independently of behavior, attention, and general arousal, we recorded S1 responses to aperture-width stimuli over five sessions in three anesthetized rats (see Aperture whisker stimuli delivered to anesthetized rats). One of the rats had never been exposed to the aperture stimuli (anesthetized phase 2 data set, n = 274 units), and the other two rats had achieved performance >80% correct during waking discrimination sessions (anesthetized phase 3 data set, n = 239 units).
Using the same methods as for the awake rats, we constructed aperture-width tuning curves for all the recorded units. The fraction of wide- and narrow-preferring tuning curves did not vary significantly between anesthetized rats in phase 2 versus phase 3 (χ2 test, degrees of freedom = 1, P = 0.3). There was no significant difference in the median tuning curve slopes in phase 2 versus phase 3 animals (P = 0.9; exact median test).
Single-unit single-trial stimulus predictions improve with learning
To what extent could changes in neuronal response properties with discrimination learning actually inform successful behavioral discriminations? To address this question, we used a classification algorithm (OLVQ, see methods) that predicts the stimulus on each trial based on a single neuron's firing during that trial. The percent of trials correctly classified by the algorithm gives a measure of discrimination performance that could be supported by this neuron's firing. Figure 6 shows that mean single-unit stimulus discrimination in S1 neurons increases over the course of discrimination training. Hence, changes in S1 single-neuron responses could contribute to improvements in the animal's discrimination performance with learning.
We chose to analyze single-unit predictions instead of ensemble stimulus predictions to facilitate comparison across phase 2 and phase 3 sessions in which different numbers of units were recorded. Note that, although the improvement in average single unit stimulus predictions to 53% correct with learning does not approach the animals' behavioral discrimination performances in excess of 75% correct, this reflects the distributed nature of the representation of aperture width across S1 neurons. However, with population sizes of order 20 or fewer S1 neurons, OLVQ performance better than 70% is typical within several hundred milliseconds of stimulus contact (see, for example, Fig. 7 of Pantoja et al. 2007; and Supplementary Fig. S1 of Krupa et al. 2004). These population performances indicate that, although the average single unit carries relatively little information about aperture width, nonredundant information from relatively few neurons can add up to levels sufficient to account for the animal's behavior.
Analysis of behavior
As described in methods, we acquired video data from four rats to determine whether the rats' behavior changed as they learned the task (Fig. 7). From each image, we extracted the position of the center of the rat's head, the direction in which the head was oriented, and the angle of the left and right whiskers with respect to the head, data that we represented as a behavioral vector b (Fig. 7B; see methods). We acquired behavioral data from three sessions before the rats learned the task and three after they learned the task. We captured the images at 50 Hz, and each pixel covered ∼150 μm2. An individual rat whisker is ∼100 μm in diameter at its base (personal observations).
Qualitatively, the movement of the rat's whiskers was the same as described previously (Krupa et al. 2001). Namely, the rats entered the chamber with their whiskers protracted and moved quickly to the rear of the chamber, retracting their whiskers as they approached the central nose poke (the mean whisker angle on entering the chamber was 56° and just before entering the nose poke was 28°, a significant difference: P < 0.001, 2-sample t-test with unpooled variance estimates). Also, as observed before, during the task, the rats usually did not perform whisking motions that are common in other behavioral contexts (some whisking occurred in ∼15% of the trials in rats both before and after learning). This is consistent with previous results showing that rats' performance on the discrimination is unaffected by cutting the motor facial nerve (Krupa et al. 2001).
Our main goal with the video analysis was to determine whether the rats' behavior changed while learning the task. We looked at this question using two different measures of behavior: the full behavioral trajectory and the behavior at the time of whisker contact with the discriminandum.
First, we examined the full trajectory of the rats' behavior before and after learning. We binned the data along the y-direction and represented the rat's behavior in each bin as the mean behavioral vector within that bin (Fig. 7C). We used an ANN classifier to estimate learning phase based on behavioral trajectory and applied a χ2 test applied to the classifier output to test the hypothesis that behavior and learning are independent (see methods).
Because there were four independent comparisons, the α level was 0.05/4 = 0.0125 (see methods). In two of the four rats, we found strong evidence against the null hypothesis that behavior and learning are independent. In a third rat, the P value was exactly 0.05, so it approached significance. The mean behavioral trajectories before and after learning for the rats with the lowest and highest P values (∼1 × 10−6 and 0.25, respectively) are shown in Fig. 7, C and D. Typically the changes were in the average position of the rats' heads.
We restricted our analysis to the initial video frames in which the whiskers contacted the discriminanda. Specifically, for each rat, we applied the same ANN-based analysis to test the null hypothesis that the rats' behavior during contact was independent of learning phase. We performed this analysis for both left and right whisker contacts (see Behavioral analysis). By this measure, only one rat showed a significant dependence of behavior on the learning phase (P < .001 for both left and right whisker contact). This was the same rat that showed the most significant dependence of behavioral trajectory on learning phase. Post hoc regression analysis of individual variables on learning phase with this rat showed that its mean head orientation was different before and after learning [P < 0.001 for both left F(68,2) and right F(54,2) whisker contacts], and the y-position of its head during left whisker contact was different before and after learning [P < 0.001; F(68,2)]. Specifically, it acquired the habit of aiming its head to the right as it sampled the discriminandum, and its head was located ∼5 mm farther along the y direction after learning.
Because binning the data does not show how quickly the rats run through the discrimination chamber, we also examined whether the speed of the rats depended on learning phase. We calculated their mean speed through the discrimination chamber on each trial, and used the same LVQ analysis described above to examine the null hypothesis that speed is independent of learning phase. One of the four rats showed a significant dependence of speed on learning phase (P = 0.006, χ2 test). This was the same rat that showed a significantly different behavioral trajectory and behavior during whisker contact. On learning the task, its average speed decreased from 825 to 667.5 mm/s.
DISCUSSION
Our goal was to determine whether previously observed differences between S1 neuronal responses in active versus passive contexts, as discussed in the Introduction, emerged over the course of training on a tactile discrimination task. By recording single-neuron activity in S1 layer V at different phases of learning, we found that the qualitative active tactile response profile (characterized by long duration responses, inhibited responses, and prestimulus responses) was already present before the start of discrimination training as the rat navigated the behavioral chambers during phase 2 of the task. We also observed significant changes in S1 neural response properties over the course of discrimination learning.
Active profile of prediscrimination responses
Even before the rats had learned to discriminate aperture-width using their facial whiskers (i.e., during phase 2 of training), S1 neurons exhibited larger and longer responses and more inhibited and anticipatory responses than during passive stimulation of the awake head-fixed rat. These results suggest that many of the gross differences between active and passive responses of neurons in the infragranular layers of S1 barrel cortex are caused by performance of a rewarded task in an active context. This was reinforced by our observation of similar neuronal responses in the absence of the aperture stimuli, during trials in which the rats received water reward simply for entering the discrimination chamber in which no stimulus was actually presented (Fig. 2B).
The diverse neuronal tactile responses we observed in these active conditions in the absence of whisker-guided discrimination could be driven by inputs from motor areas during locomotion, or they could be driven by expectations of reward from other areas. Although motor activity can facilitate tactile responses (Lee et al. 2008), others have shown behavioral suppression of tactile responses (Coulter 1974; Ghez and Lenzi 1971; Shin and Chapin 1989, 1990). Such motor-related modulation could account for the increased incidence of response-suppression in the active context compared with passive stimulation.
Other studies have shown that reward expectation can shape neural activity in primary sensory areas (Gavornik et al. 2009; Hui et al. 2009; Ifuku et al. 2006; Pantoja et al. 2007; Pleger et al. 2008; Shuler and Bear 2006), so the active response profile we observed before discrimination training could result from reward-related inputs to S1. In general reward-related inputs may interact with movement-related inputs to generate a diversity of neural responses in S1.
Response duration
Although the qualitative differences between active and passively stimulated whiskers are present even before the rat learns the task, we did subsequently observe effects of learning on the neuronal tactile responses recorded in the rat S1 cortex. There was a further increase in the percentage of responsive neurons, response durations, and magnitudes once the stimuli became relevant for the behavioral task.
Interestingly, on the first day of exposure to the aperture stimuli, neural response magnitudes and durations were greater than in subsequent exposure without discrimination training. This finding suggests that the novelty of the stimuli on the first day of exposure drives increases in response magnitude and duration, whereas their salience is extinguished by further training sessions in which the stimuli are unrelated to reward.
Studies of visual perceptual learning indicate that neural response magnitudes in primary visual cortex tend to be relatively large in early phases of learning but decrease in overtrained experts who have learned to process the relevant stimuli more efficiently (Little and Thulborn 2006; Yotsumoto et al. 2008). Although our results seem to contradict these findings, it is possible that the animals we studied did not reach asymptotic overtrained performance during the period when we recorded their S1 neural responses.
Response latency
We observed a shift of excited response latencies closer to the time of whisker stimulation. This shift may be another indication of the increased salience of the aperture stimulus as the discrimination task is learned. Similarly, we observed a clustering of inhibited latencies around the time of whisker stimulation as discrimination learning progressed. This clustering may reflect a trend toward increasing the signal to noise ratio for important stimuli (i.e., the aperture stimulus) by reducing background activity around the time an important stimulus is expected (Butovas and Schwarz 2007). This latency shift may be caused by intrinsic mechanisms, behavioral sampling strategy, or both.
Stimulus estimation
S1 single-neuron responses predicted the stimuli with significantly greater accuracy after the rats reached criterion on the discrimination. This may be partly caused by the increase in the steepness of width tuning curves that we observed at advanced stages of training.
To determine whether tuning changes were specific to the awake state, we also measured width tuning curves in anesthetized rats before discrimination training and after reaching criterion (>75% correct in phase 3). We did not observe a learning-related increase in the steepness of width tuning curves in the anesthetized animals. We can understand this result in terms of the involvement of feedback projections from higher-order brain regions in learning-related improvements to the S1 representation of aperture-width. That is, it is likely that top-down inputs to S1 evolved with learning to increase the steepness of aperture-width tuning curves to optimize discrimination performance. However, because anesthesia tends to specifically reduce the activity of feedback projections (Alkire et al. 2008), we might expect these changes in steepness to be less pronounced or absent in our anesthetized data set. In addition, some of the changes observed in awake animals between phase 2 and phase 3 may be caused by adaptation of behavioral strategies during learning. Because the rats had undergone operant conditioning before discrimination training (phase 2) and because the number of trials performed by rats did not vary significantly with discrimination training, we consider it unlikely that changes in arousal or motivational state account for the observed learning-related tuning changes.
Top-down inputs to S1 and the role of motor refinements
We hypothesized that the response changes we observed during learning are caused by changes in sensory processing resulting from alterations in top-down inputs to S1. Alternatively, the changes in tactile responses could be caused by changes in the rats' behavior, leading to changes in tactile inputs.
For example, longer response durations might be explained by longer sampling of the aperture stimuli by well-trained rats. However, the fact that the duration of whisker contacts with discriminanda did not change with discrimination training (Fig. 4) argues against this possibility. As for other behavior changes, although the difference between trajectories before discrimination training and after reaching criterion was subtle (e.g., a head-location change of 5 mm; Fig. 7), shifts in average trajectory and whisker configuration could possibly contribute to the observed changes.
Also, cutting the facial nerve of rats trained in the task, abolishing all whisker movements relative to the face, leads to no appreciable drop in behavioral performance (Krupa et al. 2001), suggesting that subtle whisker movements cannot be the sole basis of increased neuronal discrimination of the stimuli. Similarly, tactile neural responses recorded during discrimination in the peripheral trigeminal ganglion of a well-trained rat were transient and phase-locked to the stimulus (E. Thomson and M. C. Wiest, unpublished observations), suggesting that the increased duration of variable latency responses in S1 was not driven solely from the periphery. Hence, it is unlikely that motor refinements account for all of the discrimination learning-related response plasticity that we observed, but further experiments involving simultaneous videography and neuronal recordings would help resolve these questions conclusively.
Conclusions
The classical feed-forward hierarchical picture of the cortex portrays primary sensory cortices as context-independent relays for purely sensory information. However, there is a growing consensus that this is an inaccurate portrait. For example, primary sensory cortices have been found to be involved in working memory (Harris et al. 2001), memory retrieval (Goldberg et al. 2006), reward processing (Gavornik et al. 2009; Hui et al. 2009; Ifuku et al. 2006; Pantoja et al. 2007; Pleger et al. 2008; Shuler and Bear 2006), and task-specific neural plasticity based on classical and operant conditioning (Bao et al. 2004; Butt et al. 2009; Crist et al. 2001; Polley et al. 2004, 2006; Recanzone et al. 1993; Schoups et al. 2001; Weinberger 1998, 2007; Weinberger and Bakin 1998; Weinberger et al. 1993). Similarly, our previous work has shown that S1 responses to identical stimuli critically depend on the behavioral state of the animal (Fanselow and Nicolelis 1999; Krupa et al. 2004; Nicolelis and Fanselow 2002). This study extends such results, showing that infragranular S1 generates qualitatively different neuronal responses in a voluntarily moving animal engaged in goal-directed behavior compared with a passively immobilized animal, even in the absence of stimulus discrimination. Moreover, we observed refinements of the S1 representation of aperture width over discrimination learning that may partly account for improvements in the animals' behavioral discrimination performance.
GRANTS
This work was supported by National Institutes of Health Grants NS-055522 to E. Thomson and R01 DE-011451 to M.A.L. Nicolelis.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institute of Dental and Craniofacial Research, or National of Institutes of Health. No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank G. Lehew and J. Meloy for assistance in developing and constructing electrodes and general expert assistance in all technical matters; J. Lou, A. McDonough, and S. Tica for assisting with the video analysis; and S.Halkiotis.
Present address of M. C. Wiest: Neuroscience Program, Wellesley College, Wellesley, MA. 02841.
REFERENCES
- Abdi, 2007.Abdi H. (Editor). Bonferroni and Šidák Corrections for Multiple Comparisons. Thousand Oaks, CA: Sage, 2007 [Google Scholar]
- Alkire et al., 2008.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Sciences NY 322: 876–880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao et al., 2004.Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci 7: 974–981, 2004 [DOI] [PubMed] [Google Scholar]
- Butovas and Schwarz, 2007.Butovas S, Schwarz C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. Eur J Neurosci 25: 2161–2169, 2007 [DOI] [PubMed] [Google Scholar]
- Butt et al., 2009.Butt AE, Chavez CM, Flesher MM, Kinney-Hurd BL, Araujo GC, Miasnikov AA, Weinberger NM. Association learning-dependent increases in acetylcholine release in the rat auditory cortex during auditory classical conditioning. Neurobiol Learn Mem 92: 400–409, 2009 [DOI] [PubMed] [Google Scholar]
- Coulter, 1974.Coulter JD. Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol 37: 831–845, 1974 [DOI] [PubMed] [Google Scholar]
- Crist et al., 2001.Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci 4: 519–525, 2001 [DOI] [PubMed] [Google Scholar]
- Faggin et al., 1997.Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci USA 94: 9428–9433, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow and Nicolelis, 1999.Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci 19: 7603–7616, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman and Brecht, 2005.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Sciences NY 310: 810–815, 2005 [DOI] [PubMed] [Google Scholar]
- Fox, 2008.Fox K. Barrel Cortex. Cambridge, UK: Cambridge, 2008 [Google Scholar]
- Gavornik et al., 2009.Gavornik JP, Shuler MG, Loewenstein Y, Bear MF, Shouval HZ. Learning reward timing in cortex through reward dependent expression of synaptic plasticity. Proc Natl Acad Sci USA 106: 6826–6831, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez and Lenzi, 1971.Ghez C, Lenzi GL. Modulation of sensory transmission in cat lemniscal system during voluntary movement. Pfluegers 323: 273–278, 1971 [DOI] [PubMed] [Google Scholar]
- Goldberg et al., 2006.Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. J Neurosci 26: 4917–4921, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris et al., 2001.Harris JA, Harris IM, Diamond ME. The topography of tactile working memory. J Neurosci 21: 8262–8269, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris et al., 1999.Harris JA, Petersen RS, Diamond ME. Distribution of tactile learning and its neural basis. Proc Natl Acad Sci USA 96: 7587–7591, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui et al., 2009.Hui GK, Wong KL, Chavez CM, Leon MI, Robin KM, Weinberger NM. Conditioned tone control of brain reward behavior produces highly specific representational gain in the primary auditory cortex. Neurobiol Learn Mem 92: 27–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson, 2007.Hutson AD. An ‘exact’ two-group median test with an extension to censored data. J Nonparametric Stat 19: 103–112, 2007 [Google Scholar]
- Ifuku et al., 2006.Ifuku H, Nakamura T, Hirata S, Ogawa H. Neuronal activities in the reward phase in primary and higher-order gustatory cortices of monkeys. Neurosci Res 55: 54–64, 2006 [DOI] [PubMed] [Google Scholar]
- Kohonen, 1997.Kohonen T. Self Organizing Maps. Berlin: Springer-Verlag, 1997 [Google Scholar]
- Krupa et al., 2001.Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci 21: 5752–5763, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa et al., 2004.Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layer-specific somatosensory cortical activation during active tactile discrimination. Sciences NY 304: 1989–1992, 2004 [DOI] [PubMed] [Google Scholar]
- Laubach et al., 2000.Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature 405: 567–571, 2000 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2008.Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci 11: 1430–1438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little and Thulborn, 2006.Little DM, Thulborn KR. Prototype-distortion category learning: a two-phase learning process across a distributed network. Brain Cogn 60: 233–243, 2006 [DOI] [PubMed] [Google Scholar]
- Martinez and Martinez, 2002.Martinez W, Martinez AR. Computational Statistics Handbook with Matlab. Boca Raton, FL: Chapman and Hall, 2002 [Google Scholar]
- Minka, 2000.Minka TP. Automatic choice of dimensionality for PCA MIT Media LabTech Report 514, 2000 [Google Scholar]
- Moore and McCabe, 1993.Moore DS, McCabe GP. Introduction to the Practice of Statistics. New York: Freeman, 1993 [Google Scholar]
- Nicolelis and Fanselow, 2002.Nicolelis MA, Fanselow EE. Dynamic shifting in thalamocortical processing during different behavioural states. Philos Trans R Soc Lond B Biol Sci 357: 1753–1758, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis et al., 1993.Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature 361: 533–536, 1993 [DOI] [PubMed] [Google Scholar]
- Oba et al., 2003.Oba S, Sato MA, Takemasa I, Monden M, Matsubara K, Ishii S. A Bayesian missing value estimation method for gene expression profile data. Bioinformatics (Oxford) 19: 2088–2096, 2003 [DOI] [PubMed] [Google Scholar]
- Pantoja et al., 2007.Pantoja J, Ribeiro S, Wiest M, Soares E, Gervasoni D, Lemos NA, Nicolelis MA. Neuronal activity in the primary somatosensory thalamocortical loop is modulated by reward contingency during tactile discrimination. J Neurosci 27: 10608–10620, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger et al., 2008.Pleger B, Blankenburg F, Ruff CC, Driver J, Dolan RJ. Reward facilitates tactile judgments and modulates hemodynamic responses in human primary somatosensory cortex. J Neurosci 28: 8161–8168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley et al., 1999.Polley DB, Chen-Bee CH, Frostig RD. Two directions of plasticity in the sensory-deprived adult cortex. Neuron 24: 623–637, 1999 [DOI] [PubMed] [Google Scholar]
- Polley et al., 2004.Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci USA 101: 16351–16356, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley et al., 2006.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci 26: 4970–4982, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone et al., 1993.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13: 87–103, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups et al., 2001.Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature 412: 549–553, 2001 [DOI] [PubMed] [Google Scholar]
- Shin and Chapin, 1989.Shin HC, Chapin JK. Mapping the effects of motor cortex stimulation on single neurons in the dorsal column nuclei in the rat: direct responses and afferent modulation. Brain Res Bull 22: 245–252, 1989 [DOI] [PubMed] [Google Scholar]
- Shin and Chapin, 1990.Shin HC, Chapin JK. Modulation of afferent transmission to single neurons in the ventroposterior thalamus during movement in rats. Neurosci Lett 108: 116–120, 1990 [DOI] [PubMed] [Google Scholar]
- Shuler and Bear, 2006.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Sciences NY 311: 1606–1609, 2006 [DOI] [PubMed] [Google Scholar]
- Szwed et al., 2006.Szwed M, Bagdasarian K, Blumenfeld B, Barak O, Derdikman D, Ahissar E. Responses of trigeminal ganglion neurons to the radial distance of contact during active vibrissal touch. J Neurophysiol 95: 791–802, 2006 [DOI] [PubMed] [Google Scholar]
- Thomson and Kristan, 2005.Thomson EE, Kristan WB. Quantifying stimulus discriminability: a comparison of information theory and ideal observer analysis. Neural Comput 17: 741–778, 2005 [DOI] [PubMed] [Google Scholar]
- Troyanskaya et al., 2001.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics (Oxford) 17: 520–525, 2001 [DOI] [PubMed] [Google Scholar]
- Victor and Purpura, 1996.Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol 76: 1310–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Weinberger, 1998.Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem 70: 226–251, 1998 [DOI] [PubMed] [Google Scholar]
- Weinberger, 2007.Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res 229: 54–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger and Bakin, 1998.Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol 3: 145–167, 1998 [DOI] [PubMed] [Google Scholar]
- Weinberger et al., 1993.Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA 90: 2394–2398, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker et al., 1992.Welker E, Rao SB, Dorfl J, Melzer P, van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J Neurosci 12: 153–170, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest et al., 2007.Wiest M, Thomson EE, Meloy J. Multielectrode recordings in the somatosensory system. In: Methods for Neural Ensemble Recordings, edited by Nicolelis MA. Boca Raton, FL: CRC, 2007 [PubMed] [Google Scholar]
- Wiest et al., 2005.Wiest MC, Bentley N, Nicolelis MA. Heterogeneous integration of bilateral whisker signals by neurons in primary somatosensory cortex of awake rats. J Neurophysiol 93: 2966–2973, 2005 [DOI] [PubMed] [Google Scholar]
- Yotsumoto et al., 2008.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57: 827–833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar, 1999.Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1999 [Google Scholar]