Abstract
Alzheimer's disease is a chronic, age-related neurodegenerative disorder. Neurofibrillary tangles are among the pathological hallmarks of Alzheimer's disease. Neurofibrillary tangles consist of abnormal protein fibers known as paired helical filaments. The accumulation of paired helical filaments is one of the most characteristic cellular changes in Alzheimer's disease. Tau protein, a microtubule-associated protein, is the major component of paired helical filaments. Tau in paired helical filaments is hyperphosphorylated, truncated, and aggregated. What triggers the formation of paired helical filaments is not known, but neuroinflammation could play a role. Neuroinflammation is an active process detectable in the earliest stages of Alzheimer's disease. The neuronal toxicity associated with inflammation makes it a potential risk factor in the pathogenesis of Alzheimer's disease. Determining the sequence of events that lead to this devastating disease has become one of the most important goals for the prevention and treatment of Alzheimer's disease. In this review, we focus on the pathological properties of tau thought to play a role in neurofibrillary tangle formation and summarize how central nervous system inflammation might be a critical contributor to the pathology of Alzheimer's disease. A better understanding of the mechanisms that cause neurofibrillary tangle formation is of clinical importance for developing therapeutic strategies to prevent and treat Alzheimer's disease. One of the major challenges facing us is singling out neuroinflammation as a therapeutic target for the prevention of Alzheimer's disease neurodegeneration. The challenge is developing therapeutic strategies that prevent neurotoxicity linked to inflammation without compromising its neuroprotective role.
Keywords: aggregation, Alzheimer's disease, inflammation, neurofibrillary tangles, tau protein
Alzheimer's disease (AD) is histopathologically characterized by extracellular β-amyloid–containing plaques and intracellular tau-containing neurofibrillary tangles (NFTs).1 The main component of NFTs is tau, a highly soluble microtubule-associated protein whose major function is to stabilize microtubules, specifically in axons, in a phosphorylation-dependent manner.2 Tau promotes the assembly of microtubules into evenly spaced bundles in the axons and regulates the growing and shortening dynamics of individual microtubules.3 Tau is a highly soluble protein that is heat- and acid-stable, and it is rich in polar and charged amino acids with a basic character.2 Five residues (glycine, lysine, proline, serine, and threonine) make up half of the tau sequence, and this justifies its high solubility and the unfolded nature of the protein.
The main component of neurofibrillary tangles (NFTs) is tau, a highly soluble microtubule-associated protein whose major function is to stabilize microtubules, specifically in axons, in a phosphorylation-dependent manner.
The tau protein has 4 different regions (Figure 1), which range from the C-terminus to the N-terminus:
Fig 1.
Schematic representation of the 6 tau isoforms generated by alternative RNA splicing. The N-terminus contains zero, one, or two 29-aa inserts (pink) and, together with the proline-rich domain, forms the projection domain, which protrudes away from MTs. The C-terminus includes either three or four 18-aa-long imperfect repeats (blue) separated by 13- to 14-aa-long inter-repeats (green), and together they form the MT-binding domain.
Abbreviations: aa, amino acid; MT, microtubule.
The C-terminal domain with acidic and basic sub-regions, which indirectly control tau binding to microtubules via regulated phosphorylation.
The microtubule-binding domain with 3 or 4 imperfect repeats.
The positively charged proline-rich domain, which indirectly regulates the association between tau and microtubules via regulated phosphorylation.
The N-terminal domain, which contains 0, 1, or 2 negatively charged inserts.2
The proline-rich domain and the N-terminus compose the projection domain, which extends outward from the microtubule surface.3
There are 6 tau isoforms in the human brain, and they are products of alternative RNA splicing at exons 2, 3, and 10.4 The 6 different tau isoforms can be designated 3R0N, 3R1N, 3R2N, 4R0N, 4R1N, and 4R2N, the designation depending on the number of N-terminal inserts and C-terminal repeats. The microtubule-binding region contains 3 (3R) or 4 (4R) imperfect 18-amino acid-long repeats with a characteristic Pro-Gly-Gly-Gly motif separated from one another by an inter-repeat that is 13 to 14 amino acids long.2,5 The fetal human brain expresses only 3R tau, whereas the adult brain expresses approximately equal amounts of 3R tau and 4R tau. Mutations in the tau gene lead to extensive neuronal cell death and dementia, which is manifested in diseases such as hereditary Pick's disease and frontotemporal dementia with parkinsonism linked to chromosome 17.3 Exonic mutations involve amino acid substitutions in the microtubule-binding repeat region or close to it, and this suggests that they impair tau binding to microtubules. Intronic mutations located near the splice-donor site of the intron following exon 10 are regulatory and affect tau RNA splicing without affecting the amino acid composition of the protein.6
Neurodegenerative diseases collectively designated tauopathies are linked to tau mutations and/or tau posttranslational modifications. Accordingly, tau hyperphosphorylation and cleavage are important events leading to tau intracellular accumulation, aggregation, and neuronal cell death.7
Neurodegenerative diseases collectively designated tauopathies are linked to tau mutations and/or tau posttranslational modifications.
Tau Phosphorylation
An important characteristic of tau is that it possesses a large number of potential phosphorylation sites. Kinases that phosphorylate tau are distributed into 2 groups8:
Proline-directed serine-threonine protein kinases that phosphorylate Ser-Pro or Thr-Pro tau motifs. These include glycogen synthase kinase 3β (GSK3β), cyclin-dependent kinase 5 (cdk5), and stress kinases such as c-Jun N-terminal kinase (JNK) and p38.
Non–proline-directed protein kinases that phosphorylate serine or threonine residues not followed by proline. These include protein kinases A and C, calcium calmodulin–dependent kinase II, serum and glucocorticoid-dependent kinase, protein kinase B (Akt), microtubule-affinity regulating kinase, and synapses of amphids defective kinases, which are also known as brain-specific serine/threonine kinases.
The phosphorylation of tau to physiological levels is a way in which tau function is regulated.9 The physiological role of tau phosphorylation includes regulation of microtubule dynamics, neurite outgrowth, and axonal transport.9 Under normal physiological conditions, there is a balance between tau phosphorylation and dephosphorylation. Phosphatases such as protein phosphatase 1 (PP1), PP2A, PP2B, and PP2C are known to reverse tau phosphorylation, with PP2A postulated to be the phosphatase that dephosphorylates most tau phosphorylation sites.10 Abnormal hyperphosphorylation of tau within a consensus sequence for proline-directed kinases is an important factor in the conversion of normal tau into paired helical filaments (PHFs).3 For example, combinations of the kinases cdk5/GSK3 and calcium calmodulin kinase II/GSK3β are involved in the rapid phosphorylation of tau at Thr231 and Ser235, which is required for PHF formation in AD.11,12
Dephosphorylation of tau by PP2A inhibits its aggregation into PHFs and restores its ability to bind to microtubules. However, rephosphorylation of tau by different combinations of protein kinase A, calcium calmodulin kinase II, GSK3β, and cdk5 promotes its self-assembly into PHFs, and this leads to NFT formation.12
When tau is abnormally hyperphosphorylated, it loses its biological activity, becomes resistant to degradation, and goes through conformational changes that render it insoluble and aggregation-prone. Abnormal hyperphosphorylation of tau leads to its aggregation into PHFs, which are the main components of NFTs. Understanding PHF assembly and how to prevent it is relevant to AD and other neurodegenerative diseases in which tau plays a critical role, such as frontotemporal dementia with parkinsonism linked to chromosome 17.
When tau is abnormally hyperphosphorylated, it loses its biological activity, becomes resistant to degradation, and goes through conformational changes that render it insoluble and aggregation-prone.
Protein Interacting with NIMA (Never in Mitosis Gene A) 1 Restores Tau Function
The large family of proline-directed serine-threonine protein kinases, which include GSK3β, cdk5, and stress kinases such as JNK, plays a critical role in cellular growth regulation, stress responses, and neuronal survival.13 These kinases specifically recognize serine and threonine residues that precede proline. Because of its unique stereochemistry, proline can adopt 2 different conformational states: cis-isomeric and trans-isomeric states.13 Although the uncatalyzed isomerization of proline is slow, it can be accelerated by peptidyl-prolyl cis/trans-isomerases, which are enzymes able to change protein conformation. One of these enzymes, a peptidyl-prolyl isomerase called protein interacting with NIMA (never in mitosis gene A) 1 (PIN-1), regulates phosphorylation signaling by proline-directed serine-threonine protein kinases.13
The peptidyl-prolyl isomerase PIN-1 has been linked to AD because it has been detected in NFTs from AD brains14 and is thought to be neuroprotective against AD neurodegeneration.15 PIN-1 possesses an N-terminal WW domain responsible for mediating the binding of PIN-1 to tau, specifically at the pT231 residue. PIN-1 promotes tau dephosphorylation at the pThr231-Pro motif by the trans-specific phosphatase PP2A. Notably, PIN-1 binds to tau only after its mitosis-specific phosphorylation.14 Microtubule polymerization assays in conjunction with PIN-1 localization studies in normal and AD brains have suggested that one of the functions of PIN-1 is to bind directly to pThr231 on tau, promote its dephosphorylation, and restore the ability of tau to bind to microtubules.14
The peptidyl-prolyl isomerase that interacts with never-in-mitosis-A 1 has been linked to Alzheimer's disease (AD) because it has been detected in NFTs from AD brains and is thought to be neuroprotective against AD neurodegeneration.
Neurons are postmitotic cells, but some studies support the view that aberrant neuronal cell cycle re-entry plays an important role in AD neurodegeneration.16–18 Aberrant neuronal reactivation of mitosis is associated with tau hyperphosphorylation. A reduction of PIN-1 protein levels, as observed in AD, will result in higher levels of hyperphosphorylated tau; this compromises its ability to promote microtubule polymerization and increases tau aggregation and NFT formation. PIN-1 and the associated prolyl isomerization process are thus potential therapeutic targets for preventing AD neurodegeneration.19
Tau Cleavage
The independent cleavage of tau by caspases and calpain is an important posttranslational modification that, together with hyperphosphorylation, plays a critical role in the formation of PHFs.12 Caspase-cleaved tau is detected in NFTs, and this supports the view that the apoptosis cascade is involved in the formation of NFTs.20 It is thought that tau cleavage at its C-terminus by caspases renders tau prone to hyperphosphorylation and the formation of NFTs.2 The N-terminal of tau is cleaved by calpain, which induces a change in the tau conformation from an unfolded state to a β-sheet structure that renders its C-terminus susceptible to cleavage by caspases.12
Caspase-cleaved tau is detected in NFTs, and this supports the view that the apoptosis cascade is involved in the formation of NFTs.
The role of apoptosis in AD neurodegeneration is still unclear, although caspases seem to be activated early in the progression of AD.21 The 2 main apoptotic pathways are (1) the death receptor pathway involving the initiator caspase 8 and (2) the mitochondrial pathway involving the initiator caspase 9.22 Both pathways converge on pro-caspase 3 cleavage, which leads to a cascade that triggers caspase 3 activation, tau cleavage, and tau pathological aggregation. In AD brains, a colocalization of caspase 823 and caspase 924 with NFTs has been observed, and this suggests that both apoptotic pathways occur within the same neuronal populations in AD brains. However, it is not clear whether caspase 8 or 9 activation precedes or coincides with NFT formation.24
Tau is cleaved by multiple caspases at a highly conserved aspartic acid residue (Asp421) in its C-terminus, and this produces the N-terminal product Asp421Tau.20,25 Cleavage assays of full-length tau in the presence of various caspases have shown that tau is more susceptible to cleavage by executioner caspases (caspase-3 and caspase-7) than by initiator caspases (caspase-1, caspase-4, caspase-5, caspase-8, and caspase-10).26 Tau aggregation assays have indicated that Asp421Tau, generated by caspase 3 or caspase 9, aggregates faster and has a stronger seeding effect for aggregation than full-length tau.24
In AD brains, Asp421Tau is widespread in the cornu ammonis 1 region of the hippocampus. This distribution colocalizes with intraneuronal and extraneuronal amyloid beta peptide (Aβ) deposits and correlates with cognitive impairment.22 Asp421Tau has also been detected in other tauopathies, such as Pick's disease,27 supranuclear palsy, cortical degeneration, and dementia with Lewy bodies.28
There seems to be a correlation between Aβ, the major component of senile plaques in AD, and NFT formation. Aβ may lead to the activation of apoptosis through the death receptor as well as the mitochondrial pathways. Studies with E18 rat primary cortical neurons have shown that upon treatment with Aβ, tau is cleaved to Asp421Tau.22,24 Other studies have demonstrated that tau cleavage induced by Aβ treatment is prevented when the cultures are pre-incubated with caspase inhibitors.20 Furthermore, the treatment of hippocampal neurons with Aβ induces neurite degeneration and microtubule collapse only when tau is present. Tau-depleted neurons show no signs of degeneration in the presence of Aβ, and this supports a role for tau in Aβ-induced neurodegeneration.29
Correlation between Tau Hyperphosphorylation and Caspase Cleavage
The relationship between tau hyperphosphorylation and its cleavage by caspases remains poorly defined. Some studies have suggested that phosphorylation precedes cleavage in tangle evolution.23 In vitro phosphorylation of tau at Ser422 renders tau more resistant to caspase 3 proteolysis, and this supports the notion that phosphorylation at Ser422 prevents caspase cleavage some time during the progression of AD.23
The JNK family is involved in processes such as cell differentiation, proliferation, apoptosis, and neurodegeneration.30 JNKs are activated under stress conditions, such as those induced by reactive oxygen species and ultraviolet radiation.31 Studies using cell culture models32 have established that JNKs induce tau hyperphosphorylation leading to caspase activation and thus promote tau cleavage. The JNK signaling pathway can be activated by a number of stress factors, including oxidative stress and pro-inflammatory cytokines.33 JNK pathways are altered in AD; this causes abnormal phosphorylation of proteins that, under normal homeostatic conditions, would not be JNK targets.30 There are many potential substrates for JNK, but there is great interest in determining whether JNK activation is involved in tau phosphorylation and if this process occurs before or after caspase cleavage and tau aggregation. Tau phosphorylation by JNK primes tau for phosphorylation by GSK3β, and this results in tau hyperphosphorylation. Only then will tau form toxic aggregates that will in turn activate caspases and induce neuronal death. This sequence of events is further supported by the colocalization of phospho-JNK with tau inclusions.34
Tau Ubiquitination
Whether tau is ubiquitinated and degraded in vivo by the ubiquitin proteasome pathway remains controversial. In addition, it is not clear if the tau present in NFTs is ubiquitinated. However, it is well established that proteasome activity is impaired in AD brains.35
There are least 2 mechanisms associated with AD that could be responsible for proteasome impairment. The first mechanism is associated with a frameshift mutant of ubiquitin B (UbB+1) found to colocalize with NFTs and senile plaques in the cerebral cortex of patients with sporadic AD.36 The UbB+1 mutant lacks the C-terminal glycine, which is a critical amino acid for ubiquitination and the formation of polyubiquitin chains. UbB+1 impairs the degradation of ubiquitinated proteins by competing with wild-type ubiquitin for binding to the 26S proteasome.37 The second mechanism linked to AD is related to the direct binding of PHFs to the proteasome, an event that impairs proteasome activity.38
A direct link between tau aggregation and proteasome impairment is missing, although PHF-tau has been found to be ubiquitinated at its microtubule-binding domain, and this suggests that tau ubiquitination may be an early pathological event in the AD cascade.39 Some in vitro and cell culture studies have suggested that tau is degraded by the proteasome.40 Tau degradation by the proteasome seems to be dependent on tau ubiquitination with K63-polyubiquitin chains. These chains are required for tau interaction with sequestosome 1/p62, a shuttling partner for some proteasome substrates.41 Other studies using rat primary hippocampal neurons42 and human neuroblastoma SH-SY5Y cells43 have reported that tau degradation is independent of proteasome activity. This controversy remains to be solved.
A direct link between tau aggregation and proteasome impairment is missing, although paired-helical-filament tau has been found to be ubiquitinated at its microtubule-binding domain, and this suggests that tau ubiquitination may be an early pathological event in the AD cascade.
Similar to ubiquitination, sumolation is a posttranslational modification consisting of the addition of small ubiquitin-like modifiers to lysine residues on target proteins. Protein sumolation is known to prevent proteasomal degradation and/or to change protein function.44 Tau sumolation has been found to be up-regulated in cells treated with phosphatase inhibitors, and this suggests a new regulatory modification of tau that may have an implication for its pathology.39
Role in Tau Degradation of Heat Shock Protein 70-Interacting Protein/Heat Shock Protein 90 Complex
Tau undergoes hyperphosphorylation before its aggregation. One of the hypotheses for the neuronal accumulation of fibrillar tau in neurodegenerative disorders exhibiting NFTs is that chaperones fail to prevent the aggregation of hyperphosphorylated tau. Hyperphosphorylated tau is recognized and ubiquitinated by the U-box protein called carboxyl terminus of heat shock protein 70–interacting protein (CHIP).45 CHIP is a cochaperone with intrinsic ubiquitin ligase activity, which allows its chaperone function to switch from protein folding to protein degradation.44 CHIP interacts with heat shock protein 70 (Hsp70) and inhibits its ATPase activity, and this suggests that it has another function besides protein refolding. CHIP also binds to Hsp90 and forms a complex that selectively ubiquitinates hyperphosphorylated tau along with Ubiquitin-conjugating enzyme H5B (UbcH5B).45 The levels of CHIP are inversely proportional to insoluble tau accumulation in AD brains.46 In addition, mice lacking CHIP exhibit high levels of insoluble tau in their brains.47 These results suggest that CHIP complexed with Hsp90 delays the formation of tau aggregates and may thus play a protective role. The serine/threonine kinase Akt is another protein that is ubiquitinated by the CHIP/Hsp90 complex and then targeted for proteasomal degradation.48 Akt is one of the kinases that hyperphosphorylates tau and promotes tau accumulation/aggregation.49 Studies using a variety of cell models have indicated that under stress conditions the CHIP/Hsp90 complex ubiquitinates both Akt and tau for proteasomal degradation.48 Low Akt levels cause down-regulation of CHIP expression either by direct transcriptional regulation or via a feedback mechanism.48 However, with aging or under disease conditions, the levels of Akt increase, and because the CHIP/Hsp90 complex has a higher affinity for Akt than for hyperphosphorylated tau, Akt is preferentially ubiquitinated and degraded. As a result, hyperphosphorylated tau fails to be degraded, and its levels increase; this promotes tau accumulation/aggregation.48,50 Higher levels of tau increase its chances of being hyperphosphorylated by kinases such as Akt and microtubule-affinity regulating kinase/Prader-Willi/Angelman region 1,51 the latter being a microtubule-affinity regulating kinase that plays a role in phosphorylating tau during AD neurodegeneration. Notably, Prader-Willi/Angelman region 1 phosphorylation of tau at S262/S356 sharply decreases tau affinity for the CHIP/Hsp90 complex and, therefore, enhances tau accumulation.52 In conclusion, the role played by Akt in tau accumulation supersedes its role in tau phosphorylation.
The carboxyl terminus of heat shock protein 70-interacting protein complexed with heat shock protein 90 delays the formation of tau aggregates and may thus play a protective role.
Inflammation and Tau Pathology
Inflammation is implicated in AD.53 The association between inflammation and AD manifests itself through (1) the presence of activated microglia and astrocytes surrounding senile plaques and (2) higher levels of inflammatory mediators in the brains of AD patients versus age-matched controls.54 Current animal models of AD fail to address the mechanisms by which products of inflammation produced by activated microglia and astrocytes induce AD neurodegeneration. This is a very important issue because some products of inflammation are neuroprotective and others are neurotoxic.
A recent study using P301S mutant human tau transgenic mice established that hippocampal synaptic pathology and microgliosis could be the earliest manifestations of neurodegeneration related to tauopathies.55 Prominent microglial activation preceded tangle formation, and the immunosuppression of young P301S transgenic mice diminished tau pathology and increased their lifespan. It was concluded that neuroinflammation is linked to the early progression of tauopathies.55
What triggers the formation of NFTs is not known, but neuroinflammation could play a critical role. Neuroinflammation is an active process detectable in the earliest stages of AD. The neuronal toxicity associated with inflammation makes it a potential risk factor in the pathogenesis of chronic neurodegenerative diseases such as AD. Determining the sequence of events that lead to this devastating disease has become one of the most important goals for AD prevention and treatment. We recently reviewed the link between inflammation and tau pathology.56
The neuronal toxicity associated with inflammation makes it a potential risk factor in the pathogenesis of chronic neurodegenerative diseases such as AD.
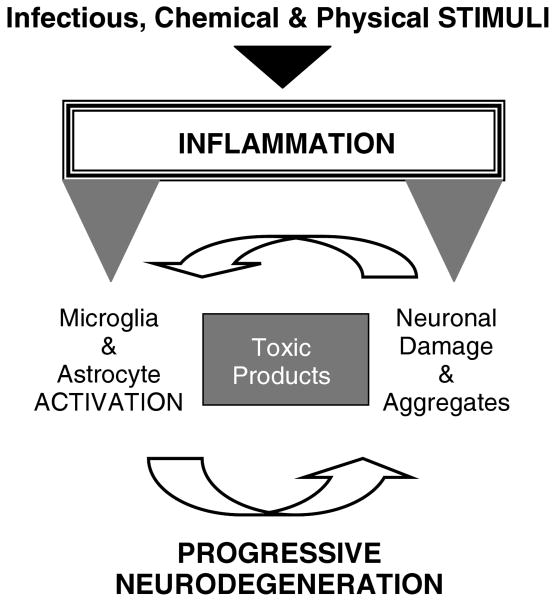
We propose a model (Figure 2) in which any stimulus (physical, chemical, or infectious) capable of inducing inflammation in brain areas affected in AD directly activates microglia and astrocytes. These stimuli can also directly induce neuronal injury, which through yet unknown factors triggers the reactive response of glial cells. In either case, the activated glia surround and drench neurons with their toxic products, such as prostaglandins, nitric oxide, interleukin 1β, interleukin 6, tumor necrosis factor alpha, and reactive oxygen species (eg, superoxide anion). All these cytotoxic agents must work in concert to induce synergistic neurotoxicity leading to neurodegeneration. This sequence of events could explain many pathological features of the AD neurodegenerative process. The elucidation of the mechanisms mediated by neurotoxic products of inflammation that lead to AD neurodegeneration will open up new and important possible targets for the pharmacological treatment of AD.57
Fig 2.
Model for the relation between neuroinflammation and tau pathology in Alzheimer's disease. We propose a sequence of events initiated by stimuli (physical, chemical, or infectious) that induce inflammation. Activated glia (microglia and astrocytes) release inflammatory factors, such as some prostaglandins (eg, prostaglandin J2), nitric oxide, interleukin 6, tumor necrosis factor alpha, and reactive oxygen species (eg, superoxide anion), that work in concert to induce neurotoxicity. One of the consequences is the impairment of the ubiquitin/proteasome pathway, which leads to an accumulation of ubiquitinated proteins. If these proteins cannot be cleared, the cells activate apoptosis. Caspase-mediated proteolysis results in the cleavage of tau (and other proteins), and this generates aggregation-prone protein fragments that, if not cleared by the autophagy/lysosomal pathway, aggregate and promote neurodegeneration. Injured neurons, in turn, initiate the production of signals (eg, extracellular adenosine triphosphate and β-amyloid plaques) that further propel glial activation. Over time, this feed-forward cycle of glial activation and neuronal injury results in progressive neurodegeneration leading to the development of symptomatic Alzheimer's disease.
Late-Breaking Studies
Two recent studies address the proteolytic events leading to tau pathology. One study58 proposes that an impairment of the ubiquitin/proteasome pathway leading to the accumulation of ubiquitinated proteins is an early response to neuronal damage. If cells cannot clear the accumulated ubiquitinated proteins, apoptosis starts and triggers caspase activation, which leads to tau cleavage. A failure to remove these aggregation-prone tau fragments by cathepsins promotes tau pathology. The other study59 suggests that chaperone-mediated autophagy is involved in the delivery of cleaved tau to lysosomes for additional cleavage. The impaired translocation of cleaved tau across the lysosomal membrane seems to promote tau oligomerization and tau aggregation at the membrane and thus interferes with lysosomal functioning. Both studies conclude that disruption of the lysosomal pathway plays a role in the final stages of tau pathology. However, as suggested by the first study, impairment of the ubiquitin/proteasome pathway, followed by caspase activation, seems to be an earlier event in the proteolytic cascade involved in tau pathology.
Impairment of the ubiquitin/proteasome pathway, followed by caspase activation, seems to be an earlier event in the proteolytic cascade involved in tau pathology.
Conclusion
AD is an age-related neurodegenerative disorder that is associated with neuroinflammation. Even though studies, models, and hypotheses have flourished over the last 2 decades, little is known about the beginning of the pathology, and when symptoms are detected, the neurodegeneration is so advanced that little can be done. NFTs are a pathological hallmark of AD. The major component of NFTs is tau, a microtubule-associated protein that is abundant in neurons and is highly soluble, yet in AD, it appears as abnormal aggregates. Tau is hyperphosphorylated, is truncated at Asp421, and aggregates into insoluble PHFs. The elucidation of the sequence of these and other tau posttranslational modifications and their contribution to neuronal cell death is highly significant for identifying the upstream steps that can be therapeutically targeted to prevent neurons from reaching a point of no return.
The dynamics of tau phosphorylation/dephosphorylation are a main focus of attention for scientists trying to find a way to regulate this process to avoid tau hyperphosphorylation and aggregation, which impairs its clearance. However, because tau is a target for so many kinases and phosphatases, understanding this process has proven to be very difficult. The challenge is to dissect which pathways render tau prone to hyperphosphorylation and aggregation, such as its cleavage by caspases or priming by a cascade of kinases. Resolving the pathways that lead to tau hyperphosphorylation and aggregation will provide a basis for designing more effective therapeutic strategies for AD.
Neuroinflammation with the ensuing gliosis could be an early manifestation of AD neurodegeneration. A better understanding of the mechanisms by which products of inflammation mediate neuronal injury may lead to more effective anti-inflammatory therapeutic strategies for preventing or treating AD neurodegeneration, which is associated with chronic inflammation.
Acknowledgments
This work was supported by the National Institutes of Health [NINDS-NS41073 through the Specialized Neuroscience Research Program (Maria E. Figueiredo-Pereira is the head of the subproject) and NCRR-RR03037 (infrastructure) to Hunter College, City University of New York].
Abbreviations
- aa
Amino acid
- Aβ
Amyloid beta peptide
- AD
Alzheimer's disease
- Akt
Protein kinase B
- cdk5
Cyclin-dependent kinase 5
- CHIP
Carboxyl terminus of heat shock protein 70–interacting protein
- GSK
Glycogen synthase kinase
- Hsp
Heat shock protein
- JNK
c-Jun N-terminal kinase
- MT
Microtubule
- NFT
Neurofibrillary tangle
- PHF
Paired helical filament
- PIN-1
Protein interacting with NIMA (never in mitosis gene A) 1
- PP
Protein phosphatase
- UbB+1
Frameshift mutant of ubiquitin B
Footnotes
Disclosures: Potential conflict of interest: Nothing to report.
References
- 1.Gotz J, Schild A, Hoerndli F, Pennanen L. Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. 2004;22:453–465. doi: 10.1016/j.ijdevneu.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer's disease. Brain Pathol. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg KJ, Ross JL, Feinstein HE, et al. Complementary dimerization of microtubule-associated tau protein: implications for microtubule bundling and tau-mediated pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedert M, Spillantini MG, Jakes R, et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 5.Crowther T, Goedert M, Wischik CM. The repeat region of microtubule-associated protein tau forms part of the core of the paired helical filament of Alzheimer's disease. Ann Med. 1989;21:127–132. doi: 10.3109/07853898909149199. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- 7.Corsetti V, Amadoro G, Gentile A, et al. Identification of a caspase-derived N-terminal tau fragment in cellular and animal Alzheimer's disease models. Mol Cell Neurosci. 2008;38:381–392. doi: 10.1016/j.mcn.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Ramos A, Smith MA, Perry G, Avila J. Tau phosphorylation and assembly. Acta Neurobiol Exp (Wars) 2004;64:33–39. doi: 10.55782/ane-2004-1489. [DOI] [PubMed] [Google Scholar]
- 9.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 10.Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer's disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta A, Kabat J, Novak M, et al. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys. 1998;357:299–309. doi: 10.1006/abbi.1998.0813. [DOI] [PubMed] [Google Scholar]
- 12.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 14.Lu PJ, Wulf G, Zhou XZ, et al. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784–788. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 15.Lu KP, Liou YC, Vincent I. Proline-directed phosphorylation and isomerization in mitotic regulation and in Alzheimer's disease. Bioessays. 2003;25:174–181. doi: 10.1002/bies.10223. [DOI] [PubMed] [Google Scholar]
- 16.Illenberger S, Zheng-Fischhofer Q, Preuss U, et al. The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol Biol Cell. 1998;9:1495–1512. doi: 10.1091/mbc.9.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323(pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosik KS, Qiu WQ, Greenberg S. Cellular signaling pathways and cytoskeletal organization. Ann N Y Acad Sci. 1996;777:114–120. doi: 10.1111/j.1749-6632.1996.tb34409.x. [DOI] [PubMed] [Google Scholar]
- 19.Maudsley S, Mattson MP. Protein twists and turns in Alzheimer disease. Nat Med. 2006;12:392–393. doi: 10.1038/nm0406-392. [DOI] [PubMed] [Google Scholar]
- 20.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert A, Keil U, Marques CA, et al. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer's disease. Biochem Pharmacol. 2003;66:1627–1634. doi: 10.1016/s0006-2952(03)00534-3. [DOI] [PubMed] [Google Scholar]
- 22.Rissman RA, Poon WW, Blurton-Jones M, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillozet-Bongaarts AL, Cahill ME, Cryns VL, et al. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem. 2006;97:1005–1014. doi: 10.1111/j.1471-4159.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- 24.Rohn TT, Rissman RA, Davis MC, et al. Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 25.Berry RW, Abraha A, Lagalwar S, et al. Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. doi: 10.1021/bi027348m. [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondragon-Rodriguez S, Mena R, Binder LI, et al. Conformational changes and cleavage of tau in Pick bodies parallel the early processing of tau found in Alzheimer pathology. Neuropathol Appl Neurobiol. 2008;34:62–75. doi: 10.1111/j.1365-2990.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 28.Wray S, Saxton M, Anderton BH, Hanger DP. Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J Neurochem. doi: 10.1111/j.1471–4159.2008.05321.x. Available at: http://www3.interscience.wiley.com. [DOI] [PubMed]
- 29.Rapoport M, Dawson HN, Binder LI, et al. Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 31.Song JJ, Lee YJ. Differential activation of the JNK signal pathway by UV irradiation and glucose deprivation. Cell Signal. 2007;19:563–572. doi: 10.1016/j.cellsig.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Sahara N, Murayama M, Lee B, et al. Active c-jun N-terminal kinase induces caspase cleavage of tau and additional phosphorylation by GSK-3beta is required for tau aggregation. Eur J Neurosci. 2008;27:2897–2906. doi: 10.1111/j.1460-9568.2008.06258.x. [DOI] [PubMed] [Google Scholar]
- 33.Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- 34.Pei JJ, Braak E, Braak H, et al. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- 35.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer's disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 36.van Leeuwen FW, de Kleijn DP, van den Hurk HH, et al. Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer's and Down patients. Science. 1998;279:242–247. doi: 10.1126/science.279.5348.242. [DOI] [PubMed] [Google Scholar]
- 37.Lam YA, Pickart CM, Alban A, et al. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keck S, Nitsch R, Grune T, Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 39.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem. 2006;281:9919–9924. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 40.David DC, Layfield R, Serpell L, et al. Proteasomal degradation of tau protein. J Neurochem. 2002;83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 41.Babu JR, Geetha T, Wooten MW. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem. 2005;94:192–203. doi: 10.1111/j.1471-4159.2005.03181.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown MR, Bondada V, Keller JN, et al. Proteasome or calpain inhibition does not alter cellular tau levels in neuroblastoma cells or primary neurons. J Alzheimers Dis. 2005;7:15–24. doi: 10.3233/jad-2005-7103. [DOI] [PubMed] [Google Scholar]
- 43.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004;279:4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 44.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 45.Sahara N, Murayama M, Mizoroki T, et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 46.Goryunov D, Liem RK. CHIP-ping away at tau. J Clin Invest. 2007;117:590–592. doi: 10.1172/JCI31505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7:736–741. doi: 10.1038/ncb0805-736. [DOI] [PubMed] [Google Scholar]
- 48.Dickey CA, Koren J, Zhang YJ, et al. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci U S A. 2008;105:3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal K, Alonso AC, Grundke-Iqbal I. Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J Alzheimers Dis. 2008;14:365–370. doi: 10.3233/jad-2008-14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandelkow EM, Thies E, Trinczek B, et al. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004;167:99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickey CA, Kamal A, Lundgren K, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflamm. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Arnaud L, Robakis NK, Figueiredo-Pereira ME. It may take inflammation, phosphorylation and ubiquitination to ‘tangle’ in Alzheimer's disease. Neurodegener Dis. 2006;3:313–319. doi: 10.1159/000095638. [DOI] [PubMed] [Google Scholar]
- 57.Klegeris A, McGeer EG, McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol. 2007;20:351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- 58.Arnaud LT, Myeku N, Figueiredo-Pereira ME. Proteasome-caspase-cathepsin sequence leading to tau pathology induced by prostaglandin J2 in neuronal cells. J Neurochem. 2009;110:328–342. doi: 10.1111/j.1471-4159.2009.06142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Martinez-Vicente M, Kruger U, et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]