Abstract
Dopaminergic neurons are subject to a significant background GABAergic input in vivo. The presence of this GABAergic background might be expected to inhibit dopaminergic neuron firing. However, dopaminergic neurons are not all silent but instead fire in single-spiking and burst firing modes. Here we present evidence that phasic changes in the tonic activity of GABAergic afferents are a potential extrinsic mechanism that triggers bursts and pauses in dopaminergic neurons. We find that spontaneous single-spiking is more sensitive to activation of GABA receptors than phasic N-methyl-d-aspartate (NMDA)-mediated burst firing in rat slices (P15–P31). Because tonic activation of GABAA receptors has previously been shown to suppress burst firing in vivo, our results suggest that the activity patterns seen in vivo are the result of a balance between excitatory and inhibitory conductances that interact with the intrinsic pacemaking currents observed in slices. Using the dynamic clamp technique, we applied balanced, constant NMDA and GABAA receptor conductances into dopaminergic neurons in slices. Bursts could be produced by disinhibition (phasic removal of the GABAA receptor conductance), and these bursts had a higher frequency than bursts produced by the same NMDA receptor conductance alone. Phasic increases in the GABAA receptor conductance evoked pauses in firing. In contrast to NMDA receptor, application of constant AMPA and GABAA receptor conductances caused the cell to go into depolarization block. These results support a bidirectional mechanism by which GABAergic inputs, in balance with NMDA receptor–mediated excitatory inputs, control the firing pattern of dopaminergic neurons.
INTRODUCTION
Dopamine released by the activity of midbrain dopaminergic neurons plays an important role in Parkinson's disease (Blandini et al. 2000), reinforcement learning (Schultz 1998), and schizophrenia (Grace 1991). At least 70% of all inputs onto dopaminergic neurons are GABAergic, the majority of which arise from the striatum, globus pallidus (GP) and substantia nigra pars reticulata (SNr) (Bolam and Smith 1990; reviewed in Tepper and Lee 2007). Because spontaneously active neurons in the GP and SNr fire at rates as high as 50 and 60 Hz, respectively (Celada et al. 1999; Deniau et al. 1978; Guyenet and Aghajanian 1978; Kita and Kitai 1991), the activity of dopaminergic neurons is subject to a large GABAergic input. Dopaminergic neurons under the influence of this GABAergic inhibition would be expected to be mostly silent. Although some dopaminergic neurons are silent (Grace et al. 2007; but see Dai and Tepper 1998), many of them are spontaneously active in anesthetized or in awake, behaving animals (Freeman et al. 1985; Hyland et al. 2002; Kiyatkin and Rebec 1998; Schultz et al. 1997). They typically fire single spikes with varying degrees of regularity and can generate high-frequency bursts by a mechanism activating N-methyl-d-aspartate (NMDA) receptors (Chergui et al. 1993; Deister et al. 2009; Overton and Clark 1992, 1997; Zweifel et al. 2009). Dopamine is released either tonically or phasically depending whether the neuron is in a single-spiking or burst-firing mode (Goto et al. 2007; Grace and Bunney 1984a,b; Wilson et al. 1977). Does tonic GABAergic input simply hinder these firing modes or could it play a more integral role in their generation?
Dopaminergic neurons receive a combination of tonic inhibitory and excitatory inputs in vivo. Dopaminergic neurons are bombarded by chloride-mediated inhibitory postsynaptic potentials (IPSPs) in vivo (Grace and Bunney 1985). Local application of GABAA receptor (GABAAR) antagonists shifts the firing pattern of dopaminergic neurons from a single-spike mode into a burst-firing mode, whereas application of GABAB receptor antagonists regularizes the firing pattern (Brazhnik et al. 2008; Engberg 1993; Paladini and Tepper 1999). These results were interpreted to mean that tonic activation of GABAA receptors inhibits burst firing and that the action of GABAB receptors is mostly presynaptic. The shift into the burst-firing mode suggests that dopaminergic neurons are also subject to tonic excitation. Local application of NMDA receptor (NMDAR) antagonists, but not an AMPA receptor (AMPAR) antagonist, significantly reduced burst firing (Chergui et al. 1993; Overton and Clark 1992), suggesting that the majority of the tonic excitation driving bursts is NMDAR mediated.
Here we show that spontaneous, single-spike firing is more sensitive to activation of both GABAA and GABAB receptors than phasic, NMDAR-mediated burst firing evoked by either iontophoresis or dynamic clamp. This suggests that the activity seen in dopaminergic neurons in vivo is the result of a balance between excitatory and inhibitory conductances. Single spiking continued after application of balanced, constant NMDAR/GABAAR, but not AMPAR/GABAAR, conductances by dynamic clamp. Applying tonic NMDAR and GABAAR conductances, we show that phasic removal of the GABAAR conductance causes burst firing and phasic increases in the GABAAR conductance causes a pause in firing. Bursts generated by disinhibition have a higher frequency than bursts generated by identical excitation alone. Our results provide evidence for an extrinsic mechanism by which phasic changes in GABAergic drive can generate bursts and pauses in firing in midbrain dopaminergic neurons.
METHODS
Slice preparation and recordings
Electrophysiological experiments were performed on slices obtained from Sprague-Dawley rats (Charles River Laboratories) 15–31 days of age. Although dopaminergic neurons recorded in vivo do show changes in firing pattern with age (Tepper et al. 1990), we found no difference among neurons from 15- or 31-day-old animals during in vitro recordings. All experimental procedures were approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee. Rats were anesthetized with ketamine/xyzaline and decapitated, and the brains were rapidly removed and cooled. Horizontal slices (240 μm) were cut using a vibrating microtome (Microm HM 650V) in oxygenated, cold artificial cerebrospinal fluid (ACSF) containing (in mM) 110 cholineCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 10 dextrose, 25 NaHCO3, 1.3 ascorbic acid, and 2.4 sodium pyruvate. Slices were transferred to an incubation chamber containing (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 4 MgCl2, 2 CaCl2, 10 dextrose, 25 NaHCO3, 1.3 ascorbic acid, 2.4 sodium pyruvate, and 0.05 glutathione. Slices were incubated at 32°C for at least 1 h before recording and kept at room temperature thereafter. Before recording, a slice was held in a submerged chamber filled with ACSF similar to the incubating solution except that 2 mM MgCl2 was used, and glutathione was not added. The slice was superfused at a rate of 2 ml/min by a gravity feed system and heated to 32–34°C with an inline heater.
SNc neurons were visualized with a gradient contrast imaging system. Perforated-patch or whole cell recordings were made from presumed SNc dopaminergic neurons. Perforated patch recordings were made with the whole cell internal described below or an internal solution containing (in mM) 140 KMeSO4, 0.2 EGTA, 7 NaCl, and 10 HEPES. The antibiotic gramicidin A or D (dissolved in DMSO, 100 μg/ml of internal solution) was used to maintain natural intracellular Cl− levels (Kyrozis and Reichling 1995) and to reduce run-down. Accidental break-in was determined by a large, instantaneous jump in spike height. Cells in which accidental break-in occurred were rejected. Whole cell recordings were made with an internal solution containing (in mM) 138 K-gluconate, 10 HEPES, 2 MgCl2, 0.2 EGTA, 0.0001 CaCl2, 4 Na-ATP, and 0.4 Na-GTP. All internal solutions were adjusted to a pH of 7.3 using 1 M KOH and an osmolarity of 270–275 mOsm. Recordings were acquired with a Multiclamp 700B and digitized (Instrutech) under command of the AxographX software program.
Dopaminergic neurons were identified by a slow, spontaneous firing rate (usually 1–4 Hz), a prominent spike afterhyperpolarization, and a large Ih current on passage of a hyperpolarizing voltage step. The presence of a large mGluR1-mediated hyperpolarizing response to iontophoresis of glutamate after a burst (Morikawa et al. 2003) was also used to identify dopaminergic neurons (Marino et al. 2001).
Dynamic clamp
Dynamic clamp experiments were conducted in whole cell mode as previously described (Deister et al. 2009). The equations used to calculate the applied current are
where [Mg] = 1.5 mM, ENMDA = 0 mV, EGABAA = −60 mV (−63 mV was the mean reversal potential for GABAA receptors in perforated patch; Gulásci et al. 2003), and EGABAB = −100 mV unless otherwise stated. An AMPAR-mediated current was created from the INMDA equation by setting [Mg] to 0. All recordings were done with a balanced bridge in continuous current clamp (Bridge Mode). A junction potential of −6 mV was corrected on-line in these experiments.
Evoked responses
Iontophoresis was chosen over electrical stimulation of the slice to evoke phasic bursts that did not contain a GABA-mediated component, which would be concomitantly activated on electrical stimulation. Iontophoresis was also chosen over bath application of NMDA (Komendantov et al. 2004; Paladini et al. 1999b) to isolate the effects of GABA receptor activation on single-spiking and burst firing. Bursts were generated by iontophoresis of glutamate (typically onto dendrites 50 μm from the recording electrode; 50–250 ms pulses, holding +1 to 10 nA, ejection −50 to −300 nA; Dagan ION-100) as previously described (Deister et al. 2009). The iontophoretic pipette contained 1 M glutamate at pH 7–9. All experiments involving iontophoresis were performed in the presence of the AMPA receptor blockers, NBQX, or GYKI-52466.
Drugs
All drugs were applied to the slice via superfusion. Isoguvacine hydrochloride (1–100 μM), (R)-baclofen (0.01–10 μM), picrotoxin (100 μM), NBQX (25 μM), GYKI-52466 (20–50 μM), and CGP-55845 (1–2 μM) were purchased from Tocris.
Data analysis
Action potentials were detected using a derivative threshold in AxographX (1–5 V/s), and changes in spiking frequency were measured. Analysis was done with Mathematica 7 (Wolfram Research). A burst was defined as a series of the action potentials that occurred within 1 s after the onset of the iontophoretic pulse. Bursts evoked by dynamic clamp were analyzed for the entire time window that the NMDA conductance was on. In the case of bursts evoked by disinhibition, the window was defined as the period in which the GABAAR conductance was phasically turned off. Maximum burst frequency was determined as the reciprocal of the minimum interspike interval (ISI) for all spikes in the burst. Mean burst frequency was determined as the reciprocal of the mean intraburst ISI. For spontaneous single spiking, mean frequency was determined as the reciprocal of the mean ISI measured over a time window of 20 s in which no current was injected to the cell. Average membrane potential was calculated as the mean of all sampled voltages, including spikes.
Statistics
In several experiments, inhibition of single-spike or burst firing frequency by GABA receptor activation was measured. Because one of the factors (GABAAR activation) was quantitative rather than categorical, ANOVA was not appropriate. Instead, regression analysis was used to fit each curve. The data were fit with a quadratic model. This was implemented in SAS (SAS Institute) using the equation
where
yi = dependent variable representing inhibited firing frequency in terms of percent of control, τi = effect of firing frequency type (Figs. 1 and 2, B and E: maximum and mean burst frequency and mean single spiking frequency) or receptor type (Fig. 2D: GABAA and GABAB), β1, β2, (τβ2)i, (τβ1)i = regression coefficients, x = continuous independent variable for GABAAR activation in terms of concentration of agonist (μM) or conductance applied (nS), and ε = residuals, ε ∼ N(0,σ2).
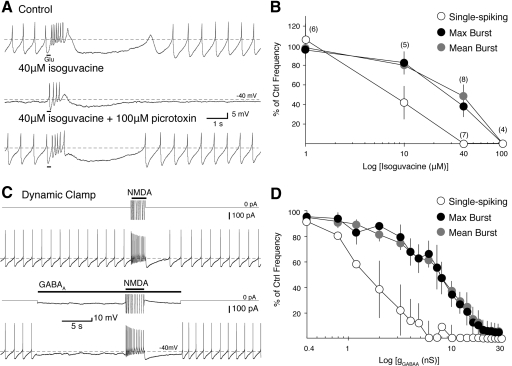
Fig. 1.
Single-spiking is more sensitive to suppression by GABAA receptor (GABAAR) activation than N-methyl-d-aspartate receptor (NMDAR)-mediated burst firing. A: a phasic burst (15 Hz maximum burst frequency) was evoked in a perforated patch recording of an identified dopaminergic neuron by iontophoresis of glutamate (Glu; black bars beneath trace, top) in 25 μM NBQX. The GABAAR agonist isoguvacine (40 μM) was applied to the bath (middle). Single-spike firing was completely suppressed while the burst frequency was decreased by 62%. The effect of isoguvacine was reversed by subsequent application of the chloride channel blocker, picrotoxin (100 μM, bottom). B: summarized data show the percent inhibition of maximum burst frequency (filled circles), mean burst frequency (gray circles), and mean spontaneous firing frequency (empty circles) from control frequencies after bath application of isoguvacine (1, 10, 40, and 100 μM). Numbers in parentheses indicate n for each concentration. C: application of a 40 nS NMDAR conductance using dynamic clamp elicits a burst (20 Hz maximum) in a whole cell somatic recording of an identified dopaminergic neuron (top). Application of a 2.8 nS GABAAR conductance (EGABAA = −60 mV) suppresses single-spike activity while the burst remains (18 Hz maximum; bottom). Above each voltage trace is the truncated current applied from the dynamic clamp (downward represents an outward current). D: summarized data (n = 4) show the percent of inhibition of maximum burst frequency (filled circles), mean burst frequency (gray circles), and mean spontaneous firing frequency (empty circles) to application of a series of GABAAR conductances in dynamic clamp (as in C).
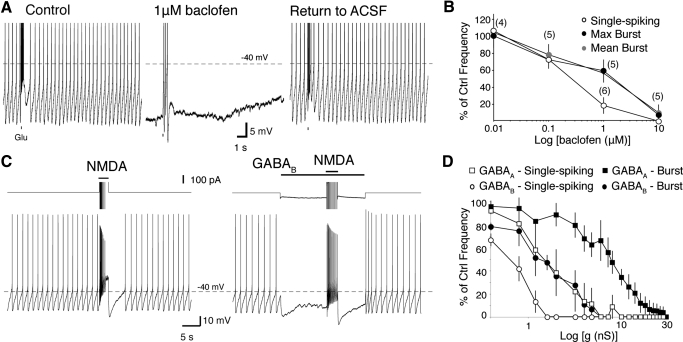
Fig. 2.
Single-spike firing is more sensitive to GABABR activation than NMDAR-mediated burst firing. A: a phasic burst was evoked in a perforated patch recording of an identified dopaminergic neuron by iontophoresis of glutamate (Glu; black bars beneath trace; top) in 25 μM NBQX. Bath application of 1 μM baclofen abolished single-spike activity and reduced the burst firing frequency. This effect was reversible on removal of baclofen [return to ACSF]. Spike heights are truncated. B: summarized data for the percent of inhibition of maximum burst frequency (filled circles), mean burst frequency (gray circles), and mean spontaneous firing frequency (empty circles) from control after bath application of baclofen (0.01, 0.1, 1, and 10 μM). Numbers in parentheses represent number of cells. C: a representative recording showing inhibition of single-spike and burst firing by application of a 1.2 nS GABABR conductance (EGABAB = −100 mV). The total current (truncated) applied by the dynamic clamp is shown above each voltage trace. D: summarized data (n = 4) show the effectiveness of GABAAR- and GABABR-mediated inhibition in suppressing maximum burst frequency (filled squares and circles, respectively) and mean spontaneous firing frequency (empty squares and circles, respectively). Conductances were applied into the same group of cells.
For dynamic clamp experiments in which a range of GABAAR conductances were applied to the same cell, an additional random effect ck was added to yi to account for within-cell effects.
After each curve was fit, multiple comparisons of firing or receptor type (τi) given GABAAR conductance were made using the Scheffe adjustment (P < 0.05). These comparisons permit us to compare, for example, whether the inhibition of single-spike firing was statistically different from the inhibition of burst firing at a specific GABAAR conductance.
For all other statistical tests, Prism (Graphpad Software) was used.
All effects are given in terms of means ± SE unless stated otherwise. In all experiments presented here, statistical significance is considered at P < 0.05.
RESULTS
GABAA receptor activation
We first studied the inhibition of spontaneous single-spiking and NMDAR-mediated burst firing by activation of GABAA receptors. Identified dopaminergic cells fired spontaneously (typically 1–4 Hz) in a regular, single-spike firing pattern during perforated patch recordings. Bursts of action potentials (mean, 22 Hz maximum burst frequency; similar to in vivo, e.g., Grace and Bunney 1984a) were evoked every 30 s by iontophoretic application of glutamate onto the recorded neuron in the presence of an AMPA receptor antagonist, NBQX (25 μM), or GYKI-52466 (20–50 μM). We applied the GABAAR agonist, isoguvacine (1, 10, 40, or 100 μM), to the bath and measured its effect on single-spike and burst firing frequency (Fig. 1). Regression analysis of the concentration-response data showed that isoguvacine inhibited single-spike firing at significantly lower concentrations than burst firing (P < 0.05, 15–75 μM isoguvacine, regression analysis). Application of 100 μM isoguvacine abolished both single-spike and burst firing (Fig. 1B). The addition of the GABAAR antagonist, picrotoxin (100 μM), reversed the effects of isoguvacine (Fig. 1A; P < 0.05, repeated-measures ANOVA with Tukey's multiple comparison test; control single-spike firing frequency: 2 ± 0.57 Hz, 40 μM isoguvacine: 0 ± 0 Hz, picrotoxin: 1.9 ± 0.68 Hz, n = 5). Spontaneous and burst firing frequencies in the presence of picrotoxin were not significantly different from control (P > 0.05; repeated-measures ANOVA with Tukey's multiple comparison test). These results suggest that spontaneous, single-spiking is more sensitive to GABAAR activation than burst firing.
We obtained similar results by applying a range of GABAAR conductances (0–30 nS) into cells using dynamic clamp (Fig. 1, C and D; Robinson and Kawai 1993; Sharp et al. 1993). Bursts (20.6 Hz maximum burst frequency on average) were evoked by application of a moderate NMDAR conductance (35 nS; range, 20–40 nS). These conductances were high enough to cause sustained high-frequency firing but were generally not high enough to cause the cell to go into a state of depolarization block by the end of the conductance pulse (which typically occurred at conductances >60 nS; data not shown). Again, single-spiking was more sensitive to GABAAR activation than burst firing (P < 0.05, 0–24 nS, regression analysis). Both single-spike and burst firing were abolished in all cells at a GABAAR conductance of 30 nS. The average GABAAR conductance at which single-spike firing was suppressed was 25% of the conductance needed to suppress burst firing (Fig. 1D; single-spike 4.8 ± 2.1 nS; burst, 19.5 ± 4.5 nS; n = 4). Together, these results show that in the presence of a tonic GABAAR conductance, single-spike firing is more sensitive to GABAAR activation than burst firing.
GABAB receptor activation
Tonic activity in GABAergic afferents may also activate postsynaptic GABAB receptors on midbrain dopaminergic neurons. Cells were recorded in perforated patch and NMDAR-mediated bursting was evoked by iontophoresis at regular intervals as above. The GABABR receptor agonist, baclofen (0.01, 0.1, 1, or 10 μM), suppressed single-spike and burst firing in a dose-dependent manner (Fig. 2). Single-spike firing was inhibited at lower concentrations than burst firing (P < 0.05, 0.6–2 μM baclofen, regression analysis).
Inhibition of single-spike and burst firing by application of a GABABR conductance was also investigated in dynamic clamp (Fig. 2, C and D; EGABAB = −100 mV). Bursts were evoked by application of a NMDAR conductance as before (Fig. 1 C–D). There was a significant difference in the regressions of the single-spike inhibition curves and both of the burst firing inhibition curves (P < 0.05, 0–8 nS, regression analysis).
We also tested whether GABABR activation was more effective at suppressing firing than GABAAR activation. The use of dynamic clamp allowed for direct comparison of these two receptor types in the same sample (from Fig. 1D). We found that there was a significant difference between GABAAR and GABABR conductances for inhibition of burst firing (P < 0.05, 0–24 nS, regression analysis) and single-spike firing (P < 0.05, 0–7.2 nS, regression analysis).
Together these results extend our findings with GABAAR to show that with activation of either GABAA or GABAB receptors single-spike firing will be abolished before the suppression of burst firing mediated by the activation of an NMDAR conductance. These results further show that GABAB receptors are more effective at suppressing both single-spike and burst firing than GABAAR. Because the conductances used in Fig. 2D were equal, this indicates that GABABR are more effective because of the more hyperpolarized reversal potential for potassium.
Bursting by disinhibition
Previous studies have suggested that bursts are suppressed by tonic activation of GABAAR in vivo (Brazhnik et al. 2008; Paladini and Tepper 1999). However, here we show that single-spike firing is more sensitive than burst firing to activation of either GABAA or GABAB receptors (Figs. 1 and 2). Therefore tonic activation of GABA receptors in vivo would be expected to suppress single spiking, causing dopaminergic neurons to be silent. However, single spiking is seen in vivo (Grace and Bunney 1984b; Paladini and Tepper 1999; Wilson et al. 1977). This suggests that the firing pattern recorded in vivo is caused by a combination of excitatory and inhibitory conductances in addition to its pacemaking currents. These results also suggest that phasic removal of this inhibitory conductance can evoke a burst and that a phasic increase in this inhibitory conductance can evoke a pause in firing.
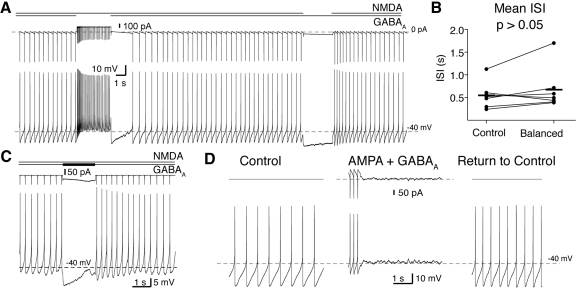
A control burst was evoked by application of a constant NMDAR conductance in whole cell recordings, as shown above (Figs. 1C and 2C). The same NMDAR conductance was applied in the presence of a constant GABAAR conductance (EGABAA = −60 mV; −63 mV measured in perforated patch recordings in Gulácsi et al. 2003) sufficient to counteract the NMDAR conductance-mediated burst and return single-spike firing back to control levels (Fig. 3, A and B; P > 0.05; control mean ISI, 0.55 ± 0.11 s; balanced ISI, 0.67 ± 0.18 s; paired t-test, n = 10). Overall, the ratio of NMDAR/GABAAR conductance used was 3.2 ± 0.4 (n = 7). In similar experiments using an AMPAR conductance instead of NMDAR, background firing could not be restored. Instead, the cell quickly went into depolarization block (Fig. 3D; 4/4 cells). Block in the AMPA/GABAA configuration occurred at much smaller conductances than those used in the balanced NMDAR/GABAAR configuration (typical NMDAR/GABAAR conductance: 25/8nS; AMPA/GABAAR conductances were <4 nS each).
Fig. 3.
Bursts and pauses can be generated by phasic changes in input. A: in a whole cell recording, balanced GABAAR and NMDAR conductances were applied into a dopaminergic neuron. A disinhibition burst could be evoked by phasic removal of the GABAAR conductance. A pause in firing could be evoked by phasic removal of the NMDAR conductance (A) or by a phasic increase in the GABAAR conductance (C, increase of 2 nS). The total current applied by the dynamic clamp is shown above each voltage trace. The total current in C has been truncated. B: single-spiking frequency in the presence of tonic NMDAR and GABAAR (Balanced) conductances was not significantly different from spontaneous, single-spiking (Control; P > 0.05, paired t-test). Horizontal lines indicate the means for the sample. D: single spiking is not maintained with application of balanced GABAAR and AMPAR conductances.
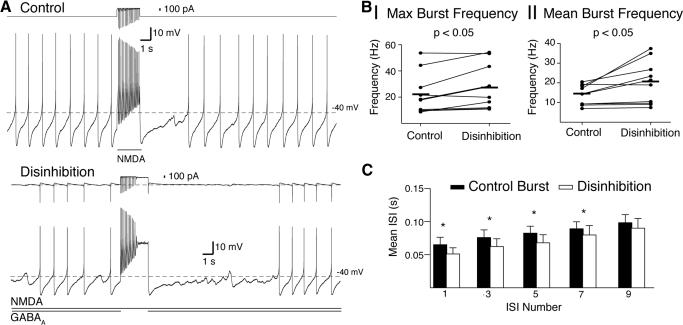
In the NMDAR/GABAAR balanced configuration, a burst of action potentials could be evoked by briefly turning off the GABAAR conductance (Fig. 3A). A pause in firing was evoked by transiently turning off the NMDAR conductance (Fig. 3A) or by increasing the GABAAR conductance (Fig. 3C). Bursts produced by disinhibition had a greater frequency than control bursts with an identical NMDAR conductance (Fig. 4, A and B; P < 0.05; control maximum burst frequency, 22.2 ± 4.9 Hz; disinhibition, 27.8 ± 5.4 Hz; paired t-test, n = 10; 1st 5 spikes mean burst frequency; control, 13.8 ± 1.6 Hz; disinhibition, 20.0 ± 3.4 Hz; paired t-test, n = 10). Both disinhibition and excitation-only bursts displayed prominent spike frequency adaptation (Fig. 4A). The increase in burst firing frequency was sustained over the first eight spikes of the burst (Fig. 4C). This suggests that the mechanism by which disinhibition bursts are faster occurs on the order of hundreds of milliseconds.
Fig. 4.
Disinhibition bursts have a greater frequency than phasic NMDA bursts of the same conductance. A: representative example of a burst produced by phasic activation of NMDA receptors (Control, top trace; gGABAA = 0; 18 Hz maximum) and by disinhibition (bottom trace; removal of gGABAA = 5.6 nS; 29 Hz maximum). The total current applied by the dynamic clamp is shown above each voltage trace. In both cases (control and disinhibition), an NMDA conductance of 20 nS was used. B: summary data show a significant increase in maximum (I) and mean (II) burst frequencies of disinhibition bursts compared with control bursts. Horizontal lines indicate the means for the sample. C: summary data show that the interspike interval (ISI) for disinhibition bursts was significantly shorter than control bursts for the 1st 7 ISIs (* = P < 0.05).
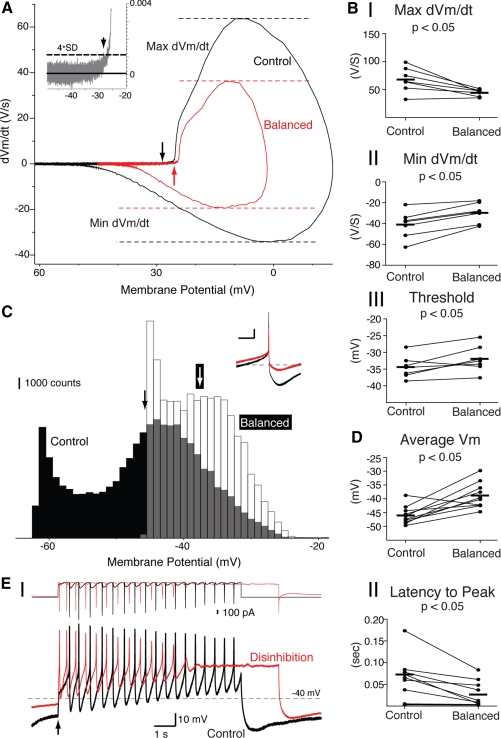
The mechanism by which disinhibition bursts are faster than control bursts may lie in differences in the availability of spiking currents just before the removal of the GABA conductance (Fig. 5). To test this, we constructed phase plots of both spontaneous single-spiking (Fig. 5A, black) and balanced conductance single-spiking modes (Fig. 5A, red). There was a significant decrease in maximum dVm/dt (Fig. 5BI; P < 0.05; control 67.8 ± 8.3, balanced 44.4 ± 2.9, paired t-test, n = 7). There was also a significant increase in minimum dVm/dt (Fig. 5BII; P < 0.05; control −41.0 ± 4.9, balanced −29.7 ± 3.6, paired t-test, n = 7). Balanced spiking had a more depolarized threshold than control spiking (Fig. 5BIII; P < 0.05; control −34.4 ± 1.3 mV, balanced −32.0 ± 1.5 mV, paired t-test, n = 7). This suggests that an increase in sodium channel availability is not the cause of the increased burst frequency of disinhibition bursts.
Fig. 5.
Disinhibition bursts may be faster because of depolarization. A: phase plots of spontaneous single-spiking (black) and balanced single-spiking (red). Threshold was defined as the voltage in which there was a significant break (4 × SD) of dVm/dt from baseline (shown in inset for control) and is shown on the control and balanced phase plots by black and red arrows, respectively. B: there was a significant decrease in maximum dVm/dt (I) during balanced spiking. There was also a significant increase in minimum dVm/dt (II) and spiking threshold (III) during balanced spiking. C: overlay of all-points histograms for sampled membrane potentials during spontaneous firing (Control, black bars) and balance between excitation and inhibition (Balanced, white bars). Black and white arrows represent the average membrane potential for Control and Balanced, respectively. The histograms were generated from 9 s of single spike firing in each configuration. Ten superimposed single spikes (aligned at the peak) are shown as an inset for Control (black) and Balanced configurations (red). Scale bars for inset are 10 mV (ordinate) and 100 ms (abscissa). Dashed line in inset shows −40 mV. D: average membrane potential for balanced spiking was more depolarized than control spiking. E: overlay of control (black) and disinhibition (red) bursts (I; same bursts as shown in Fig. 4A). The total current applied by the dynamic clamp is shown above each voltage trace. Summary data show a significant decrease in the time to the peak of the 1st action potential (II) from application of an NMDAR conductance (Control, black) or removal of a GABAAR conductance (Disinhibition, red). Arrow indicates the time of conductance change. Horizontal lines indicate means for the sample in each panel.
Histograms of control and balanced spiking showed that the average membrane potential was significantly more depolarized for balanced than control spiking (Fig. 5, C and D, also Fig. 5A; example histogram taken from cell shown in Fig. 4A; P < 0.05; control −46 ± 1.0 mV, balanced −39 ± 1.5 ms, paired t-test, n = 10). There was also a significant decrease in the latency from the time of conductance change to the peak of the first action potential in the burst (Fig. 5E; P < 0.05; control 58 ± 16 ms, disinhibition 27 ± 9 ms, paired t-test, n = 10). This suggests that a current that inactivates with depolarization (e.g., an A-type potassium current) may be the cause of the increased burst frequency of disinhibition bursts.
DISCUSSION
High conductance state of the dopaminergic neuron
Dopaminergic neurons recorded in vivo display a variety of firing patterns: silent, regular single-spiking, irregular single-spiking, and bursty (reviewed in Tepper and Lee 2007). Dopaminergic neurons recorded in slices, however, fire only in the regular, single-spiking mode. This difference in firing patterns of dopaminergic neurons recorded in slices and in vivo is often ascribed to the loss of afferent input in slices (Overton and Clark 1997). This suggests that single-spiking in vivo is generated by an in vitro–like pacemaking mechanism in which spikes can be advanced or delayed because of afferent input (hereafter referred to as the pacemaker mechanism). Under such a scheme, bursts are caused by phasic excitatory inputs and pauses in firing are caused by phasic inhibitory inputs. Such a mechanism may underlie the reward-related responses of dopaminergic neurons described by Schultz (1998). However, additional mechanisms for generating phasic bursts and pauses are likely for dopaminergic neurons in the high conductance state (reviewed in Destexhe et al. 2003).
There is much evidence that suggests the presence of a tonic inhibitory drive onto dopaminergic neurons. Dopaminergic neurons are bombarded by chloride-mediated IPSPs in vivo (Grace and Bunney 1985). These IPSPs are most likely caused by spontaneously active afferent cells in the globus pallidus (GP) or substantia nigra pars reticulata (SNr), which fire at ∼50 Hz (Celada et al. 1999; Deniau et al. 1978; Guyenet and Aghajanian 1978; Kita and Kitai 1991). The presence of this tonic inhibitory drive can also be shown by the local application of GABA antagonists in vivo. Paladini and Tepper (1999); and Brazhnik et al. (2008) found that local application of a variety of GABAA antagonists by pressure ejection onto the recorded neuron shifted the firing pattern of the dopaminergic neuron from a single-spiking mode to a bursting one, indicating that tonic GABAAR activation suppresses burst firing.
Our results, showing that NMDA-mediated burst firing is suppressed at greater levels of both GABAA and GABAB receptor activation than single spiking, also suggest that dopaminergic neurons are tonically inhibited in vivo. However, if single spiking in vivo is generated by a pacemaker mechanism, our results suggest that both single-spiking and bursting would be suppressed and there would be no tonic level of dopamine in efferent structures. However, many dopaminergic neurons recorded in vivo are not silent (Grace and Bunney 1984a,b; Paladini and Tepper 1999; Paladini et al. 1999a; Wilson et al. 1977), and tonic levels of dopamine are observed at target loci (Floresco et al. 2003; Gonon 1988). Therefore our results suggest that dopaminergic neurons receive tonic excitatory input.
Local application of NMDAR antagonists but not an AMPAR antagonist significantly reduce spontaneous burst firing (Chergui et al. 1993; Overton and Clark 1992; see also Charlety et al. 1991), suggesting that tonic excitation is present and is NMDAR mediated. Similar results were obtained with genetic NMDAR inactivation (Zweifel et al. 2009), and lesion of the subthalamic nucleus (STN), a spontaneously active glutamatergic afferent, regularized the firing pattern of dopaminergic cells (Smith and Grace 1992). Incomplete antagonism of NMDAR activation may explain why the strongly bursting cells of Chergui et al. (1993) do not become silent, as would be expected for a dopaminergic neuron in the high conductance state.
Are the GABAAR and NMDAR conductances applied here with dynamic clamp comparable to the conductances activated by tonic GABAergic synaptic input in vivo? Using a GABAA receptor time constant of 6 ms (τ; Brancucci et al. 2004), a single channel conductance of 5–30 pS (Δg; Guyon et al. 1999; Macdonald and Olsen 1994), and a 50 Hz input rate (F), we calculate that 887–5,330 GABAA receptors must be activated by globus pallidus and substantia nigra pars reticulata inputs to achieve the 8 nS steady-state conductance (gss) used in the balanced configuration in Fig. 1D (gss = Δg/(1 − e[−1/(τFn)]); Wilson et al. 2004). Assuming 12 receptors per synapse [∼5 immunogold particles per synapse (Fujiyama et al. 2002) times 2.5 GABAA receptors per gold particle (Nusser et al. 1997)], this corresponds to a minimum ∼1–8% of the total GABAergic synapses onto an SNc neuron (∼5,600 GABAergic synapses total; Henny et al. 2009). Similarly, we calculate that 2,300 NMDA receptors from 348 synapses must be activated for the NMDAR conductance used in the balanced configuration (gss = 25 nS; τ = 43 ms, Schilström et al. 2006; Δg = 50 pS, Edmonds et al. 1995; F = 5 Hz for subthalamic nucleus, Chergui et al. 1994; 2.9 immunogold particles per synapse, Chatha et al. 2000; assuming 2.3 channels per synapse, similar to AMPA, Nusser 1999). This corresponds to ∼15% of the available glutamateric synapses (∼30% of synapses are VGluT2+, Henny et al. 2009). Thus the NMDAR/GABAAR conductances used in this study are easily approachable in the intact network. These results also suggest that mechanisms that affect the properties of NMDAR and GABAAR (e.g., conductance, desensitization, or kinetics) may also have significant effects on the balance achieved by the dopaminergic neuron and hence influence firing pattern.
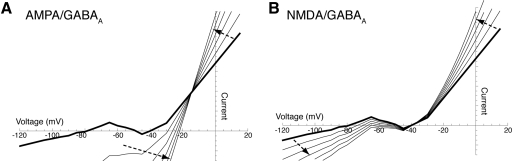
We propose that many dopaminergic neurons in vivo may be in the high conductance state generated by tonic GABAAR and NMDAR conductances. Activation of both AMPA and GABAA receptors decrease the input resistance of the cell and shunt the pacemaker and burst-generating currents (Fig. 6A). Thus the negative slope region of the IV curve, created by voltage-gated sodium and calcium channels, disappears. Similar results are expected for all other excitatory conductances such as nicotinic acetylcholine receptors. However, activation of NMDAR is unique because its negative slope conductance produces an increase in input resistance (Koch 1999). This negative slope conductance is caused by the magnesium sensitivity of the NMDA receptor. The increase in input resistance with activation of NMDA receptors counteracts the decrease in input resistance caused by activation of GABAA receptors, and the negative slope region of the IV curve persists (Fig. 6B). This explains why spikes can still be produced in the balanced NMDA/GABAA configuration. Differences in input resistance measurements in vivo (31 MΩ; Grace and Bunney 1983) and in slices (∼20 0MΩ; 70–250 MΩ, Kita et al. 1986; 135 MΩ, Grace and Onn 1989; 384 MΩ, Paladini et al. 1999b) suggest that 27.3 nS of the total input conductance in vivo (32.3 nS measured in vivo − 5 nS measured in slices = 27.3 nS) are caused by tonic NMDA and GABAA conductances. We found that cells went into block at AMPA/GABAA conductances around 4 nS and thus cannot account for the 27.3 nS difference in input conductance. The 27.3 nS input conductance difference in the NMDA/GABAA configuration may be underestimated because of the increase in input resistance with NMDAR activation. The drop in input resistance caused by impalement with sharp electrode recordings will overestimate this difference in input conductance.
Fig. 6.
The high conductance state of the dopaminergic neuron. The effect of tonic AMPA/GABAA (A) and NMDA/GABAA (B) conductances on the steady-state IV curve of a fictive dopaminergic neuron. Note that the negative slope region between −65 and −50 mV persists at much higher NMDA/GABAA conductances than AMPA/GABAA conductances. The thick black line represents the control condition where gAMPA, gNMDA, and gGABAA are set to 0. The thin lines represent a 5 nS increase in gAMPA (A) or gNMDA (B) and a 1.6 nS increase in gGABAA. Dashed arrows represent displacements of the IV curve as these conductances are increased.
An imbalance in tonic inputs may drive the cell into hyperpolarization or depolarization block in some proportion of dopaminergic neurons (Dai and Tepper 1998; Grace et al. 2007). For example, silent cells are cells whose membrane potential (−65 to −70 mV; Grace and Bunney 1984b) is near the chloride reversal potential (−68 mV; Grace and Bunney 1985). They represent the case where there is a tonic GABAA conductance sufficient to shunt intrinsic pacemaker currents (Grace et al. 2007) but lack a strong enough NMDA conductance to return the cell to the spiking regime. Silent cells, like other shunting configurations, may still be able to produce spikes in response to input fluctuations.
A dopaminergic neuron firing mainly under the influence of a balance between excitatory and inhibitory inputs would be more sensitive to dynamic fluctuations in rate and gain of inputs within the basal ganglia network (Carvalho and Buonomano 2009; Destexhe et al. 2003; Vogels and Abbott 2009) and adopt a more irregular single-spike firing pattern, as normally seen in vivo (Paladini et al. 1999a).
Tipping the scales: generating pauses and bursts
NMDA receptors play an important role in the generation of bursts (Chergui et al. 1993, 1994; Deister et al. 2009; Overton and Clark 1992; Tong et al. 1996; Zweifel et al. 2009). The simplest mechanism by which bursts are generated in vivo is phasic activation of NMDA receptors. However, in the presence of tonic synaptic conductances, this requires that the NMDA receptor currents overcome a substantially reduced input resistance (≤90% from in vitro estimates) and an increased Mg2+ block on the NMDA receptor (Paladini and Tepper 1999). These limitations can be overcome by an alternative mechanism where bursting is triggered by disinhibition (Hong and Hikosaka 2008; Jhou et al. 2009; Matsumoto and Hikosaka 2009).
Here we show that not only can bursts be produced by disinhibition but that disinhibition bursts have a greater firing frequency than bursts evoked by NMDA receptors alone. The mechanism by which disinhibition bursts are faster than excitation-only bursts is probably caused by a more depolarized voltage in the balanced state compared with spontaneous, single-spiking (Fig. 5C). This depolarization is a result of the total current produced from the application of NMDA and GABAA conductances with an effective synaptic reversal potential that is a conductance-weighted average of ENMDA (0 mV) and EGABAA (−60 mV). A depolarized membrane potential may inactivate A-type potassium channels (Gentet and Williams 2007; Khaliq and Bean 2008; Liss et al. 2001). The time to first spike measurement of Fig. 5EII is influenced by both spike-generating sodium channels and A-type potassium channels, which play an important role in the determination of the interspike interval. However, in control conditions, more sodium channels are available, which would overestimate the contribution of IA. This suggests that A-type potassium channels are inactivated in the balanced configuration and may be responsible for the increase in burst frequency. Inactivation of A-type potassium channels would also increase single-spiking frequency (Liss et al. 2001). However, an increase is not seen here (Fig. 3B) because the procedure by which we obtain the balanced configuration was designed to keep the firing rate similar to the control, spontaneous firing rate. These results also suggest that disinhibition bursts may be more precisely timed to rewarding stimuli than bursts by NMDA receptor activation alone.
Recent studies have shown that synaptic plasticity may enhance synaptically triggered burst firing and therefore underlie the acquisition of the burst response to a reward-predicting stimulus (Harnett et al. 2009). Although changes in plasticity can be effected within minutes, changes in the balance between excitatory and inhibitory inputs are capable of modulating bursts quicker. Thus scaling of intraburst firing rate can occur according to the probability of reward associated with a stimulus within a single trial (Morris et al. 2004) or on successive trials of a learning task (Pan et al. 2005).
Phasic increases in GABAergic drive activate postsynaptic GABAA and/or GABAB receptors to induce a pause in firing. The length of the pause would be proportional to the increase in drive. GABAB receptors would be expected to produce a longer pause than GABAA receptors and may be recruited by synaptic spillover (Galvan et al. 2006). This suggests that the role of GABAB receptors may not be only presynaptic. Pauses in firing may also be initiated or augmented by removal of excitation (Fig. 4A, e.g., the STN).
The firing pattern of dopaminergic neurons is a behaviorally important signal necessary for normal reward learning (Schultz 1998; Zweifel et al. 2009). Bursts and pauses in firing encode reward prediction error. Our results provide an extrinsic mechanism by which both reward-related responses could be evoked by phasic changes in GABAergic drive. These results suggest that the role of tonic GABAergic inhibition is not simply to suppress single-spiking and burst firing. On the contrary, the presence of tonic background inhbition allows for bidirectional changes in the firing rate and pattern in dopaminergic neurons. Thus GABAergic inhibition plays a fundamental role in the generation of the reward prediction error signal.
ACKNOWLEDGMENTS
We thank D. Polhamus for statistics help and C. Deister and members of the University of Texas at San Antonio, Specialized Neuroscience Research Program for comments on this manuscript.
GRANTS
This work was supported by National Institutes of Health Grants MH-084494 to C. J. Lobb and MH-079276 and NS-060658 to C. A. Paladini.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Blandini et al., 2000.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol 62: 63–88, 2000 [DOI] [PubMed] [Google Scholar]
- Bolam and Smith, 1990.Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res 529: 57–78, 1990 [DOI] [PubMed] [Google Scholar]
- Brancucci et al., 2004.Brancucci A, Berretta N, Mercuri NB, Francesconi W. Presynaptic modulation of spontaneous inhibitory postsynaptic currents by gamma-hydroxybutyrate in the substantia nigra pars compacta. Neuropsychopharmacology 29: 537–543, 2004 [DOI] [PubMed] [Google Scholar]
- Brazhnik et al., 2008.Brazhnik E, Shah F, Tepper JM. GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J Neurosci 28: 10386–10398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho and Buonomano, 2009.Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron 61: 774–785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada et al., 1999.Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience 89: 813–825, 1999 [DOI] [PubMed] [Google Scholar]
- Charlety et al., 1991.Charlety PJ, Grenhoff J, Chergui K, De la Chapelle B, Buda M, Svensson TH, Chouvet G. Burst firing of mesencephalic dopamine neurons is inhibited by somatodendritic application of kynurenate. Acta Physiol Scand 142: 105–112, 1991 [DOI] [PubMed] [Google Scholar]
- Chatha et al., 2000.Chatha BT, Bernard V, Streit P, Bolam JP. Synaptic localization of iontotropic glutamate receptors in the rat substantia nigra. Neuroscience 101: 1037–1051, 2000 [DOI] [PubMed] [Google Scholar]
- Chergui et al., 1994.Chergui K, Akaoka H, Charléty PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport 5: 1185–1188, 1994 [DOI] [PubMed] [Google Scholar]
- Chergui et al., 1993.Chergui K, Charléty PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci 5: 137–144, 1993 [DOI] [PubMed] [Google Scholar]
- Dai and Tepper, 1998.Dai M, Tepper JM. Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience 85: 1089–1099, 1998 [DOI] [PubMed] [Google Scholar]
- Deister et al., 2009.Deister CA, Teagarden M, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci 29: 15888–15897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau et al., 1978.Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res 32: 409–422, 1978 [DOI] [PubMed] [Google Scholar]
- Destexhe et al., 2003.Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4: 739–751, 2003 [DOI] [PubMed] [Google Scholar]
- Edmonds et al., 1995.Edmonds B, Gibb AJ, Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Ann Rev Physiol 57: 495–519, 1995 [DOI] [PubMed] [Google Scholar]
- Engberg et al., 1993.Engberg G, Kling-Petersen T, Nissbrandt H. GABAB-receptor activation alters the firing pattern of dopamine neurons in the rat substantia nigra. Synapse 15: 229–238, 1993 [DOI] [PubMed] [Google Scholar]
- Floresco et al., 2003.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973, 2003 [DOI] [PubMed] [Google Scholar]
- Freeman et al., 1985.Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci 36: 1983–1994, 1985 [DOI] [PubMed] [Google Scholar]
- Fujiyama et al., 2002.Fujiyama F, Stephenson FA, Bolam JP. Synaptic localization of GABA(A) receptor subunits in the substantia nigra of the rat: effects of quinolinic acid lesions of the striatum. Eur J Neurosci 15: 1961–1975, 2002 [DOI] [PubMed] [Google Scholar]
- Galvan et al., 2006.Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience 143: 351–375, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet and Williams, 2007.Gentet LJ, Williams SR. Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J Neurosci 27: 1892–1901, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon, 1988.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24: 19–28, 1988 [DOI] [PubMed] [Google Scholar]
- Goto et al., 2007.Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53: 583–587, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, 1991.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41: 1–24, 1991 [DOI] [PubMed] [Google Scholar]
- Grace and Bunney, 1983.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience 10: 301–315, 1983 [DOI] [PubMed] [Google Scholar]
- Grace and Bunney, 1984a.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1984b.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1985.Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res 333: 271–284, 1985 [DOI] [PubMed] [Google Scholar]
- Grace et al., 2007.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227, 2007 [DOI] [PubMed] [Google Scholar]
- Grace and Onn, 1989.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9: 3463–3481, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulácsi et al., 2003.Gulácsi A, Lee CR, Sík A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J Neurosci 23: 8237–8246, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet and Aghajanian, 1978.Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res 150: 69–84, 1978 [DOI] [PubMed] [Google Scholar]
- Guyon et al., 1999.Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugène D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J Physiol 516: 719–737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett et al., 2009.Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron 62: 826–838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny et al., 2009.Henny P, Brown MTC, Magill PJ, Bolam JP.A quantitative analysis of the glutamatergic and GABAergic somatodendritic innervation of individual dopaminergic neurons of the rat substantia nigra. Soc Neurosci Abstr 56615, 2009 [Google Scholar]
- Hong and Hikosaka, 2008.Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron 60: 720–729, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland et al., 2002.Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopaminergic cells in the freely moving rat. Neuroscience 114: 475–492, 2002 [DOI] [PubMed] [Google Scholar]
- Jhou et al., 2009.Jhou TC, Fields HL, Baxter MB, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61: 786–800, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq and Bean, 2008.Khaliq ZM, Bean BP. Dynamic, nonlinear feedback regulation of slow pacemaking by A-type potassium current in ventral tegmental area neurons. J Neurosci 28: 10905–10917, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita and Kitai, 1991.Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res 564: 296–305, 1991 [DOI] [PubMed] [Google Scholar]
- Kita et al., 1986.Kita T, Kita H, Kitai ST. Electrical membrane properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Res 372: 21–30, 1986 [DOI] [PubMed] [Google Scholar]
- Kiyatkin and Rebec, 1998.Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recordings and iontophoresis in awake, unrestrained rats. Neuroscience 85: 1285–1309, 1998 [DOI] [PubMed] [Google Scholar]
- Koch, 1999.Koch C. Biophysics of Computation New York: Oxford, 1999 [Google Scholar]
- Komendantov et al., 2004.Komendantov AO, Komendantova OG, Johnson SW, Canavier CC. A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons. J Neurophysiol 91: 346–357, 2004 [DOI] [PubMed] [Google Scholar]
- Kyrozis and Reichling, 1995.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods 57: 27–35, 1995 [DOI] [PubMed] [Google Scholar]
- Liss et al., 2001.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. EMBO J 20: 5715–5724, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald and Olsen, 1994.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci 17: 569–602, 1994 [DOI] [PubMed] [Google Scholar]
- Marino et al., 2001.Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci 21: 7001–7012, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto and Hikosaka, 2009.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 12: 77–84, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa et al., 2003.Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci 23: 149–157, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris et al., 2004.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43: 133–143, 2004 [DOI] [PubMed] [Google Scholar]
- Nusser, 2000.Nusser Z. AMPA and NMDA receptors: similarities and differences in their synaptic distribution. Curr Opin Neurobiol 10: 337–341, 2000 [DOI] [PubMed] [Google Scholar]
- Nusser et al., 1997.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron 19: 697–709, 1997 [DOI] [PubMed] [Google Scholar]
- Overton and Clark, 1992.Overton P, Clark D. Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10: 131–140, 1992 [DOI] [PubMed] [Google Scholar]
- Overton and Clark, 1997.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev 25: 312–334, 1997 [DOI] [PubMed] [Google Scholar]
- Paladini et al., 1999a.Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience 89: 799–812, 1999a [DOI] [PubMed] [Google Scholar]
- Paladini et al., 1999b.Paladini CA, Iribe Y, Tepper JM. GABAA receptor stimulation blocks NMDA-induced bursting of dopaminergic neurons in vitro by decreasing input resistance. Brain Res 832: 145–151, 1999b [DOI] [PubMed] [Google Scholar]
- Paladini and Tepper, 1999.Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse 32: 165–176, 1999 [DOI] [PubMed] [Google Scholar]
- Pan et al., 2005.Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci 25: 6235–6242, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson and Kawai, 1993.Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J Neurosci Methods 49: 157–165, 1993 [DOI] [PubMed] [Google Scholar]
- SchilströM et al., 2006.SchilströM B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci 26: 8549–8558, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, 1998.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27, 1998 [DOI] [PubMed] [Google Scholar]
- Schultz et al., 1997.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997 [DOI] [PubMed] [Google Scholar]
- Sharp et al., 1993.Sharp AA, O'Neil MB, Abbott LF, Marder E. The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci 16: 389–394, 1993 [DOI] [PubMed] [Google Scholar]
- Smith and Grace, 1992.Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse 12: 287–303, 1992 [DOI] [PubMed] [Google Scholar]
- Tepper et al., 1990.Tepper JM, Damlama M, Trent F. Postnatal development of the electrical activity of rat nigrostriatal dopmaminergic neurons. Brain Res Dev Brain Res 54: 21–33, 1990 [DOI] [PubMed] [Google Scholar]
- Tepper and Lee, 2007.Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res 160: 189–208, 2007 [DOI] [PubMed] [Google Scholar]
- Tong et al., 1996.Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm 103: 889–904, 1996 [DOI] [PubMed] [Google Scholar]
- Vogels and Abbott, 2009.Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci 12: 483–491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 2004.Wilson CJ, Weyrick A, Terman D, Hallworth NE, Bevan MD. A model of reverse spike frequency adaptation and repetitive firing of subthalamic nucleus neurons. J Neurophysiol 91: 1963–1980, 2004 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 1977.Wilson CJ, Young SJ, Groves PM. Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res 136: 243–260, 1977 [DOI] [PubMed] [Google Scholar]
- Zweifel et al., 2009.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDA-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 106: 7281–7288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]