Abstract
A set of brain regions known as the default network increases its activity when focus on the external world is relaxed. During such moments, participants change their focus of external attention and engage in spontaneous cognitive processes including remembering the past and imagining the future. However, the functional contributions of the default network to shifts in external attention versus internal mentation have been difficult to disentangle because the two processes are correlated under typical circumstances. To address this issue, the present study manipulated factors that promote spontaneous cognition separately from those that change the scope of external attention. Results revealed that the default network increased its activity when spontaneous cognition was maximized but not when participants increased their attention to unpredictable foveal or peripheral stimuli. To examine the nature of participants' spontaneous thoughts, a second experiment used self-report questionnaires to quantify spontaneous thoughts during extended fixation epochs. Thoughts about one's personal past and future comprised a major focus of spontaneous cognition with considerable variability. Activity correlations between the medial temporal lobe and distributed cortical regions within the default network predicted a small, but significant, portion of the observed variability. Collectively, these results suggest that during passive states, activity within the default network reflects spontaneous, internally directed cognitive processes.
INTRODUCTION
People spend a great deal of time engaged in internally directed thought (Singer 1966). Spontaneous mental explorations, sometimes referred to as “mind-wandering,” punctuate the most demanding tasks and become frequent when people are at rest or performing monotonous activities. Contrary to earlier Freudian notions, spontaneous thought is dominated by typical life events and is minimally focused on fantasy (Singer and Antrobus 1963). Based on these observations, Singer (1966) and Klinger (1971) hypothesized that spontaneous cognition serves an adaptive function by helping us to mentally explore and prepare for upcoming situations.
Multiple lines of evidence suggest that a distributed cortical network, referred to as the “default network,” plays an important role in spontaneous cognition. The default network increases its activity during passive tasks when spontaneous thoughts are at their peak (Binder et al. 1999; Mazoyer et al. 2001; Shulman et al. 1997; also see Christoff et al. 2004), attenuates its activity when externally demanding tasks are made more difficult (Mazoyer et al. 2002; McKiernan et al. 2003, 2006), and is associated with stimulus-independent thoughts (Mason et al. 2007; McGuire et al. 1996; McKiernan et al. 2006; for reviews, see Buckner et al. 2008; Gusnard and Raichle 2001). Additionally, activity within the default network is linked to slowed response time and errors on externally directed tasks (Eichle et al. 2008; Polli et al. 2005; Weissman et al. 2006), presumably because such trials are associated with mind-wandering (Christoff et al. 2009). Lesions to the medial prefrontal cortex (MPFC) sometimes lead to reported loss of thought (e.g., Damasio and Van Hoesen 1983), and metabolic reductions in the MPFC and the posterior cingulate cortex (PCC) are characteristics of physiological states marked by loss of consciousness (reviewed in Baars et al. 2003).
The possibility that the default network is involved in internal mentation is controversial. A prominent alternative account of the default network is that it, or a subset of the regions that comprise the network, play a role in “the representation (monitoring) of the world around us” (Gusnard and Raichle 2001). In almost all situations where attention to the external environment is relaxed and spontaneous cognition increases, a concurrent shift in the focus of external attention occurs such that our awareness broadens, possibly reflecting the anticipation of unpredictable events (Gilbert et al. 2006, 2007).
Several lines of evidence lend credibility to the default network's possible role in some aspect of external attention or monitoring. Shulman et al. (1997) noted in the first meta-analysis of the default network that modulation of the network was most pronounced when task blocks involved foveal stimuli. In addition, parietal lesions extending along the posterior midline can lead to simultanagnosia, a common feature of Balint's syndrome where patients lack the ability to perceive multiple objects in their environment (Hécaen and de Ajuriaguerra 1954). However, Balint's syndrome is often linked to posterior lateral lesions and the relationship between lesion loci and their corresponding functional deficits is unclear (for discussion, see Rizzo and Vecera 2002).
Support for the role of the MPFC and PCC in broad external attention comes from functional magnetic resonance imaging (fMRI) studies of attention to external stimuli. In particular, MPFC and PCC—nexuses of connectivity within the default network (Andrews-Hanna et al. 2010; Buckner et al. 2008; Fransson and Marrelec 2008; see also Hagmann et al. 2008)—increase their activity during perceptual tasks in which targets appear randomly at one of multiple locations compared with a cued location (Hahn et al. 2007). Hahn and colleagues (2007) also found that activity within certain regions of the default network was correlated with trial-by-trial variability in performance, such that faster responses during peripheral target detection were linked to activity increases. A similar finding was observed when participants performed a low-demanding foveal baseline task, leading to the suggestion that the default network supports stimulus-oriented thoughts, or “watchfulness” for upcoming stimuli (Gilbert et al. 2006, 2007). Note that these results contrast with those that demonstrate inverse relationships between default network activity and behavioral performance on externally directed tasks (e.g., Weissman et al. 2006).
Existing studies have thus linked activity within the default network to two opposing functions: 1) internal mentation and 2) attention to or monitoring of the external environment. Although the two hypotheses differ with respect to their emphases on external compared with internal processing modes, they have been difficult to disentangle because of the inherent challenge associated with measuring cognition during unconstrained states that lack a behavioral response. The main goal of the present studies was to adjudicate between these two plausible, but opposing, hypotheses about the functions of the default network.
In an initial study, we used fMRI to indirectly measure activity within the default network across three conditions that manipulated spontaneous cognition separately from the scope of external attention. Critically, stimuli were held constant in all three conditions. The subjects simply fixated on a central crosshair while expectations differentially modulated the direction and scope of their attention across the conditions. Results were consistent with a role of the default network in spontaneous cognition but not broad or focal attention to the external environment. In a second study, we probed the content of participants' spontaneous thoughts and found that participants reported frequent thoughts about the past and future. Using a large sample of participants (n = 139), we demonstrated that the propensity for such thoughts was associated with functional coupling between the medial temporal lobe (MTL) and distributed cortical regions that are within the default network. Collectively, these results are most consistent with the role of the default network in spontaneous, internally directed cognition.
METHODS
Overview
The first objective of the present set of experiments was to distinguish between two prominent, but opposing, hypotheses implicating the default network in external attention or spontaneous cognition. The distinct hypotheses have been difficult to disentangle because the two variables often track each other and because of the inherent challenge associated with measuring spontaneous cognition during unconstrained task states. Here we tested the two hypotheses by contrasting fMRI signal changes as an indirect measure of neural activity during fixation blocks that varied with respect to scope of external attention (broad and focal) and frequency of spontaneous thoughts as a measure of internal mentation. During conditions designed to encourage external attention, participants detected brief flickers occurring in either peripheral (broad) or central (focal) locations. In contrast, during a condition designed to encourage spontaneous cognition, participants passively fixated on a crosshair. To ensure that variables were manipulated as expected, an independent group of participants completed an identical paradigm in a mock MRI simulator. For these participants, randomly presented tones were placed throughout the tasks to gauge participants' frequency of spontaneous thoughts.
Three different hypotheses about the expected results were entertained. If the default network plays a prominent role in external attention in general (i.e., watchfulness for upcoming stimuli; Gilbert et al. 2007), the contrast between the external attention conditions and the passive condition should isolate regions within the default network. If the default network plays a specific role in broad external attention, as suggested by a number of prior studies, the contrast between the broad and focal external attention conditions should elicit activity within the default network. If, on the other hand, the default network functions to support internal mentation, activity within the network should track reported measures of spontaneous cognition and should be weakest in the external attention conditions (both broad and focal). Of course an additional possibility is that distinct regions within the default network may enable broad attention and spontaneous cognition, respectively.
Our second objective was to explore the nature of participants' spontaneous thoughts and to examine whether the individual variability underlying such thoughts was predicted by functional coupling between regions comprising the default network. Since prior studies have established that the MTL and distant cortical regions including the retrosplenial cortex (Rsp), posterior inferior parietal lobule (pIPL), and MPFC become engaged when participants remember their past and imagine their future, we investigated whether the tendency to experience spontaneous episodic thoughts about the past and future predicted functional coupling between the MTL and the default network. A significant relationship would strengthen support for the network's role in certain forms of spontaneous cognition.
Participants
In all, 199 right-handed young adults participated in the study (22.2 yr, range: 18–35 yr, 97 male; Table 1). Participants were native English speakers recruited from Harvard University and the greater Boston community. Exclusion criteria included a history of psychiatric or neurological illness as well as use of psychoactive medications. Procedures were carried out according to the guidelines put forth by either the Harvard University Committee on the Use of Human Subjects in Research (experiment 1: behavioral) or the Review Board of Partners Healthcare at Massachusetts General Hospital (experiment 1: fMRI and experiment 2).
Table 1.
Participant demographics
| Study | n | Age, yr (mean) | Age Range, yr | Number of Males |
|---|---|---|---|---|
| Total* | 199 | 22.2 | 18–35 | 97 |
| Study 1 | 60 | 23.1 | 18–35 | 34 |
| Mock MRI through sampling study | 30 | 24.5 | 18–35 | 17 |
| Functional MRI study | 30 | 21.7 | 18–31 | 17 |
| Study 2 | 139 | 21.8 | 18–32 | 63 |
Within study 1, the participants who completed the thought sampling study were distinct from those who completed the functional MRI study (indicated by boldface entries). However, 19 participants comprising study 2 also volunteered for the functional MRI study comprising study 1; thus n = 199 represents the number of data sessions (there were 180 independent participants).
Task paradigms and questionnaires
Experiment 1 (Overview).
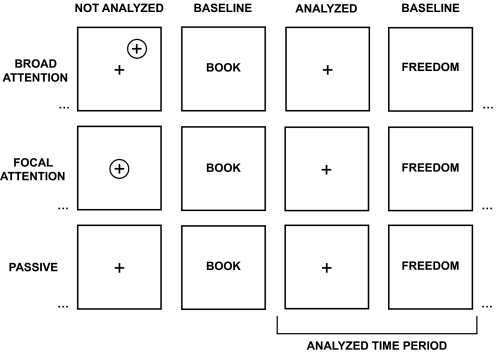
To test between the two prominent hypotheses about the function of the default network, three conditions were explored that varied the direction and scope of attention by manipulating expectancies: 1) broad attention, 2) focal attention, and 3) passive fixation. During critical task blocks used for analysis, stimuli were held constant and no responses were made in any condition (Fig. 1). Only expectancies and ancillary processes linked to focus of attention and spontaneous cognition differed.
Fig. 1.
Experimental paradigm to dissociate external attention from spontaneous cognition. The experimental paradigm contained 3 conditions that manipulated focus of attention through subject expectations. In the broad attention condition (top row), participants fixated and simultaneously pressed a button whenever a peripherally located flicker was detected. In the focal attention condition (middle row), participants detected centrally located flickers. In the passive condition (bottom row), participants remained fixated but did not expect flickers, thus increasing the tendency to engage in spontaneous cognition. Critically, embedded blocks within the broad and focal attention conditions contained no flickers. These analyzed blocks matched stimuli across all conditions (see blocks labeled analyzed time period). Thus only the expectations of the subject differed between conditions with subjects expecting peripheral events in the broad attention condition (top), foveal events in the focal attention condition (middle), or no events in the passive condition (bottom). The baseline condition for each critical fixation condition was abstract/concrete semantic classification of words.
A block-designed paradigm was used where blocks of fixation across the three conditions alternated with baseline blocks consisting of an abstract/concrete semantic classification task on visually presented words. Note that the baseline here is different from most prior neuroimaging studies; the active task block serves as a common baseline for the three critical fixation conditions that provide the test of the hypotheses. Participants completed a total of six task runs, each comprised of six 30-s fixation blocks flanked by seven 20-s blocks of the baseline semantic classification task. Participants performed one condition at a time across two consecutive runs and condition order was counterbalanced across participants.
In the broad attention condition, participants were instructed to remain fixated on a central fixation crosshair for the duration of each fixation block and to concurrently press a button whenever a brief flicker was detected somewhere in the periphery of the visual display. A peripheral flicker was marked by a gray X that appeared for ~30 ms at one of three eccentricities (corresponding to visual angles: 1.4, 2.8, 4.2°) and eight angles (0, 45, 90, 135, 180, 225, 270, 315°), yielding 24 possible locations for each flicker. Importantly, unbeknown to the participants, flickers were omitted from four blocks and these blocks lacking flickers were the targets of analysis to compare across conditions. In the focal attention condition, participants were told that flickers would occur directly on top of the central fixation crosshair (marked by a gray X that appeared for 67 ms). Again, flickers were omitted from four unpredictable blocks. In the passive fixation condition, participants were instructed to fixate on the fixation crosshair but not to respond during these blocks because no flickers would occur.
To encourage top-down modulation of attention, participants were told that flickers would occur infrequently, were informed of the importance of detecting each flicker as fast as possible, and were discouraged from detecting false positives. Flickers appeared only briefly and were difficult to detect. Four lists that varied in the total number of blocks containing flickers (some lists included flickers in the abbreviated fixation block inserted to allow for intensity stabilization of the blood oxygenation level dependent [BOLD] signal), the total number of flickers per run (three to six), and the onset of the flickers within the run were created and counterbalanced across participants to ensure that the broad and focal conditions did not systematically differ in the onset of flickers. As mentioned earlier, four fixation blocks within each run were matched across conditions with respect to stimuli since flickers did not appear in these blocks. These blocks were the targets of analysis. Thus for the purpose of the fMRI analysis, only expectations and focus of external attention differed between conditions. The same onsets used for fixation blocks extracted from the focal attention runs were also extracted from the passive runs. Blocks in which false-positive key-press responses occurred were removed from analysis in all conditions.
Experiment 1 (Behavioral Study).
An underlying assumption of the study was that the passive condition would encourage more spontaneous cognition than the broad or focal external attention conditions. To test this assumption, we conducted a separate behavioral thought sampling study during which an independent group of participants (n = 30, 24.5 yr, range: 18–35 yr, 17 male) completed the same six task runs while spontaneous thoughts were sampled throughout each run. Five tones within different fixation blocks were sounded during each run. Lists were created that varied the placement of the tones within the run and these lists were counterbalanced across subjects to ensure that the broad and focal conditions did not systematically differ in tone placement. Participants were asked to classify their thoughts whenever a tone sounded by stating aloud the word “thought” if they experienced a spontaneous thought unrelated to the task at hand immediately prior to the tone or “nothing” if a task-unrelated thought was not experienced. The specific instructions are listed below:
Now, there is one additional component to this experiment. At random times while the fixation crosshair is on the screen, a brief tone will occur that sounds like a ring. Here is an example: [sample tone played]. When you hear the tone, we would like you to classify what you were thinking about immediately before the tone sounded. If your mind was wandering and you were thinking about something unrelated to the task at hand, please say the word “thought” out loud. For example, you may have been thinking about something that you did last night or something you should do on your way home. Or you may have been thinking how tired or hungry you are. If you were thinking about either of these types of things or about something else that was not related to the task at hand, please say the word “thought” out loud. You should not press anything.
On the other hand, if your mind was not wandering and you were thinking about the flickers, the fixation crosshair, or nothing at all, you should say the word “nothing” out loud. For example, you may have been wondering where or when the flicker is going to appear or thinking about the features of the fixation crosshair—for example, its color or size. Or you may have not been thinking about anything at all, meaning your mind was completely blank. What all these types of activities have in common is that they are not thoughts that are unrelated to the task. Therefore if you experience something that falls into this category, you should say the word “nothing” out loud. Again, you should not press anything.
Flickers will not occur during or immediately after a tone—therefore you should not rush through your response to the tone for the sole purpose of being able to detect flickers afterward. Instead, think carefully about your response before you say it because you will be allowed to state only one of the two options, either “thought” or “nothing.” At the same time, you should remember that your primary task in this experiment is to determine whether words are abstract or concrete and to detect the flickers.
An important aspect of the behavioral study was that participants performed the experiment while lying in a mock MRI simulator (Psychology Software Tools, Pittsburg, PA). A mock scanner ensured that a similar environment was present across the two experiments (including simulated scanner noise, earplugs, a button box, and a head coil) while simultaneously allowing us to sample spontaneous thoughts throughout each run. In addition to thought sampling using probes, participants completed a postexperimental questionnaire to assess subjective reports of mind-wandering frequencies for each condition as well as the self-relevancy and goal-oriented nature of such thoughts (see following text). Questions were answered using the scale 1 = never to 7 = always. In addition, participants were encouraged to list examples of any spontaneous thoughts that occurred during the experiment, providing further insight into the nature of their content. The questions were as follows.
During the task that involved detecting flickers that occurred in the periphery, to what extent did your mind wander away from thoughts about the task at hand?
During the task that involved detecting centrally located flickers, to what extent did your mind wander away from thoughts about the task at hand?
During the task where you stared at the fixation crosshair for blocks of 30 s, to what extent did your mind wander away from thoughts about the task at hand?
If your mind wandered away from the task at hand during any of the tasks, to what degree did your thoughts revolve around self-relevant concerns (i.e., things that are important to you)?
If your mind wandered away from the task at hand during any of the tasks, to what degree were your thoughts directed toward a particular goal (e.g., trying to figure something out; planning something in your future)?
Any examples of thoughts that occurred during any of the tasks would be extremely helpful to us. Please use the space below to describe a few of these thoughts.
Administering a retrospective thought sampling questionnaire allowed us to assess frequencies of task-unrelated thoughts using a second method. Given the ambiguities in the validity of various thought sampling procedures, we chose to explore multiple convergent techniques. Although the on-line thought sampling method required less reliance on long-term memory compared with the retrospective questionnaire, it is possible that the on-line method caused participants to monitor the nature of their thoughts, bringing more thoughts into explicit awareness and leaving fewer thoughts as unaware (for discussion, see Smallwood and Schooler 2006). In addition, since participants may have experienced more thoughts relating to monitoring during the on-line thought probe study, they may have encountered difficultly determining whether these thoughts were task-unrelated. We reasoned that by introducing a small number of tones per run (n = 5) as well as emphasizing in the task instructions that the thought probe aspect of the study was secondary to the primary flicker/fixation task, participants would dedicate minimal time to monitoring their thoughts. Importantly, the retrospective thought sampling questionnaire was also given to the independent sample of 30 young adults who completed the experimental task in an MRI scanner (see following text).
Experiment 1 (fMRI Study).
The fMRI study was conducted in an independent sample of participants matched on age and gender (n = 30, 21.7 yr, range: 18–31 yr, 17 male) using the identical experimental paradigm as outlined earlier, with the exception that the on-line thought probes were not present, nor were participants instructed to monitor the nature of their spontaneous thoughts so as not to interfere with performance on the primary task. As in the behavioral study, surprise postscanning questionnaires were distributed to gather subjective reports of spontaneous cognition for each condition.
Experiment 2.
Low-frequency functional correlations (coupling) were examined between distributed brain regions in addition to spontaneous thoughts. To obtain a large sample size, we added this study to ongoing studies in our laboratory where time permitted. For this “tag on” study, a large number of participants (n = 139, 21.8 yr, range: 18–32 yr, 63 male) fixated on a crosshair for between two and four runs. The duration of each run varied between 4 min 20 s and 6 min 30 s depending on the context of the existing study.
In all cases, thought content was probed immediately after the participants exited the MRI scanner using a surprise questionnaire. The goal of the questionnaire was to estimate the content of spontaneous cognition using the best approach available. The approach has limitations including the ability of the participants to remember their thoughts and the possibility that they may not have conscious access to all aspects of their thought content. Nonetheless, motivated by past successes using self-report questionnaires (e.g., Andrews-Hanna et al. 2010; Kirchhoff and Buckner 2006; Mason et al. 2007), we used such an approach here recognizing these potential biases and limitations.
For the assessment, participants were asked to a divide a blank circle to reflect the total amount of time they spent thinking about each of 14 a priori categories of thought during the fixation runs (see categories in Table 2). This approach encouraged the participants to make relative judgments about whether one category was represented more than another and also encouraged them to allocate the total time during fixation. The resulting pie chart percentages were measured with a protractor. Three versions of the questionnaire were randomly distributed to participants, each with a different order of categories. The nontemporal category was described as “thinking about something with no particular temporal domain (e.g., problem solving an idea; thinking about what the purpose of the experiment is).” If individuals chose the category “other” (represented in Table 2 as “uncategorized”) and were unable to categorize their response, they were further asked to write examples of such types of thoughts that could not be identified by the remaining categories. This allowed us to leave open the possibility that our categories were inadequate. The most frequent response for the uncategorized domain included “covertly singing.” The pie chart shown in Fig. 8 collapsed categories into the composites in bold in Table 2.
Table 2.
Thought content/frequency questionnaire
| Category | Mean, % | SD |
|---|---|---|
| Past | 19.2 | 26.6 |
| Earlier today | 7.3 | 9.5 |
| Yesterday to a week ago | 8.1 | 9.8 |
| Past year or several years | 3.8 | 7.3 |
| Future | 28.7 | 32.1 |
| Remainder of the day | 12.7 | 13.1 |
| Tomorrow to next week | 11.5 | 10.8 |
| Next year or several years | 4.5 | 8.2 |
| Nontemporal | 15.4 | 16.0 |
| Stimuli | 10.3 | 14.0 |
| Fixation crosshair | 6.3 | 8.0 |
| Additional stimuli | 4.0 | 6.0 |
| Mind Blank | 5.0 | 10.2 |
| Other | 21.4 | 42.5 |
| Counting | 7.4 | 15.0 |
| Focused meditation | 5.6 | 9.3 |
| Sleeping | 3.3 | 6.6 |
| Uncategorized | 5.1 | 11.6 |
Listed in boldface are the composite measures used for Fig. 8. Below some composite measures are the categories comprising each composite.
Fig. 8.
Spontaneous cognition is prominent during passive fixation. Postscanning questionnaires reveal that participants spend the majority of their time engaged in internal mentation during extended fixation blocks that prominently activate the default network. Suggesting an adaptive role, participants spend approximately half of the allotted time thinking about their past and future (particularly the recent past and near future).
For purposes of the present experiment, total thoughts pertaining to the past and the future were calculated for each participant and were regressed against participants' low-frequency functional correlation maps. See the following text for details.
MRI data acquisition parameters
Anatomical and functional data were acquired using a 3 Tesla Siemens Tim Trio scanner (Siemens, Erlangen, Germany) and a 12-channel phased-array head coil. Three-dimensional T1-weighted, high-resolution anatomical images were acquired using a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (repetition time [TR] = 2,530 ms, time to echo [TE] = 3.39 ms, 1 × 1 × 1 mm voxel size). A gradient echo, echo-planar pulse sequence (TR = 2,500 ms, TE = 43 ms, 3 mm slice thickness, 36 axial oblique slices with a 0.5 mm gap aligned to the anterior commissure/posterior commissure plane) sensitive to BOLD contrast was used to acquire functional data across all experiments, except for one data set comprising experiment 2 (TR = 5,000 ms, TE = 30 ms, 2 mm slice thickness, 55 contiguous axial oblique slices). All participants wore MRI-compatible glasses (either corrective or noncorrective lens), were given ear plugs to dampen scanner noise, and responded to visual stimuli (created using Psychophysics Toolbox software; Brainard 1997), visible through an MRI-compatible mirror with a button box placed in their left hand.
MRI data processing
A combination of FSL (FMRIB, Oxford, UK) and SPM2 (Wellcome Department of Cognitive Neurology, London, UK) tools were used to prepare functional data for task and connectivity analysis. These steps included 1) removal of the first four frames of each run to eliminate signal variation caused by T1-stabilization (FSL), 2) correction for slice timing to remove differences in acquisition time across slices within each TR (SPM2), 3) motion correction within and across runs using a rigid-body motion correction algorithm that adjusts functional images based on movement calculated across three separate translations and three separate rotations (FSL), 4) registration of functional data to an EPI target template in the space of the Montreal Neurological Institute atlas (SPM2), 5) whole brain reslicing at 2-mm cubic voxels (SPM2), and 6) spatial smoothing using a 6-mm full-width half-maximum Gaussian kernel (SPM2).
Experiment 1 (fMRI Study).
For fMRI analysis of block-designed task-related data, SPM2 was used to convolve a canonical hemodynamic response function and its temporal derivatives with a boxcar function lasting the length of the experimental task blocks (30 s). A high-pass filter was applied to remove low frequencies commonly associated with scanner drift or other properties unrelated to the tasks. The regressors of interest included the onsets of the four blocks from each condition in which no flickers appeared. The onsets of the remaining blocks were included in the general linear model but were not analyzed. Each subject's fixed-effects contrasts were entered into a between-subject random-effects analysis using one-sample t-tests.
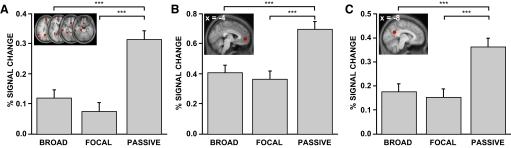
The 11 regions comprising the default network used for region of interest (ROI) analyses were defined in a separate study (Andrews-Hanna et al. 2010) and include the dorsal MPFC (dMPFC), anterior MPFC (aMPFC), ventral MPFC (vMPFC), PCC, Rsp, left pIPL (pIPL), left temporoparietal junction (TPJ), left lateral temporal cortex (LTC), left temporal pole (TempP), left parahippocampal cortex (PHC), and left hippocampal formation (HF). All 11 regions were combined into a single region representing the larger default network (shown in Fig. 4A). Additionally the activity within the aMPFC (Fig. 4B) and PCC (Fig. 4C) was examined separately.
Fig. 4.
Hypothesis-driven analyses reveal increased activity within the default network during the passive condition. Significantly increased fMRI blood oxygenation level-dependent (BOLD) signal (an indirect measure of activity) was observed during the passive condition compared with either the broad or focal attention conditions. BOLD signal is measured as percentage signal change compared with baseline. This pattern was observed in A, a large region of interest comprising multiple regions within the default network; as well as in B, the anterior medial prefrontal cortex; and in C, the posterior cingulate cortex. The image insets (top left in each panel) show the regions plotted in either transverse (A) or sagittal (B and C) views. Error bars reflect SE. ***P < 0.001.
MRI correlation analyses (experiment 2)
Functional correlation analysis procedures used in experiment 2 are based on those introduced by Biswal et al. (1995) and are described in detail in our previous studies (e.g., Van Dijk et al. 2010). A series of preprocessing steps were used to prepare the data for correlation analysis (Fox et al. 2005; Vincent et al. 2006). Data were first temporally filtered to retain only frequencies <0.08 Hz. Next, a series of “nuisance regressors” were created that captured physiological or other nonneural sources of variance. Regressors corresponding to the six translation/rotation parameters from the rigid-body motion correction captured variance related to the participants' movement over the course of the runs. A regressor corresponding to the signal averaged within a white matter ROI and another regressor corresponding to the signal averaged within a ventricular ROI were also defined. The signal averaged over a whole brain mask was used to capture nonspecific sources of variance including respiration. Finally, the first temporal derivative was included for each regressor to capture temporal shifts in the BOLD signal. The regressors were simultaneously entered into a regression analysis with the voxelwise, whole brain BOLD signal across time. The residual, representing the voxelwise time series controlled for physiological or spurious nonneural sources of variance, was retained for correlation analysis.
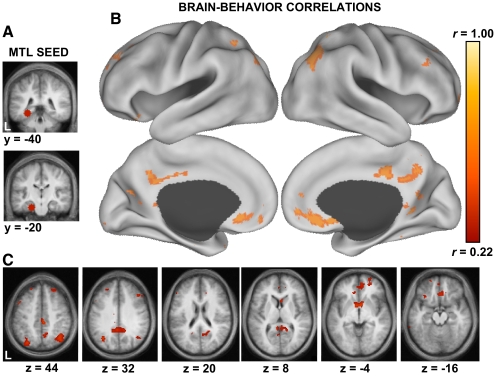
Seed-based correlation analyses were accomplished in two ways. First, a linear regression was performed between the time series averaged across all voxels within a combined 8-mm-radius PHC (−28, −40, −12) and 8-mm-radius HF (−22, −20, −26) a priori ROI shown in Fig. 9 (seeds defined in Andrews-Hanna et al. 2010) and the time series within every other voxel in the brain. The regression yielded correlation maps for individual subjects reflecting the strength of correlation between the MTL seed and each brain voxel. These individual subject maps were then entered into a regression analysis with the subjects' percentage of temporally oriented thoughts to examine which regions functionally coupled to the MTL predicted individual variability in the frequency of such thoughts (Fig. 9).
Fig. 9.
Frequency of thoughts about the past and future predicts medial temporal lobe (MTL)-cortical functional correlations. Whole brain functional correlations with the MTL were extracted from 139 participants and regressed against their reported frequency of temporally oriented thoughts (past + future). A: the MTL seed is shown on coronal slices and includes a combined hippocampal formation (HF) and parahippocampal cortex (PHC) region of interest. B: a surface projection (Caret software; Van Essen 2005) shows voxels that exhibit a correlation of r > 0.22 (P < 0.01) for the brain–behavior relationships. In other words, individuals that report a greater frequency of temporally oriented thoughts exhibit greater functional correlation between the MTL and regions within the default network that are associated with an MTL subsystem (see Andrews-Hanna et al. 2010; Buckner et al. 2008). Note that the topography of the correlations overlaps those associated with the default network as traditionally defined. C: the same analysis is shown on transverse slices.
In addition to whole brain measures of functional correlation, we also used a seed-target approach by examining the correlation strength between all a priori regions comprising an “MTL subsystem” within the default network (for detailed descriptions of the MTL subsystem, see Andrews et al. 2010; Buckner et al. 2008). These regions included HF, PHC, vMPFC, Rsp, and pIPL and represented a subset of those analyzed in experiment 1. As mentioned earlier, the regions were defined in a previous study that demonstrated the role of the MTL and its functionally correlated cortical regions for episodic thought (for coordinates and details, see Andrews-Hanna et al. 2010). When computing correlations between these multiple a priori regions, the averaged sets of time series within each region were cross-correlated with each other. In all cases, correlations were converted to Fisher's z correlation coefficients for statistical analyses because they are approximately normally distributed.
RESULTS
The default network is sensitive to changes in spontaneous cognition but not scope of external attention
Behavioral results.
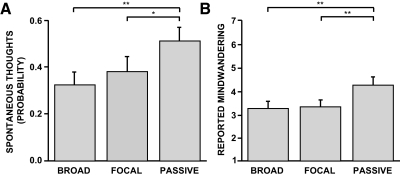
To confirm that the passive condition was characterized by increases in spontaneous cognition compared with the broad and focal external attention conditions, data from the behavioral thought sampling study were analyzed (similar to Binder et al. 1999). As predicted, participants experienced significantly more spontaneous thoughts when probed with tones during the passive condition (51.7 ± 6.1%) than during the broad (33.0 ± 5.5%) and focal (38.7 ± 6.3%) conditions (Fig. 2A; one-way repeated-measures ANOVA: F = 5.40, P < 0.01; passive vs. broad paired t-test: t = 3.25, P < 0.01; passive vs. focal paired t-test: t = 2.05, P < 0.05). No difference was observed between broad and focal conditions (broad vs. focal paired t-test, t = −1.07, P = 0.29). Postexperimental subjective mind-wandering reports for each condition mirrored the thought sampling results (Fig. 2B; one-way repeated-measures ANOVA: F = 11.1, P < 0.001; passive vs. broad paired t-test: t = 3.53, P < 0.01; passive vs. focal paired t-test: t = 3.56, P < 0.01; broad vs. focal paired t-test: t = −0.47, P = 0.65).
Fig. 2.
Thought sampling and poststudy probes demonstrate differences in spontaneous cognition between conditions. To confirm that conditions varied with respect to spontaneous cognition, an independent sample of 30 participants completed the experimental paradigm in a mock magnetic resonance imaging (MRI) scanner that allowed spontaneous thought sampling during the paradigm itself and follow-up self-report questionnaires after the study was completed. A: significantly more task-unrelated thoughts were observed during the passive condition compared with that of either the broad or focal attention conditions. B: participants also reported mind-wandering significantly more during the passive condition in a postexperimental questionnaire (1 = spontaneous thoughts were never experienced to 7 = spontaneous thoughts were always experienced). These results confirm the passive condition was associated with an increased tendency to engage in spontaneous cognition. Error bars reflect SE. *P < 0.05; **P < 0.01.
Participants detected a majority of the flickers, with no significant differences in accuracy and response time (RT) between the external attention conditions (broad accuracy: 81.2 ± 3.2%, focal accuracy: 76.4 ± 3.7%, paired t-test: t = 1.4, P = 0.18; broad RT: 613 ± 34 ms, focal RT: 613 ± 24 ms, paired t-test: t = 0.00, P = 0.99). Across all conditions, participants reported that their spontaneous thoughts were personally significant (mean = 5.26 ± 0.27; scale from 1 to 7, where 1 = never and 7 = always) and moderately goal-oriented in nature (mean = 4.16 ± 0.30).
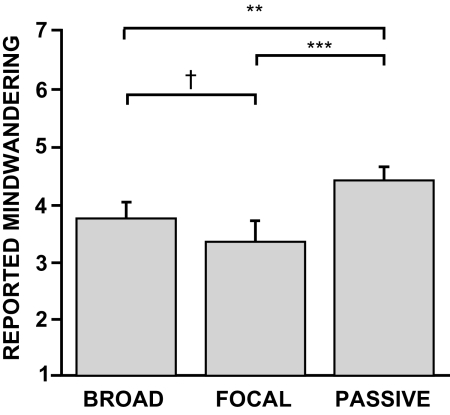
Of importance, the 30 young adults who performed the same experimental paradigm while lying in the MRI scanner (minus the thought probes) also reported mind-wandering significantly more during the passive condition compared with either the broad or focal external attention conditions (Fig. 3; one-way repeated-measures ANOVA: F = 10.6, P < 0.001; passive vs. broad paired t-test: t = 3.25, P < 0.01; passive vs. focal paired t-test: t = 3.82, P < 0.001). A nonsignificant trend toward mind-wandering more during the broad than focal condition was also observed (broad vs. focal: t = 1.88, P = 0.07). Across all conditions, participants again reported spontaneous thoughts that were personally significant (mean = 5.40 ± 0.24; scale from 1 to 7, where 1 = never and 7 = always) and moderately goal-oriented in nature (mean = 4.23 ± 0.31).
Fig. 3.
Poststudy questionnaires confirm differences in spontaneous cognition during the functional magnetic resonance imaging (fMRI) study. The same surprise postexperimental questionnaire administered to the participants that completed the thought sampling study (Fig. 2) was also administered to the independent group of participants that completed the experimental task while in the MRI scanner. Following the MRI study, participants reported mind-wandering significantly more during the passive condition in a postscanning questionnaire. A nonsignificant trend was observed between the broad and focal conditions, with numerically more reports of mind-wandering during the broad condition. Error bars reflect SE. **P < 0.01, ***P < 0.001, †P = 0.07.
Participants detected a majority of the peripheral and central flickers, with no difference in accuracy between the two conditions (broad accuracy: 87.6 ± 3.1%; focal accuracy: 88.2 ± 3.6%; paired t-test: t = −0.15, P = 0.88). A trend toward longer RTs was observed in the broad condition (broad RT: 656 ± 25 ms) compared with the focal condition (focal RT: 605 ± 21 ms; paired t-test: t = 1.97, P = 0.06).
Regional fMRI results.
Hypothesis-driven ROI analyses were performed using three a priori regions within the default network that were defined in a previous study (Andrews-Hanna et al. 2010) and overlap previous fMRI meta-analyses using our scanning and analysis procedures (e.g., Buckner et al. 2008, 2009). These ROIs included 1) a large composite region (mask) comprised of 11 identically sized midline and left-lateralized default network regions, 2) a region located in the aMPFC, and 3) a region located in the PCC. The aMPFC and PCC are considered “hubs” of the default network (Andrews-Hanna et al. 2010; Buckner et al. 2008) and have also been implicated in broad external attention in previous studies (see Hahn et al. 2007).
In support of the internal mentation hypothesis, activity within the default network ROI was significantly higher during the passive condition compared with that during the broad and focal conditions (Fig. 4A; one-way repeated-measures ANOVA: F = 46.2, P < 0.001; passive vs. broad paired t-test: t = 7.98, P < 0.001; passive vs. focal paired t-test: t = 9.22, P < 0.001). In contrast, we did not observe significant differences between the broad and focal conditions (broad vs. focal paired t-test: t = 1.54, P = 0.13). Similarly the aMPFC and PCC ROIs, paralleling the behavioral observations, exhibited increased activity during the passive condition with no difference between the broad and focal conditions (aMPFC: Fig. 4B, one-way repeated-measures ANOVA: F = 33.4, P < 0.001; passive vs. broad paired t-test: t = 7.14, P < 0.001; passive vs. focal paired t-test: t = 7.90, P < 0.001; broad vs. focal paired t-test: t = 0.89, P = 0.38; PCC: Fig. 4C; one-way repeated-measures ANOVA: F = 24.2, P < 0.001; passive vs. broad paired t-test: t = 6.34, P < 0.001; passive vs. focal paired t-test: t = 6.50, P < 0.001; broad vs. focal paired t-test: t = 0.59, P = 0.56). Thus initial results are consistent with the idea that the default network supports spontaneous cognition.
Temporal fMRI results.
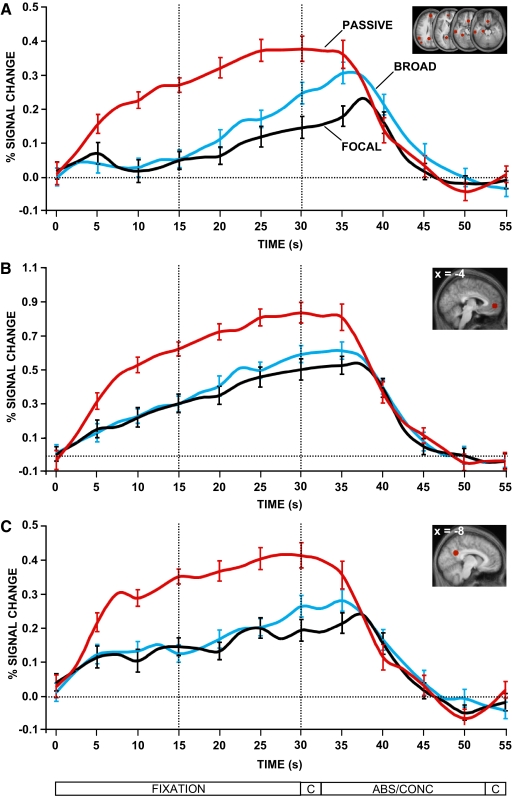
Next, we examined the time course of activity for the three conditions (Fig. 5). Activity within the passive condition increased rapidly as contrasted with either of the external attention conditions. More detailed inspection revealed that activity within the default network increased for both the broad and focal attention conditions as the epochs elapsed in time, more so for the broad condition compared with the focal condition (Fig. 5A). With the consideration that both conditions were associated with mind-wandering, these results are consistent with the possibility that participants tended toward mind-wandering as time progressed, with a slightly greater tendency in the broad condition. Critically, the time courses revealed no difference between the broad and focal attention conditions during the first 15 s of the fixation blocks when task motivation was presumably greatest. During this initial time period, there was an increase in default network activity in the passive condition compared with that in either of the two external attention conditions. Here, there was no evidence of a difference between the broad and focal conditions even at the level of a trend. Similar observations were observed for the time course of activity within the aMPFC (Fig. 5B) and the PCC (Fig. 5C).
Fig. 5.
The time course of activity within the default network across conditions. The time course of the BOLD signal is plotted for the critical fixation epochs for each condition. BOLD signal is measured as percentage signal change compared with the abstract/concrete baseline task. The timing of each block is outlined below (C = cue). Dotted lines separate fixation blocks into 2 distinct 15 s epochs. In all regions, there was an increase in activity in the passive condition compared with either the broad or focal attention conditions. This difference emerged rapidly with the onset of the manipulation. What is also apparent is that the activity observed in the broad and focal conditions in Fig. 4 is largely accounted for by activity increases that occur late in the task epochs. This is most clear in A where the activity in the broad and focal conditions is absent in the first 15 s of the block. Error bars reflect SE.
Broad attention demonstrated effects distinct from the default network
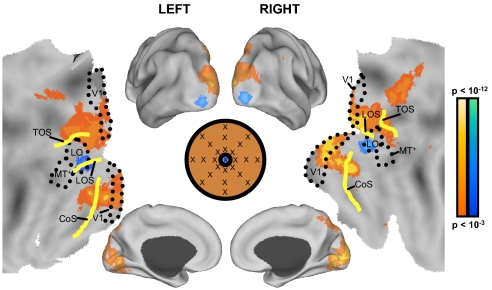
Next, whole brain exploratory analyses were conducted to directly examine the effects of broad external attention. Although previous ROI analyses did not reveal a role for the larger default network in broad attention, it is possible that smaller regions within the large ROI, or perhaps regions adjacent to those examined, support broad attention. As illustrated in Fig. 6, greater activity during the broad attention condition was observed in the dorsal and ventral aspects of the medial occipital cortex, including the putative human homolog of macaque area V6, the transverse occipital sulcus (TOS), collateral sulcus (CoS), parietotemporal sulcus (POS), as well as the posterior aspect of the precuneus (at or near area 7m). In contrast, a region near the lateral occipital area (LO) exhibited greater activity during the focal attention condition. Such findings correspond to those of previous studies examining differences in brain activity when viewing peripheral stimuli separately from focal stimuli (e.g., compare Fig. 6 in this study with Fig. 5 in Levy et al. 2004), but are inconsistent with studies highlighting the role of the default network in broad external attention (e.g., Hahn et al. 2007). Area V6 contains neurons with wide receptive fields (Pitzalis et al. 2006) and a region near LO is recruited during foveal stimulation (Hasson et al. 2002; Levy et al. 2001, 2004; Malach et al. 2002). Additionally, reports from patients with amblyopia, a condition marked by loss of foveal vision, highlight reduced fMRI activity in a similar LO region as that observed in the present study (Lerner et al. 2006).
Fig. 6.
Whole brain exploratory analyses demonstrate that the default network does not support broad external attention. Increased activity during broad attention compared with focal attention was observed in primary visual cortex, medial and lateral extrastriate cortex near the transverse occipital sulcus (TOS), and precuneus at or near area 7m (shown in red). In contrast, the focal attention condition elicited increased activity in the lateral occipital area (LO) and the lateral occipital sulcus (LOS) (shown in blue). Activity exceeding a threshold of P < 0.001 uncorrected is plotted on an inflated surface using Caret software (Van Essen 2005) as well as a flattened cortical map. Estimated areal boundaries are marked by a dotted line, whereas relevant sulci are highlighted in yellow.
The fMRI studies cited earlier have all concerned bottom-up, stimulus-driven attention. The conditions that were analyzed here included the fixation blocks from each run in which there were neither flickers nor false-positive responses. The consistency between the patterns of activation observed in the present study and the previous studies suggests that the conditions effectively modulated attention via top-down biasing mechanisms (also see Brefczynski and DeYoe 1999). Thus in addition to performance measures, results of the present functional analyses suggest that the manipulation of attentional scope was successful.
The whole brain analyses also yielded a result potentially relevant to reconciling past assertions that the default network is involved in some aspect of external attention. The default network is often loosely described as involving all of the precuneus, including regions along the dorsal extent of the midline that likely encroach on area 7m. Based on careful examination of macaque connectional anatomy and task activation in humans we have previously hypothesized that the region at or near the precuneus area 7m is likely not part of the default network (Buckner et al. 2008). Note that we use the term “at or near” because we cannot be certain of homology or areal boundaries in humans. Putative area 7m in humans, which is likely a component of the dorsal visual stream, is contiguous with regions of the posterior cingulate that receive widespread connections from distributed regions of cortex (for further discussion, see Marguelis et al. 2009). Thus we expect that the posterior cingulate, which is a prominent component of the default network, should dissociate from putative area 7m in the precuneus.
To test this possibility in the present data, we examined low-frequency spontaneous correlations with the putative area 7m in the precuneus in the rest state fixation runs acquired in the same group of participants (procedures analogous to Kahn et al. 2008; Vincent et al. 2006, 2008). The seed region centered at or near area 7m was defined as an 8-mm sphere surrounding the peak coordinate (16, −78, 56) extracted from the broad > focal contrast. Results revealed a strong dissociation. The precuneus region near putative area 7m was positively correlated with extrastriate and superior parietal regions, but was negatively correlated with the regions comprising the default network (Fig. 7). This result reinforces the possibility that putative area 7m within the precuneus is likely distinct from the regions comprising the default network. Moreover, this particular region is correlated with a network of regions involved in visual perception and attention. Thus it is important to study the functional properties of this region separately when analyzing the functions of the default network, especially in contexts that examine external attention.
Fig. 7.
Functional connectivity of the precuneus region at or near area 7m reveals it is not a component of the default network. An 8 mm sphere seed region centered at or near area 7m (16, −78, 56) from the broad > focal attention whole brain contrast in Fig. 6 was created (seed shown to the left). This region was used as a seed to examine low-frequency functional correlations during rest fixation runs in the same group of participants (similar to Kahn et al. 2008; Vincent et al. 2006, 2008). Warm colors represent voxels that exhibit positive correlations with the precuneus region at or near putative area 7m at a threshold of P < 0.0001 uncorrected, whereas cool colors represent voxels exhibiting negative correlations with the seed region. Note that a majority of the default network is negatively correlated with the precuneus, thus suggesting that precuneus area 7m and the default network belong to distinct brain systems (also see Buckner et al. 2008 for discussion).
Spontaneous cognition is frequently directed toward the past and the future
The previous analyses support the hypothesis that the default network participates in spontaneous cognition and is not primarily modulated by attention to the external environment, even when unpredictable stimuli in the periphery were the targets. Although past observations suggest that individuals engage in “freely wandering past recollection, future plans, and other personal thoughts and experiences” (Andreasen et al. 1995) and construction of “alternative hypothetical behavioral patterns to be ready for what may happen” (Ingvar 1979) during passive states, a quantitative characterization of what individuals think about under typical fMRI rest state conditions is presently lacking.
To explore the content associated with such spontaneous processes, we conducted another fMRI study in which a large sample of participants classified the categories and frequency of their mental activity after fixating on a crosshair for an extended duration (between 10 and 30 min). Participants completed a quantitative questionnaire, attributing the percentage of total time they spent thinking about each of 14 a priori categories of thought on a blank pie chart (Table 2). Overall, participants reported experiencing a diverse array of thought content during the passive epochs (mean = 7.1, SD = 2.5: different forms of spontaneous thought during the epochs of passive fixation). The selected categories and corresponding percentages, however, varied across individuals (Table 2). Participants spent very little time thinking about the actual fixation stimulus (mean = 6.3%, SD = 8.0%) or any other stimuli in the study (mean = 4.0%, SD = 6.0%). Additionally, participants reported their mind being blank only a small percentage of the time (mean = 5.0%, SD = 10.2%). Stimulus-independent thoughts associated with active mental activity were reported the majority of time, with a preference toward thinking about the past (mean = 19.2%, SD = 26.6%) and the future (mean = 28.7%, SD = 32.1%; Fig. 8; Table 2). Consistent with previous behavioral studies examining the temporal trajectory of autobiographical thoughts (D'Argembeau and Van der Linden 2004, 2006; Spreng and Levine 2006), the recent past and immediate future were attributed the highest percentage. These results suggest that personal thoughts about the recent past and near future dominate the focus of spontaneous cognition in fMRI paradigms that elicit activity across the default network.
Thoughts about the past and future correlate with functional interactions between the medial temporal lobe and cortical regions within the default network
The medial temporal lobe contains structures necessary for autobiographical recall (Scoville and Milner 1957; Squire et al. 2004) and recent observations suggest a role in imagination and prospection (Buckner 2010; Hassabis et al. 2007; Klein et al. 2002; Schacter and Addis 2009). Stark and Squire (2001) noted that hippocampal activity during passive states renders fixation a particularly poor experimental control condition for memory encoding tasks. Similarly, Christoff et al. (2004) reasoned that robust MTL activity during passive rest suggests that “long-term memory processes may form the core of spontaneous thought flow.” Furthermore, the MTL functionally correlates in a selective manner with components of the default network (Andrews-Hanna et al. 2010; Greicius et al. 2004; Kahn et al. 2008; Vincent et al. 2006) and tasks that require subjects to actively remember the past and envision the future show increased activity in the hippocampus and adjacent medial temporal structures as well as a number of core default network regions (Buckner et al. 2008; Schacter et al. 2007). Given the involvement of the MTL in the default network, we reasoned that the frequency with which participants engaged in temporally oriented thoughts would predict the nature of the interaction between the MTL and the default network.
To explore this relationship in the present experiment, we extracted individual functional correlation (fcMRI) maps with a seed region in the MTL in 139 participants. We then performed a whole brain regression of participants' fcMRI maps and their respective measures of thoughts about the past and future. Individuals who reported a higher percentage of such thoughts exhibited increased functional correlations between the MTL and several regions that fall within the default network, including vMPFC, Rsp, and pIPL (Fig. 9). Functional correlation with the pIPL highlighted in our previous studies (Kahn et al. 2008; Vincent et al. 2006) exhibited the most robust relationship. We recently demonstrated that the pIPL, vMPFC, and Rsp tightly couple together, comprising an MTL-correlated subsystem within the default network that supports episodic memory retrieval and future prediction (Andrews-Hanna et al. 2010). Interpreted in this context, these results provide support for a functional contribution of the default network in spontaneous episodic thoughts. However, it should be noted that the results are somewhat limited by their correlational nature. For example, episodic thoughts may covary with other forms of cognition such as retrieval of semantic information or overall frequency of spontaneous thoughts (e.g., Binder et al. 1999, 2009).
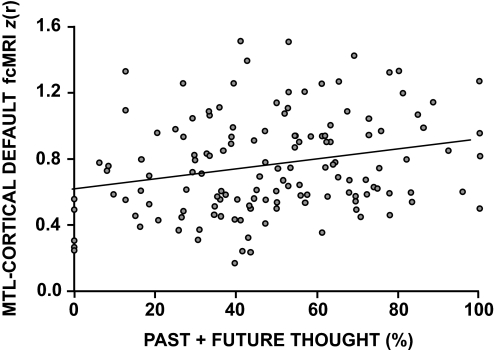
To confirm the brain–behavior relationship between frequency of spontaneous thoughts and functional coupling within the default network, all pairwise z-transformed correlation coefficients between regions comprising the MTL subsystem (vMPFC, Rsp, pIPL, PHC, and HF: regions defined in Andrews-Hanna et al. 2010) were averaged into a composite measure of subsystem strength. This composite measure was then regressed against the percentage of time individuals reported experiencing spontaneous thoughts about the past and future. The predicted positive relationship was observed between MTL fcMRI and temporally oriented thoughts (r = 0.18; P < 0.05; two-tailed t-test; Fig. 10). Note that the amount of variance accounted for in the low-frequency spontaneous BOLD fluctuation is small. As Fig. 10 shows, five subjects reported no past or future oriented thoughts (falling on the y-axis line) and showed among the lowest levels of MTL functional correlations. The group correlation remained significant when these individuals were removed from analysis.
Fig. 10.
Exploratory analysis of medial temporal lobe functional correlations. For each participant, the mean z-transformed correlation coefficients between all pairs of regions comprising the medial temporal lobe and its correlated cortical regions (HF, PHC, Rsp, pIPL, vMPFC) is plotted along with the percentage of time participants reported thinking about the past and the future during extended fixation epochs. A significant (but modest) positive relationship is observed between the 2 variables (r = 0.18; P < 0.05), suggesting that participants who think more about the past and/or the future exhibit increased functional coupling within the “MTL subsystem” of the default network.
Spontaneous low-frequency fluctuations are present during sleep and anesthesia, suggesting that the major contribution is likely unrelated to the cognitive state of the participant and more likely constrained by invariant anatomical connectivity (for discussion, see Fox and Raichle 2007; Van Dijk et al. 2010). The observation that changes in task state can modulate the strength of default-network coupling (e.g., Buckner et al. 2009; Fransson 2006; J Sepulcre, H Liu, T Talukdar, I Martinocorena, BTT Yeo, and RL Buckner, unpublished data) suggests that a portion of intrinsic default-network activity is related to cognitive processes that fluctuate over short timescales. The present results show that within the portion of spontaneous activity that is linked to awake, cognitive operations, a significant (but modest) amount of the variance across individuals is accounted for by participants' tendencies to think about the past and future.
DISCUSSION
Here we reveal multiple lines of evidence that implicate the brain's default network in spontaneous cognition. We first used fMRI to dissociate two plausible cognitive processes occurring during passive states that have previously been linked to the default network. Results revealed robust activity increases in the default network when participants were engaged in a passive task that maximized spontaneous cognition, compared with tasks that maximized focal and broad external attention. Next, to explore individual variability in spontaneous cognition and the neural correlates of this individual variability, we examined the relationship between functional activity correlations within the default network and spontaneous thoughts. Episodic thoughts about the past and the future were associated with increased functional coupling between the MTL and specific cortical regions within the default network. Collectively, our results provide evidence that the default network functions to support spontaneous cognition, particularly about significant personal past events and future goals.
Internally guided thought involves spontaneous projection of oneself into alternative settings
Our results suggest that humans use available moments to engage in spontaneous, internally directed thought. Spontaneous cognition (or “mind-wandering”) is a common and likely adaptive phenomenon (Klinger and Cox 1987; Singer and Antrobus 1963; Singer and McCraven 1961; for reviews see Buckner et al. 2008; Smallwood and Schooler 2006). We demonstrate here that even short epochs were sufficient to elicit prominent spontaneous cognition (>50% of time). Our results are consistent with prior reports using thought sampling techniques during blocks of passive fixation (Binder et al. 1999) and behavioral findings that note reciprocal relationships between attentional demands and spontaneous thoughts (Antrobus et al. 1966; McKiernan et al. 2006; Smallwood et al. 2008; Teasdale et al. 1995).
The content of participants' spontaneous thought speaks to its possible function. Klinger (1971) proposed the “current concern hypothesis,” suggesting that humans mind-wander primarily about matters of self-importance. Here we show that during short epochs, a majority of thoughts were personally significant in nature and more than half were oriented toward a particular goal. One participant reported thinking about “events that happened during the weekend [and] what's for dinner,” whereas another reported “what I'd do after leaving here—either go meet up with [my] significant other at his place or run home and get work done for tomorrow.” Still another wrote, “Well, I am moving in two days, so I find myself writing mental ‘to do’ lists and lists of things I had still to pack, and also just imagining life in a new apartment, new city, etc.” Consistent with these examples, quantitative analysis of the nature of participants' thoughts during longer passive epochs revealed that participants spent a majority of their time thinking about their past and future, especially the recent past and immediate future. Pondering over our recent past may allow us to consolidate significant events, whereas envisioning our personal future enables us to entertain plausible future scenarios, “experiencing” them before they happen (Atance and O'Neill 2001; Gilbert and Wilson 2007). Broadly speaking, these likely adaptive phenomena deserve additional scientific attention.
Activity increases within the default network are associated with spontaneous cognition
Given that humans spend a great deal of time engaged in spontaneous cognition, we suspected the presence of a brain system to support it. Although passive states encourage spontaneous cognition, they may also encourage widely different cognitive processes, including exploratory monitoring of the external environment or enhanced watchfulness for upcoming stimuli. Such externally oriented processes have been previously linked to the default network (Gilbert et al. 2005, 2006, 2007; Hahn et al. 2007). Because of the difficulty associated with measuring both external attention and spontaneous cognition during unconstrained tasks, the contribution of the default network to each of these processes has been difficult to disentangle (for discussion, see Buckner et al. 2008; Gilbert et al. 2007; Mason et al. 2007).
To help resolve this debate, the present study examined default network activity across three fixation conditions manipulated with respect to propensity for spontaneous cognition and attention to the external environment. Stimuli and responses were held constant—only expectations differed across conditions. Results revealed that the default network was sensitive to spontaneous cognition, but not to the scope of one's external attention, as would be predicted by hypotheses focused on broad attention. Thus our results are most consistent with the idea that the default network underlies the neural correlates of spontaneous cognition. However, it remains yet to be determined whether attentional scopes even broader than the confines of the visual display, attention to modalities other than vision, or diffuse background attention rather than goal-directed attention are better predictors of default network activity.
Medial temporal lobe and functionally correlated regions are associated with spontaneous episodic thought
The present results provide support for the role of the MTL and a wider cortical brain system in spontaneous episodic thought. Results from functional correlation analyses reveal a positive relationship between temporally oriented thoughts and activity correlations between the MTL and other regions comprising the default network. These regions include bilateral vMPFC, pIPL, and Rsp, all corresponding to the MTL subsystem within the default network that may support memory retrieval and prospection (Andrews-Hanna et al. 2010; Buckner et al. 2008). Task activation paradigms reveal similar brain regions when participants recall their personal past and envision their possible future (e.g., Addis et al. 2007; Andrews-Hanna et al. 2010; Botzung et al. 2008; D'Argembeau et al. 2010; Okuda et al. 2003; Szpunar et al. 2007). Our results suggest that the MTL subsystem is used spontaneously, in a manner that relates to episodic thoughts about the past and the future. Combined with a number of behavioral demonstrations revealing similarities between the two temporal domains (Addis et al. 2008; D'Argembeau and Van der Linden 2006; Spreng and Levine 2006), these results are consistent with the notion that individuals draw on details from their past and flexibly recombine these details into novel future events.
Early work conducted by the psychologist Edward Tolman highlighted the flexible recombination of past events in the rat (Tolman and Gleitman 1949; Tolman et al. 1946). More recently, spontaneous activity patterns within the hippocampus have been dissociated from the rats' immediate local cues, reminiscent of the findings in the present study. Consistent with the role of the MTL in memory and prediction, hippocampal activity patterns elicited during stopped periods reflect prior environmental input and, under certain conditions, correlate with the animal's future choices, including errors in behavior (Buckner 2010; Diba and Buzsáki 2007; Foster and Wilson 2006; Johnson and Redish 2007; Lisman and Redish 2009; Pastalkova et al. 2008). An interesting speculation is that these activity patterns emitted spontaneously in the rodent may be a protoform of the more complex and controlled human skills such as creativity and innovation. These complex, yet flexible cognitive processes likely rely on continual interplay between the MTL and the cortical regions that have come to be known as the “default network.”
An interesting line for future research will be to examine the putative role of the default network in particular aspects of internal mentation (i.e., autobiographical memory and prospection) in relation to other more basic functions such as retrieval of semantic information (Binder et al. 2009), integration of imagery-based information into a coherent scene (Andrews-Hanna et al. 2010; Hassabis and Maguire 2007), or rapid retrieval of contextual associations (Bar 2007). Many such processes are likely at play during periods of rest and may represent the building blocks of more complex processes such as reminiscing and planning. Interpreted in this context, thoughts about the past and the future measured in experiment 2 may index participants' tendency toward semantic retrieval or other such functions mentioned earlier, making the brain–behavior correlations difficult to interpret. Thus although the present results lend support to a link between the default network and spontaneous thoughts—especially about the past and the future—these thoughts may be a correlate of other cognitive processes. Again, a broad implication of the present results is a call for detailed and systematic study of spontaneous cognition and its adaptive functions.
GRANTS
This work was supported by National Institute on Aging Grant AG-021910 and the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank T. Hedden and R. Poulin for assistance with data collection; D. Schacter, M. Bar, I. Kahn, J. Vincent, F. Krienen, and three anonymous reviewers for helpful discussion; and A. Snyder for generously providing assistance in implementing the fcMRI processing tools.
REFERENCES
- Addis et al. 2007.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45: 1363–1377, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis et al. 2008.Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychol Sci 19: 33–41, 2008 [DOI] [PubMed] [Google Scholar]
- Andreasen et al. 1995.Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152: 1576–1585, 1995 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna et al. 2010.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron 65: 550–562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus et al. 1966.Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: experimental enhancement and suppression of spontaneous cognitive processes. Percept Mot Skills 23: 399–417, 1966 [Google Scholar]
- Atance and O'Neill 2001.Atance CM, O'Neill DK. Episodic future thinking. Trends Cogn Sci 5: 533–539, 2001 [DOI] [PubMed] [Google Scholar]
- Baars et al. 2003.Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci 26: 671–675, 2003 [DOI] [PubMed] [Google Scholar]
- Bar 2007.Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci 11: 280–289, 2007 [DOI] [PubMed] [Google Scholar]
- Binder et al. 2009.Binder JR, Desai RV, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder et al. 1999.Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93, 1999 [DOI] [PubMed] [Google Scholar]
- Biswal et al. 1995.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Res Med 34: 537–541, 1995 [DOI] [PubMed] [Google Scholar]
- Botzung et al. 2008.Botzung A, Denkova E, Manning L. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn 66: 202–212, 2008 [DOI] [PubMed] [Google Scholar]
- Brainard 1997.Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Brefczynski and DeYoe 1999.Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci 2: 370–374, 1999 [DOI] [PubMed] [Google Scholar]
- Buckner 2010.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol 61: 27–48, 2010 [DOI] [PubMed] [Google Scholar]
- Buckner et al. 2008.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function and relevance to disease. Ann NY Acad Sci 1124: 1–38, 2008 [DOI] [PubMed] [Google Scholar]
- Buckner et al. 2009.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29: 1860–1873, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff et al. 2009.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA 106: 8719–8724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff et al. 2004.Christoff K, Ream JM, Gabrieli JDE. Neural basis of spontaneous thought processes. Cortex 40: 623–630, 2004 [DOI] [PubMed] [Google Scholar]
- Damasio and Van Hoesen 1983.Damasio AR, Van Hoesen GW. Emotional disturbances associated with focal lesions of the limbic frontal lobe. In: Neuropsychology of Human Emotion, edited by Heilman KM, Satz P. New York: Guilford Press, 1983, p. 85–110 [Google Scholar]
- D'Argembeau et al. 2010.D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Salmon E. Modulation of medial prefrontal and inferior parietal cortices when thinking about past, present, and future selves. Soc Neurosci 5: 187–200, 2010 [DOI] [PubMed] [Google Scholar]
- D'Argembeau and Van der Linden 2004.D'Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: influence of valence and temporal distance. Conscious Cogn 13: 844–858, 2004 [DOI] [PubMed] [Google Scholar]
- D'Argembeau and Van der Linden 2006.D'Argembeau AD, Van der Linden M. Individual differences in the phenomenology of mental time travel: the effect of vivid visual imagery and emotion regulation strategies. Conscious Cogn 15: 342–350, 2006 [DOI] [PubMed] [Google Scholar]
- Diba and Buzsáki 2007.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci 10: 1241–1242, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele et al. 2008.Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA 105: 6173–6178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster and Wilson 2006.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440: 680–683, 2006 [DOI] [PubMed] [Google Scholar]
- Fox and Raichle 2007.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007 [DOI] [PubMed] [Google Scholar]
- Fox et al. 2005.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson 2006.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44: 2836–2845, 2006 [DOI] [PubMed] [Google Scholar]
- Fransson and Marrelec 2008.Fransson P, Marrelec G. The precuneus/posterior cingulate plays a pivotal role in the default mode network: evidence from a partial correlation analysis. NeuroImage 42: 1178–1184, 2008 [DOI] [PubMed] [Google Scholar]
- Gilbert and Wilson 2007.Gilbert DT, Wilson TD. Prospection: experiencing the future. Science 317: 1351–1354, 2007 [DOI] [PubMed] [Google Scholar]
- Gilbert et al. 2007.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought.” Science 317: 43b, 2007 [DOI] [PubMed] [Google Scholar]
- Gilbert et al. 2005.Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur J Neurosci 21: 1423–1431, 2005 [DOI] [PubMed] [Google Scholar]
- Gilbert et al. 2006.Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform 32: 45–58, 2006 [DOI] [PubMed] [Google Scholar]
- Greicius et al. 2004.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard and Raichle 2001.Gusnard D, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694, 2001 [DOI] [PubMed] [Google Scholar]
- Hagmann et al. 2008.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of the human cerebral cortex. PLoS Biol 6: e159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al. 2007.Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex 17: 1664–1671, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis et al. 2007.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA 104: 1726–1731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis and Maguire 2007.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci 11: 299–306, 2007 [DOI] [PubMed] [Google Scholar]
- Hasson et al. 2002.Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron 34: 479–490, 2002 [DOI] [PubMed] [Google Scholar]
- Hécaen and de Ajuriaguerra 1954.Hécaen H, de Ajuriaguerra J. Balint's syndrome (psychic paralysis of visual fixation) and its minor forms. Brain 77: 373–400, 1954 [DOI] [PubMed] [Google Scholar]
- Ingvar 1979.Ingvar DH. “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness: on the functional anatomy of the conscious state. Acta Neurol Scand 60: 12–25, 1979 [DOI] [PubMed] [Google Scholar]
- Johnson and Redish 2007.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci 27: 12176–12189, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn et al. 2008.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100: 129–139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff and Buckner 2006.Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron 51: 263–274, 2006 [DOI] [PubMed] [Google Scholar]
- Klein et al. 2002.Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: the effects of episodic memory loss on an amnesiac patient's ability to remember the past and imagine the future. Soc Cogn 20: 353–379, 2002 [Google Scholar]
- Klinger 1971.Klinger E. Structure and Functions of Fantasy New York: Wiley, 1971 [Google Scholar]
- Klinger and Cox 1987.Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imag Cogn Pers 7: 105–128, 1987 [Google Scholar]
- Lerner et al. 2006.Lerner Y, Hendler T, Malach R, Harel M, Leiba H, Stolovitch C, Pianka P. Selective fovea-related deprived activation in retinotopic and high-order visual cortex of human amblyopes. NeuroImage 33: 169–179, 2006 [DOI] [PubMed] [Google Scholar]
- Levy et al. 2001.Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci 4: 533–539, 2001 [DOI] [PubMed] [Google Scholar]
- Levy et al. 2004.Levy I, Hasson U, Harel M, Malach R. Functional analysis of the periphery effect in human building related areas. Hum Brain Mapp 22: 15–26, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman and Redish 2009.Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philos Trans R Soc Lond B Biol Sci 364: 1193–1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach et al. 2002.Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn Sci 6: 176–184, 2002 [DOI] [PubMed] [Google Scholar]
- Margulies et al. 2009.Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106: 20069–20074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason et al. 2007.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science 315: 393–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer et al. 2002.Mazoyer P, Wicker B, Fonlupt P. A neural network elicited by parametric manipulation of the attention load. Neuroreport 13: 2331–2334, 2002 [DOI] [PubMed] [Google Scholar]
- Mazoyer et al. 2001.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive function sustain the conscious resting state in man. Brain Res Bull 54: 287–298, 2001 [DOI] [PubMed] [Google Scholar]
- McGuire et al. 1996.McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport 7: 2095–2099, 1996 [PubMed] [Google Scholar]
- McKiernan et al. 2006.McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage 29: 1185–1191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan et al. 2003.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408, 2003 [DOI] [PubMed] [Google Scholar]
- Okuda et al. 2003.Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Sukuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and the past: the roles of the frontal pole and the medial temporal lobes. NeuroImage 19: 1369–1380, 2003 [DOI] [PubMed] [Google Scholar]
- Pastalkova et al. 2008.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science 321: 1322–1327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis et al. 2006.Pitzalis S, Galletti C, Huang R-S, Patria F, Committeri G, Galati G, Fattori P, Sereno MI. Wide-field retinotopy defines human cortical visual area v6. J Neurosci 26: 7962–7973, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli et al. 2005.Polli FE, Barton JJS, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci USA 102: 15700–15705, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo and Vecera 2002.Rizzo M, Vecera SP. Psychanatomical substrates of Balint's syndrome. J Neurol Neurosurg Psychiatry 72: 162–178, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter and Addis 2009.Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci 364: 1245–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter et al. 2007.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 8: 657–661, 2007 [DOI] [PubMed] [Google Scholar]
- Scoville and Milner 1957.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman et al. 1997.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663, 1997 [DOI] [PubMed] [Google Scholar]
- Singer 1966.Singer JL. Daydreaming: An Introduction to the Experimental Study of Inner Experience New York: Random House, 1966 [Google Scholar]
- Singer and Antrobus 1963.Singer JL, Antrobus JS. A factor-analytic study of daydreaming and conceptually-related cognitive and personality variables. Percept Mot Skills 17: 187–209, 1963 [DOI] [PubMed] [Google Scholar]
- Singer and McCraven 1961.Singer JL, McCraven VG. Some characteristics of adult daydreaming. J Psychol Interdisc Appl 51: 151–164, 1961 [Google Scholar]
- Smallwood et al. 2008.Smallwood J, McSpadden M, Schooler JW. When attention matters: the curious incident of the wandering mind. Mem Cogn 36: 1144–1150, 2008 [DOI] [PubMed] [Google Scholar]
- Smallwood and Schooler 2006.Smallwood J, Schooler JW. The restless mind. Psychol Bull 132: 946–958, 2006 [DOI] [PubMed] [Google Scholar]
- Spreng and Levine 2006.Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Mem Cogn 34: 1644–1651, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire et al. 2004.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Ann Rev Neurosci 27: 279–306, 2004 [DOI] [PubMed] [Google Scholar]
- Stark and Squire 2001.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98: 12760–12765, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar et al. 2007.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA 104: 642–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale et al. 1995.Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Mem Cogn 23: 551–559, 1995 [DOI] [PubMed] [Google Scholar]
- Tolman and Gleitman 1949.Tolman E, Gleitman H. Studies in learning and motivation: I. Equal reinforcements in both end-boxes, followed by shock in one end-box. J Exp Psychol 39: 810–819, 1949 [DOI] [PubMed] [Google Scholar]
- Tolman et al. 1946.Tolman E, Ritchie B, Kalish D. Studies in spatial learning. I. Orientation and the short-cut. J Exp Psychol 36: 13–24, 1946 [DOI] [PubMed] [Google Scholar]
- Van Dijk et al. 2010.Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103: 297–321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen 2005.Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage 28: 635–662, 2005 [DOI] [PubMed] [Google Scholar]
- Vincent et al. 2008.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent et al. 2006.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96: 3517–3531, 2006 [DOI] [PubMed] [Google Scholar]
- Weissman et al. 2006.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention Nat Neurosci 9: 971–978, 2006 [DOI] [PubMed] [Google Scholar]