Abstract
Taste cells use multiple signaling mechanisms to generate appropriate cellular responses to discrete taste stimuli. Some taste stimuli activate G protein coupled receptors (GPCRs) that cause calcium release from intracellular stores while other stimuli depolarize taste cells to cause calcium influx through voltage-gated calcium channels (VGCCs). While the signaling mechanisms that initiate calcium signals have been described in taste cells, the calcium clearance mechanisms (CCMs) that contribute to the termination of these signals have not been identified. In this study, we used calcium imaging to define the role of sodium-calcium exchangers (NCXs) in the termination of evoked calcium responses. We found that NCXs regulate the calcium signals that rely on calcium influx at the plasma membrane but do not significantly contribute to the calcium signals that depend on calcium release from internal stores. Our data indicate that this selective regulation of calcium signals by NCXs is due primarily to their location in the cell rather than to the differences in cytosolic calcium loads. This is the first report to define the physiological role for any of the CCMs utilized by taste cells to regulate their evoked calcium responses.
INTRODUCTION
Taste receptor cells are housed in taste buds in the oral cavity and detect chemicals found in potential food items. These taste stimuli activate distinct receptors on the taste cells that turn on specific signaling pathways (see reviews Lindemann 2001; Medler 2008). Sour taste stimuli rely on the detection of ions that activate ion-gated channels to cause cell depolarization and calcium influx through voltage-gated calcium channels (VGCCs) (Richter et al. 2003). These taste cells, also called type III cells, form conventional chemical synapses with afferent gustatory neurons (Medler et al. 2003; Yang et al. 2000; Yee et al. 2001). The chemical ligands involved in bitter, sweet, and umami tastes activate a G protein coupled receptor (GPCR) pathway to release calcium from internal stores via activation of phospholipase C β2 (PLC β2) (Zhang et al. 2003). The type II taste cells that use this GPCR pathway do not express VGCCs and lack conventional, chemical synapses (Clapp et al. 2006; DeFazio et al. 2006; Medler et al. 2003). In these taste cells, neurotransmitter is released onto afferent gustatory neurons or other potential targets via hemichannels that are activated by cell depolarization and a rise in intracellular calcium (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007, 2008). We recently identified a third population of taste cells that are bitter sensitive but express VGCCs. These taste cells produce bitter-evoked calcium responses that are significantly larger than the bitter responses found in type II cells and appear to use a PLC β3 signaling pathway to cause calcium release from stores (Hacker et al. 2008).
Regardless of the signaling mechanism used, all taste cells increase cytosolic calcium to produce normal synaptic signals (DeFazio et al. 2006; Huang et al. 2007; Romanov et al. 2007), but to date no studies have focused on how these calcium signals are terminated. We recently found that even in the absence of cell stimulation, cytosolic calcium in taste cells is regulated by mitochondrial calcium transport (Hacker and Medler 2008) and sodium calcium exchanger (NCX) activity (Laskowski and Medler 2009). While NCXs contribute to the regulation of basal calcium in taste cells, their potential role in the cell's recovery from stimulus-induced calcium elevations has not been investigated.
We isolated mouse taste receptor cells and recorded cytosolic calcium changes with fura 2-AM. External sodium was replaced with lithium to inhibit sodium/calcium exchanger (NCX) activity and taste cells were stimulated with either 50 mM KCl to depolarize the cell and cause calcium influx through VGCCs or with a tastant that causes calcium release from internal stores. Lithium cannot substitute for sodium in exchangers but will pass through sodium channels. As a result, replacing sodium with lithium allows for the selective inhibition of NCX activity (Blaustein and Santiago 1977). We found that inhibiting exchangers significantly changed the calcium influxes due to opening VGCCs but did not affect taste stimulus-induced calcium release from stores. This study provides the first evidence that NCXs selectively contribute to the regulation of evoked calcium signals in taste cells and provides new insight into their physiological role in these cells.
METHODS
Taste cell isolation
Mice were cared for in compliance with the University at Buffalo Animal Care and Use Committee procedures and were killed with CO2 and cervical dislocation. Taste receptor cells were harvested from circumvallate (CV) and foliate (Fol) papillae of C57BL/6 mice (n = 36) as previously described and were identified based on their characteristic morphology (Hacker et al. 2008). Taste cells have a bipolar morphology with their apical end extending microvilli into the oral cavity through a taste pore (Finger and Simon 2000). For our experiments, taste buds are aspirated from the surrounding epithelium and individual taste receptor cells are isolated from the taste buds. All experiments were performed on these isolated taste cells, which prevent any potential communication from other taste cells that may have otherwise influenced the results. An example taste receptor cell that has been loaded with Fura 2-AM is shown in Fig. S1.1
Calcium imaging
Isolated taste cells from the CV and Fol papillae were plated onto coverslips precoated with Cell Tak (BD Bioscience, San Jose, CA). Cells were loaded with 2 μM fura 2-AM (Molecular Probes, Invitrogen) containing the nonionic dispersing agent Pluronic F-127 (Molecular Probes, Invitrogen). Loaded cells were visualized using an Olympus IX71 microscope with ×40 oil immersion lens, and images were captured using a SensiCam QE camera (Cooke, Romulus, MI) and Imaging Workbench 5.2 (Indec Biosystems, Santa Clara, CA). During experiments, cells were kept under constant perfusion with Tyrode's solution followed by alternating changes in solutions. All solutions were bath applied using a gravity flow perfusion system (Automate Scientific, San Francisco, CA) and laminar flow perfusion chambers (RC-25F, Warner Scientific, Hamden, CT). Control experiments determined that there is approximately a 3 s delay between the onset of stimulus application and the stimulus contact with the target cell in our experimental set up. Stimulus application time is reported in the figures with no compensation for the delay due to stimulus delivery.
An evoked response was defined as an increase in fluorescence that was >2 SD above baseline. Data were graphed and analyzed using OriginPro 7.5 software. The amplitudes of the cytosolic calcium elevations were calculated as [(peak-baseline)/baseline]⁎100 and were reported as percent increases over baseline. We compared the percent increases over baseline to not confound the data due to differences in baseline calcium values. Response duration was measured as the length of time between the initial calcium increase of the response and the subsequent fall of the calcium response as it returns to baseline. This response duration was measured at half-peak height to determine how the initial kinetics of the response was affected when NCXs were not functioning. Most taste cells had baseline calcium values ranging from 50–150 nM and were analyzed based on previously described criteria (Laskowski and Medler 2009). Taste cells with a baseline calcium level >200 nM were not used in the analysis. Statistical comparisons were made using a one-way ANOVA with a Bonferroni's post hoc analysis or Student's t-test, either independent or paired as appropriate. The limit of significance was set at P < 0.05 and SE was reported.
Solutions
All chemicals were purchased from Sigma. Lithium Tyrode's was composed of Tyrode's solution with LiCl substituted for NaCl. Additional solutions were made by dilution with Tyrode's or lithium Tyrode's: bitter (10 mM denatonium benzoate), sweet (2 mM saccharin), umami (20 mM monopotassium glutamate), high-K [Tyrode's solution replacing 50 mM NaCl or 50 mM LiCl (in lithium Tyrode's) with 50 mM KCl], and low-K [Tyrode's solution replacing 20 mM NaCl or 20 mM LiCl2 (in lithium Tyrode's) with 20 mM KCl].
RESULTS
In an earlier study, we demonstrated that NCXs contribute to the regulation of basal cytosolic calcium in >90% of taste cells. When NCXs are inhibited by the replacement of external sodium with lithium, cytosolic calcium increases, even if the taste cell is not stimulated in any other way. This increase in cytosolic calcium is not due to the reverse mode activity of the exchangers but is the result of a constitutive calcium influx that is normally regulated by the exchangers. When the exchangers are inhibited, this constitutive calcium influx causes an increase in cytosolic calcium that cannot be compensated for until the exchangers are again functional (Laskowski and Medler 2009). Due to their physical contact with the external environment, taste cells can be exposed to variable ionic conditions. This may have created a need for these cells to be able to increase as well as decrease cytosolic calcium to routinely maintain physiologically appropriate basal calcium levels.
The fact that just inhibiting NCX activity elevates cytosolic calcium in taste cells was a complication that we had to consider in our analyses. To determine if the exchangers directly influence the stimulus-induced calcium signal, we had to account for the NCX effect on basal calcium levels within the overall calcium response that occurred when we inhibited the NCX activity and also applied the stimulus. We could then compare the adjusted calcium response to the stimulus-evoked calcium response that was measured while the NCXs were functional and determine if NCXs were significantly contributing to the clearance of the cytosolic calcium elevations generated by the different stimuli. While it is possible that the cytosolic calcium elevation due to inhibiting the exchangers could lead to calcium-dependent inactivation of the stimulus-evoked responses, within the time frame of these experiments, there was not a large increase in cytosolic calcium before the stimulus was applied. Therefore it is unlikely that there was a significant level of calcium dependent inactivation that would affect the evoked calcium responses.
NCXs significantly regulate calcium influx through VGCCs
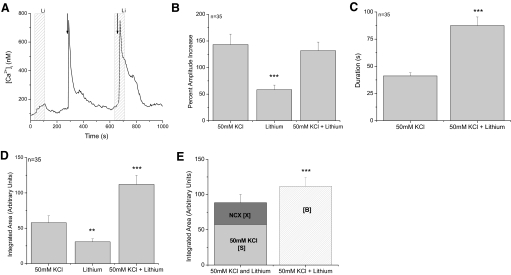
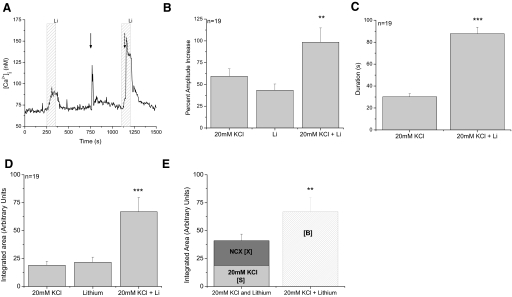
We first tested the effect of inhibiting NCXs on the taste cells' ability to recover from calcium influx through open VGCCs (Fig. 1). In the taste cells that express VGCCs, applying 50 mM KCl (10 s) to the cells caused a membrane depolarization that opened VGCCs and caused a calcium influx (Fig. 1A). The peak amplitudes of the 50 mM KCl-evoked calcium responses did not significantly change when NCXs were inhibited (Fig. 1B, P = 0.27, n = 35); however, inhibiting NCXs significantly increased the duration of the initial phase of the evoked calcium influx (Fig. 1C, P < 0.001). The duration of the response was only measured at half-peak height to get a view of the initial response kinetics and to determine if the NCXs affected this part of the calcium signal. Our findings indicate that NCXs contribute to the taste cell's ability to recover from the initial large calcium influxes on a relatively slow time scale but do not significantly contribute to the immediate, short-term buffering of the calcium influx that affects the peak amplitude.
Fig. 1.
Sodium-calcium exchangers (NCXs) significantly regulate calcium influx through voltage-gated calcium channels (VGCCs). A: replacing external sodium with lithium (light gray column) caused a reversible increase in cytosolic calcium levels while a 10 s application of 50 mM KCl (arrow) depolarized the taste cells and opened VGCCs. The calcium influx through the VGCCs generated a large increase in cytosolic calcium that slowly returned to baseline levels. B: inhibiting NCX activity alone generated a small increase in cytosolic calcium that was significantly smaller than the peak amplitude of the calcium response due to opening VGCCs (triple asterisk, P < 0.001, 1-way ANOVA with Bonferroni's post hoc analysis). Opening VGCCs while NCXs were inhibited did not affect the amplitude of the evoked response (P = 0.78). Data were plotted as average percent increase (with SE) over baseline calcium values. C: inhibiting NCX activity significantly increased the duration of the calcium response due to opening VGCCs (triple asterisk, P < 0.001, paired Student's t-test). Duration was measured at half-peak height. D: the area under the curve for each condition was integrated and compared using the 1-way ANOVA with a Bonferroni's post hoc analysis. 50 mM KCl + lithium was significantly larger than either 50 mM KCl or lithium alone (triple asterisk, P < 0.001). The area of the lithium induced response was significantly smaller than either 50 mM KCl or 50 mM KCl + lithium (double asterisk, P < 0.01). E: the integrated area under the 50 mM KCl + lithium elevation was significantly larger than the combination of the integrated areas reported in D for the calcium elevations that occurred when 50 mM KCl and lithium were applied individually (triple asterisk, P < 0.001, paired Student's t-test). Adding the area of the 50 mM KCl-evoked calcium response (light gray column, labeled [S]) to the integrated area of the calcium response when NCXs were inhibited (dark gray column, labeled [X]) was only 79% of the integrated area of the calcium response that was generated when VGCCs were open while NCXs were inhibited (striped column, labeled [B]).
Because these calcium responses are complex wave forms, we integrated the area under the curve to better understand how NCXs may influence the cytosolic calcium response due to the opening of VGCCs. Integrating the response provides a proportional measure of the amount of calcium inside the taste cell in response to stimulation. For simplicity, we will refer to this integrated area as the calcium load on the cell. The area under the curve was measured beginning at the onset of the calcium elevation until the calcium levels returned to baseline values. This differs from the peak amplitude of the response that can be used to measure two different aspects of the calcium signal: the peak calcium concentration that is reached in response to cell stimulation and a relative change in the peak amplitude compared with baseline calcium values. Because baseline calcium levels were somewhat variable (most baseline values ranged from 50 to 150 nM), we did not use absolute peak calcium concentration in our analysis because this value may be influenced by the baseline calcium values. Instead we compared the percent increase over baseline calcium levels; this allowed us to measure a relative change in cytosolic calcium levels. The measure of the percent calcium amplitude increase differs from the measure of the overall calcium load. A stimulus may not elicit a large peak increase in calcium levels but may still generate a relatively large calcium load if the calcium remains elevated for a longer period of time. Analyzing both the peak amplitude and the evoked calcium load allows us to define and compare different aspects of these calcium signals.
Even if the exchangers are not terminating the stimulus-induced calcium signal, they are still contributing to the regulation of basal calcium levels and will generate a cytosolic calcium load if inhibited. If NCXs are contributing to the removal of the stimulus-induced calcium load, their activity will increase when a stimulus is applied because they would now be compensating for the new calcium load on the cell in addition to their role in the maintenance of basal calcium. Under these conditions, the stimulus-induced calcium load (S) plus the calcium load due to inhibiting NCXs (X) would be less than the calcium load that resulted from concurrently applying the stimulus and inhibiting NCXs (B) because the exchangers would be contributing to the removal of the evoked calcium signal as well as regulating basal calcium levels. If the NCXs are not making a significant contribution to the termination of the stimulus-induced calcium signal, then the sum of the calcium load due to the stimulus (S) and the calcium load due to inhibiting NCXs (X) will be approximately equal to the calcium load when both events happen at the same time (B) because they are two separate events. We can summarize these ideas as: S + X ≅ B: NCXs are not terminating the stimulus signal but are still regulating basal calcium levels. S + X < B: NCXs contribute to both the termination of the stimulus signal and the regulation of basal calcium levels.
In Fig. 1, the average calcium load due to only inhibiting NCXs (X) was significantly smaller than the average calcium loads when VGCCs were opened (Fig. 1D, P < 0.01, n = 35). We also found that the average calcium load when both NCXs were inhibited and VGCC were open (B) was significantly larger than just opening VGCCs (S) (Fig. 1D, P < 0.001). Adding the average X [31 arbitrary units (AUs)] to the average S (58 AUs) generated a total calcium load that was only 79% of the average B (112 AUs). Therefore the calcium load on the taste cell when both events occurred at the same time (calcium influx through open VGCCs and calcium increase due to inhibiting NCXs) was significantly larger than the sum of the individual calcium loads that were due to either opening VGCCs alone or inhibiting NCXs alone (Fig. 1E, P < 0.001). These data suggest that NCX activity increases when there is a calcium influx through VGCCs and the exchangers significantly contribute to the clearance of this additional stimulus-induced calcium load on the cell.
NCXs do not significantly contribute to bitter-evoked calcium responses
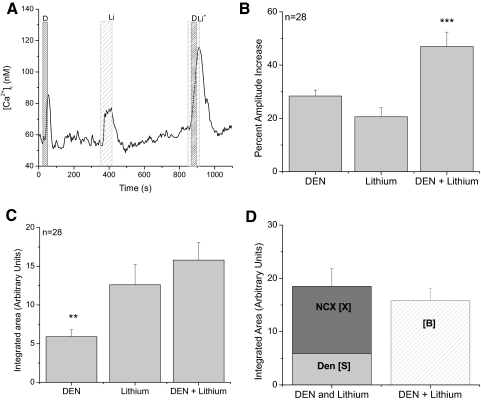
We used the same analysis paradigm to measure the effect of inhibiting NCXs on the denatonium-evoked calcium response (Fig. 2). Denatonium was used as a representative bitter stimulus which activates GPCRs to cause calcium release from internal stores (Akabas et al. 1988; Margolskee 2002; Ogura et al. 1997; Zhang et al. 2003). We chose to use 10 mM denatonium to maximally activate the evoked calcium response. In an earlier study, we found that 10 mM denatonium generated a maximum calcium response that was specific in its actions. Higher concentrations of denatonium (>10 mM) did not generate a larger calcium response, whereas lower concentrations generated smaller calcium elevations which were harder to detect. Compared with the calcium signal due to opening VGCCs with a strong depolarization, these calcium responses are significantly smaller (Hacker et al. 2008). Analysis using a one-way ANOVA determined that the peak amplitude of the response was significantly larger when denatonium-sensitive taste cells were activated in the absence of external sodium (Fig. 2B, P < 0.001, n = 28); this initially suggested that NCXs contribute to the formation of the bitter signal. Because these bitter responses are relatively slow and variable in their recovery times, we did not analyze the duration of the responses.
Fig. 2.
NCXs do not significantly regulate bitter-evoked calcium responses. A: replacing external sodium with lithium (light gray column labeled Li) caused a reversible increase in cytosolic calcium levels while a 30 s application of 10 mM denatonium benzoate (dark gray column labeled D) caused calcium release from internal stores. When bitter receptors were activated while NCXs were inhibited, the cytosolic calcium response increased. B: analysis of the average peak increases for denatonium, external lithium, and denatonium + external lithium revealed that denatonium + lithium was significantly larger than the amplitude of the responses to lithium or denatonium alone (ANOVA, triple asterisk, P < 0.001). C: the area under the curve for each condition was integrated and compared using the 1-way ANOVA with a Bonferroni's post hoc analysis. Denatonium alone generated a significantly smaller response than lithium alone or lithium + denatonium (double asterisk, P < 0.01). No differences between the areas of lithium or lithium + denatonium were found. D: the integrated area under the lithium + denatonium response (Li + Den, striped column, labeled [B]) was approximately equal to the combination of the integrated areas reported in C for the calcium signals that occurred when denatonium (light gray column, labeled [S]) and lithium (dark gray column, labeled [X]) were applied individually.
We also integrated the calcium response area to determine if the larger signal seen in Fig. 2B was due to an additive effect of the denatonium-induced calcium load (S) and the calcium load due to inhibiting NCXs (X) or if the differences in the peak values reflected a significant effect of inhibiting NCX activity on the cell's ability to recover from the denatonium-evoked calcium load. When we compared the calcium loads for S, X, and B, there was no significant difference between the average calcium loads for X and B (calcium load due to inhibiting NCXs and applying denatonium; Fig. 2C, P = 0.1) while the average denatonium-induced calcium load (S) was significantly smaller (Fig. 2C, P < 0.01). Adding S (6 AUs) and X (13 AUs) was ≅B (16 AUs; Fig. 2D). Therefore the increase in peak amplitude seen in Fig. 2B is likely due to the additive effects of two separate events: the denatonium-induced calcium release from internal stores and the inhibition of the NCXs at the plasma membrane. Based on these data, we concluded that NCXs do not play a significant role in the cell's recovery from the denatonium-induced calcium response.
NCXs do not significantly contribute to sweet- or umami-evoked calcium responses
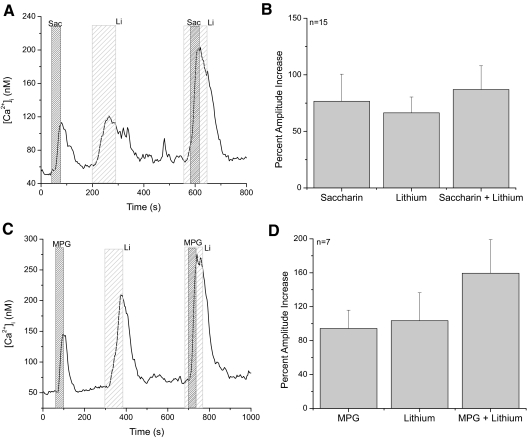
We repeated these experiments with a sweet stimulus (2 mM saccharin) and an umami stimulus (20 mM monopotassium glutamate, MPG) (Maruyama et al. 2006) to determine if our results for denatonium were specific to this stimuli or if this was a general trend for taste stimuli that depend on calcium release from internal stores. Figure 3A shows a representative calcium response in a saccharin-sensitive taste cell. There were no significant differences among the amplitudes of the saccharin-evoked responses, the lithium-evoked responses, or the saccharin + lithium responses (Fig. 3B, ANOVA, P = 0.78). Comparisons of the calcium loads for these responses also found no differences (data not shown, ANOVA, n = 14, P = 0.16). Analysis of the umami-evoked calcium responses in the presence and absence of functional NCXs (Fig. 3C) also found no significant differences in either the amplitudes of the responses (Fig. 3D, ANOVA, P = 0.33) or the calcium loads (data not shown, ANOVA, n = 7, P = 0.14). Taken together, these data support the conclusion that NCXs do not significantly contribute to the termination of taste responses that depend on calcium release from internal stores.
Fig. 3.
NCXs do not significantly contribute to sweet- or umami-evoked calcium responses. A: in sweet-sensitive taste cells, saccharin (2 mM, 30 s, dark gray striped column labeled Sac) caused an elevation in cytosolic calcium that was comparable to the calcium response due to inhibiting NCX activity (light gray striped column). Activating a saccharin response while NCXs were inhibited did not significantly increase the amplitude of the response (B, ANOVA, n = 14, P = 0.78). C: in umami-sensitive taste cells, application of monopotassium glutamate (MPG) for 30 s (20 mM, dark gray column) elevated cytosolic calcium. Replacement of external sodium with lithium (light gray striped column) elevated cytosolic calcium due to the inhibition of NCX activity. MPG in the presence of external lithium caused a larger increase in cytosolic calcium compared with either condition alone. D: analysis using 1-way ANOVA of the average peak increases for MPG, external lithium and MPG + external lithium found no significant differences in the response amplitudes (P = 0.33).
NCXs do not contribute to the termination of bitter-evoked calcium responses in dual-responsive taste cells
These data demonstrate that exchangers significantly contribute to the regulation of calcium influx through VGCCs but do not significantly affect calcium signals that depend on calcium release from internal stores. However, it was not clear if these differences were due to the physiological differences of the calcium loads or if there was something specific about the taste cell type that was influencing the different roles for the exchangers. The different taste cell types have many distinctive physiological characteristics. For example, the type II cells that are sensitive to bitter, sweet, or umami depend on calcium release from internal stores but do not express VGCCs. We have also found that there are significant differences in the amplitude of the calcium elevation due to inhibiting NCX activity in unstimulated taste cells that significantly correlates with taste cell type (Laskowski and Medler 2009). This finding led us to hypothesize that the different roles for exchangers in this study could be due to differences between the taste cell types. To test this hypothesis, we examined a subset of taste cells that express VGCCs but also release calcium from internal stores in response to bitter stimuli. These dual-responsive cells share some functional characteristics of type III cells and may be a subset of type III cells that we could use to determine if differences in cell type affect the role of the NCXs in the removal of the evoked calcium load.
Until recently, it was thought that the taste cells that are sensitive to bitter, sweet, or umami tastants and release calcium from internal stores in response to these tastants were only the type II cells that do not express VGCCs or chemical synapses (DeFazio et al. 2006; Zhang et al. 2003). However, we recently identified a population of taste cells that are sensitive to some bitter stimuli but also express VGCCs. These taste cells do not express the PLCβ2/IP3R3 signaling pathway and are not type II taste cells. While the signaling pathways in these taste cells have not been completely characterized, our initial studies indicate that these bitter responses are activating a PLCβ3/IP3R1 signaling pathway and depend on calcium release from internal stores even though these taste cells also express VGCCs (Hacker et al. 2008).
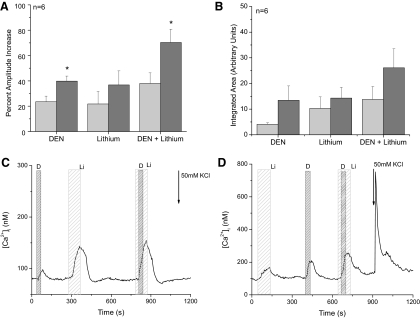
To determine if there were any taste cell type differences that could explain why NCXs were contributing to the termination of the calcium signals due to VGCCs but not to the calcium responses that depended on calcium release from stores, we measured the role of NCXs in this population of dual-responsive taste cells that can generate both types of calcium signals: calcium influx through VGCCs and calcium release from internal stores. When we compared the peak amplitude of the denatonium-evoked calcium responses in these dual-responsive taste cells (express VGCCs) to bitter-sensitive type II taste cells (which do not express VGCCs), we found that the amplitude of the denatonium-evoked calcium response was significantly larger in the dual-responsive taste cells (Fig. 4A, P < 0.05) which agrees with our earlier findings (Hacker et al. 2008). When NCXs were inhibited in the absence of cell stimulation for these two populations of taste cells, the average peak responses were larger in the dual-responsive taste cells but not significantly so (Fig. 4A, P = 0.36); this agrees with our earlier findings (Laskowski and Medler 2009). Finally, comparisons of the responses between these two cell populations when denatonium was applied while NCXs were inhibited found that the amplitude of the calcium responses in the dual responsive taste cells was significantly larger than in the type II taste cells (Fig. 4A, P < 0.05).
Fig. 4.
NCXs do not contribute to the termination of denatonium-evoked calcium responses in dual-responsive taste cells. A: comparison of the amplitude of the evoked calcium responses in type II taste cells (bitter sensitive and lack VGCCs, light gray columns) and dual-responsive taste cells (bitter sensitive and express VGCCs, dark gray columns) found that dual responsive taste cells generated significantly larger responses to denatonium compared with type II cells (Student's t-test, aterisk, P < 0.05). There were no differences in the amplitude of the lithium-induced calcium elevations (P = 0.86). In dual responsive taste cells, the amplitude of the denatonium + lithium response was significantly higher than the calcium response that was generated in type II taste cells under the same conditions (asterisk, P < 0.05). B: comparisons of the averaged integrated area for the type II (light gray columns) and dual-responsive taste cells (dark gray columns) in response to either denatonium, lithium external, or denatonium + lithium revealed no significant differences (P = 0.72). C: an example of lithium effects on a bitter-evoked calcium elevation in a taste cell that releases calcium from internal stores in response to bitter stimuli but does not express VGCCs. D: an example of the effect of inhibiting exchanger activity on the bitter-evoked calcium elevation in a dual responsive taste cell that releases calcium from internal stores in response to bitter stimuli and also expresses VGCCs.
Analysis of the peak responses for the denatonium, lithium, and denatonium + lithium responses in the dual-responsive taste cells generated comparable results to the findings reported in Fig. 2. As in the type II cells, the denatonium + lithium-evoked calcium response was significantly larger than the denatonium only response in the dual-responsive taste cells (n = 6, P < 0.05, data not shown). In addition, adding the peak amplitudes of the calcium responses to denatonium (S) and lithium (X) were approximately equal to the amplitude of the response when denatonium was applied while NCXs were inhibited in these cells (B). Further analysis of the response calcium loads found no significant differences between the evoked responses from either the type II taste cells or the dual-responsive taste cells (Fig. 4B, P = 0.72). These data indicate that the denatonium-evoked calcium signal and the calcium elevation that occurs when NCXs are inhibited are two separate events that both contribute to the overall increase in cytosolic calcium when they occur simultaneously. Therefore the NCXs do not significantly contribute to the termination of the stimulus-evoked calcium signal that depends on calcium release from internal stores, in either type II cells or in other populations of bitter-sensitive taste cells. Example traces of bitter only taste cells and dual-responsive taste cells are shown in Fig. 4, C and D. As stated in methods, all experiments were performed on isolated taste cells; this prevented the cell from receiving any signals from neighboring taste cells.
NCXs significantly contribute to the termination of calcium elevations due to influx through VGCCs regardless of calcium load
Because taste cell type did not appear to account for the differences in NCX activity that we had identified, it was still unclear what was driving this selectivity in the NCX activity. It could be that NCXs do not regulate the calcium signals that depend on internal stores because the calcium stores are not located in close proximity to the NCXs, which are restricted to the plasma membrane. Another possibility was the calcium load due to emptying calcium stores was too small to activate the NCXs. We designed the next set of experiments to answer the issue of load magnitude versus signal location. Taste cells were depolarized with 20 mM KCl (10s) instead of 50 mM KCl to open VGCCs. The calcium responses to 20 mM KCl were smaller than the calcium responses to 50 mM KCl (compare responses in Fig. 1A to Fig. 5A) but comparable in size to the calcium response due to release from internal stores. Therefore these experiments measured the effects of NCX activity on a small calcium load that originated at the plasma membrane. When 20 mM KCl was applied while NCXs were inhibited, the peak amplitude got significantly larger (Fig. 5, A and B, P < 0.01) and the duration of the response also significantly increased (Fig. 5C, P < 0.001).
Fig. 5.
NCXs significantly contribute to the termination of calcium elevations due to influx through VGCCs, regardless of calcium load. A: replacement of external sodium with lithium (light gray column) caused an elevation in cytosolic calcium, whereas a 10 s application of 20 mM KCl (arrow) slightly depolarized the taste cells to open VGCCs and generated a small calcium influx. This response was much larger when it was repeated while NCXs were inhibited. B: analysis using 1-way ANOVA of the average peak increases for 20 mM KCl, external lithium, and 20 mM KCl + external lithium revealed that the peak amplitude of the response for 20 mM KCl + lithium was significantly larger than the amplitude of the responses to 20 mM KCl or lithium alone (double asterisk, P < 0.01). C: inhibiting NCX activity significantly increased the duration of the calcium response due to opening VGCCs (triple asterisk P < 0.001, paired Student's t-test). Duration was measured at half-peak height. D: the area under the curve for each condition was integrated and compared using the 1-way ANOVA with Bonferroni's post hoc analysis. 20 mM KCl + lithium was significantly larger than either 20 mM KCl or lithium alone (triple asterisk, P < 0.001). E: the integrated area under the 20 mM KCl + lithium elevation was significantly larger than the combination of the integrated areas reported in D for the calcium elevations that occurred when 20 mM KCl and lithium were applied individually (double asterisk, P < 0.01, paired Student's t-test). Adding the area of the 20 mM KCl-evoked calcium response (light gray column, labeled [S]) to the integrated area of the calcium response when NCXs were inhibited (dark gray column, labeled [X]) was 60% of the integrated area of the calcium response that was generated when VGCCs were opened while NCXs were inhibited (striped column, labeled [B]).
When the taste cells were depolarized with only 20 mM KCl, the calcium load due to 20 mM KCl was comparable to the calcium load for the bitter-evoked calcium release from internal stores shown in Fig. 2C. When the taste cells were depolarized with 20 mM KCl while the NCXs were inhibited, the average calcium load for this response (B) was significantly larger than the individual responses (Fig. 5D, P < 0.001). Adding the average calcium loads for 20 mM KCl (S, 19 AUs) and lithium external (X, 22 AUs) generated a total area that was only 60% of the calcium load due to opening VGCCs while NCXs were inhibited (B, 67 AUs, double asterisks, P < 0.01, Student's t-test, Fig. 5E). These results parallel the findings when the cells underwent a stronger depolarization with 50 mM KCl. These data further support the conclusion that NCXs significantly contribute to the regulation and termination of the cytosolic calcium response due to calcium influx and suggest that this physiological role is due primarily to location within the cell and not to calcium load per se.
DISCUSSION
Taste cells are heterogeneous, both in their anatomy and in their function (DeFazio et al. 2006; Finger 2000; Kinnamon et al. 1985, 1988; Royer and Kinnamon 1988; Tomchik et al. 2007). Some taste cells depolarize and open VGCCs to activate neurotransmitter release through conventional chemical synapses while other taste cells lack VGCCs (Clapp et al. 2006; DeFazio et al. 2006; Medler et al. 2003) and rely on calcium release from internal stores to open a hemichannel and release neurotransmitter (Huang et al. 2007; Romanov et al. 2007). These functionally distinct taste cell populations coexist within a taste bud and have unique roles in the detection of taste stimuli (Clapp et al. 2004, 2006; DeFazio et al. 2006; Hacker et al. 2008; Medler et al. 2003; Roper 2007). It is well established that these two taste cell populations use different cellular mechanisms to increase cytosolic calcium when stimulated. However, nothing is known about their signal termination mechanisms even though calcium clearance mechanisms (CCMs) are critical to a cell's ability to form the appropriate cellular response when stimulated (Augustine et al. 2003; Berridge et al. 1998; Blaustein 1988; Bootman et al. 2002). Our study demonstrates that CCMs, specifically NCXs, have distinct roles in the regulation of evoked calcium signals within these two populations of taste cells.
This study is the first to describe a physiological role for any CCM in the termination of evoked calcium signals in taste cells. Previously we reported that exchangers are important in the routine regulation of basal calcium levels in taste cells (Laskowski and Medler 2009), and in this study we show that NCXs are also important in removing cytosolic calcium due to influx through VGCCs. This agrees with studies in other cell types that have established the importance of NCXs in the removal of cytosolic calcium after VGCCs opening (Blaustein and Lederer 1999; Gleason et al. 1994; Kim et al. 2005; Sher et al. 2008). In contrast, evoked calcium release from internal stores was not significantly regulated by NCXs in taste cells. These findings were consistent for all three types of taste stimuli that depend on calcium release from internal stores even though the amplitudes of the evoked responses for the bitter, sweet, and umami stimuli were variable. The average denatonium (bitter) peak response was only a 29% increase over baseline levels, while the average saccharin (sweet) response was 71% greater than baseline values and the MPG (umami) peak response was 94% larger than baseline calcium levels. Regardless of the calcium load that was placed on the taste cells, stimulus-evoked calcium release from internal stores did not appear to be regulated by NCXs.
The different roles for exchangers in taste cells with VGCCs versus taste cells that rely on calcium release from internal stores may be due to the selectivity of NCX isoform expression in these different taste cell populations. In fact, our earlier study found that the size of the calcium response due to inhibiting NCX activity significantly correlated with the different taste cell populations (Laskowski and Medler 2009). While we do not currently know if these findings are due to differences in specific NCX isoform expression or due to differences in the constitutive calcium leak channel that contribute to this calcium elevation (Laskowski and Medler 2009), this earlier study does demonstrate that functional differences in NCX activity likely exists between the different taste cell populations.
Our data indicate that neither taste cell type nor the magnitude of the calcium load was significantly influencing the physiological role of NCXs in taste cells. Instead it appears that the site of origin for the calcium signal in the cell is the primary factor determining if NCXs significantly regulate the calcium response due to cell stimulation. These data suggest that the NCX activity is a membrane limited effect and that these transporters are only able to sense localized calcium elevations that originate at the plasma membrane. This is somewhat surprising because taste cells do not have complex morphological compartmentalizations compared with many neurons. While taste cells are elongate polarized cells, they do not have axons or elaborate dendritic specializations (Finger and Simon 2000) that could easily compartmentalize the calcium signal and generate a microdomain that would restrict the site of calcium elevations within the cell. Taste receptor cells do extend microvillar structures into the oral cavity that may have functional microdomains that affect the initiation of the taste-evoked calcium signal. However, once the calcium release from internal ER calcium stores has been initiated, the calcium signal likely becomes more widespread within the cell. In fact, many taste cells that depend on calcium release from internal stores have subsurface cisternae (SSC) of smooth endoplasmic reticulum (ER) associated at close appositions between the taste cells and the adjoining nerve processes (Clapp et al. 2004). Therefore at least part of the ER that houses much of the internal calcium store is closely associated with the plasma membrane. Even though calcium release from internal stores is generally a global signal within cells, our data indicate that despite the physical proximity between the ER and plasma membrane, this calcium signal is sufficiently restricted within the taste cell to limit which CCMs contribute to its termination.
These findings are consistent with other studies in neurons that have shown that calcium signaling is usually restricted to microdomains to prevent the nonselection activation of calcium-dependent processes. The localization of a calcium signal within the cell is due to the diffusion limitations of the calcium signal and the proximity between the calcium channels where the signal originates and the calcium sensors that bind to the calcium ions and generate the cellular response (see review, Augustine et al. 2003). The importance of a particular calcium clearance mechanism in the formation of the cellular response will depend on where the CCMs are expressed relative to the channels that initiate the response. We have not yet localized where NCXs are expressed in taste cells, but based on their contribution to the calcium influx signals, we predict they are closely associated with VGCCs at presynaptic nerve terminals as has been reported in other neurons (Juhaszova et al. 1996; Luther et al. 1992; Reuter and Porzig 1995).
Our findings also suggest that the exchangers are not closely associated with the calcium stores that are responsible for the bitter-, sweet-, or umami taste-evoked calcium release from internal calcium stores and that other CCMs must regulate the termination of the calcium release signals. It is possible that NCX activity is important in the indirect regulation of these calcium responses because they may play a role in the loading and unloading calcium stores that has been suggested in other cell types (Blaustein and Lederer 1999; Blaustein et al. 2002). But these indirect effects reported in the other studies are longer-term effects than what we were measuring in this study. Future studies are needed to determine if the exchangers are co-localized with the calcium stores and if they affect the loading of calcium stores in these cells.
Finally, our data suggest that the presence of a particular calcium signaling mechanism influences the expression and physiological role of the calcium clearance mechanisms in taste cells. The heterogeneity of taste cells extends not only to the mechanisms that are responsible for initiating a calcium response but is also present in the mechanisms used by the cells to terminate the response.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grant DC-006358 to K. F. Medler.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Drs. E. Gleason and S. Medler for insightful comments.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Akabas et al. 1988.Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science 242: 1047–1050, 1988 [DOI] [PubMed] [Google Scholar]
- Augustine et al. 2003.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron 40: 331–346, 2003 [DOI] [PubMed] [Google Scholar]
- Berridge et al. 1998.Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature 395: 645–648, 1998 [DOI] [PubMed] [Google Scholar]
- Blaustein 1988.Blaustein MP. Calcium transport and buffering in neurons. Trends Neurosci 11: 438–443, 1988 [DOI] [PubMed] [Google Scholar]
- Blaustein et al. 2002.Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann NY Acad Sci 976: 356–366, 2002 [DOI] [PubMed] [Google Scholar]
- Blaustein and Lederer 1999.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999 [DOI] [PubMed] [Google Scholar]
- Blaustein and Santiago 1977.Blaustein MP, Santiago EM. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J 20: 79–111, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman et al. 2002.Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol 12: R563–565, 2002 [DOI] [PubMed] [Google Scholar]
- Clapp et al. 2006.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol 4: 7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp et al. 2004.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol 468: 311–321, 2004 [DOI] [PubMed] [Google Scholar]
- DeFazio et al. 2006.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26: 3971–3980, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger et al. 2005.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005 [DOI] [PubMed] [Google Scholar]
- Finger and Simon 2000.Finger TE, Simon SA. Cell biology of taste epithelium. In: The Neurobiology of Taste and Smell, edited by Finger TE, Silver WL, Restrepo D. New York: Wiley-Liss, 2000, p. 287–314 [Google Scholar]
- Gleason et al. 1994.Gleason E, Borges S, Wilson M. Control of transmitter release from retinal amacrine cells by Ca2+ influx and efflux. Neuron 13: 1109–1117, 1994 [DOI] [PubMed] [Google Scholar]
- Hacker et al. 2008.Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol 99: 1503–1514, 2008 [DOI] [PubMed] [Google Scholar]
- Hacker and Medler 2008.Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of Basal calcium levels in mouse taste cells. J Neurophysiol 100: 2177–2191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. 2007.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova et al. 1996.Juhaszova M, Shimizu H, Borin ML, Yip RK, Santiago EM, Lindenmayer GE, Blaustein MP. Localization of the Na(+)-Ca2+ exchanger in vascular smooth muscle, and in neurons and astrocytes. Ann NY Acad Sci 779: 318–335, 1996 [DOI] [PubMed] [Google Scholar]
- Kim et al. 2005.Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J Neurosci 25: 6057–6065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon et al. 1988.Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds. III. Patterns of synaptic connectivity. J Comp Neurol 270: 1- 10: 56–17, 1988 [DOI] [PubMed] [Google Scholar]
- Kinnamon et al. 1985.Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol 235: 48–60, 1985 [DOI] [PubMed] [Google Scholar]
- Laskowski and Medler 2009.Laskowski AI, Medler KF. Sodium/calcium exchangers contribute to the regulation of cytosolic calcium levels in mouse taste cells. J Physiol 587: 4077–4089, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann 2001.Lindemann B. Receptors and transduction in taste. Nature 413: 219–225, 2001 [DOI] [PubMed] [Google Scholar]
- Luther et al. 1992.Luther PW, Yip RK, Bloch RJ, Ambesi A, Lindenmayer GE, Blaustein MP. Presynaptic localization of sodium/calcium exchangers in neuromuscular preparations. J Neurosci 12: 4898–4904, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee 2002.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277: 1–4, 2002 [DOI] [PubMed] [Google Scholar]
- Maruyama et al. 2006.Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci 26: 2227–2234, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler 2008.Medler K. Signaling mechanisms controlling taste cell function. Crit Rev Eukaryot Gene Expr 18: 125–137, 2008 [DOI] [PubMed] [Google Scholar]
- Medler et al. 2003.Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci 23: 2608–2617, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura et al. 1997.Ogura T, Mackay-Sim A, Kinnamon SC. Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus. J Neurosci 17: 3580–3587, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter and Porzig 1995.Reuter H, Porzig H. Localization and functional significance of the Na+/Ca2+ exchanger in presynaptic boutons of hippocampal cells in culture. Neuron 15: 1077–1084, 1995 [DOI] [PubMed] [Google Scholar]
- Richter et al. 2003.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol 547: 475–483, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov et al. 2007.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. Embo J 26: 657–667, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov et al. 2008.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol 132: 731–744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper 2007.Roper SD. Signal transduction and information processing in mammalian taste buds. Pfluegers 454: 759–776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer and Kinnamon 1988.Royer SM, Kinnamon JC. Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers. J Comp Neurol 270: 11- 24: 58–19, 1988 [DOI] [PubMed] [Google Scholar]
- Sher et al. 2008.Sher AA, Noble PJ, Hinch R, Gavaghan DJ, Noble D. The role of the Na+/Ca2+ exchangers in Ca2+ dynamics in ventricular myocytes. Prog Biophys Mol Biol 96: 377–398, 2008 [DOI] [PubMed] [Google Scholar]
- Tomchik et al. 2007.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27: 10840–10848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. 2000.Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol 424: 205–215, 2000 [DOI] [PubMed] [Google Scholar]
- Yee et al. 2001.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 440: 97–108, 2001 [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2003.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]