Abstract
Attempts have been made in various studies to identify and trace changes in function in the aging visual system. Some results are conflicting and we report here a unique approach in an attempt to resolve selected issues. We have estimated neurometabolic coupling in the central visual pathway in young and old cats. Our technique provides high resolution simultaneous measurements of neuronal activity and changes in concentration of tissue oxygen in the thalamus of young and old cats. Following visual stimulation, we find shorter latency and time to peak in tissue oxygen responses in old compared with young animals. Estimates of local activity induced initial negative oxygen response show substantial reductions in older animals. Measurements of neural activity in the form of multiple unit activity are similar in the two age groups. To investigate the mechanisms underlying the changes in tissue oxygen response in older animals, we measured vascular capillary density and found it to be substantially lower in old than that in young animals. Together, these findings suggest that the changes in metabolic responses with age may be largely accounted for by alterations in the cerebral microvasculature rather than by changes in neural activity.
INTRODUCTION
Different techniques have been used in attempts to identify the nature and causes of deterioration of visual function with advanced age. These include behavioral (Mata et al. 2009; Solbakk et al. 2008; Verhaeghen et al. 2003) and single cell studies in the thalamus (Spear et al. 1994) and visual cortex (Schmolesky et al. 2000). There have also been anatomical studies of changes in microvascular density, ultrastructure, and shapes of arterioles and capillaries (Farkas and Luiten 2001; Riddle et al. 2003). In recent work, humans of advanced ages have been examined with application of noninvasive imaging techniques, mainly functional magnetic resonance imaging (fMRI) (Ances et al. 2009; Buckner et al. 2000; D'Esposito et al. 1999; Handwerker et al. 2007; Hesselmann et al. 2001; Riecker et al. 2003; Ross et al. 1997; Tekes et al. 2005). Although fMRI offers the clear advantage of examination of different parts of the brain of behaving human subjects, interpretation of the blood oxygen level dependent (BOLD) signal used in fMRI does not enable direct assessment of neural activity. When changes in the BOLD signal are observed, it is important to know whether the underlying basis is primarily neural or metabolic. A fundamental question then is whether age-related changes in visual function have a vascular or metabolic basis or are a consequence of neural changes or a combination of all these factors.
It has been shown in previous work that vasculature and metabolic processes are altered in the aged brain. Specifically, cerebral metabolic rate of oxygen (CMRO2) has been reported to decrease significantly with age in cortical and subcortical regions (Leenders et al. 1990; Takada et al. 1992; Yamaguchi et al. 1986). Age-related changes in microvasculature include reduced microvascular density, irregular ultrastructure, and altered arterial and capillary morphology (for a review, see Farkas and Luiten 2001). These changes could cause substantial hemodynamic and rheological alterations by inadequate movement of critical components between blood and parenchymal cells of the brain. A tentative conclusion from these studies is that age-related changes in cerebral blood flow (CBF), CMRO2, and microvascular density and morphology may underlie functional decreases in the aging brain.
What is missing in the work described earlier is combined measurements in a given preparation of both metabolic and neural activity in the aged brain. In recent work, we have employed a dual microelectrode device which enables simultaneous colocalized measurement of oxygen tension in brain tissue and extracellular neural activity (Li and Freeman 2007; Thompson et al. 2003, 2004, 2005). Oxygen tension, which is linearly related to tissue oxygen concentration (Shaw et al. 2002), is measured by one of the two microelectrodes. The other electrode measures extracellular activity simultaneously in a colocalized area of the brain, in this case the visual pathway. Brain tissue oxygen is related to the BOLD signal in fMRI as follows. Sensory stimulation, in this case through visual activation, causes an increase in oxygen metabolism because active neurons use oxygen for energy. Increased CBF is required to replenish the oxygen to support the activated neurons. This increases the concentration of tissue oxygen. The BOLD signal measures deoxyhemoglobin changes that occur in response to elevated neuronal activity as tissue oxygen is increased. Note, however, that an increase of tissue oxygen is directly related to a positive BOLD response, although a decrease in tissue oxygen is not necessarily associated with a negative BOLD response.
In previous work, we have determined that tissue oxygen responses in the visual cortex generally exhibit biphasic responses. Initially, there is a small negative component that is followed by a secondary large amplitude peak (Thompson et al. 2003). We assume that the initial dip reflects the first change in metabolic demand, which is an increase in oxygen metabolism. The secondary positive peak would then reflect the increase in blood flow required along with oxygen demand. Because the two phases of the biphasic response overlap in time, the amplitude and time course of each component are influenced by those of the other. By use of the precise retinotopic organization and small receptive field structure of lateral geniculate nucleus (LGN) neurons, we have been able to conduct separate studies of the negative and positive components of the oxygen response (Li and Freeman 2007; Thompson et al. 2004). This provides estimates of amplitudes and temporal properties for each component of the tissue oxygen response. In the current study, we have used these approaches to obtain simultaneous measurements of oxygen concentration and neural activity in the LGN of young and old cats. This provides a direct comparison of each related change in neural-metabolic coupling. We have used LGN instead of visual cortex because responses there are more robust and suitable for extended physiological study in aged subjects.
METHODS
We studied 32 young cats (6.2 ± 1.4 SD months of age; range, 4–10 mo) and 11 old animals (117 ± 16 SD months of age; range, 100–154 mo). From extensive previous tests, we have observed that cats of ≥3 mo postnatal exhibit neural responses in visual pathways that are mature and close to those of adults (DeAngelis et al. 1993). Cats >9 yr of age are considered senescent (Griffiths 1968). A cat aged 9 yr is roughly equal to a human of 60 yr of age.
Physiological preparation
Procedures were carried out in compliance with guidelines of the National Institutes of Health for the care and use of laboratory animals. All studies and examinations were conducted in accordance with a protocol for the Use of Animals in Research, approved by the Animal Care and Use Committee at University of California, Berkeley. Prior to each experiment cats were examined ophthalmoscopically to rule out obvious pathology or optical problems.
Anesthesia was induced with isoflurane (2–2.5%) and femoral veins were cannulated. Isoflurane was discontinued and anesthesia was continued with thiopental sodium delivered intravenously, combined with fentanyl (10 μg·kg−1·h−1). A tracheostomy was performed and a tracheal cannula was positioned. The animal was then artificially ventilated (25% O2-75% N2O) at a rate adjusted to maintain expired CO2 at 4–5%. Anesthesia was sufficient to allow the ventilator to control the breathing apparatus. A craniotomy was then performed at 6 mm anterior and 9 mm lateral to Horsley–Clarke zero. The dura was resected and the cortical surface was covered with agar and wax to reduce pulsation and create a closed chamber. During surgical procedures, a bolus of thiopental sodium (10 mg/ml) was administered as required. At the completion of surgery, fentanyl was discontinued and thiopental was set for continuous infusion as determined individually for each animal (generally 1–2 mg·kg−1·h−1). Eye movements were blocked with a continuous intravenous infusion of pancuronium bromide (0.2 mg·kg−1·h−1). Throughout each experiment, electroencephalogram (EEG), electrocardiogram (ECG), heart rate, temperature, intratracheal pressure, and end-tidal CO2 were monitored.
Tissue oxygen measurement
Oxygen responses were recorded by a Clark style polarographic oxygen sensor. The outer tip diameter of the sensor was about 30 μm. The spherical sensing region was about 60 μm in diameter (Unisense, Aarhus, Denmark). Before each use, the sensor was calibrated in a bath of 0.9% saline at 38°C and responses were observed for progressive changes in oxygen concentration for which there is a linear relationship. During each experiment, a micromanipulator was used to advance the sensor vertically from the cortical surface into the LGN via Horsley–Clarke coordinates A6L9. The oxygen sensor was connected to a high-impedance picoammeter that was polarized at −0.8 V. The currents generated by the sensor were amplified and sampled at 10 Hz. Recording sites were separated by ≥200 μm and data were collected when response oscillations in oxygen signals were minimal. Around 30% of potential recording sites were excluded because of large oscillations.
Neural activity
Neural activity was recorded by a platinum microelectrode housed adjacent to the oxygen sensor within one of the two barrels of the micropipette. Impedance of the neural microelectrode was generally 0.2–1 MΩ at 1 kHz in 0.9% NaCl solution. Neural signals were processed via an amplifier through a high impedence headstage. Neural activity was then filtered to isolate extracellular action potentials (0.25 to 8 kHz) and local field potentials (LFPs, 0.7–170 Hz). Action potentials were isolated by setting a threshold above background noise and collecting the resulting multiple-unit activity (MUA). The sampling rates for LFPs and MUA data were 500 Hz and 25 kHz, respectively. Time-dependent frequency analysis was applied to the LFP signal. The 60 Hz line noise and 85 Hz monitor refresh rate were removed by applying a Matlab function of noise removal (rmlinesc.m, Chronux toolbox). LFP spectrograms were then calculated on a 500-ms window and a 50-ms step size with a multitaper spectral estimation (Thomson 1982). The power of baseline activity in the prestimulus period (2 s) was subtracted from the LFP spectrograms. The power at each frequency and time bin was then normalized by the average power for that frequency in the baseline. For each recording site, modulation of LFP power was averaged over trials.
Visual stimuli
Drifting sinusoidal gratings were used as visual stimuli. After preliminary estimates of optimal stimulus parameters, tuning functions were obtained quantitatively by use of drifting sinusoidal gratings (mean luminance = 45 cd/m2). Contrast was kept at 100% and spatial and temporal frequencies were optimized. Stimuli were presented via optical control to the dominant eye. Each stimulus was presented for 4 s. During each test the unstimulated eye viewed a blank screen with the same mean luminance as that for the dominant eye. Receptive field positions were checked periodically and remapped around every hour. If a receptive field position changed, stimuli were adjusted and new data were collected.
Capillary morphometry
At the end of each experiment, an overdose of pentobarbital sodium was delivered and the animal was perfused with saline to remove circulatory fluids. Next, perfusions were made first with 1.5% formaldehyde, and then with saline, each for 10 min, to displace any excess in fixative. After overnight storage in formaldehyde, LGN was extracted with an adjustable angle blade mounted to a stereotaxic device. Tissue was stored overnight in gum-sucrose at 20°C. Sections (60 μm) were made of the tissue on a freezing microtome. Tissue was then stained using alkaline-phosphatase according to the following protocol adapted from procedures described previously (Kiernan 1999). Sections were exposed to a wash of distilled water and then incubated for 20 min at room temperature in a solution of 0.05 M Tris, α-naphthyl acid phosphate, MgCl2, and Fast Blue RR Salt. The sections were neutralized in 1% acetic acid and rinsed in water. Sections were then mounted onto slides and coverslips were positioned. Sections with poor stain quality (no clear capillary networks) were excluded from this analysis. For each section showing the track of the microsensor, a sampling region roughly 1,050 × 790 μm, close to the track, was selected for analysis. Digital images were collected with a camera mounted on a microscope system. An objective lens was used for analysis of all sections to maintain constant depth of field. Digital images were processed by use of Photoshop and each image was converted into a grayscale format. A threshold function was used to generate binary data and dust and scratch filters in Photoshop were used to remove impurities in the image. Capillary density was determined as the ratio of the number of black pixels, which represent alkaline phosphatase active capillaries, to the total number of pixels in the area of the image (Moody et al. 2004). All data were processed in a similar manner. The technician who did the analysis did not know the origin of the tissue, i.e., whether the data came from young or old animals.
Data analysis
For a given stimulus condition, multiple trials (16–64) were averaged to obtain oxygen responses at each recording site. Averaged responses were obtained for different interstimulus intervals, varied randomly between 30 and 44 s to obviate synchronization with oscillations in the oxygen signal. Main oxygen signals, prior to stimulus onset (10 s), were taken as the baseline. These values were subtracted from individual oxygen responses. Oxygen responses were averaged across all trials and normalized by use of mean oxygen levels. This process yields percentages of oxygen change (Thompson et al. 2003). An oxygen response is considered significant (P < 0.05, t-test) if there is a statistically significant change in peak or dip compared with the baseline signal. Similarly, an MUA or LFP response is considered significant if it is statistically different from spontaneous neural activity (P < 0.05, t-test). The response time of the oxygen sensor causes a delay of 0.2–0.5 s, which was individually subtracted in our data analysis. The time course of oxygen responses is considered monophasic if there is a significant change in one polarity without one in the opposite direction during the first 10 s after stimulus onset.
We used a permutation statistical test (9,999 resamples; Hesterberg et al. 2005) to estimate significance of differences between young and old groups in this study. The permutation test has a significant advantage because it does not rely on any theoretical probability models that may or may not be appropriate for the oxygen data. Error estimates in this report are in the form of SEs unless otherwise noted.
RESULTS
Oxygen responses
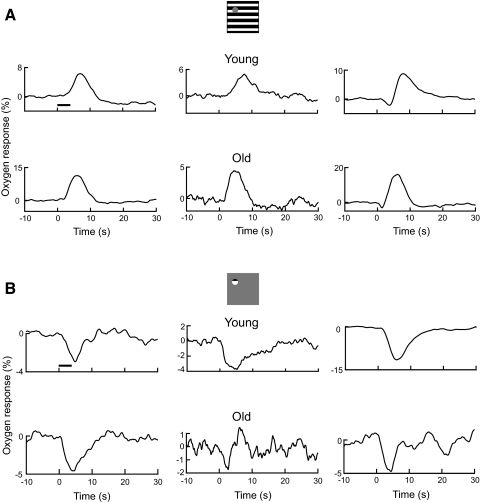
We studied 137 and 61 recording sites, respectively, in young and old cats. Out of all our samples, 14 and 8 recording sites, respectively, in young and old cats did not exhibit significant changes in oxygen responses to our visual stimuli patterns. Responses from these recording sites are therefore excluded from further analysis. In total, 123 and 53 recording sites respectively in young and old cats are included in the current study. About 50% of the recording sites in young cats are from our database. Two visual stimulus patterns were used to induce oxygen responses from the cat's LGN, as depicted in Fig. 1. The first stimulus, Fig. 1A, consists of a full field grating with a mask centered on the receptive field of the recording site. Because of precise retinotopic organization of the LGN, the stimulus activates responses in a large region except for a small population of cells proximal to the tip of the sensor. We have found in previous work that oxygen responses to a large stimulus are typically biphasic, with an initial negative dip component followed by a positive peak (Thompson et al. 2003). However, the stimulus with a small central mask, depicted in Fig. 1A, generally elicits a monophasic positive oxygen response (Li and Freeman 2007; Thompson et al. 2004, 2005). Examples of response patterns found for this visual target are shown in the remainder of Fig. 1A for young and old cats. Figures in the top panels depict representative oxygen responses for three recording sites in young cats. Compared with the baseline oxygen signal, all three examples show significant positive responses to the large stimulus (t-test, P = 0, 0.02, 0, respectively). The peak amplitudes and latencies in oxygen responses for the three examples are 6.3, 4.9, and 8.8% and 7.2, 7.8, and 8.1 s, respectively. Two (top left and top middle) of the three examples show a pattern of monophasic positive oxygen response, in which no initial dip is observed. The third example (top right) exhibits a biphasic oxygen response that consists of a small but significant initial dip (P = 0.03) followed by a large positive peak. This is a typical response pattern that we have observed previously in the LGN when a large visual stimulus without a central mask was presented (Thompson et al. 2004, 2005). Figures in the bottom panels of Fig. 1A represent oxygen responses for three recording sites in old cats. The peak amplitudes and latencies for the three examples are 11.3, 4.4, and 16.0% and 6.1, 4.5, and 6.3 s, respectively. Two examples (bottom left and bottom middle) exhibit a monophasic positive oxygen response (P = 0.01 and P = 0.047, respectively). For the third example (bottom right), a biphasic oxygen response is shown with a dip and peak, both of which are significant (P < 0.04 and P = 0, respectively).
Fig. 1.
Examples of tissue oxygen responses to visual stimuli. A: oxygen responses to large stimulus for young (top panels) and old (bottom panels) cats. Each curve represents an average oxygen response across multiple trials for a recording site. The horizontal line represents stimulus onset and duration. B: oxygen responses to small stimulus for young (top panels) and old (bottom panels) cats.
The second type of visual stimulus is depicted in Fig. 1B. In this case, the stimulus is a small grating, 1–2°, which is centered on the receptive field of the recording site. This stimulus activates a small population of cells and generally elicits monophasic negative oxygen responses in the LGN (Li and Freeman 2007; Thompson et al. 2004, 2005). Examples of response patterns to this stimulus are shown below the stimulus icon for young (top panels) and old cats (bottom panels) in Fig. 1B. Data shown here are from the same recording sites as those in Fig. 1A. All three examples from young cats demonstrate substantial and significant monophasic negative oxygen responses (P = 0.017, 0.005, and 0, respectively). The dip amplitudes and latencies for the three examples are −3.0, −3.8, and −11.5% and 5.0, 5.3, and 5.8 s, respectively. For old cats, the dip amplitudes and latencies for the three examples are −4.6, −1.7, and −4.7% and 4.2, 2.8, and 4.5 s, respectively. Significant monophasic negative oxygen responses (P < 0.01 and P = 0.02, respectively) are observed for two example recording sites (Fig. 1B, bottom left and bottom right). A dip is also observed in the other example (bottom middle) after the presentation of a visual stimulus. However, the response is not statistically significant (P = 0.3) due to large oscillations in the baseline oxygen signal. Note that for this recording site, there is a significant positive oxygen response to the large stimulus (Fig. 1A, bottom middle).
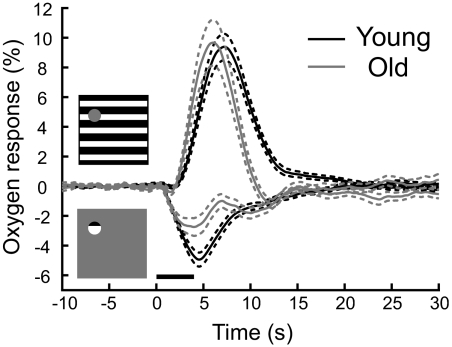
A summary of oxygen response patterns for young and old groups is shown in Fig. 2. The baseline oxygen tension for each recording site was subtracted to obtain the population-averaged oxygen responses for each age group. For young and old animals, the average baseline oxygen tensions are (mean ± SD) 40.8 ± 29.5 and 34.1 ± 25.8 mmHg, respectively. These values are not significantly different (P = 0.3). In Fig. 2, population-averaged oxygen responses to the large stimulus for young and old cats are represented by black and gray curves, respectively. Young and old cats exhibit similar mean amplitudes (9.4 ± 0.9 vs. 9.7 ± 1.5%, P = 0.9). However, for old cats, there is a relatively early latency prior to the peak value compared with that for young animals (7.3 and 6.2 s for young and old cats, respectively). For the second visual stimulus, different amplitudes and time courses are observed in the negative oxygen responses for young and old groups. Amplitudes of average negative oxygen responses are −4.9 ± 0.4% for young and −2.7 ± 0.6% for old cats (P < 0.01). Latency time periods to initial dips of average negative oxygen responses for young and old cats are 4.6 and 4.1 s, respectively.
Fig. 2.
Population-averaged oxygen responses in the lateral geniculate nucleus (LGN) are shown for 2 stimulus patterns. Black and gray curves represent oxygen responses for young and old cats, respectively. Dashed lines depict ±1SE. The population sizes for young and old cats are 123 and 53 sites, respectively.
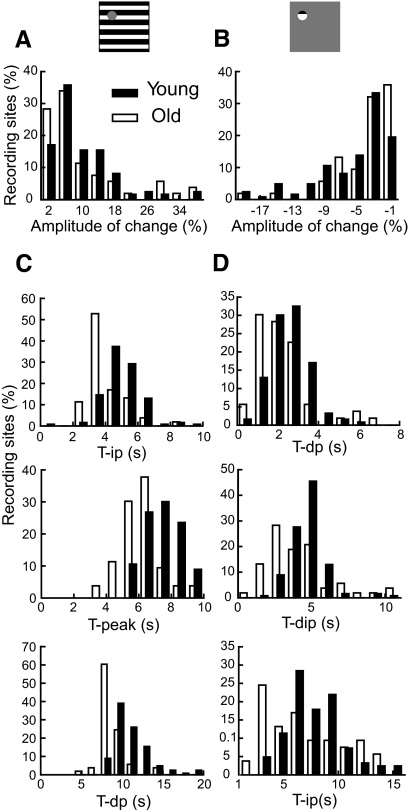
Oxygen responses vary substantially in amplitude and time course across recording sites for both age groups. In Fig. 3, A and B, histograms are shown that represent amplitudes of oxygen responses to the two visual stimulus patterns for young and old animals. In each case a normalization process was used for the total number of recording sites to facilitate comparisons for different sample sizes of young and old cats. For a given recording site, amplitude is defined as the maximum change in oxygen signal that occurs after stimulus onset. For both groups of cats, similar amplitudes of oxygen responses to the large stimulus were observed (10.5 ± 0.9% for young and 10.5 ± 1.5% for old cats, P = 0.99, Fig. 3A). However, the small stimulus elicits significantly different amplitudes in oxygen responses (−5.7 ± 0.4% for young and −3.9 ± 0.6% for old cats, P = 0.02, Fig. 3B). To evaluate time course of different aspects of the oxygen response, we define temporal points for the positive oxygen response at half the peak of increasing phase (tip), peak (tpeak), and half the peak of decreasing phase (tdp). For the negative oxygen response, we define temporal points at half the dip of decreasing phase (tdp), the dip (tdip), and half the dip of increasing phase (tip). For recording sites that do not exhibit monophasic positive or negative oxygen responses, the positive phase or negative phases are used, respectively, for responses to the large stimulus or small stimulus, to determine time parameters. For young and old animals in our study, four recording sites in each group do not exhibit monophasic positive oxygen responses to the large stimulus. For monophasic negative oxygen responses, 5 and 18 recording sites in young and old animals, respectively, do not show this type of response to the small stimulus. The histograms in Fig. 3, C and D illustrate time parameters for young and old cats for both stimulus patterns. The histograms associated with the large stimulus, depicted in Fig. 3C, show significant age associated temporal latency decreases in oxygen response patterns. Specifically, old animals exhibit earlier oxygen responses to the large stimulus than young cats. Averaged values for tip, tpeak, and tdp, for young cats, are 5.0 ± 0.1, 7.5 ± 0.1, and 11.1 ± 0.2 s, respectively. These are all significantly longer (P < 0.0001) than those for old cats (4.1 ± 0.1, 6.2 ± 0.1, and 8.7 ± 0.2 s for tip, tpeak, and tdp, respectively). For the negative oxygen response to the small stimulus, the difference in response times between the two age groups is also significant [Fig. 3D, tdp: 2.6 ± 0.1 s (young) and 2.3 ± 0.2 s (old), P = 0.03; tdip: 4.8 ± 0.1 s (young) and 4.2 ± 0.3 s (old), P = 0.02; tip: 7.9 ± 0.2 s (young) and 8.1 ± 0.5 s (old), P = 0.05].
Fig. 3.
Histograms are presented of amplitude and temporal latency values for tissue oxygen responses to the two stimulus patterns used in this study. Plotted is the mean percentage of all recording sites at each value of amplitude and latency. A: amplitude of the positive oxygen response to the large stimulus. B: amplitude of the negative oxygen response to the small stimulus. C: latencies of the positive oxygen response to the large stimulus. Top: T-ip; middle: T-peak; bottom: T-dp. D: latencies of the negative oxygen response to the small stimulus. Top: T-dp; middle: T-dip; bottom: T-ip.
Neural activity
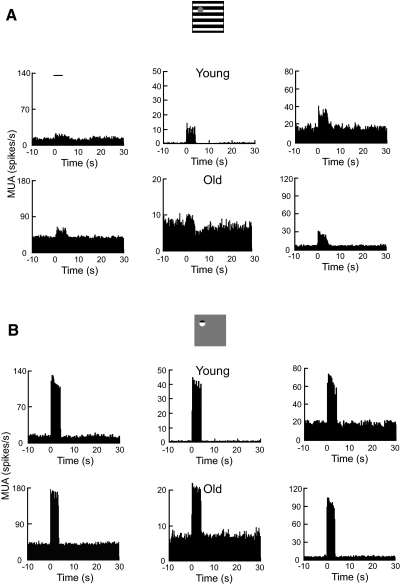
The analysis of oxygen responses described earlier clearly shows that temporal latencies are significantly shorter in the old animals. To gain a more complete understanding of this effect, we examined the neural responses that are associated with the oxygen measurements. Examples of neural data in the form of multiple-unit activity (MUA) are shown in Fig. 4 for young and old cats. Note that neural responses shown here are from the same recording sites presented in Fig. 1. For the three recording sites in young cats (Fig. 4A, top panels), average MUAs to the large stimulus are 6.4, 8.0, and 15.5 spikes/s, respectively. For the three examples in old cats (bottom panels), averaged MUAs are 6.8, 1.3, and 14.3 spikes/s, respectively. One example recording site in each age group (Fig. 4A, top left and middle bottom) did not exhibit significant MUA (t-test, P > 0.05) to the masked stimulus. All significant MUAs for both age groups peaked at about 0.2 s after the presentation of visual stimulus. For neural responses to the small stimulus, all example recording sites in both age groups fired vigorously and significantly (Fig. 4B). Average MUAs for the three examples in young cats are 101.3, 30.1, and 39.7 spikes/s, respectively (top panels). For the three examples in old cats, average MUAs are 103.8, 12.1, and 76.9 spikes/s, respectively (bottom panels). Similar to data shown in Fig. 4A, MUAs for all examples in Fig. 4B (top) were at about 0.2 s after stimulus onset.
Fig. 4.
Examples of neural responses (multiple-unit activity [MUA]) to visual stimuli. Data are from the same recording sites in Fig. 1. A: MUAs to the large stimulus for young (top panels) and old (bottom panels) cats. Spike responses are quantified as the average spike rate across trials during the stimulus. Abscissa zero represents the stimulus onset. The horizontal line represents stimulus onset and duration (4 s). Histograms before and after presentation of the visual stimulus represent spontaneous spike activities. B: MUAs to the small stimulus for young (top panels) and old (bottom panels) cats.
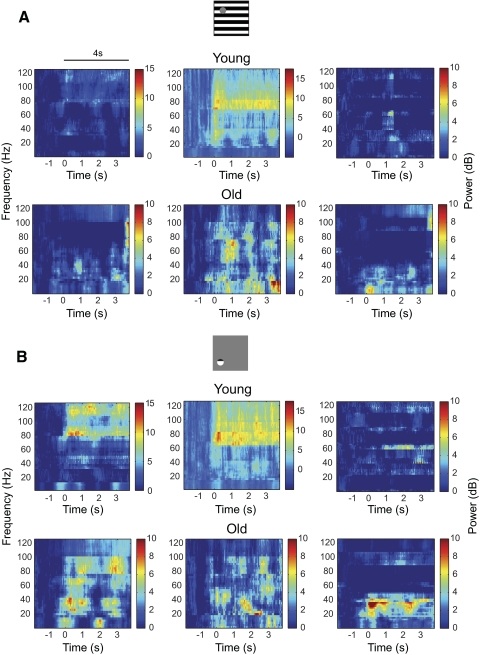
Figure 5 illustrates LFP spectrograms for the same recording sites shown in Figs. 1 and 4 for young and old cats. Graphs show average LFP power across trials. To estimate the significance of LFP responses, average LFP power across the frequency band (1–125 Hz) for stimulus duration of 4 s was compared with the baseline LFP signal 2 s prior to stimulus onset. For the three recording sites in young cats (Fig. 5A, top panels), averaged LFPs to the large stimulus are 0.6, 1.9, and 0 dB, respectively. Significant LFP response was observed for one example recording site (top middle, P = 2.9 × 10−5) but not for the other two sites (top left and top right, P = 0.3 and P = 0.5, respectively, t-test). For the three recording sites in old cats (Fig. 5A, bottom panels), two exhibited significant LFP responses (left and middle panels) while the other one showed insignificant LFP response to the stimulus. The average LFP responses are 1.7 (bottom left, P = 0.001), 2.1 (bottom middle, P = 0.01), and 0.3 (bottom right, P = 0.8), respectively. Figure 5B illustrates LFP responses to the small stimulus. For three recording sites in young cats (top panels), average LFP responses are 3.0 (top left, P = 5.1 × 10−7), 3.1 (top middle, P = 1.4 × 10−9), and 0.1 (top right, P = 0.6) dB, respectively. For three recording sites in old cats (bottom panels), average LFP responses are 2.8 (bottom left, P = 9 × 10−8), 1.6 (bottom middle, P = 0.04), and 0.9 (bottom right, P = 0.02) dB, respectively.
Fig. 5.
Examples of local field potential (LFP) spectrograms from the LGN to visual stimulation. Data are from the same recording sites as in Figs. 1 and 4. Spectrograms were obtained by taking the average modulated power across trials. The horizontal line represents stimulus onset and duration. A: LFPs to the large stimulus for young (top panels) and old (bottom panels) cats. B: LFPs to the small stimulus for young (top panels) and old (bottom panels) cats.
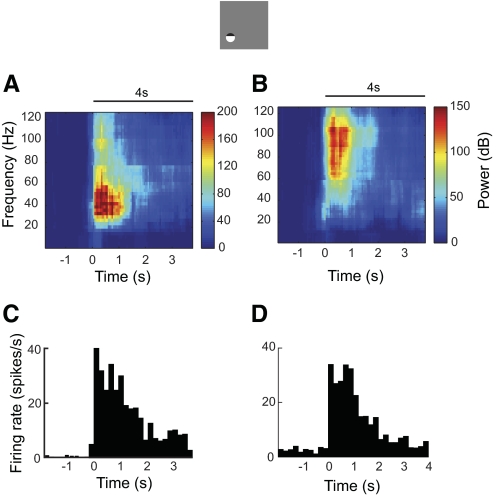
In general, our analysis shows that LFP measurements in the LGN of the thalamus tend to be noisy, weak, and variable, as reported in previous work (Rasch et al. 2009). For a recording site in each age group, we observe significant MUAs (Fig. 1A, top right and bottom right) and tissue oxygen responses (Fig. 4A, top right and bottom right) but insignificant LFPs (Fig. 5A, top right and bottom right) to the large stimulus. For a recording site in young animals, significant MUA (Fig. 4B, top right) and tissue oxygen responses (Fig. 1B, top right) were elicited by the small stimulus. However, no significant LFPs were observed (Fig. 5B, top right). We also note that LFP response in the LGN exhibits different power distributions than those from cortex. Figure 6 illustrates two example recording sites in area 17 in the cat. Both recording sites exhibit significant LFPs (Fig. 6, A and B) and MUA (Fig. 6, C and D). The average MUAs are 16.6 (Fig. 6C, P = 0) and 14.3 (Fig. 6D, P = 0) spikes/s, respectively. The average LFPs are 39.8 (P = 2.8 × 10−4) and 30.2 (P = 2.1 × 10−12) dB, respectively. Note that the major increase in power is between 25 and 70 Hz (Fig. 6A) or between 55 and 110 Hz (Fig. 6B). This is consistent with results in previous studies (Henrie and Shapley 2005; Logothetis et al. 2001; Viswanathan and Freeman 2007). LFP response in the LGN, however, is spread over frequencies between 1 and 125 Hz. Because LFP is believed to reflect the superposition of synchronized dendritic currents that are averaged over a large region of tissue (Mitzdorf 1987), the difference in LFPs between LGN and cortex may be accounted for by variations in excitatory and inhibitory connections in and around these two brain regions.
Fig. 6.
Two examples of neural responses to visual stimulation in area 17. Neural activity was recorded by the dual-purpose microsensor. Electrode penetrations were made along the medial bank of the postlateral gyrus from Horsley–Clarke coordinates P4L2 at an angle of 10° medial and 20° anterior. Sinusoidal drifting gratings at preferred orientation, spatial frequency, temporal frequency, and size were presented to the dominant eye while the other eye viewed a blank screen. Stimulus contrast was 50%. Horizontal lines represent stimulus onset and duration. (A, B) LFP spectrograms and (C, D) MUAs for 2 example recording sites.
Population-averaged neural data are shown together with oxygen responses from young and old cats in Fig. 7 for the two types of stimulus pattern. Spontaneous MUA is subtracted individually for each recording site. The average spontaneous MUAs are 11.6 ± 1.0 and 13.5 ± 1.7 spikes/s for young and old cats, respectively. The difference is not significant (P = 0.3). For the large stimulus (Fig. 7A), averaged MUAs are closely similar in the two age groups (14.5 ± 1.4 and 13.4 ± 2.3 spikes/s for young and old cats, respectively) (permutation test, P = 0.7). This finding is consistent with the result that young and old cats show similar amplitudes in the positive oxygen response to the large stimulus. However, the age associated temporal alteration in positive oxygen response is not matched by analogous temporal changes in neural response. Averaged MUAs for young and old cats have identical time delays from stimulus onset (0.2 s). Results for the small stimulus (Fig. 7B) are similar to those for the large grating, i.e., no significant differences are found between young and old cats in amplitude and response time in averaged neural activity. Averaged MUAs for young and old cats are 64.9 ± 3.3 and 59.5 ± 6.3 spikes/s, respectively. These results are not significantly different (P = 0.4). As in the case of the large stimulus, MUAs for the small grating show a 0.2 s time delay from stimulus onset. Clearly, the time advance in tissue oxygen response is not matched by a similar temporal change in neural activity.
Fig. 7.
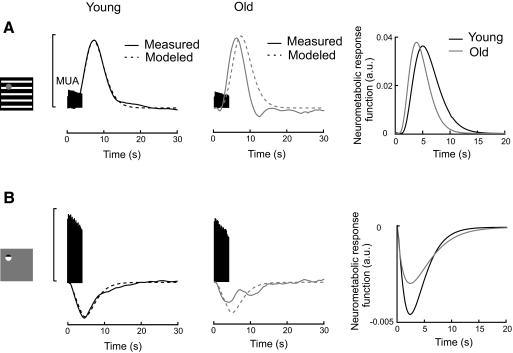
Changes in neurometabolic coupling with extended age. A: changes in neurometabolic coupling for the large stimulus. Population-averaged peristimulus MUAs are plotted with measured (solid lines) and modeled (dashed lines) positive oxygen responses (left and middle). For young cats (left), the modeled oxygen response is generated by convolving the MUA with a neurometabolic response function. The neurometabolic response function is estimated as a gamma function with which the averaged MUA is convolved to best fit the averaged oxygen response. Vertical scale bar represents normalized MUA (70 spikes/s) and percentage change of oxygen signal (10%). For old cats (middle), the modeled oxygen response is generated by convolving the MUA from old cats and the neurometabolic response function from young animals. Figure on right illustrates neurometabolic response functions for young (black curve) and old (gray curve) cats. Each neurometabolic response function is modeled from the oxygen response and MUA observed in each group. B: changes in neurometabolic coupling for the small stimulus in the same format as for A. Neurometabolic response functions on right in A and B are in arbitrary units (a.u.).
Neurometabolic coupling
Our results show clear age-related differences in oxygen responses but not in neural activity in the LGN. To examine this apparent change of neurometabolic coupling with age, we have examined a model of oxygen response. For the neurometabolic response function, we have chosen to use a gamma function that well models hemodynamic responses (Boynton et al. 1996) and tissue oxygen responses (Li and Freeman 2007). Averaged neural activity is convolved with the gamma function to derive averaged fits of oxygen responses. Data fitting is accomplished with the fminsearch function in Matlab. The function we use for young animals is then convolved with MUAs of young and old cats to obtain modeled oxygen responses. These are then compared with corresponding measured oxygen responses as illustrated in Fig. 7, which shows averaged neural and oxygen responses for young and old cats for small and large visual stimuli. Results for the large visual stimulus, shown in Fig. 7A (left), indicate close approximation between modeled (dashed black curve) and measured (solid black curve) oxygen responses for young cats. The goodness of fit R2 is 1.0. To estimate how neurometabolic coupling changes with age, the neurometabolic response function for young cats is then convolved with MUA data of old animals to yield a modeled oxygen estimation for aged cats. The modeled oxygen response for old animals is expected to match that of the measured one if neurometabolic coupling does not change with age. In this case the modeled oxygen response (Fig. 7A, middle panel, dashed gray curve) exhibits a time delay in comparison with the measured data (Fig. 7A, middle panel, solid gray curve) (R2 = 0.45). This implies that the neurometabolic response is faster in old compared with that in young animals for large visual stimuli that activate cells in a large region of LGN. Results for the small visual stimulus, illustrated in Fig. 7B, show somewhat analogous differences between modeled and measured oxygen responses for young and old cats. In this case, measured and modeled responses for young animals are closely similar and R2 = 0.95. However, for the older animals, there is again a difference with R2 = 0.43.
As already noted, small and large visual stimuli produced negative and positive oxygen responses, respectively. We now compare directly neurometabolic response functions for small and large stimui for young and old cats. Each neurometabolic response function is obtained from measured oxygen response and neural activity for each group and expressed in arbitrary units (a.u.). For the large stimulus, the neurometabolic response function for old cats (Fig. 7A, right, gray curve) exhibits an earlier peak compared with that for young animals (Fig. 7A, right, black curve) (3.8 vs. 5.0 s). Response amplitudes for young and old animals are well matched (0.036 vs. 0.038). This suggests that a robust neural response in a large region of LGN is accompanied by a relatively early increase in CBF for old cats. For the small stimulus, old cats exhibit relatively weaker response amplitudes compared with those for young animals (−0.0026 vs. −0.0048). In other words, for a given level of neural response, we observe a smaller initial dip of tissue oxygen response in old animals.
Capillary density
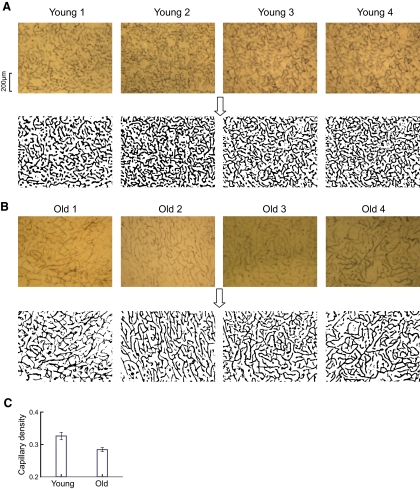
There are, of course, numerous factors associated with changes in the aging brain. One area that has been studied concerns microvascular density. Vascular density in a specific area of the brain appears to be directly related to local rates of metabolic activity (Cavaglia et al. 2001; Malonek et al. 1997). It is therefore relevant for the current study to examine microvascular structure in young and old animals. Previous work has shown that microvascular density is reduced in aged human and animal brains at the cortical surface, internal cerebral cortex, and hippocampus (for review, see Riddle et al. 2003). In the current study, we were interested in possible microvascular differences in the LGN of the thalamus. We have examined this in young and old animals by measurements of microvascular densities of capillary beds in the LGN. Examples of eight sections of tissue with alkaline-phosphatase stained capillary networks are shown in Fig. 8, A and B for young and old animals, respectively. For analysis, these images are converted to binary black-and-white versions as shown below the tissue sections in Fig. 8. In the case of the eight samples shown, capillary densities are 0.31, 0.38, 0.4, and 0.32 (Fig. 8A, young animals) and 0.24, 0.25, 0.27, and 0.28 (Fig. 8B, old cats), respectively. This trend of lower capillary density in old compared with young cats is confirmed in our population data. We have analyzed 21 and 47 samples, respectively, from four young (5.3 ± 0.9 SD months postnatal; range, 4.4–6 mo) and six old cats (134.7 ± 12.7 SD months old; range, 116–154 mo). The histogram of Fig. 8C shows the average capillary densities for young (0.33 ± 0.01) and old (0.28 ± 0.01) animals. The difference is significant (P < 0.01) and we conclude that vascular density is lower in old compared with that in young thalamus in the cat. This is consistent with data from previous studies that microvascular density decreases with normal aging (for review, see Riddle et al. 2003).
Fig. 8.
Changes in capillary bed density with extended age. A and B: representative alkaline phosphatase-stained capillary networks of the LGN for 4 young (A, top) and 4 old cats (B, top) and their binary images (bottom). Their ages are 4, 6, 5, and 6 mo for young cats and 141, 123, 141, and 116 mo for old cats, respectively. Black pixels in binary images represent areas occupied by capillaries. Scale bar: 200 μm. C: comparison of mean capillary densities in the LGN between young and old cats. Error bars represent ±1SE.
DISCUSSION
In the study described here, we have made simultaneous colocalized measurements in the LGN of the thalamus of the cat, of tissue oxygen responses and neural activity that are elicited by presentation of visual stimuli. The object was to see whether there are clear differences in old compared with young animals. Our findings indicate no significant differences in neural activity, but two clear age-related changes in oxygen responses. A typical oxygen response in young animals is biphasic, i.e., there is an initial negative component followed by a substantial positive peak. In old animals, the positive peak, which is associated with neural activity over a large volume of tissue (Thompson et al. 2005), has a relatively short temporal onset. The second change concerns the initial negative component that is associated with neural activity in a small population of neurons (Thompson et al. 2005). In this case, the amplitude for young animals is significantly greater than that for old cats.
Before considering these differences in our current data, we should note that many physiological and anatomical changes with aging have been documented in previous work. Prior areas of study are wide ranging and include, for example, diverse regions from brain stem to cerebral cortex (Ito et al. 2002; Solbakk et al. 2008; Yamaguchi et al. 1986). We have selected the LGN instead of visual cortex in our current work because of the relatively robust responses from cells. We found from preliminary studies that older cats are more difficult to record from in the extended physiological sessions that are required, so we assumed we would get more substantial experimental information from LGN. We also focus on two specific types of measurement: metabolic and neural in the form of oxygen partial pressure and spike data. There are other relevant measurements that could be made, such as Doppler assessments, to obtain direct information about blood flow. This is not technically practical in the case of LGN and we assume here that the two basic measurements of metabolic and neural activity will yield important insights regarding aging effects.
Biphasic oxygen response
The microelectrode used in the current study measures partial pressure of oxygen, which reflects a balance between local consumption and supply. In previous work with this microelectrode, we have determined that tissue oxygen responses in the visual cortex and LGN are generally of a biphasic nature, including an initial negative component or dip followed by a relatively large positive peak (Thompson et al. 2003, 2004). This biphasic profile is similar to that found in previous studies in which optical imaging and fMRI techniques have been used (Kim et al. 2000; Malonek and Grinvald 1996). We assume that the initial dip reflects mainly localized oxygen consumption, whereas the secondary positive component represents an increased blood flow to the activated area (Thompson et al. 2003), although some overlap in these processes is likely. It is possible to emphasize either the negative or the positive oxygen component, as recorded in the LGN, by control of the parameters of the visual stimulus (Li and Freeman 2007; Thompson et al. 2004, 2005). When a very small stimulus is used to activate a recording site, the typical response is a robust increase in neural activity and a monophasic negative oxygen response. In some cases, this type of response occurs without a late positive component. We assume this type of response is mostly a reflection of increased CMRO2. For the stimulus condition in which a large visual display with a mask over the receptive field is used, a monophasic positive oxygen response is generally observed. In this case the visual stimulus elicits activity over a large region of the LGN, excluding that portion surrounding the electrode tip. Because the sensing region for the oxygen sensor is small, tissue oxygen consumption by neurons is probably restricted to a relatively small volume of tissue. However, increased CBF is more diffuse and could enhance tissue oxygen levels in a relatively large activated region. Therefore the monophasic positive oxygen response may mostly reflect an increase in CBF. Based on these assumptions, the differences in monophasic oxygen response characteristics between young and old animals could be due to age-related differences in CMRO2 and CBF in the LGN. Alternatively, the two components in tissue oxygen response may alter the amplitudes and the peak (or dip) times of each other. An early change in the positive component could reduce the amplitude and time of the initial dip of tissue oxygen response. On the other hand, a large initial dip could delay the peak time and reduce the amplitude of the positive peak. Therefore different interactions between peak and dip components may contribute to the differences in tissue oxygen response between the two age groups.
A technical issue should be considered in relation to these findings. Like most microelectrode measurements, it is necessary to consider the possibility that the placement procedure may cause an artificial vasodilated condition, such as that reported in previous work (Fukuda et al. 2006). In the experiments reported here, we have used a specially designed long microelectrode (∼3 cm). This is considerably longer than the distance from the cortical surface to the LGN and it allows for flexibility to minimize tissue damage during placement of the electrode in the thalamus. In previous work we have found no difference in baseline oxygen tension between LGN and visual cortex, suggesting that tissue damage is minimal (Li and Freeman 2007). Baseline oxygen tensions in LGN in the current study for young and old animals are consistent with the results in previous studies from our lab and those of other investigators (Duong et al. 2001; Li and Freeman 2007; Offenhauser et al. 2005).
Short temporal latency of the positive oxygen response and low amplitude of the negative oxygen response in old cats
Our finding that old animals exhibit a significant early temporal component is counterintuitive because relative delays in reaction times are generally observed in aged subjects (Porciatti et al. 1999; Solbakk et al. 2008). There are various factors involved in the measurement of oxygen tension. Oxygen tension is proportional to its diffusion coefficient (Fatt 1976). Since we measure oxygen tension over time, it is possible that there are differences in the oxygen diffusion coefficient of brain tissue between young and old animals and this may account for some of the effects we have observed. We also know from our current result that capillary density in the LGN is substantially reduced in aged animals. This is consistent with previous studies of aging (Abernethy et al. 1993; Amenta et al. 1995; Sonntag et al. 1997). Oxygen function models include a mitochondrial buffer that is regulated by CBF (Buxton 2001; Mayhew et al. 2001). Since the aged brain has low capillary density, there may also be a minimum of oxygen buffers in the capillary bed. In this case, the vascular response may start relatively early to maintain the oxygen diffusion gradient between the capillary bed and the mitochondria. This notion may be tested by direct simultaneous measurements of blood flow and oxygen, although that is technically challenging for deep brain structures.
Changes in baseline CBF have been reported to alter both the magnitude and temporal characteristics of the functional hemodynamic response in humans (Behzadi and Liu 2006; Cohen et al. 2002; Liu et al. 2004). By changing the arterial partial pressure of carbon dioxide, the global CBF baseline could be increased or decreased during hypercapnia or hypocapnia. A larger amplitude and longer peak time of BOLD signal was observed during hypercapnia, whereas a lower amplitude and shorter peak time of BOLD signal was seen during hypocapnia (Cohen et al. 2002). The vasoactive agent caffeine was also used to reduce the baseline CBF in human subjects. A significant decrease in the time to peak was found for postdose BOLD responses (Behzadi and Liu 2006; Liu et al. 2004). Note that the effects of a decrease in baseline CBF on BOLD responses are consistent with the aging effects on the positive tissue oxygen response reported here. A number of studies have demonstrated an age-related decrease in baseline CBF (Ances et al. 2009; Bentourkia et al. 2000; Berman et al. 1988; Inoue et al. 2005; Leenders et al. 1990; Marchal et al. 1992; Martin et al. 1991). Therefore the decrease in baseline CBF may be associated with the earlier positive oxygen response in old cats.
Oxygen metabolism is known to change with advanced age. Previous studies using positron emission tomography have demonstrated that resting CMRO2 decreases significantly with age in cortical and subcortical regions (Leenders et al. 1990; Takada et al. 1992; Yamaguchi et al. 1986). Results in our current study demonstrate a significant age-related reduction in the amplitude of the negative oxygen response to a visual stimulus. Several mechanisms could contribute to this result. First, compared with young cats, activity-induced CMRO2 might be significantly lower in the old group. It has been suggested that vascular density in a specific area of the brain appears to be directly related to local rates of metabolic activity (Cavaglia et al. 2001; Malonek et al. 1997). Therefore the reduction in capillary density with age in the LGN reported here could be related to a decrease of oxygen consumption. Second, other processes, such as a reduction in neuron density (Ahmad and Spear 1993) and deterioration in mitochondrial numbers and functions (Ames et al. 1995), may also contribute to the reduction in CMRO2 in old cats. Third, it is possible that a relatively fast microvascular response in aged animals may account for this result. The small stimulus generally elicits a monophasic negative oxygen response that presumably reflects an activity-induced reduction in partial pressure of tissue oxygen. An early microvascular response might increase oxygen level relatively quickly in old animals. This in turn would cause an attenuated negative oxygen response.
In conclusion, simultaneous measurements of tissue oxygen and neural activity in the LGN of young and old cats demonstrate that neurometabolic coupling changes substantially in aged animals. Although there is no significant change in neural activity with age, tissue oxygen responses in old cats exhibit a relatively low initial dip, although oxygenation levels are attained more quickly.
GRANTS
This work was supported by National Eye Institute Grants EY-01175 and EY-03716.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank M. Peterson for help with data collection and discussions regarding this project, J. K. Thompson and D. Pal for help with data collection, and M. Jacob for help with histology.
REFERENCES
- Abernethy et al., 1993.Abernethy WB, Bell MA, Morris M, Moody DM. Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp Neurol 121: 270–274, 1993 [DOI] [PubMed] [Google Scholar]
- Ahmad and Spear, 1993.Ahmad A, Spear PD. Effects of aging on the size, density, and number of rhesus monkey lateral geniculate neurons. J Comp Neurol 334: 631–643, 1993 [DOI] [PubMed] [Google Scholar]
- Amenta et al., 1995.Amenta F, Cavallotti D, Del Valle M, Mancini M, Naves FJ, Vega JA, Zeng YC. Age-related changes in brain microanatomy: sensitivity to treatment with the dihydropyridine calcium channel blocker darodipine (PY 108–068). Brain Res Bull 36: 453–460, 1995 [DOI] [PubMed] [Google Scholar]
- Ames et al., 1995.Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta 1271: 165–170, 1995 [DOI] [PubMed] [Google Scholar]
- Ances et al., 2009.Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp 30: 1120–1132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi and Liu, 2006.Behzadi Y, Liu TT. Caffeine reduces the initial dip in the visual BOLD response at 3 T. NeuroImage 32: 9–15, 2006 [DOI] [PubMed] [Google Scholar]
- Bentourkia et al., 2000.Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci 181: 19–28, 2000 [DOI] [PubMed] [Google Scholar]
- Berman et al., 1988.Berman RF, Goldman H, Altman HJ. Age-related changes in regional cerebral blood flow and behavior in Sprague–Dawley rats. Neurobiol Aging 9: 691–696, 1988 [DOI] [PubMed] [Google Scholar]
- Boynton et al., 1996.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner et al., 2000.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 12: 24–34, 2000 [DOI] [PubMed] [Google Scholar]
- Buxton, 2001.Buxton RB. The elusive initial dip. NeuroImage 13: 953–958, 2001 [DOI] [PubMed] [Google Scholar]
- Cavaglia et al., 2001.Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res 910: 81–93, 2001 [DOI] [PubMed] [Google Scholar]
- Cohen et al., 2002.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab 22: 1042–1053, 2002 [DOI] [PubMed] [Google Scholar]
- DeAngelis et al., 1993.DeAngelis GC, Ohzawa I, Freeman RD. Spatiotemporal organization of simple-cell receptive fields in the cat's striate cortex. I. General characteristics and postnatal development. J Neurophysiol 69: 1091–1117, 1993 [DOI] [PubMed] [Google Scholar]
- D'Esposito et al., 1999.D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. NeuroImage 10: 6–14, 1999 [DOI] [PubMed] [Google Scholar]
- Duong et al., 2001.Duong TQ, Iadecola C, Kim SG. Effect of hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow. Magn Reson Med 45: 61–70, 2001 [DOI] [PubMed] [Google Scholar]
- Farkas and Luiten, 2001.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 64: 575–611, 2001 [DOI] [PubMed] [Google Scholar]
- Fatt, 1976.Fatt I. Polarographic Oxygen Sensors Cleveland, OH: CRC Press, 1976 [Google Scholar]
- Fukuda et al., 2006.Fukuda M, Moon CH, Wang P, Kim SG. Mapping iso-orientation columns by contrast agent-enhanced functional magnetic resonance imaging: reproducibility, specificity, and evaluation by optical imaging of intrinsic signal. J Neurosci 26: 11821–11832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, 1968.Griffiths BC. The geriatric cat. J Small Anim Pract 9: 343–355, 1968 [DOI] [PubMed] [Google Scholar]
- Handwerker et al., 2007.Handwerker DA, Gazzaley A, Inglis BA, D'Esposito M. Reducing vascular variability of fMRI data across aging populations using a breath holding task. Hum Brain Mapp 28: 846–859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrie and Shapley, 2005.Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol 94: 479–490, 2005 [DOI] [PubMed] [Google Scholar]
- Hesselmann et al., 2001.Hesselmann V, Zaro Weber O, Wedekind C, Krings T, Schulte O, Kugel H, Krug B, Klug N, Lackner KJ. Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett 308: 141–144, 2001 [DOI] [PubMed] [Google Scholar]
- Hesterberg et al., 2005.Hesterberg T, Moore DS, Monaghan S, Clipson A, Epstein R. Bootstrap methods and permutation tests. In: Introduction to the Practice of Statistics (5th ed.), edited by Sorensen L. New York: W.H. Freeman, 2005, p. 1141–70 [Google Scholar]
- Inoue et al., 2005.Inoue K, Ito H, Goto R, Nakagawa M, Kinomura H, Sato T, Sato K, Fukuda H. Apparent CBF decrease with normal aging due to partial volume effects: MR-based partial volume correction on CBF SPECT. Ann Nucl Med 19: 283–290, 2005 [DOI] [PubMed] [Google Scholar]
- Ito et al., 2002.Ito H, Kanno I, Ibaraki M, Hatazawa J. Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab 22: 997–1003, 2002 [DOI] [PubMed] [Google Scholar]
- Kiernan, 1999.Kiernan J. Histological and Histochemical Methods: Theory and Practice Oxford, UK: Butterworth-Heinemann Medical, 1999 [Google Scholar]
- Kim et al., 2000.Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci 3: 164–169, 2000 [DOI] [PubMed] [Google Scholar]
- Leenders et al., 1990.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RSJ. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990 [DOI] [PubMed] [Google Scholar]
- Li and Freeman, 2007.Li B, Freeman RD. High-resolution neurometabolic coupling in the lateral geniculate nucleus. J Neurosci 27: 10223–10229, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2004.Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB. Caffeine alters the temporal dynamics of the visual BOLD response. NeuroImage 23: 1402–1413, 2004 [DOI] [PubMed] [Google Scholar]
- Logothetis et al., 2001.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- Malonek et al., 1997.Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA 94: 14826–14831, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek and Grinvald, 1996.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554, 1996 [DOI] [PubMed] [Google Scholar]
- Marchal et al., 1992.Marchal G, Rioux P, Petit-Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 49: 1013–1020, 1992 [DOI] [PubMed] [Google Scholar]
- Martin et al., 1991.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11: 684–689, 1991 [DOI] [PubMed] [Google Scholar]
- Mata et al., 2009.Mata R, Wilke A, Czienskowski U. Cognitive aging and adaptive foraging behavior. J Gerontol B Psychol Sci Soc Sci 64: 474–481, 2009 [DOI] [PubMed] [Google Scholar]
- Mayhew et al., 2001.Mayhew J, Johnston D, Martindale J, Jones M, Berwick J, Zheng Y. Increased oxygen consumption following activation of brain: theoretical footnotes using spectroscopic data from barrel cortex. NeuroImage 13: 975–987, 2001 [DOI] [PubMed] [Google Scholar]
- Mitzdorf, 1987.Mitzdorf U. Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int J Neurosci 33: 33–59, 1987 [DOI] [PubMed] [Google Scholar]
- Moody et al., 2004.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology 233: 883–890, 2004 [DOI] [PubMed] [Google Scholar]
- Offenhauser et al., 2005.Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol 565: 279–294, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti et al., 1999.Porciatti V, Fiorentini A, Morrone MC, Burr DC. The effects of ageing on reaction times to motion onset. Vision Res 39: 2157–2164, 1999 [DOI] [PubMed] [Google Scholar]
- Rasch et al., 2009.Rasch M, Logothetis NK, Kreiman G. From neurons to circuits: linear estimation of local field potentials. J Neurosci 29: 13785–13796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle et al., 2003.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev 2: 149–168, 2003 [DOI] [PubMed] [Google Scholar]
- Riecker et al., 2003.Riecker A, Grodd W, Klose U, Schulz JB, Groschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab 23: 565–573, 2003 [DOI] [PubMed] [Google Scholar]
- Ross et al., 1997.Ross MH, Yurgelun-Todd DA, Renshaw PF, Maas LC, Mendelson JH, Mello NK, Cohen BM, Levin JM. Age-related reduction in functional MRI response to photic stimulation. Neurology 48: 173–176, 1997 [DOI] [PubMed] [Google Scholar]
- Schmolesky et al., 2000.Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci 3: 384–390, 2000 [DOI] [PubMed] [Google Scholar]
- Shaw et al., 2002.Shaw AD, Li Z, Thomas Z, Stevens CW. Assessment of tissue oxygen tension: comparison of dynamic fluorescence quenching and polarographic electrode technique. Crit Care 6: 76–80, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbakk et al., 2008.Solbakk AK, Fuhrmann Alpert G, Furst AJ, Hale LA, Oga T, Chetty S, Pickard N, Knight RT. Altered prefrontal function with aging: insights into age-associated performance decline. Brain Res 1232: 30–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag et al., 1997.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 138: 3515–3520, 1997 [DOI] [PubMed] [Google Scholar]
- Spear et al., 1994.Spear PD, Moore RJ, Kim CB, Xue JT, Tumosa N. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys. J Neurophysiol 72: 402–420, 1994 [DOI] [PubMed] [Google Scholar]
- Takada et al., 1992.Takada H, Nagata K, Hirata Y, Satoh Y, Watahiki Y, Sugawara J, Yokoyama E, Kondoh Y, Shishido F, Inugami A, Fujita H, Ogawa T, Murakami M, Iida H, Kanno I. Age-related decline of cerebral oxygen metabolism in normal population detected with positron emission tomography. Neurol Res 14: 128–131, 1992 [DOI] [PubMed] [Google Scholar]
- Tekes et al., 2005.Tekes A, Mohamed MA, Browner NM, Calhoun VD, Yousem DM. Effect of age on visuomotor functional MR imaging. Acad Radiol 12: 739–745, 2005 [DOI] [PubMed] [Google Scholar]
- Thompson et al., 2003.Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 299: 1070–1072, 2003 [DOI] [PubMed] [Google Scholar]
- Thompson et al., 2004.Thompson JK, Peterson MR, Freeman RD. High-resolution neurometabolic coupling revealed by focal activation of visual neurons. Nat Neurosci 7: 919–920, 2004 [DOI] [PubMed] [Google Scholar]
- Thompson et al., 2005.Thompson JK, Peterson MR, Freeman RD. Separate spatial scales determine neural activity-dependent changes in tissue oxygen within central visual pathways. J Neurosci 25: 9046–9058, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, 1982.Thomson D. Spectrum estimation and harmonic analysis. Proc IEEE 70: 1055–1096, 1982 [Google Scholar]
- Verhaeghen et al., 2003.Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: a meta-analysis. Psychol Aging 18: 443–460, 2003 [DOI] [PubMed] [Google Scholar]
- Viswanathan and Freeman, 2007.Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10: 1308–1312, 2007 [DOI] [PubMed] [Google Scholar]
- Yamaguchi et al., 1986.Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 17: 1220–1228, 1986 [DOI] [PubMed] [Google Scholar]