Abstract
The pedunculopontine nucleus (PPN) is involved in the activated states of waking and paradoxical sleep, forming part of the reticular activating system (RAS). The studies described tested the hypothesis that single unit and/or population responses of PPN neurons are capable of generating gamma band frequency activity. Whole cell patch clamp recordings (immersion chamber) and population responses (interface chamber) were conducted on 9- to 20-day-old rat brain stem slices. Regardless of cell type (I, II, or III) or type of response to the nonselective cholinergic receptor agonist carbachol (excitation, inhibition, biphasic), almost all PPN neurons fired at gamma band frequency, but no higher, when subjected to depolarizing steps (50 ± 2 Hz, mean ± SE). Nonaccommodating neurons fired at 18–100 Hz throughout depolarizing steps, while most accommodating neurons exhibited gamma band frequency of action potentials followed by gamma band membrane oscillations. These oscillations were blocked by the sodium channel blocker tetrodotoxin (TTX), suggesting that at least some are mediated by sodium currents. Population responses in the PPN showed that carbachol induced peaks of activation in the theta and gamma range, while glutamatergic receptor agonists induced overall increases in activity at theta and gamma frequencies, although in differing patterns. Gamma band activity appears to be a part of the intrinsic membrane properties of PPN neurons, and the population as a whole generates different patterns of gamma band activity under the influence of specific transmitters. Given sufficient excitation, the PPN may impart gamma band activation on its targets.
INTRODUCTION
During activated states (waking and paradoxical sleep), electroencephalographic (EEG) responses are characterized by low-amplitude, high-frequency oscillatory activity in the gamma band range (∼20–100 Hz). Gamma frequency oscillations have been proposed to participate in sensory perception, problem solving, memory, and rapid eye movement (REM) sleep (Eckhorn et al. 1988; Gray and Singer 1989; Palva et al. 2009; Phillips and Takeda 2009; Voss et al. 2009), and it has been suggested that such coherent events occur at cortical or thalamocortical levels (Llinas et al. 1991; Singer 1993). Similar oscillations, however, also are present in the hippocampus (Whittington et al. 1997) and cerebellum (Middleton et al. 2008). The mechanisms behind such activity include the presence of inhibitory cortical interneurons that exhibit intrinsic oscillatory activity in the gamma band frequency (Llinas et al. 1991; Steriade 1999), many of which are electrically coupled (Gibson et al. 1999), as well as of fast rhythmic bursting pyramidal neurons that are also electrically coupled (Cunningham et al. 2004).
The pedunculopontine nucleus (PPN) is most active during waking and paradoxical sleep (Steriade and McCarley 1990), is part of the reticular activating system (RAS) that modulates ascending projections through the thalamus and descending projections through the pons and medulla, and is composed of different populations of cholinergic, glutamatergic and GABAergic neurons (Wang and Morales 2009). Extracellular recordings of PPN neurons in vivo identified six categories of thalamic projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital (PGO) wave generation (Steriade et al. 1990b). Some of these neurons had low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing (20–80 Hz). PPN neurons increase firing during REM sleep (“REM-on”) or both waking and REM sleep (“wake/REM-on”) but decrease during slow-wave sleep (SWS) (Datta and Siwek 2002; Sakai et al. 1990; Steriade et al. 1990a), suggestive of increased excitation during activated states. Stimulation of the PPN potentiated the appearance of fast (20- 40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s (Steriade et al. 1991). Injections of glutamate into the PPN increased waking and paradoxical sleep (Datta et al. 2001b), while injections of the glutamatergic receptor agonist N-methyl-d-aspartic acid (NMDA) increased only waking (Datta et al. 2001a), and injections of the glutamatergic receptor agonist kainic acid (KA) increased only paradoxical sleep (Datta 2002). In addition, the PPN has recently been found to exhibit electrical coupling (Garcia-Rill et al. 2007), along with groups of cells in one of its ascending targets, the parafascicular intralaminar nucleus, that indirectly participates in cortical activation, and in one of its descending targets, the subcoeruleus nucleus, that participates in paradoxical or REM sleep regulation (Garcia-Rill et al. 2008; Heister et al. 2007). These findings suggest that PPN outputs can indirectly induce gamma band activity at the level of the cortex, but does it do so by triggering such activity in its ascending targets such as the parafascicular nucleus or by driving these regions with such activity? Does the PPN have the mechanisms necessary for generating its own gamma band activity?
The PPN contains three basic cell types based on intrinsic membrane properties (Kamondi et al. 1992; Leonard and Llinas 1990; Takakusaki and Kitai 1997). Type I PPN neurons display Ca2+ mediated low threshold spikes (LTS) following a return from hyperpolarizion. Type II PPN neurons have a hyperpolarization activated potassium current (IA) that delays the return to baseline following a hyperpolarizing current step. Type III PPN neurons have both LTS and IA currents. The present study was carried out to determine if PPN neurons exhibit gamma band activity in terms of action potential frequency or membrane oscillations in single cells and if the population as a whole shows gamma band activity when pharmacologically activated.
METHODS
Slice preparation
Pups aged 7–20 days from adult timed-pregnant Sprague-Dawley rats (280–350 g) were anesthetized with ketamine (70 mg/kg im) until tail pinch reflex was absent. This age range was selected due to the developmental decrease in REM sleep of the rat that occurs between 10 and 30 days (Jouvet-Mounier et al. 1970). This period of investigation enabled sampling from a baseline period (7–12 days) as well as the epoch of the greatest transitions between 12 and 20 days as determined by our previous body of work on the PPN (Garcia-Rill et al. 2008). Pups were decapitated, and the brain was rapidly removed and cooled in oxygenated sucrose-artificial cerebrospinal fluid (sucrose-ACSF). The sucrose-ACSF consisted of (in mM) 233.7 sucrose, 26 NaHCO3, 3 KCl, 8 MgCl2, 0.5 CaCl2, 20 glucose, 0.4 ascorbic acid, and 2 sodium pyruvate. Sagittal sections (400 μm) containing the PPN were cut, and slices were allowed to equilibrate in normal ACSF at room temperature for 1 h. The ACSF was composed of (in mM) 117 NaCl, 4.7 KCl, 1.2 MgSO4, 2.5 CaCl2, 1.2 NaH2PO4, 24.9 NaHCO3, and 11.5 glucose. Some experiments were done using low Mg2+ ACSF with the following composition (in mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 20 glucose, and 24.9 NaHCO3. Slices were recorded at 30°C while perfused (1.5 ml/min) with oxygenated (95% O2-5% CO2) ACSF in either an immersion chamber for patch clamp studies or in an interface chamber for population response studies. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and were in agreement with the National Institutes of Health guidelines for the care and use of laboratory animals.
Recordings
Differential interference contrast optics was used to visualize neurons using an upright microscope (Nikon FN-1, Nikon). Whole cell recordings were performed using borosilicate glass capillaries pulled on a P-97 puller (Sutter Instrument, Novato, CA) and filled with a solution of (in mM): 124 K-gluconate, 10 HEPES, 10 phosphocreatine di tris, 0.2 EGTA, 4 Mg2ATP, and 0.3 Na2GTP. For histological purposes, some recordings were conducted with 0.02% Lucifer yellow added to the intracellular solution, while 0.3% neurobiotin (Vector Laboratories, Burlingame, CA) was used in the remaining recordings. Osmolarity was adjusted to ∼270–290 mosM and pH to 7.4. Holding potentials (HPs) were set close to resting membrane potential at −60 mV. The pipette resistance was 5–10 MΩ. All recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) in both current and voltage clamp mode. Analog signals were low-pass filtered at 2 kHz and digitized at 5 kHz using a Digidata-1440A and pClamp10 software (Molecular Devices). Our normal pipette solution is designed to mimic the normal intracellular electrolyte concentration. Within a short time after rupturing the membrane, the intracellular solution will reach equilibrium with the pipette solution. In all of our experiments, we did repetitive testing of depolarization-induced firing, and we did not find any significant rundown of the responses. This further confirmed our observation that if there is any change or rundown in the membrane properties of the neurons due to intracellular dialysis, it should have occurred in the first few minutes immediately after patching the cells.
The following intrinsic membrane properties were characterized: resting membrane potential (RMP, in spontaneously firing cells this was determined in the presence of tetrodotoxin, TTX), action potential (AP) threshold (determined by the 1st AP that appeared in I-V steps), AP amplitude, AP half-width duration, input resistance (Rin), membrane time constant (tau, determined by fitting an exponential decay curve to the initial portion of the hyperpolarizing current step that induced a hyperpolarization potential of about −40 mV), membrane capacitance, rebound inward current density (normalized to membrane capacitance, pA/pF; determined by a hyperpolarizing step from −55 to −105 mV under voltage clamp), hyperpolarization-activated cation Ih current density (determined in the same way as rebound current). The preceding values have been reported in previous studies (Ye et al. 2010), but in the present case, we specifically analyzed the responses to depolarizing steps (500 ms duration, 2.5 s intertrial interval) in current clamp mode to determine the maximal or close to maximal firing frequencies of PPN cells.
For population studies, we used a BSC-1 interface chamber (Automate Science, Berkeley, CA), and the slice was visualized with a Wild dissection microscope. Recordings were made with borosilicate glass capillaries pulled to a 1–2 μ tip with 1–2 MΩ resistance and filled with ACSF. Recordings were amplified with a Grass Instrument (Quincy, MA) P511 amplifier, filtered at 1 Hz to 1 kHz, digitized with a Digidata-1332A at a rate of 10 kHz, and stored on computer hard drive, and averages and power spectra were analyzed using pClamp10 software. Further analysis was conducted using MatLab software (The MathWorks, Natick, MA). Plots of the event related spectral perturbation (ERSP) for each population response were generated with the EEGLAB MatLab Toolbox (Delorme and Makeig 2004). An ERSP represents a measure of event related brain dynamics induced in the EEG spectrum by a stimulus or event. It basically measures average dynamic changes in amplitude of the broad band EEG frequency spectrum as a function of time relative to an experimental event (Delorme and Makeig 2004). These analyses generated power spectra for continuous points in time, e.g., during and after application of an agent or washout. These graphs plot frequency of activity over time, and the amplitude of the frequency shown is color-coded such that background (control) appears light green, and higher amplitudes appear progressively more yellow, then red. A convenient way of reading these graphs is to view a power spectrum plot of a single point in time, e.g., at minute 4 after carbachol (CAR) perfusion, such as that shown in Fig. 6A right side, as a vertical line at minute 4 in the ERSP shown in Fig. 6C. The various peaks in the power spectrum at a single time point (Fig. 6A) are represented as more intense colors at only one time point in the ERSP plot (C) and can be thought of as a running power spectrum over time.
Fig. 6.
CAR induced specific peaks at gamma frequency. A: 1 s sample data (left) and power spectrum (right) shows a 20 s recording of oscillatory activity (peaks at 17 and 34 Hz) induced by 30 μM CAR (red record), compared with control (black record) and wash (yellow record). Control, CAR, and wash were tested in 4 different slices. B: autocorrelation of CAR recording vs. control clearly shows that rhythmic oscillations were induced in PPN neurons when stimulated with CAR. C: the MatLab graph of ERSP shows that peaks at 17 and 34 Hz were induced beginning at minute 4 after the start of CAR perfusion. The effect persisted after the end of CAR perfusion (minute 10), but returned to control levels by 15 min of wash (not shown). The power spectrum in A was taken at minute 5 after the beginning of CAR application and represents a vertical sample of the ERSP at minute 5.
Drug application
Bath-applied drugs were administered to the slice via a peristaltic pump (Cole-Parmer, Vernon Hills, IL) and a three-way valve system such that solutions reached the slice after 1.5 min after the start of application. The nonspecific cholinergic agonist CAR (10–50 μM), sodium channel blocker tetrodotoxin citrate (TTX, 1–3 μM), and glutamatergic receptor agonists kainic acid (KA, 0.2–2 μM) and N-methyl-d-aspartic acid (NMDA, 1–10 μM), were all purchased from Sigma (St. Louis, MO). The input resistance was calculated by measuring the initial current produced by a hyperpolarizing voltage step from −60 to −100 mV, and depolarizing/hyperpolarizing steps used to derive an I-V curve in voltage clamp mode. The liquid-junction potential was ∼9 mV for pipettes filled with K-gluconate intracellular solution and the reported voltages were not corrected for this value.
Data analysis
Off-line analyses were performed using Clampfit software (Molecular Devices). Power spectra were composed for maximal or close to maximal firing frequency during depolarizing steps as well as membrane oscillations during the steps in accommodating cells. Analysis conditions (power spectra) for population responses consisted of 20 s windows every 1 min prior to drug application, during the peak effect, and after the agent had washed. These analyses generated power spectra for a particular point in time, e.g., at the peak of a response. Basically, amplitudes of power spectra for each group of four slices were tabulated at 0–55 Hz, and a mean of the amplitudes at each frequency calculated for each group of slices, e.g., control, neuroactive agent, wash. A repeated-measures ANOVA model was fit for each response using SAS Proc Mixed software (SAS Institute, Cary, NC). Because different concentrations and frequencies were determined in each group of slices, a covariance structure existed for measurements within groups of slices. Concentration, frequency, and concentration-by-frequency standard errors were estimated using White's empirical covariance structure estimation method. If concentration-by-frequency interaction terms for a specific response were significant at the 5% level, the focus of the differences among concentration levels was assessed according to specific levels of frequency. The Tukey approach was employed to control for multiple comparisons. F values and degrees of freedom were reported for all linear regression ANOVAs. Differences were considered significant at values of P ≤ 0.05. All results are presented as means ± SE.
RESULTS
Whole cell recordings
Whole cell patch clamp recordings were performed in PPN neurons (n = 50). Cells were held at −60 mV in current clamp and increasing depolarization current steps were used to induce gamma band frequency firing of APs (Fig. 1A). This protocol used nine current steps with an increase of 30 pA for each step. Each step was 500 ms in duration with 2.5 s interval between steps so that the records shown were truncated and spliced together to show only the current steps. The final current step was 280 pA greater than the current injection required for holding the cell at −60 mV. During the current steps, the cells were depolarized and fired APs when above threshold. Firing frequency was determined by measuring the interspike interval (ISI) between the first two, middle two, and final two APs in each current step. Higher amplitude current injections increased the amplitude of the depolarization and the frequency of APs until the cells fired maximally at gamma frequency (Fig. 1B). Only three cells (6%) fired below gamma frequency during the beginning of the stimulus. The average maximal firing frequency occurred during the beginning of the 180 pA current step when the average firing frequency of the 50 recorded neurons was 50 ± 2 Hz. Following the initial peak in firing frequency, the firing rate of these neurons decreased during the middle and end of the stimulus, but the firing rate was still within the gamma range (peak middle ISI: 31 ± 2 Hz; peak end ISI: 27 ± 2 Hz).
Fig. 1.
Gamma band activity in whole cell recorded pedunculopontine nucleus (PPN) cells. A: increasing steps of current (increase of 30 pA per step, each step was 500 ms in duration, 2.5 s latency between each step, and the record was truncated between current steps and spliced to show only the current steps) caused cells to fire action potentials at higher frequencies. This cell fired maximally at 54 Hz, which is within the gamma frequency range. B: graph showing the average firing frequency of the 50 recorded cells at the beginning (black), middle (red), and end (green) of each current step. The average maximal firing frequency was at the 180 pA current step when cells fired at the average rate of 50 ± 2 Hz at the beginning of the current step. Cell firing frequency then decreased during the middle and end of the current step, and there was no significant difference between the firing rate during the middle and end of the stimulus (ns, P > 0.05). Significant compared with baseline is represented by an asterisk when P < 0.05, double asterisk when P < 0.01, and triple asterisk when P < 0.0001. C: graph showing the average firing frequency of each cell type at the beginning, middle, and end of the 180 pA current step. At the beginning of the current step, type I neurons (n = 17) fired significantly faster than type II (n = 16) or III (n = 16) cells (asterisk, P < 0.05), but type II and III neurons did not fire significantly faster than one another (ns, P > 0.05). Furthermore, there was no significant difference between the firing frequencies of the 3 cells types during the middle and end of the current step (ns, P > 0.05).
Next the firing frequencies of type I (LTS, no IA current), II (IA current, no LTS), and III (IA current and LTS) PPN neurons were compared during the first, middle, and end ISI. During the beginning of the current injection, type I PPN neurons (n = 17) fired at higher frequencies than type II or type III PPN neurons (Fig. 1C; type 1 vs. type 2: t = 2.41, df = 31, P = 0.02; type 1 vs. type 3: t = 2.14, df = 31, P = 0.04). The firing frequencies of type II (n = 16) and III (n = 16) PPN neurons were not significantly different (t = 0.20, df = 30, P = 0.85). Furthermore, the firing frequencies during the middle and end of the stimulus were not significantly different among the three cell types (P > 0.05). Because PPN cells can either be inhibited or excited by CAR, we compared the firing frequencies of CAR inhibited and CAR excited PPN neurons and found no significant difference [CAR inhibited (n = 22) vs. CAR excited (n = 14): t = 1.54, df = 34, P = 0.13].
All cells were held at −60 mV before the start of the protocol, and each current step was consistent for each cell. However, the voltage change created by the same current step is known to vary between cells because of the differences in input resistance. Therefore it is possible that cells with a higher input resistance would have a greater change in voltage and higher firing frequency in response to the same current step than other cells with lower input resistance. However, we found that the recorded cells had a flat distribution of input resistances, and there was no correlation between maximal firing frequency and input resistance (n = 50, r = 0.23, P = 0.1). Therefore we believe comparing the firing frequency across cells during the same amplitudes of the current steps was appropriate in this experiment.
We found that 12 of the recorded neurons were accommodating and had obvious membrane oscillations following APs during the 180 pA current step. One of these 12 cells (8%) fired APs below gamma frequency and had membrane oscillations below gamma frequency. Another cell fired APs at gamma frequency, but the following membrane oscillations were below gamma frequency. The remaining 10 cells (83%) showed gamma frequency APs and membrane oscillations. Figure 2A shows an example of an accommodating cell with membrane oscillations. At a current injection of 180 pA (which was shown to be the appropriate amount of current to induce maximal firing in PPN neurons), this cell had an initial firing frequency of 80 Hz. The frequency of membrane oscillations was determined using the power spectrum function in pClamp and was found to be in the 40–50 Hz range (Fig. 2B). These membrane oscillations were blocked by application of TTX (3 μM), which blocks sodium channels (Fig. 2C). Furthermore, there was no peak in the power spectrum (Fig. 2D) following application of TTX, further indicating that the membrane oscillations were blocked. Therefore the membrane oscillations present in accommodating neurons in the PPN appeared to be sodium channel dependent and occurred at gamma frequency.
Fig. 2.
Membrane oscillations in PPN cells. A: some cells showed membrane oscillations during depolarizing current pulses. This is a PPN cell recording during a 500 ms, 180 pA current step, which caused membrane oscillations in this accommodating neuron. B: this power spectrum shows the frequency of the membrane oscillations. There was a clear peak at 35 Hz, indicating that these oscillations were in the gamma range. C: application of TTX (3 μM) blocked the membrane oscillations, indicating that they may be sodium channel dependent. D: there was no peak in the power spectrum following application of TTX, indicating that the membrane oscillations were blocked.
Because gamma frequency firing of APs and membrane oscillations could be induced in PPN neurons by depolarizing current steps, we hypothesized that gamma oscillations could also be induced by receptor agonists. To test this hypothesis, we bath applied NMDA, KA, and CAR under voltage clamp with a holding potential of −60 mV. Prior to and following application of these agents, no membrane oscillations were observed. To compare the oscillations induced by drug application to baseline, we compared the highest peak in the power spectrum between 0 and 20, 21 and 40, and 41 and 60 Hz to baseline. Application of NMDA (4 μM) significantly increased the power at gamma frequencies [Fig. 3A, blue; 21–40 (n = 4, t = −3.13, df = 6, P = 0.02) and 41–60 (n = 4, t = −9, df = 6, P < 0.01) Hz], and membrane oscillations were observed in the recording (blue record). However, NMDA did not significantly increase the power of the oscillations at lower frequencies [0–20 (n = 4, t = −2.02, df = 6, P = 0.09)]. Furthermore, KA (1 μM) significantly increased the oscillations at low frequencies, as evident in the recording and in the power spectrum [Fig. 3B, green record; 0–20 Hz (n = 4, t = −2.96, df = 6, P = 0.03)]. However, KA did not increase the oscillations in the gamma range [21–40 Hz (n = 4, t = −1, df = 6, P = 0.36) and 41–60 Hz (n = 4, t = −1, df = 6, P = 0.36)]. CAR (30 μM) increased the power of the oscillations at higher gamma band (41–60 Hz) but also showed peaks in the theta range, similar to the effects of KA (Fig. 3C, red record), but not at lower gamma band (21–40 Hz) [0–20 (n = 4, t = −4.60, df = 6, P < 0.01), 21–40 (n = 4, t = −1.63, df = 6, P = 0.15), and 41–60 Hz (n = 4, t = −2.83, df = 6, P = 0.03)].
Fig. 3.
Gamma band activity induced in PPN cells by cholinergic and glutamatergic inputs. A: power spectrum and 1 s recordings of a whole cell patched PPN neuron prior to N-methyl-d-aspartate (NMDA; black record), during the NMDA peak effect (blue record), and following wash (yellow record). Application of NMDA induced oscillations as can be seen in the blue recording. However, the oscillations were not at a specific frequency and the power was increased at almost every frequency (including gamma). B: power spectrum and 1 s recordings of a PPN cell prior to kainic acid (KA; black record), during KA peak effect (green record), and following wash (yellow record). Application of KA induced oscillations in the theta range (green record, green line in power spectrum). C: power spectrum and 1 s recordings of a PPN neuron prior to carbachol (CAR; black record) during CAR peak effect (red record), and following wash (yellow record). Application of CAR increased the power of the oscillations at almost every frequency (red line), but there were also specific peaks in the theta and gamma range.
Population responses
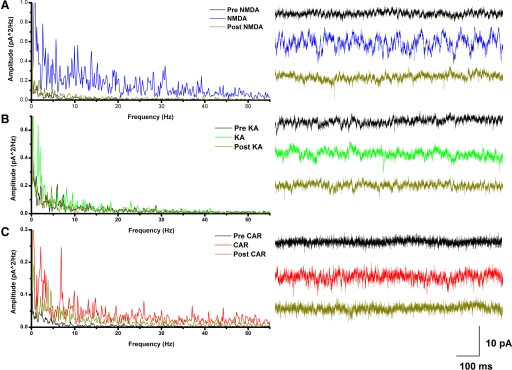
We used an interface chamber to record PPN population responses such as those that have recently been reported in cortex (Cunningham et al. 2004; Metherate and Cruikshank 1999; Sukov and Barth 2001), hippocampus (Fisahm et al. 1998), and cerebellum (Middleton et al. 2008). The electrode was inserted into the pars compacta in the posterior PPN with the tip approximately in the middle of the thickness of the slice to record from as large a number of neurons as possible. Multiple predrug control recordings were carried out to establish a background level of activity. The power spectrum of 20 s windows of control activity generally consisted of small peaks of activity at 0–5 Hz and very little, if any, activity at higher frequencies (Fig. 4).
Fig. 4.
NMDA induced dose-dependent overall increase in PPN neuron activity. A: 20 s recordings of population responses at the beginning of each minute during control (black record), 10 μM NMDA (peak activity, blue record), and wash artificial cerebrospinal fluid (ACSF), yellow record], which were used to create a power spectrum (right). One second samples of these recordings are shown to the left of the power spectrum. Control, NMDA, and wash were tested in 4 different slices. B: dose-dependent effects of NMDA shown in the power spectrum on the right that includes 20 s recordings during the peak activity of 1 μM (light blue record), 5 μM (medium blue record), and 10 μM NMDA (dark blue record). Control, NMDA at each concentration, and wash were tested in 4 different slices. One second sample data are shown on the left, revealing a step-wise increase by concentration in overall activity at almost all frequencies ranging from theta to gamma.
Population responses were recorded with a gap free continuous protocol using Clampfit software. Before any pharmacological agents were applied, a series of recordings were performed to ensure the background noise levels were consistent (<10 μV). Drug application time for all the pharmacological agents was 10 min and starting at minute 0, 20 s of population activity was recorded at the beginning of every minute. After each drug application, the slices were washed with ACSF until the background level returned to the control level.
To test the effects of glutamatergic input on the population of neurons in the PPN, we bath applied NMDA (10 μM; Fig. 4A). Recordings from before drug application (black record), during the peak effect of the drug (blue record), and following washout of the drug (yellow record) showed that 10 μM NMDA induced a significant increase in the oscillatory activity of these neurons (n = 4 different slices, t = 5.53, df = 6, P = 0.0) compared with control and washout. The power spectrum displays the marked changes in activity among the NMDA peak effect, control, and subsequent wash. To develop a dose-response curve, we tested three concentrations of NMDA (1, 5, and 10 μM; Fig. 4B). Recordings during application of 1 μM (light blue record), 5 μM (medium blue record), and 10 μM (dark blue record) showed a NMDA concentration-dependent increase in the oscillatory activity of these neurons. Furthermore, the power spectrum demonstrates that higher concentrations of NMDA increased the power of the oscillations at every frequency (theta through gamma; n = 4 different slices at each concentration, t = 4.61, df = 30, P = 0.009).
In a previous study, KA induced gamma frequency oscillations in vitro in cortical areas of the rat brain (Cunningham et al. 2004). We tested the effects of KA on PPN neurons using three concentrations (0.2, 1, and 2 μM) to determine the dose dependence of its effect. We compared the highest peaks across our 10 min recordings for each concentration, n = 4 different slices at each concentration. Results indicated that the increase in amplitude of the response was statistically significant compared with their controls for all three concentrations [0.2 μM (n = 4 different slices, t = −3.31, df = 6, P = 0.016), 1 μM (n = 4 different slices, t = −3.17, df = 6, P = 0.019), and 2 μM (n = 4 different slices, t = −6.02, df = 6, P < 0.001)] for peaks in the theta, low, and high gamma ranges. The results presented show the PPN population response using 2 μM KA (Fig. 5). Recordings prior to drug application (black record), during the peak effect of the drug (green record), and following washout of the drug (yellow record) show an increase in the oscillatory activity during application of KA (Fig. 5A; n = 4 different slices, t = 32.83, df = 6, P < 0.001). Furthermore, the power spectrum shows that KA, like NMDA, increased the overall activity but to a lesser extent and specifically induced oscillations at frequencies in the theta and gamma range (n = 4 different slices, t = 6.14, df = 6, P = 0.001). To determine if the oscillations induced by KA were rhythmic, we generated an autocorrelation using a 20 s sample of the recording during KA application and compared it to the control condition (Fig. 5B). The autocorrelation showed no rhythmic correlations in this slice. MatLab was used to generate a graph of ERSPs, using all (n = 11 time points) 20 s recordings taken during a 10 min perfusion with 2 μM KA (Fig. 5C). The peak effect of KA was between 4 and 8 min with a gradual decrease in effect in later records. During the peak effect, KA induced oscillations at almost every frequency in the spectrum (theta through gamma). The effects over time are shown in the ERSP (Fig. 5C), suggesting that specific peaks increased over time. Note the diagonally occurring increases in frequency of the darkest (highest amplitude) peaks over time, suggestive of a free-running increase in frequencies. For example, the highest peaks at minute 4 were at ∼5, 17, and 25 Hz, and each increased at minute 6 to ∼12, 28, and 45 Hz, then increased again at minute 8 to ∼25, 40, and higher frequency peaks, respectively.
Fig. 5.
KA increased overall activity while simultaneously increasing oscillations at gamma frequencies. A: 1 s sample of data (left) and power spectrum (right) of oscillatory activity generated by 2 μM KA (green record), and return of the oscillatory activity to normal background noise level after wash (ACSF, yellow record), similar to that in the control (black record) condition. One second sample recordings are shown to the left of the power spectrum. The power spectrum was taken at minute 4, which is the same as the minute 4 time point in the event related spectral perturbation (ERSP) below. Control, KA, and wash were tested in 4 different slices. B: autocorrelation of 20 s sample of oscillatory activity generated by 2 μM KA compared with the control condition did not show rhythmic correlation in this slice. C: MatLab graph of ERSP generated using all (n = 11 time points) 20 s recordings taken during 10 min perfusion with 2 μM KA that began at the start of the plot. Data showed that the peak effect of KA was between minute 4 (the minute 4 power spectrum shown in A above is equivalent to a vertical sample of the ERSP at minute 4) and minute 8 min after the beginning of drug application with a gradual reduction of activity in last few recordings (∼11–12 min). During the peak effect, KA induced oscillations at different frequencies, with power spectra showing a complex free-running rhythm. That is, peaks of activation present at low frequencies gradually increased to achieve higher frequencies, while peaks of activity at high frequencies also increased gradually to achieve even higher frequencies.
The effect of cholinergic input on the population of neurons in the PPN was tested using three concentrations of CAR. In previous studies, CAR induced different frequency oscillations in a concentration- and temperature-dependent manner in the hippocampus (Dickinson et al. 2003; Fellous and Sejnowski 2000). In this study, we applied three concentrations of CAR (10, 30, and 50 μM) to the PPN and discovered that rhythmic oscillations were induced by CAR in a concentration-dependent manner (n = 4 different slices at each concentration, t = 61.45, df = 2, P < 0.001). Results showed a dose-dependent increase in amplitude of the responses especially when low gamma frequency (20–40 Hz) was compared across the 10 min recording to baseline for each concentration [10 μM (n = 4 different slices, t = 3.16, df = 6, P = 0.019), 30 μM (n = 4 different slices, t = 4.44, df = 6, P = 0.004), and 50 μM (n = 4 different slices, t = −5.20, df = 6, P = 0.002)]. Recordings prior to 30 μM CAR application (black record), during application (red record), and following wash (yellow record) showed that CAR increased the oscillatory activity of these neurons (n = 4 different slices, t = 61.44, df = 6, P < 0.001). Furthermore, the power spectrum showed specific peaks of activity in the theta and low gamma range, which according to the autocorrelation (Fig. 6B) were rhythmic with a period of ∼15–18 Hz. The MatLab portion of this figure (Fig. 6C) illustrates PPN neuronal population responses during a 10 min period of CAR exposure and demonstrates that CAR (30 μM) induced oscillations at specific frequencies that were initiated after perfusion. Note that as CAR began to take effect at minutes 3 to 4, the peaks in the power spectrum increased at ∼15 Hz and at double this frequency at 30 Hz with somewhat less coherence at ∼45 Hz, then slowly increased at minutes 8 to 9 to ∼18 Hz and at double this frequency at 35 Hz and even higher frequencies. The effect persisted for several minutes after the beginning of wash, returning to control levels after 10–20 min of wash. Therefore unlike KA and NMDA, which increased overall activity, CAR produced specific peaks of rhythmic gamma oscillations.
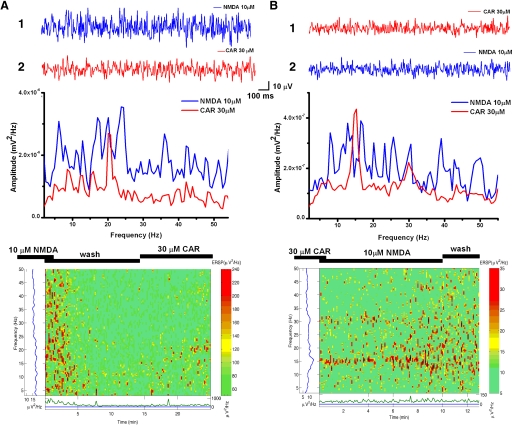
To determine the effect of sequential application of CAR and NMDA on PPN neuronal population responses, we applied NMDA and CAR interchangeably (NMDA 1st and CAR 2nd, and vice versa; Fig. 7). When 10 μM NMDA was applied first (Fig. 7A), this agent induced an overall increase in activity at almost all frequencies (blue record). Subsequent application of 30 μM CAR (red record) decreased overall activity leaving only specific peaks of activity induced at almost the same amplitude as the highest peaks during the NMDA effect. Note that the effects of previous exposure to NMDA before the beginning of the ERSP in this figure (Fig. 7A, bottom) showed that the overall increase at many frequencies decreased after 5 min of wash. Within 5 min after the beginning of addition of CAR, the same slice began firing at specific peaks at ∼10, 15, and 20 Hz. We were also able to induce gamma frequency oscillations with 30 μM CAR followed by 10 μM NMDA (Fig. 7B). Specific peaks at gamma frequencies initially induced by CAR (red record) were replaced by the usual overall increase in activity induced by NMDA (blue record). Note that previous exposure to CAR (before the beginning of this ERSP plot) had induced peaks of activity at ∼15 and 30 Hz in this slice. NMDA was then applied without wash at the beginning of this ERSP plot, and the effect of CAR began to be replaced by a more scattered spectrum by minute 7. By minute 10 of NMDA exposure, rolling peaks of activity from low to high frequencies had replaced the specific peaks induced by CAR.
Fig. 7.
NMDA and CAR generated characteristic oscillatory activities when applied successively regardless of the order of drug application. A: 1 s sample data (top) at peak of 10 μM NMDA when applied 1st (1, blue record), and at peak of 30 μM CAR when applied 2nd (2, red record). Power spectrum (middle) shows how oscillatory activities at almost all frequencies (theta through gamma) were generated at the peak of the NMDA effect (blue record) but were replaced by oscillations at specific gamma frequencies at the peak of the CAR effect (red record). The MatLab graph (bottom) shows the entire 25 min recording beginning during wash after 10 μM NMDA (which had induced overall activation) but was replaced by 30 μM CAR, which, after 5 min, induced peaks of activity in the gamma range. Basically, the tissue was washed in ACSF (min 0–14) and 30 μM CAR added at minute 14. B: 1 s sample data (top), and power spectrum (middle) show gamma oscillations (15 and 30 Hz) were induced by 30 μM CAR (red record) while addition of NMDA (blue record) without wash induced overall increases in frequencies of activity. Please note that the scales in A, 1 and 2, are higher than in B, 1 and 2, including the ERSP activity levels in the 2 slices. MatLab graph (bottom) shows a 13 min recording during which CAR (30 μM) had induced specific peaks at 15 and 30 Hz but were replaced within 4–5 min after application of NMDA (10 μM) without wash by increases in overall activity at frequencies ranging from theta to gamma. Note that the CAR (min 0–10) effect was still present at minute 6 when NMDA began increasing overall activity.
DISCUSSION
Whole cell recordings
In such structures as the striatum and subthalamic nucleus, depolarizing current steps linearly increase firing frequency to >500 and >250 Hz, respectively (Azouz et al. 1997; Barraza et al. 2009). However, our results show that when PPN neurons are depolarized with increasing current steps, firing frequency plateaus at gamma frequency (40–60 Hz). This plateau was observed in all PPN cell types regardless of channel constitution or transmitter type. These results have not been observed in similar experiments in other brain regions and are unique to the PPN. Previous studies on the PPN reported the presence of three cell types based on electrophysiological criteria (Kamondi et al. 1992; Leonard and Llinas 1990; Takakusaki and Kitai 1997), including the presence of LTS currents in type I cells (noncholinergic), of A currents in type II cells (two-thirds cholinergic), and of both A+LTS currents in type III neurons (one-third cholinergic). In addition, type II neurons exhibit a persistent sodium conductance (Leonard and Llinas 1990). However, no previous study used depolarizing pulses to determine maximal or close to maximal firing frequencies and membrane oscillations in PPN neurons. We should note that gamma band frequency of APs has been reported in cells of a descending target of the PPN, the subcoeruleus nucleus (Brown et al. 2006). The present results demonstrate almost all PPN neurons exhibit gamma band activity when activated, as well as gamma band membrane oscillations, but not at higher frequencies. In addition, electrical and pharmacological activation of the PPN induced gamma band population responses. These results suggest that the PPN can impart gamma band activity on its targets when maximally activated. This represents a novel mechanism for the induction of activated states, waking and paradoxical sleep, by PPN efferents.
These experiments demonstrate that almost all PPN neurons are able to fire APs maximally at gamma band frequency when electrically stimulated using current steps and exhibited gamma band membrane oscillations that were blocked by TTX. Therefore these results suggest that one of the mechanisms responsible for these oscillations may involve sodium channels. Furthermore, in single cells, application of NMDA increased the frequency of membrane oscillations at both theta and gamma frequencies, while KA induced membrane oscillations mainly in the theta range. Previously, sodium and calcium dependent subthreshold oscillations were reported in the PPN (Takakusaki and Kitai 1997). However, at present, it is not clear if the membrane oscillations we recorded represent subthreshold oscillations, suprathreshold oscillations, or AP failures at gamma band frequency.
In physiological conditions, the NMDA receptor is blocked by Mg2+ at resting membrane potential (Von Bohlen und Halbach and Dermietzel 2006). Following depolarization, the magnesium block is released and the channel pore is permissive to ions. The initial depolarization of the membrane is mediated through non-NMDA receptors (KA and AMPA). In this experiment, Mg2+ free ACSF solution was used to bathe the cells. Therefore the effect of NMDA in generating gamma band responses was observed without need to add other agents. The NMDA receptor is slow to activate (due to the presence of the Mg2+ block), but slower to inactivate than KA and AMPA receptors (Hille 2001). This may explain why KA generated oscillatory activity in single cells in the theta range, while NMDA generated oscillatory activity at almost every frequency. Using electrical stimulation, we found that the transition from theta to gamma frequency required a much higher level of excitation (preliminary data). Perhaps the transient nature of KA receptor activation does not allow the cell to be sufficiently activated to allow for gamma band activity. However, because the NMDA receptor desensitizes more slowly, the cell can reach sufficient depolarization to exhibit gamma frequency oscillations.
Metabotropic glutamate receptors generate slow postsynaptic responses and contribute to a delayed neuronal response (Von Bohlen und Halbach and Dermietzel 2006). In this experiment, we did not test the effects of metabotropic glutamate receptor agonists on gamma band activity, but we suspect that they may play some role. Perhaps gamma band activity is generated by the combined activity of ionotropic glutamate receptors and is maintained by the prolonged activity of metabotropic glutamate receptors, but such studies were beyond the scope of the current experiment.
Population responses
Our population response studies showed that the PPN is also capable of producing gamma frequency oscillations in population responses when pharmacologically stimulated with CAR (which induced activation at specific peaks), NMDA (which induced overall increases in activity), and KA (which induced overall activation at lower and somewhat at higher frequencies). This was the only difference between single cell and population response results, namely, the lack of activation of higher frequencies by KA on the single cells tested with some activation by KA at higher frequencies in the population recordings. A larger sample of cells may reveal if this is indeed a major difference or merely a sampling issue. It may also be that subpopulations of cells may be activated to fire at different frequencies.
Although the role of specific receptor channels behind gamma rhythms in the PPN is still unknown, the population responses following CAR, NMDA, and KA were similar to those seen in cortical and hippocampal areas. Interestingly, sequential application of glutamatergic and cholinergic agonists induced characteristic profiles of response frequencies (Fig. 7). We suspect that the interaction of these inputs at physiologically varying concentrations, and with electrically coupled cells and other populations, may reveal how sleep-wake states may be modulated.
We know from EEG studies that PPN stimulation indirectly produces gamma oscillations in the cortex (Steriade et al. 1991). Our findings suggest that gamma frequency oscillations are not just the property of cortical and hippocampal neurons but that they also belong to other regions of the brain such as the PPN. This suggests that communication in the neocortex, and perhaps also cerebellar cortex, is facilitated by gamma rhythms that act as a common spatiotemporal code (Middleton et al. 2008). We believe that gamma frequency oscillations found in PPN play a major role in communication between RAS nuclei. Our results suggest that the PPN may generate gamma band activity and project these rhythms to its ascending thalamic targets involved in cortical arousal, e.g., parafascicular (Pf) nucleus, and to its descending targets involved in paradoxical or REM sleep, e.g., subcoeruleus (SubC) nucleus.
Limitations and physiological significance
A number of essential experiments were beyond the scope of the present studies. The temperature of the chambers was uniformly maintained at 30°C, so that the effects of increasing temperature have yet to be tested. It may be that increasing temperature may increase the frequencies of activity elicited by the various treatments, bringing the range of the power spectrum to higher levels. It needs to be established if the membrane oscillations are selectively TTX sensitive, indicating the presence of sodium currents, or if in at least some cells are generated by a calcium conductance.
In the cortex, several classes of interneurons are capable of sustained subthreshold oscillatory rhythms and increase their frequency with membrane depolarization, which may allow coherent activation depending on the level of excitation (Llinas et al. 1991). Such cortical oscillations are generated by the alternating activation of a TTX-sensitive persistent sodium conductance and a delayed rectifier, similar to those found in type II PPN neurons (Leonard and Llinas 1990). The lack of LTS currents in type II cells enable these neurons to maintain elevated firing levels, and the presence of gamma band oscillations allow these neurons to sustain such activation. Such activation has been proposed to account for temporal coherence of sensory perception at the level of the cortex and thalamocortical circuits (Llinas et al. 2005; Singer 1999). However, our results show that type I and III cells also fired at gamma band frequency. Moreover, our findings suggest that type I PPN cells, which are known to be noncholinergic and have LTS currents and no A currents, exhibited the highest firing frequencies compared with types II and III, which did not differ from each other (Fig. 1).
The differential effects of CAR, NMDA, and KA suggest that the population as a whole may be modulated by both cholinergic and glutamatergic input to generate activity at different levels and peaks. It remains to be determined how these two transmitter systems may control that activity during different states such as waking versus REM sleep. One potential control schema is suggested by the effects of the three agents on single cell recordings (Fig. 3). At least in some cells, NMDA increased activity at 21–40 and 41–60 Hz, but not 0–20 Hz, while KA preferentially increased activity at 0–20 Hz, and CAR increased activity at 0–20 and 41–60 Hz. That is, CAR and KA together may preferentially induce activity at 0–20 Hz, NMDA may preferentially induce activity at 21–40 Hz (in the population studies, CAR also induced activity at this range), and CAR and NMDA together may preferentially induce activity at 41–60 Hz. This suggests that the combined influence of two transmitters may modulate separate frequencies of oscillations.
In vivo recordings have shown that PPN cells show gamma band activity but also lower levels of firing (Datta and Siwek 2002; Steriade et al. 1990b). One population of PPN cells fired spontaneously at low rates (<4 Hz), but the same cells exhibited high-frequency firing (>100 Hz) in relation to PGO waves (Steriade et al. 1990b). Another population fired at 10–40 Hz during REM sleep, but had a spontaneous rate ∼18 Hz, yet others fired at higher rates (10–20 Hz) spontaneously, but at 70–80 Hz in relation to PGO waves (Steriade et al. 1990b). There is little doubt that PPN neurons have the capacity to fire at higher rates than the low frequencies seen during passive changes in sleep-wake states. However, most of these studies record activity in relation to spontaneously changing sleep-wake states. That is, it could be that few cells are maximally activated in such passive preparations, so that these cells may fire well below gamma band frequency unless activated by arousing stimuli. Sudden sensory stimulation may maximally activate these cells to fire at gamma band frequency. Significantly, they tend not to fire at higher frequencies than gamma, suggesting that intrinsic properties dictate this level of maximal activation. Almost all PPN cells were found to fire maximally at gamma band frequency but no higher (Fig. 1). Moreover, the fact that gamma band activation was maintained throughout the depolarizing current steps and did not wane means that there is little accommodation to brief versus longer stimuli as long as they are of sufficient amplitude to elicit gamma band frequency. The significance of the plateau levels at gamma band suggests that as long as PPN activation is maintained, its targets receive continuous gamma band activation. This may result in generating gamma band activity in these targets. The observation that membrane oscillations are also limited to gamma band suggests that in the absence of maximal activation, some cells still show oscillations at these frequencies. The population as a whole, however, would show overall activity at gamma band frequencies. It may also be that accommodating cells are activated only during brief stimuli while nonaccommodating cells fire throughout, thus allowing the population to discriminate the duration of stimuli.
The present results suggest that a similar mechanism to that in the cortex for achieving temporal coherence is present in the PPN and perhaps its subcortical targets such as the parafascicular and subcoeruleus nuclei. We suggest that rather than participating in the temporal binding of sensory events, gamma band activity generated in the PPN may help stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep. Much work is needed to support this speculation, but the intriguing findings described here certainly provide a starting point for such investigations.
Conclusion
The present findings provide novel insights into the function of the PPN, demonstrating the presence of gamma band activity in whole cell recorded neurons as well as in population responses. These data suggest that this element of the RAS provides gamma band oscillatory activity in the presence of sufficient excitation, perhaps relaying these rhythms to its ascending targets indirectly involved in cortical arousal, and to its descending targets involved in paradoxical sleep.
GRANTS
This work was supported by National Institutes of Health Grants R01 NS-020246 and P20 RR0-20146 to the Center for Translational Neuroscience.
DISCLOSURES
No commercial support or off-label/investigational use.
Supplementary Material
REFERENCES
- Azouz et al. 1997.Azouz R, Gray CM, Nowak LG, McCormick DA. Phsyiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex 7: 534–545, 1997 [DOI] [PubMed] [Google Scholar]
- Barraza et al. 2009.Barraza D, Hitoshi K, Wilson C. Slow spike frequency adaptation in neurons of the rat subthalamic nucleus. J Neurophysiol 102: 3689–3697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. 2006.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience 143: 739–755, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham et al. 2004.Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Nat Acad Sci USA 101: 7152–7157, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta 2002.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol 87: 1790–1798, 2002 [DOI] [PubMed] [Google Scholar]
- Datta et al. 2001a.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res 66: 109–116, 2001a [DOI] [PubMed] [Google Scholar]
- Datta and Siwek 2002.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res 70: 79–82, 2002 [DOI] [PubMed] [Google Scholar]
- Datta et al. 2001b.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Reg Integ Comp Physiol 280: R752–R759, 2001b [DOI] [PubMed] [Google Scholar]
- Delorme and Makeig 2004.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- Dickinson et al. 2003.Dickinson R, Awaiz S, Whittington MA, Lieb WR, Franks NP. The effects of general anaesthetics on carbachol-evoked gamma oscillations in the rat hippocampus in vitro. Neuropharmacology 44: 864–872, 2003 [DOI] [PubMed] [Google Scholar]
- Eckhorn et al. 1988.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitbock HJ. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern 60: 121–130, 1988 [DOI] [PubMed] [Google Scholar]
- Fellous and Sejnowski 2000.Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz) and gamma (35–70 Hz) bands. Hippocampus 10: 187–197, 2000 [DOI] [PubMed] [Google Scholar]
- Fisahn et al. 1998.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394: 186–189, 1998 [DOI] [PubMed] [Google Scholar]
- Garcia-Rill et al. 2008.Garcia-Rill E, Charlesworth A, Heister DS, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep 31: 673–690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill et al. 2007.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep 30: 1405–1414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson et al. 1999.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gray and Singer 1989.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA 86: 1698–1702, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister et al. 2007.Heister DS, Hayar A, Charlesworth A, Yates C, Zhou YH, Garcia-Rill E. Evidence for electrical coupling in the subCoeruleus (SubC) nucleus. J Neurophysiol 97: 3142–3147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille 2001.Hille B. Ion Channels of Excitable Membraes Sunderland, MA: Sinaur, 2001 [Google Scholar]
- Jouvet-Mounier et al. 1970.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol 2: 216–239, 1970 [DOI] [PubMed] [Google Scholar]
- Kamondi et al. 1992.Kamondi A, Williams J, Hutcheon B, Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J Neurophysiol 68: 1359–1372, 1992 [DOI] [PubMed] [Google Scholar]
- Leonard and Llinas 1990.Leonard CS, Llinas R. 1990. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Brain Cholinergic Systems, edited by Steriade M, Biesold D. Oxford, UK: Oxford Science, 1990, p 205–223 [Google Scholar]
- Llinas et al. 1991.Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Nat Acad Sci USA 88: 897–901, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás et al. 2005.Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28: 325–333, 2005 [DOI] [PubMed] [Google Scholar]
- Metherate and Cruikshank 1999.Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma- band activity in auditory cortex in vitro. Exp Brain Res 126: 160–174, 1999 [DOI] [PubMed] [Google Scholar]
- Middleton et al. 2008.Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T, Schofield IS, Jenkins A, Whittington MA. High-frequency network oscillations in cerebellar cortex. Neuron 58: 763–774, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva et al. 2010.Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage 49: 3257–3268, 2010 [DOI] [PubMed] [Google Scholar]
- Philips and Takeda 2009.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol 73: 350–354, 2009 [DOI] [PubMed] [Google Scholar]
- Sakai et al. 1990.Sakai K, el Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 527: 213–223, 1990 [DOI] [PubMed] [Google Scholar]
- Singer 1993.Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol 55: 349–374, 1993 [DOI] [PubMed] [Google Scholar]
- Singer 1999.Singer W. Time as coding space? Curr Opin Neurobiol 9: 189–194, 1999 [DOI] [PubMed] [Google Scholar]
- Steriade 1999.Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Handbook of Behavioral State Control. Cellular and Molecular Mechanisms, edited by Lydic R, Baghdoyan HA. New York: CRC, 1999, p. 327–347 [Google Scholar]
- Steriade et al. 1991.Steriade M, Curro Dossi R, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci USA 88: 4396–4400, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade et al. 1990a.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci 10: 2541–2559, 1990a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade et al. 1990b.Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci 10: 2560–2579, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade and McCarley 1990.Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep New York: Plenum, 1990 [Google Scholar]
- Sukov and Barth 2001.Sukov W, Barth DS. Cellular mechanisms of thalamically evoked gamma oscillations in auditory cortex. J Neurophysiol 85: 1235–1245, 2001 [DOI] [PubMed] [Google Scholar]
- Takakusaki and Kitai 1997.Takakusaki K, Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience 78: 771–794, 1997 [DOI] [PubMed] [Google Scholar]
- Von Bohlen und Halbach and Dermietzel 2006.Von Bohlen und Halbach O, Dermietzel R. Neurotransmitters and Neuromodulators Weinheim: Wiley-VCH, 2006 [Google Scholar]
- Voss et al. 2009.Voss U, Holsmann R, Tuin I, Hobson JA. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep 32: 1191–1200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Morales 2009.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29: 340–358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington et al. 1997.Whittington MA, Stanford IM, Colling SB, Jeffreys JGR, Traub RD. Spatiotemporal patterns of gamma frequency oscillations tetanically induced in the rat hippocampal slice. J Physiol 502: 591–607, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. 2010.Ye M, Hayar A, Strotman BS, Garcia-Rill E. Cholinergic modulation of fast inhibitory and excitatory transmission to pedunculopontine thalamic projecting neurons. J Neurophysiol 103: 2417–2432, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.