Abstract
Bariatric surgery for obesity has emerged as an effective and commonly used treatment modality. This paper reviews the surgical site infections (SSIs) that occur post bariatric surgery and SSI prevention. The benefit of bariatric surgery resulting in profound weight loss brings with it consequences in the form of postoperative complications that can have profound effects on morbidity and mortality in these patients. This paper sets out to define different types of SSIs that occur following bariatric surgery and to discuss existing literature on the critical aspects of SSI prevention and the appropriate use of surgical antimicrobial prophylaxis for bariatric surgery.
Keywords: anastomotic leak, antibiotic prophylaxis, bariatric surgery, port infection, surgical site infection, wound infection
Background
Obesity is a major public health problem with the numbers of obese individuals globally increasing. In the USA, 5.2% of adults are morbidly obese (BMI >40) [1]. Despite improvements in the medical care of associated comorbid conditions, such as Type 2 diabetes and cardiovascular disease, there is a direct association between obesity and increased mortality [2]. According to a recent study, by the year 2030, approximately 90% (86.3%) of all American adults will be overweight or obese [3]. Total healthcare costs attributable to obesity are estimated to double every decade, will reach US$860.7–US$956.9 billion by 2030 and will account for 16–18% of total US healthcare costs [3]. Nonsurgical interventions are initially recommended for the management of obesity. However, bariatric surgery is being increasingly utilized for patients with a BMI of more than 35 or 40 with other comorbid conditions who are unresponsive to medical interventions.
Recent reports indicate a tenfold increase in the number of bariatric surgical procedures performed in the USA from 16,200 in 1994 to 171,000 in 2005 [4]. Furthermore, an increasing number of older adults (>60 years) are being offered bariatric surgery as a weight loss measure owing to widespread obesity and the fact that the population is living longer [5]. Many studies have demonstrated a greater reduction in weight loss and a 25–40% reduction in overall mortality in obese patients following bariatric surgery compared with patients treated nonsurgically [6–8].
Surgery performed on an obese patient is associated with an increased risk for postoperative complications including surgical site infections (SSIs). The reported incidence of SSIs following bariatric surgery is approximately 15% [9], which is similar to the rate of SSI for obese patients undergoing nonbariatric abdominal surgeries [10].
Types of bariatric surgical procedures
Historically, the three most common types of bariatric surgical procedures include an open and laparoscopic Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB). The laparoscopic sleeve gastrectomy has become a popular procedure in the past 4–5 years but the number of procedures that have been performed for this type of surgery and the number of published reports have been small in comparison with the other bariatric surgical procedures.
A recent multicenter prospective study of 4776 bariatric procedures showed that the combined end point of 30-day mortality, major thrombotic complication, re-intervention and prolonged hospitalization was 1.0% for LAGB, 4.8% for laparoscopic RYGB and 7.8% for open RYGB, with an overall mortality rate of 0.3% [11]. The major risk factors for increased complication rates in this study were obstructive sleep apnea, poor functional status or a history of prior thrombotic events. The introduction of laparoscopic RYGB has been associated with a significant reduction in perioperative mortality and complications [11,12]. The complication rate associated with LAGB is even lower than that seen with laparoscopic RYGB (1 vs 3.3%) [12].
Surgical site infections
Epidemiology, definition & classification
Surgical site infections are defined as infections occurring within 30 days after a surgical operation (or within 1 year if an implant is left in place after the procedure) that affect either the incision or tissue deep into the operation site [13]. A wound is considered infected if it meets any of the CDC definitions [14], including the isolation of pathogens from an aseptically obtained culture of fluid or tissue from the wound; purulent drainage from the incision, with or without laboratory confirmation of infection; local signs and symptoms of infection such as erythema and warmth; and diagnosis of wound infection by the surgeon.
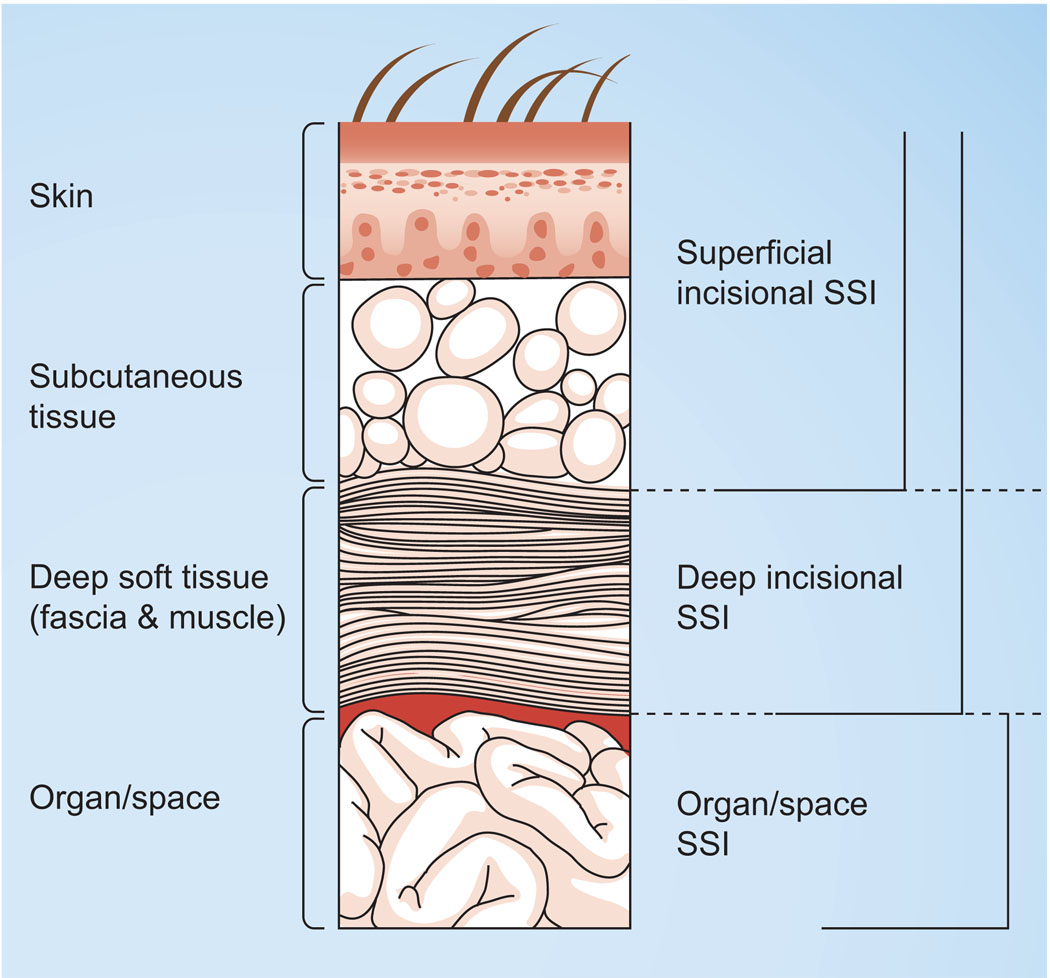
Surgical site infections may be superficial (those involving only the skin or subcutaneous tissue) or deep incisional infections (those involving deep soft tissues of an incision) or infections involving organs or body spaces (Figure 1) [14]. Organ/space SSIs are associated with both higher mortality rate and higher costs [15] than superficial SSIs.
Figure 1. Classification of surgical site infections according to CDC National Nosocomial Surveillance System.
SSI: Surgical site infection.
Reproduced from [14].
According to the CDC, SSIs are the second most frequently reported nosocomial infections and account for 22% of all healthcare-associated infections [13,16]. Among surgical patients, SSIs are the most common nosocomial infection, accounting for 38% of healthcare-associated infections. It is estimated that SSIs develop in 2–5% of the 16 million patients undergoing surgical procedures in the USA each year (i.e., one out of every 24 patients who have inpatient surgery in the USA develops a postoperative SSI) [14].
Surgical site infections in the general population are associated with a two- to three-fold increased risk of death, and a 60% increased risk of requiring a postoperative intensive care unit stay. Length of hospital stay is increased by 7–12 days, the patient is five-times more likely to require readmission, and direct healthcare costs are increased by at least US$5000 [17].
The incidence of SSIs with open bariatric surgeries can be as high as 16% [18]. At our institution (Detroit Medical Center, MI, USA) the SSI rate was 12% among the 751 patients who underwent open RYGBs [19]. However, the incidence of SSIs has decreased with the introduction of laparoscopic procedures with rates of SSI incidence following this approach at 4% [20–22], probably due to smaller incisions, shorter hospital stay and minimal blood loss, compared with open procedures [21].
Obesity as a risk factor for SSIs
Several studies in the general surgical population have reported obesity to be an independent risk factor for SSIs and for associated morbidity and mortality [10,23,24]. A prospective study by Dindo et al. found that SSIs were significantly more common in the obese population (4% in obese patients vs 3% in nonobese patients; p = 0.03) [23]. Interestingly, wound infection rates and severity were similar in morbidly obese patients who underwent bariatric surgery compared with nonbariatric abdominal operations (15 and 16%) [10].
Obesity increases the risk for SSI by several mechanisms. The primary defence against pathogens is oxidative killing by neutrophils, which is critically dependent on tissue oxygen tension. Hence, the incidence of surgical wound infections is directly related to tissue perfusion and oxygenation. Obese patients have decreased tissue oxygen tension at, and near, the incision site, which increases the risk for SSI [25]. Fleischmann et al. demonstrated that tissue oxygenation is also impaired in obese patients undergoing laparoscopic surgery as a result of hemodynamic compromise caused by pneumoperitoneum [26].
Other proposed mechanisms for increased SSI risks in obese patients are decreased serum and tissue concentrations of prophylactic antibiotics and increased rates of preoperative hyperglycemia [27,28]. In addition, obese patients have an increased frequency of other comorbid conditions, such as diabetes mellitus that increase the risk for SSIs. In addition, the perioperative skin preparation of obese patients, as well as surgical closure, can be particularly challenging compared with nonobese patients.
Risk factors for SSIs following bariatric surgery
Although the risk factors for SSIs have been well described for various types of surgical procedures, scant data exist describing risk factors for SSIs following bariatric surgery [17,18]. Christou et al. conducted a retrospective review addressing the incidence of and risk factors for SSI in 269 patients undergoing open bariatric surgery. According to their risk-stratification analysis, 10.9 (4%) SSIs were expected, but 54 (20%) were observed. The authors identified the use of epidural analgesia and delay in the appropriate timing of prophylactic antibiotics to be associated with a higher risk of SSI. Interestingly, their study found a high correlation between SSI and development of post-incisional hernia, another common complication following surgery in obese patients [18].
At the Detroit Medical Center, our group performed a case–cohort study during a 2-year study period (2006–2008) to determine the risk factors for SSI following open RYGB surgical procedures [19]. The SSI rate among the 751 RYGBs performed was 12% (n = 91). A total of 65 SSIs were categorized as superficial, 18 as deep incisional and eight as organ/space. Bivariate predictors of SSIs included morbid obesity (defined by the authors as BMI ≥50), asthma, smoking, sleep apnea, increased duration of surgery, presence of urinary incontinence in the preoperative setting and needing assistance with ambulation in the preoperative setting. In multivariate analysis, Type 2 diabetes, morbid obesity (BMI ≥50), preoperative urinary incontinence and sleep apnea were each associated with an approximate twofold increase in SSI risk. SSI following RYGB was associated with an increased risk for emergency department visits for all causes, hospital re-admissions, outpatient procedures and 30-day mortality [19].
Most SSIs following bariatric surgery occur within the first 2–3 weeks of surgery [18]. The most common source of pathogens causing SSIs post-gastrointestinal surgeries is endogenous patient flora (including staphylococcal and streptococcal species) and flora of the gastrointestinal tract (including aerobic and anaerobic Gram-negative bacilli). As is similar with other gastrointestinal surgeries, SSI following bariatric surgery can be polymicrobial. The most common organisms causing SSI following bariatric surgery include staphylococcal species such as Staphylococcus aureus and coagulase-negative staphylococci. At our institution, of the 91 SSIs in our study, the most commonly isolated bacteria were streptococci (n = 27), Enterococcus spp. (n = 9), coagulase-negative staphylococci (n = 9), Enterobacteriaceae (n = 5), S. aureus (n = 4) and Eikenella spp. (n = 3). Anaerobic cultures were sent from the operating room in 25 cases and in 15 cases (60%), anaerobes were recovered. The most common anaerobe isolated was Prevotella (n = 10), followed by Peptostreptococcus (n = 5, including one case of bacteremia), Bacteroides (n = 1) and Veillonella (n = 1).
Anastomotic leak & intra-abdominal sepsis following bariatric surgery
Anastomotic leak occurs in up to 5.8% of bariatric surgeries and is considered one of the most life-threatening complications of bariatric surgery [29]. It is reported to be even more common than pulmonary embolism [30,31] and can lead to peritonitis, severe intra-abdominal sepsis, intensive-care unit admission and high mortality [32].
Intra-abdominal sepsis, a complication often associated with anastomatic leak, is an important, life-threatening complication of any abdominal surgery. Early recognition of intra-abdominal sepsis can be a challenge in obese patients owing to the misleading absence of abdominal signs due to large masses of subcutaneous abdominal tissue [32]. A study by Kermarrec et al. reported that respiratory distress and tachycardia are early markers of abdominal sepsis in bariatric surgery patients [32]. Other investigators have demonstrated upper gastrointestinal studies to be very predictive of early leak diagnoses [33]. In patients with an anastomotic leak and severe abdominal infection, delaying surgery can lead to increased mortality [34]; hence early re-exploration in order to obtain source control of the leak (within 48 h of index surgery) should be practiced [35]. Sapala et al. described a method for ‘leak prophylaxis’ in their prospective study including 738 open RYGB surgeries and found that application of fibrin sealant at the anastomotic site can prevent leaks [36].
Port site infections in LAGB
Laparoscopic adjustable gastric banding is a very effective type of bariatric surgery performed in the modern era.
The most common type of infection associated with LAGB is infection of the implanted subcutaneous access port with an incidence ranging from 0.3 to 9% [37,38]. The diagnosis of a port infection can be made as a clinical diagnosis or following upper endoscopy. Upper endoscopy can also rule out band erosion. Isolated port infections are managed by removing the port and implanting a new port once the infection has cleared. Even though port infection is considered a relatively minor complication of LAGB, it often necessitates re-operation at the port site or at the level of the band. To circumvent this complication, Fabry et al. came up with a technique that ensures port stability by using a larger surface area for attachment of the port to the fascia [39].
Surgical-site infection prevention
General preventative measures
Many SSI prevention measures are described in detail in the current guidelines. These measures include treating all existing infections prior to surgery, minimizing hair removal preoperatively (and if required, removing hair immediately prior to surgery using clippers), achieving appropriate glucose control during the preoperative period, maintaining normothermia during the perioperative period, applying antiseptic skin preparation appropriately prior to surgery, ensuring proper hand/forearm antisepsis for surgical team members, maintaining a sterile field in the operating room and providing appropriate antimicrobial prophylaxis [13,40,41]. Expert surgical technique, including methods of wound closure can also impact SSI risk [42]. Most of these recommendations for the prevention of SSIs apply to bariatric surgery. However, some facets of prevention, such as the provision of adequate antimicrobial prophylaxis, present challenges in the obese patient population.
Antimicrobial prophylaxis: overview
The benefits of perioperative antimicrobial prophylaxis in preventing SSIs have been clearly demonstrated through numerous trials and endorsed in various guidelines [13,40,43–48]. The goal of perioperative antimicrobial prophylaxis is to ensure that adequate antibiotic levels are maintained above the minimum inhibitory concentration (MIC) from the time of incision and throughout the procedure. The selection of appropriate prophylactic antibiotic regimens requires consideration of the expected microbial flora at the surgical site, patient-specific factors such as allergy and exposure to resistant bacteria, institution-specific factors such as local antibiograms and antibiotic formulary availability and drugs. Drug considerations include bactericidal activity, pharmacokinetic and pharmacodynamic parameters to ensure the adequate delivery of antibiotics in relation to surgical incision and the maintenance of optimal drug levels throughout the procedure. The bariatric surgery population presents challenges related to optimal drug dosage, infusion time and drug disposition. Unfortunately, there are only limited data describing the pharmacokinetic properties of antimicrobials in the obese patient population.
Antimicrobial prophylaxis: agent selection for bariatric surgery
The goal of surgical antimicrobial prophylaxis is not to sterilize tissues, but to decrease bacterial burden to a level that can be controlled by host defenses. Numerous microbial species have been implicated as SSI pathogens. In bariatric surgical procedures, the predominant organisms include Gram-positive bacteria such as staphylococci, streptococci and enterococci, and Gram-negative pathogens such as Enterobacteriaceae including Proteus mirabilis, Serratia marcescens, Enterobacter and Escherichia coli, and anaerobes including Bacteroides fragilis [18,19].
Regimens for patients not allergic to β-lactams
Antimicrobial prophylaxis is delivered by the intravenous route. Historically, cephalosporins have been the dominant class of antimicrobials for surgical prophylaxis. They are well tolerated and have a low incidence of allergy. The rates of cross-reactivity with penicillin are low enough to justify the use of a cephalosporin in patients who do not have a history of IgE-mediated reaction to a penicillin [49]. The most advocated prophylactic agent for gastroduodenal procedures is cefazolin [40]. For bariatric surgeries above or including the duodenum, cefazolin is the drug of choice. For bariatric procedures below the duodenum, agent(s) with anaerobic activity are preferred, such as the cephamycins or cefazolin in combination with metronidazole. The cephamycins are a unique group of cephalosporins with good activity against anaerobic organisms and they are frequently used as prophylactic agents in bariatric surgery [40]. Available cephamycins in the USA are cefoxitin a and cefotetan. Cephamycin activity against the B. fragilis group varies significantly by agent and species. The percentage susceptibility of B. fragilis and the B. fragilis group against cefotetan re 81 and 56%, respectively [50]. Activity for cefoxitin against B. fragilis and the B. fragilis group are 94.8 and 92.6%, respectively [50]. Therefore, cefoxitin is the preferred cephamycin as it provides adequate coverage of the pathogens that are most commonly identified as causing SSI following bariatric surgery. Based on the Gram-negative susceptibility data from local surgical surveillance, nonantipseudomonal third-generation cephalosporins (such as cefotaxime or ceftriaxone) may provide excellent activity against E. coli and are an alternative to cefazolin. Enterococci are questionable pathogens in polymicrobial surgical settings [51–55]; hence, they are not routinely covered by surgical antimicrobial prophylaxis.
Alternative prophylactic regimens include the β-lactam/ β-lactamase inhibitor combiniations such as ampicillin/ sulbactam. However, there has been a significant increase in resistance of certain organisms such as E. coli to ampicillin/ sulbactam [56–58]. Ertapenem, a type 1 carbapenem, and tigecycline, a novel glycylcycline, have good activity against flora that are commonly encounterd during bariatric surgery. However, these agents have a broad spectrum of activity and should be reserved for the treatment of documented resistant pathogens rather than for routine prophylaxis. Other β-lactams used alone or in combination are also options, although they are not recommended for routine antimicrobial prophylaxis use. These agents include ceftazidime (an antipseudomonal third-generation cephalosporin), cefepime, type 2 carbapenems (such as meropenem, imipenem-cilastatin or doripenem) and other β-lactam/β-lactamase inhibitor combinations such as piperacillin/tazobactam and ticarcillin/clavulanic acid. Use of these agents should be restricted owing to their broad spectrum of activity against pathogens that do not commonly cause SSIs such as Pseudomonas aeruginosa.
Regimens for patients allergic to β-lactams
Although no formal guidelines exist for choosing a prophylaxis agent for patients with a history of IgE-mediated allergy to penicillins or cephalosporins, several potential agents are available. For example, non-β-lactam agents such as clindamycin plus ciprofloxacin, levofloxacin, or clindamycin plus an aminoglycloside (such as gentamicin, tobramycin or amikacin depending on local Gram-negative susceptibility) are options for bariatric surgeries, particularly if the ileum is not involved. If the ileum is involved in the procedure, additional anaerobic coverage can be provided by combining levofloxacin with metronidazole, or by administering moxifloxacin as a single agent. Aztreonam is a β-lactam with Gram-negative activity that does not cross-react with other β-lactams, except for ceftazidime. Hence, it may also be considered as an option in combination with agents that have activity against Gram-positive and anaerobic organisms.
Table 1 summarizes the antimicrobial recommendations for bariatric surgical prophylaxis.
Table 1.
Antimicrobial recommendations for bariatric surgical prophylaxis.
| Anatomic site involved |
First-line therapy |
Alternative | IgE-mediated penicillin/ cephalosporin allergy |
Not routinely recommended |
|---|---|---|---|---|
| Gastroduodenal | Cefazolin | Cefotaxime or ceftriaxone |
Clindamycin plus fluoroquinolone (ciprofloxacin), fluoroquinolone (levofloxacin), clindamycin plus aminoglycoside (gentamicin or tobramycin or amikacin)† or clindamycin plus aztreonam‡ |
β-lactam/β-lactamase inhibitors (ampicillin/sulbactam or piperacillin/tazobactam or ticarcillin/clavulanic acid) Cephalosporins (ceftazidime or cefepime) Carbapenems (ertapenem, meropenem, imipenem or doripenem) Tigecycline |
| Ileal involvement | Cefoxitin, or cefazolin plus metronidazole |
Cefotaxime plus metronidazole or ceftriaxone plus metronidazole |
Fluoroquinolone (moxifloxacin), metronidazole plus fluoroquinolone (levofloxacin) or clindamycin plus aztreonam plus metronidazole |
β-lactam/β-lactamase inhibitors (ampicillin/sulbactam, piperacillin/tazobactam or ticarcillin/clavulanic acid) Cephalosporins (ceftazidime or cefepime) Carbapenems (ertapenem, meropenem, imipenem or doripenem) Tigecycline |
Depending on local Gram-negative susceptibility.
Aztreonam has demonstrated in vitro cross-reactivity with ceftazidime [90].
Increasingly, the emergence of more resistant pathogens, such as methicillin-resistant S. aureus (MRSA), has complicated decisions regarding preoperative antimicrobial prophylaxis. Vancomycin has been advocated for surgical prophylaxis in certain types of surgeries for patients with severe allergies to β-lactams. Vancomycin has been increasingly considered for prophylaxis at institutions with a high rate of infections caused by MRSA or methicillin-resistant coagulase-negative staphylococci. However, there is no consensus regarding what constitutes high levels of methicillin resistance. One guideline defines a high rate of infection caused by MRSA as over 20%, based on expert panel consensus [40]. Christou et al. examined 269 patients undergoing isolated RYGB surgery and cultured S. aureus from 39% of infected wounds. However, the frequency of methicillin resistance was not reported [18]. At our institution, among 91 SSIs, S. aureus was isolated in only four cases, and two of these were MRSA [19]. Thus, MRSA does not appear to be a common SSI pathogen following bariatric surgery; therefore, in most cases, antimicrobial prophylaxis to target MRSA is unnecessary. However, decisions regarding the need to provide antimicrobial prophylaxis against MRSA should be based on local SSI surveillance data and the frequency of MRSA SSIs.
Antimicrobial prophylaxis: route of administration
Most antimicrobial agents do not achieve optimal serum levels when administered orally. Although certain oral antimicrobials have comparable bioavailability with their intravenous formulation, the time to achieve maximum serum concentration is slower due to the need for absorption through the gastrointestinal tract. Intravenous antimicrobial prophylaxis is the most extensively studied route and remains the preferred route of administration.
The application of topical and subcutaneous antimicrobials to the bariatric surgical site has also been studied. Alexander et al. examined the infusion of kanamycin 0.1% solution (1000 µg/ml), at the time of incision closure, into the subcutaneous space of 837 morbidly obese patients undergoing open, primary or revisional bariatric procedures, in addition to standard systemic antimicrobial prophylaxis (e.g., intravenous cefazolin) [59]. Kanamycin was allowed to dwell for 2 h. Overall, SSIs developed in the subcutaneous tissues of 0.72% of patients, and superficial infections developed in 2.5% of patients. Unfortunately, no control group was included in this study. A finding of concern related to this route of administration is the absorption of kanamycin into the serum of patients, as reported in another study by the same investigators [60]. Christou et al. supplemented parenteral prophylactic antibiotic with 500 mg of cefazolin powder, which was placed directly in the wound before incision closure in patients undergoing an open RYGB [18]. No difference in the rate of wound infection was observed among patients receiving cefazolin powder compared with patients who did not (20 with, 17% without local antibiotic). Although the role of topical and incisional antimicrobials in prevention of SSI is intriguing, currently, they are not the standard of care for the prevention of SSI following bariatric surgery.
Mechanical bowel preparation, which includes the preoperative administration of laxatives, enemas and sometimes oral antimicrobial agents, has not been studied for use in bariatric surgery, and should not be routinely used as prophylaxis for SSIs.
Hence, intravenous administration is preferred over topical, subcutaneous or mechanical bowel preparation as the route of delivery for bariatric antimicrobial surgical prophylaxis.
Antimicrobial prophylaxis: dosage in the bariatric population
Many factors affect antimicrobial concentrations in the surgical patient. Patient-specific factors include age, weight, body composition, renal function and volume status and surgery-specific factors include blood loss and fluid or blood replacement during surgery. Antimicrobial-specific factors include dosage, half-life, frequency of administration and the degree of protein binding. Furthermore, the effectiveness of the antibiotic is also influenced by the MICs of pathogens targeted. These various patient, surgical and antimicrobial factors influence the total versus free concentration of the antimicrobial in the blood and tissues. One of the most important determinants of antimicrobial levels in the serum and tissues at the time of surgical incision is antimicrobial dosing.
Limited pharmacokinetic studies have been conducted in obese patients, in whom multiple physiologic alterations can influence drug disposition. In many ways, pharmacokinetics are similar for obese and nonobese patients. For example, the absorption of drugs does not appear to be significantly altered by the weight of a patient [61], and in general, the fat mass should not significantly influence the distribution of hydrophilic drugs. Albumin-bound drugs are unlikely to have significantly altered protein-binding characteristics in obese versus nonobese individuals [62]. However, there are some notable differences in obese individuals; for example, the distribution of lipophilic drugs is generally increased, and obese individuals generally have an increased glomerular planar surface area and altered renal elimination [61]. Both of these factors might affect serum and tissue antimicrobial levels. The optimal dosing of prophylactic antimicrobials before bariatric surgery remains undetermined, although some experts believe that ‘more is better’ and that high antimicrobial doses are warranted. Study findings in obese patients are often drug specific and utilize different classifications to define obesity.
Studies examining the cephalosporins have reported that drug clearance (Cl) and volume of distribution (Vd) for cefotaxime and cefotiam (a third- and second-generation cephalosporin, respectively) were increased in obese patients, and both Cl and Vd were correlated with body surface area [63,64]. These findings support increasing the dose of cephalosporins when they are used for prophylaxis.
A case describing the administration of piperacillin/tazobactam dosed at 3.375 g every 4 h in an obese patient (BMI: 50) noted an increased Vd and a decreased peak serum concentration of the antibiotic [65]. The authors concluded that altered dosing regimens may be required to optimize antimicrobial activity in morbidly obese patients.
Although not advocated as first-line prophylactic agents, the carbapenems have been well studied in obese patients. Chen et al. compared the pharmacokinetic and pharmacodynamic parameters of ertapenem in normal-weight patients and obese patients [66]. They found that after adjusting for body surface area or total body weight, the Vd increased with increasing weight, while Cl decreased. Total drug exposure also decreased with weight. Pharmacodynamic modeling suggested that obesity decreased the probability of attaining the desired time above the MIC for the drug, suggesting the need for higher doses [66]. By contrast, for meropenem, no weight-based dose changes were needed because the drug’s short half-life minimized the impact of increased Vd and Cl [67].
For the aminoglycosides, both the Vd and Cl are increased in obesity, and appear to offset one another [68–73]. Since the aminoglycosides exhibit a concentration-dependent mechanism of action, achieving appropriate peak concentrations is most important. Hence, adjusted body weight (commonly accounts for 40% of excess body weight) should be used to derive doses to correct for the excess mass in obese patients. However, increased dosing must be weighted against the potential for toxicity, particularly among patients with renal insufficiency. Since less toxic agents are available for prophylaxis, aminoglycosides are not the preferred agents for use in patients with renal insufficiency.
Studies with ciprofloxacin have reported varying results. The study by Allard et al. described an increase in Vd and Cl in obese patients [74] and this was supported by a case report by Caldwell et al. [75]. By contrast, Hollenstein et al. administered a single intravenous dose of ciprofloxacin 2.85 mg/kg (based on total body weight) to 12 obese (mean BMI: 41; mean weight: 122 kg) and normal-weight patients (mean BMI: 19.8) and found that the Vd and Cl were lower in the obese subjects [76]. Although peak serum concentrations were higher in the obese subjects, tissue penetration was lower. Hence, the study suggested that higher doses should be given to improve tissue concentrations in obese patients.
Investigators have also analyzed the dose and pharmacokinetics of prophylactic antimicrobials in patients undergoing bariatric surgery. All of these studies involved a cephalosporin. In a study by Mann et al., the Vd and Cl of cefamandole increased in obese patients undergoing Roux-en-Y gastrojejunostomy when compared with historical nonobese controls [77]. When the dose was doubled, improved therapeutic tissue concentrations were attained. Forse et al. observed that in obese patients under going vertical banded gastroplasty, serum and adipose cefazolin concentrations at the time of incision and closure were similar between obese patients who received a 2-g prophylactic dose versus nonobese patients who were given a 1-g prophylactic dose [78]. Furthermore, obese patients who received a 2-g prophylactic dose of cefazolin were found to have decreased postoperative infections compared with obese patients who received cefazolin 1-g (16.5 vs 5.6% for 1 and 2 g of prophylactic cefazolin, respectively; p = 0.03). Edmiston et al. examined patients undergoing open or laparoscopic RYGB who received cefazolin 2 g intravenously 30–60 min before incision [28]. They reported decreasing concentrations of cefazolin in the serum, skin, adipose tissue and omental tissue with increasing BMI. Overall, therapeutic cefazolin tissue concentrations were achieved in only 48.1, 28.6 and 10.2% of the BMI categories of 40–50, 50–60, and 60 or higher, respectively. Before the second dose (cefazolin 2 g delivered in the third hour of operation), serum concentrations were above the cefazolin breakpoint of 32 µg/ml in 41.1, 18.2 and 0% of patients in the three BMI groups, respectively.
In bariatric surgical patients who require prophylaxis against MRSA, vancomycin can be used. There have been several reports regarding vancomycin pharmacokinetics in obesity, although they are not specific to bariatric surgery [73,79–82]. Vancomycin Cl appears to increase uniformly with obesity across studies [61]. However, the magnitude of increase in Vd is more variable [61]. Currently, the recommendation is to dose vancomycin using total body weight [83]. However, the maximum vancomycin dose that can be safely used in patients of extreme body weight is unknown. Although routine therapeutic drug monitoring does not appear to be necessary given the limited duration of vancomycin administration for surgical prophylaxis, caution must be exercised in patients who have decreased renal function, as large doses may result in unnecessarily prolonged exposure (see Table 2 for details).
Table 2.
Suggested initial dose and time to redosing for antimicrobials commonly used for bariatric surgical prophylaxis in patients with normal renal function.
| Antimicrobial | Half-life if normal renal function (h) |
Recommended infusion time (min) |
Standard intravenous dose |
Recommended intravenous dose per BMI group |
Recommended redosing interval (h) |
|---|---|---|---|---|---|
| Cefazolin | 1.2–2.5 | 3–5†
15–60‡ |
1 g | BMI ≥30 to ≤50 kg/m2: 2 g BMI >50 kg/m2: 3 g |
2–5 |
| Cefoxitin | 0.5–1.1 | 3–5†
15–60‡ |
1 g | BMI ≥30 to ≤50 kg/m2: 2 g BMI >50 kg/m2: 3 g |
2–3 |
| Ceftriaxone | 5–11 | 3–5†
15–60‡ |
1 g | BMI ≥30 to ≤50 kg/m2: 2 g BMI >50 kg/m2: 3 g |
10 |
| Cefotaxime | 1–2 | 3–5†
20–30‡ |
1 g | BMI ≥30 to ≤50 kg/m2: 2 g BMI >50 kg/m2: 3 g |
2–4 |
| Metronidazole | 6–14 | 30–60 | 500 mg | 1 g | 6–8 |
| Clindamycin | 2–5.1 | 10–60 (not to exceed 30 mg/min) |
600 mg | BMI ≥30 to ≤50 kg/m2: 900 mg BMI >50 kg/m2: 1200 mg |
6–8 |
| Gentamicin or tobramycin§ |
2–3 | 30–60 | 1.5 mg/kg¶ | See footnote¶ | 3–6 |
| Ciprofloxacin | 3–7 | 60 | 400 mg | 400 mg | 4–10 |
| Levofloxacin | 5.7–9.6 | 60 90 |
500 mg – |
– 750 mg |
– 24 |
| Moxifloxacin | 11.5–15.6 | 60 | 400 mg | 400 mg | 24 |
| Aztreonam | 1.5–2 | 3–5† | 1 g | BMI ≥30 to ≤50 kg/m2: 2 g BMI ≥50 kg/m2: 3 g |
3–5 |
| Vancomycin | 4–6 | 1 h/g | 1 g | 25 mg/kg (TBW)#
Maximum initial dose: 2.5 g Maximum redose: 1.5 g |
8–12§§ |
Dose injected directly into vein or running intravenous fluids.
Intermittent intravenous infusion.
Aminoglycosides are not recommended for patients with renal insufficiency as less toxic alternative prophylactic agents exist.
If the patient’s weight is 30% above their ideal body weight, dosing weight can be determined as follows: DW = IBW + 0.4 (TBW – IBW).
This is assuming the patient has not previously received vancomycin within 24–48 h of this dose.
Count interval of redosing from the end of infusion of the initial dose.
DW: Dosing weight; IBW: Ideal body weight; TBW: Total body weight.
In summary, although data are limited, it appears that standard doses of antimicrobial agents, particularly the cephalosporins, result in low serum and tissue levels in obese patients. Pending additional studies, we recommend that the highest dose of prophylactic antimicrobial agent that can be safely administered, after being adjusted for renal function, be used for surgical prophylaxis. Proposed doses for different antimicrobial agents that are commonly used for bariatric surgical prophylaxis are listed in Table 2 [48].
Antimicrobial prophylaxis: timing of administration
Current guidelines based on published literature suggest that infusion of the first dose of most prophylactic antimicrobial sshould begin within 30 min to 1 h before incision [13,40,84,85]. Antimicrobials such as fluoroquinolones or vancomycin require longer infusion times and thus, infusion should begin within 1–2 h prior to incision. This slower infusion can help to prevent infusion-related arrhythmias (with fluoroquinolones) or Red-man syndrome (with vancomycin). Obese patients often require higher doses of antimicrobials to achieve similar concentrations of the drug in their serum and tissues. Depending on the antimicrobial agent, higher doses may require a longer infusion time. Owing to the ability to rapidly infuse cephalosporins, increasing the prophylactic dose of this type of agent should not significantly impact the timing of administration. By contrast, current guidelines recommend extending the vancomycin infusion time to 1.5–2 h for doses of 1.5 and 2 g [83]. Unfortunately, these prolonged infusion times can greatly complicate preoperative planning. For example, surgical prophylaxis using higher doses of vancomycin that would necessitate infusion for longer than 2 h would not meet the performance measure set forth by the Centers for Medicare & Medicaid Services and the CDC under the Surgical Infection Prevention Project [84]. Situations where optimal dosing conflicts with regulatory requirements need to be re-evaluated by clinicians and regulatory agencies.
The β-lactams exhibit time-dependent killing properties. Doubling the dose of a β-lactam drug in obese patients achieves similar concentrations to those obtained in normal-weight patients with standard doses [62,77,78]. Thus, adequate levels of these drugs can be achieved in the serum and tissues of bariatric surgical patients by administering higher doses or shortening the time to redosing.
Repeating administration of the dose of prophylactic antimicrobials is necessary for prolonged procedures in which the concentration of the antimicrobial is anticipated to fall below the MIC of target pathogens. The need for redosing depends on the duration of surgery, the serum and tissue levels obtained from the initial prophylactic dose, the half-life of the drug and the MIC of the target organisms. Redosing of prophylactics is recommended if the procedure exceeds two half-lives of the drug from the time that the first dose was administered or if the operation extends beyond 3 h in duration [40,84]. Excessive bleeding [86] or fluid replacement during surgery may expedite the need to redose, while renal insufficiency would prolong the half-life of most drugs, and thus, prolong the time period before redosing is necessary. Currently, guidelines do not recommend a standardized redosing approach based on fluid loss or replacement. In general, for cefazolin-based prophylactic regimens, cefazolin should be redosed if the procedure exceeds 2–5 h, or if the procedure exceeds 2–3 h for cefoxitin, 3–5 h for aztreonam, 4–10 h for ciprofloxacin or 6–8 h for metronidazole [84].
Computerized dosing prompts can enhance adherence to prophylactic antimicrobial administration times, particularly pertaining to redosing. In a study by St Jacques et al., a timer notified the clinician of an approaching antibiotic redose time and prompted the clinician to redose the agent [87]. The use of such a system increased the rate of appropriate redosing of antimicrobials from 20 to 58%.
In summary, for bariatric procedures, the first dose of most prophylactic antimicrobials should be infused within 30 min to 1 h before incision. For fluoroquinolones, the infusion should begin within 1–2 h prior to incision. For vancomycin, the infusion time should generally be 1 h per gram of drug prior to the operation. Redosing of antimicrobials during surgery should occur if the procedure exceeds two half-lives of the drug (see Table 2 for details).
Antimicrobial prophylaxis: duration
The initial prophylactic dose, administered 1–2 h before incision, is the most important dose in terms of SSI prevention. As is the case for most procedures, the duration of antimicrobial prophylaxis for bariatric surgery should not exceed 24 h after surgery is completed [13,84].
Expert commentary
Bariatric surgery has provided a very effective treatment modality that is, in some cases, life-sustaining for obese patients. From an infection prevention perspective, there are several interventions that can be utilized to decrease the risk for SSIs. Adequately treating active infections in the preoperative period, thoroughly and carefully preparing the patient’s skin and operative site, administering antimicrobial prophylaxis in an appropriate manner, minimizing the duration of surgery and utilizing more laparoscopic approaches rather than open approaches will all help to minimize SSI risk. Gentle handling and manipulation of tissues during surgery can also decrease SSI risk.
However, several issues remain unresolved. The optimal dosing of prophylactic antimicrobials has not been well studied, particularly in the morbidly obese population. The role of periopertive glucose control, which has been extremely effective in reducing SSIs following cardiothoracic surgery [88] and perioperative warming of the patient, which has been extremely effective in reducing SSI risk following colorectal surgery, have not been explored [89]. In addition, the role of oxygen supplementation, which has been effectively used in colorectal surgical populations to reduce SSI risk, has not been explored in the bariatric population. Trials utilizing one or more of these processes would be well received and might provide much needed evidence-based data regarding SSI prevention.
Five-year view
Over the next 5 years, we anticipate that the population of patients undergoing bariatric surgery will grow immensely. Unfortunately, SSI pathogens are becoming more resistant to standard antimicrobials. In particular, the continuing spread of community-acquired MRSA and extended-spectrum β-lactamase-producing Gram-negative bacilli will make the delivery of effective antimicrobial prophylaxis more challenging, particularly in the obese population. Public reporting of infection rates and decreased insurance reimbursements for hospital-acquired infections, such as SSIs, might have a profound effect on the bariatric surgery population, as these patients are generally at ‘high risk’ for SSIs and have a relatively high frequency of comorbid conditions, increasing their risk for SSIs.
Key issues.
Given the rising incidence of obesity, bariatric surgery has proven life sustaining.
Surgical-site infections associated with bariatric surgery can be prevented with appropriate pre-operative measures including appropriate prophylactic antibiotics, adequate control of hyperglycemia and utilization of more laparoscopic methods for surgery.
Cefazolin is the preferred agent for surgical prophylaxis in bariatric procedures above or including the duodenum, while cefoxitin or cefazolin in combination with metronidazole should be used for bariatric procedures below the duodenum.
For patients with IgE-mediated hypersensitivity to penicillin or cephalosporin, antimicrobial surgical prophylaxis often includes a fluoroquinolone or clindamycin in combination with a fluoroquinolone, an aminoglycoside or aztreonam. Metronidazole is added if anaerobic activity is required for bariatric surgeries involving the ileum.
Intravenous is preferred over topical, subcutaneous or mechanical bowel preparation as the route of delivery for bariatric antimicrobial surgical prophylaxis.
Standard doses of antimicrobial agents may result in low serum and tissue concentrations in obese patients. Hence, the highest dose of prophylactic antimicrobial agent that can be safely administered should be used for bariatric surgical prophylaxis. For cefazolin and cefoxitin, this translates to a minimal initial dose of 2 g, and for metronidazole the initial dose should be 1 g.
All intravenous prophylactic antimicrobial agents should be infused within 30 min to 1 h before incision, with the exception of fluoroquinolones or vancomycin.
Antimicrobial prophylaxis should be redosed if the bariatric surgery exceeds two half-lives of the drug from the time the first dose was administered or if the operation exceeds 3 h.
The duration of antimicrobial prophylaxis for bariatric surgery should not exceed 24 h after surgery is completed.
Footnotes
Financial & competing interests disclosure
Keith Kaye is a consultant and on the speakers bureau for Merck, and has received research support. Keith Kaye is also on the speakers bureau for Pfizer and has received research support from them, he has also received a grant from the NIH (K23-AG023621-05). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N. Engl. J. Med. 2005;353(20):2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg JA, Robinson MK. Surgery: how safe is bariatric surgery? Nat. Rev. Endocrinol. 2009;5(12):645–646. doi: 10.1038/nrendo.2009.221. [DOI] [PubMed] [Google Scholar]
- 5.Wittgrove AC, Martinez T. Laparoscopic gastric bypass in patients 60 years and older: early postoperative morbidity and resolution of comorbidities. Obes. Surg. 2009;19(11):1472–1476. doi: 10.1007/s11695-009-9929-0. [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 8.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol. Assess. 2009;13(41):1–190. doi: 10.3310/hta13410. 215–357, III–IV. [DOI] [PubMed] [Google Scholar]
- 9.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann. Surg. 2004;239(5):599–605. doi: 10.1097/01.sla.0000124292.21605.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topaloglu S, Avsar FM, Ozel H, et al. Comparison of bariatric and non-bariatric elective operations in morbidly obese patients on the basis of wound infection. Obes. Surg. 2005;15(9):1271–1276. doi: 10.1381/096089205774512465. [DOI] [PubMed] [Google Scholar]
- 11.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surg. Endosc. 2008;22(12):2554–2563. doi: 10.1007/s00464-008-0074-y. [DOI] [PubMed] [Google Scholar]
- 13.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999;27(2):97–132. [PubMed] [Google Scholar]
- 14.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992;13(10):606–608. [PubMed] [Google Scholar]
- 15.Poulsen KB, Bremmelgaard A, Sorensen AI, Raahave D, Petersen JV. Estimated costs of postoperative wound infections. A case-control study of marginal hospital and social security costs. Epidemiol. Infect. 1994;113(2):283–295. doi: 10.1017/s0950268800051712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittet D, Harbarth S, Ruef C, et al. Prevalence and risk factors for nosocomial infections in four university hospitals in Switzerland. Infect. Control Hosp. Epidemiol. 1999;20(1):37–42. doi: 10.1086/501554. [DOI] [PubMed] [Google Scholar]
- 17.Anaya DA, Dellinger EP. The obese surgical patient: a susceptible host for infection. Surg. Infect. (Larchmt) 2006;7(5):473–480. doi: 10.1089/sur.2006.7.473. [DOI] [PubMed] [Google Scholar]
- 18. Christou NV, Jarand J, Sylvestre JL, McLean AP. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes. Surg. 2004;14(1):16–22. doi: 10.1381/096089204772787239.. • Only study describing the risk factors of surgical site infections (SSIs) after open bariatric surgery.
- 19.Chopra TCK, Dhar S, Deborah T, et al. Epidemiology and outcomes associated with surgical site infection (SSI) following bariatric surgery. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 September (2009); San Francisco, CA, USA. [Google Scholar]
- 20.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann. Surg. 2000;232(4):515–529. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann. Surg. 2001;234(3):279–289. doi: 10.1097/00000658-200109000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients – what have we learned? Obes. Surg. 2000;10(6):509–513. doi: 10.1381/096089200321593706. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361(9374):2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 24.Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet. Gynecol. 2002;100(5 Pt 1):959–964. doi: 10.1016/s0029-7844(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 25.Kabon B, Nagele A, Reddy D, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischmann E, Kurz A, Niedermayr M, et al. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes. Surg. 2005;15(6):813–819. doi: 10.1381/0960892054222867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czupryniak L, Strzelczyk J, Pawlowski M, Loba J. Mild elevation of fasting plasma glucose is a strong risk factor for postoperative complications in gastric bypass patients. Obes. Surg. 2004;14(10):1393–1397. doi: 10.1381/0960892042583761. [DOI] [PubMed] [Google Scholar]
- 28. Edmiston CE, Krepel C, Kelly H, et al. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery. 2004;136(4):738–747. doi: 10.1016/j.surg.2004.06.022.. • Primary research examining cefazolin serum and tissue concentrations based on BMI groups and time from antibiotic dose in order to assess the ability to achieve therapeutic targets.
- 29.Gonzalez R, Sarr MG, Smith CD, et al. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J. Am. Coll. Surg. 2007;204(1):47–55. doi: 10.1016/j.jamcollsurg.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez R, Nelson LG, Gallagher SF, Murr MM. Anastomotic leaks after laparoscopic gastric bypass. Obes. Surg. 2004;14(10):1299–1307. doi: 10.1381/0960892042583978. [DOI] [PubMed] [Google Scholar]
- 31.Goldfeder LB, Ren CJ, Gill JR. Fatal complications of bariatric surgery. Obes. Surg. 2006;16(8):1050–1056. doi: 10.1381/096089206778026325. [DOI] [PubMed] [Google Scholar]
- 32.Kermarrec N, Marmuse JP, Faivre J, et al. High mortality rate for patients requiring intensive care after surgical revision following bariatric surgery. Obes. Surg. 2008;18(2):171–178. doi: 10.1007/s11695-007-9301-1. [DOI] [PubMed] [Google Scholar]
- 33.Madan AK, Stoecklein HH, Ternovits CA, Tichansky DS, Phillips JC. Predictive value of upper gastrointestinal studies versus clinical signs for gastrointestinal leaks after laparoscopic gastric bypass. Surg. Endosc. 2007;21(2):194–196. doi: 10.1007/s00464-005-0700-x. [DOI] [PubMed] [Google Scholar]
- 34.Seiler CA, Brugger L, Forssmann U, Baer HU, Buchler MW. Conservative surgical treatment of diffuse peritonitis. Surgery. 2000;127(2):178–184. doi: 10.1067/msy.2000.101583. [DOI] [PubMed] [Google Scholar]
- 35.Koperna T, Schulz F. Prognosis and treatment of peritonitis. Do we need new scoring systems? Arch. Surg. 1996;131(2):180–186. doi: 10.1001/archsurg.1996.01430140070019. [DOI] [PubMed] [Google Scholar]
- 36.Sapala JA, Wood MH, Schuhknecht MP. Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: report on 738 gastric bypass patients. Obes. Surg. 2004;14(1):35–42. doi: 10.1381/096089204772787266. [DOI] [PubMed] [Google Scholar]
- 37.Angrisani L, Furbetta F, Doldi SB, et al. Lap band adjustable gastric banding system: the Italian experience with 1863 patients operated on 6 years. Surg. Endosc. 2003;17(3):409–412. doi: 10.1007/s00464-002-8836-4. [DOI] [PubMed] [Google Scholar]
- 38.Cadiere GB, Himpens J, Hainaux B, Gaudissart Q, Favretti S, Segato G. Laparoscopic adjustable gastric banding. Semin. Laparosc. Surg. 2002;9(2):105–114. [PubMed] [Google Scholar]
- 39.Fabry H, Van Hee R, Hendrickx L, Totte E. A technique for prevention of port complications after laparoscopic adjustable silicone gastric banding. Obes. Surg. 2002;12(2):285–288. doi: 10.1381/096089202762552791. [DOI] [PubMed] [Google Scholar]
- 40.ASHP Therapeutic Guidelines on Antimicrobial Prophylaxis in Surgery. American Society of Health-System Pharmacists. Am. J. Health Syst. Pharm. 1999;56(18):1839–1888. doi: 10.1093/ajhp/56.18.1839. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 2008;29 Suppl. 1:S51–S61. doi: 10.1086/591064. [DOI] [PubMed] [Google Scholar]
- 42.Derzie AJ, Silvestri F, Liriano E, Benotti P. Wound closure technique and acute wound complications in gastric surgery for morbid obesity: a prospective randomized trial. J. Am. Coll. Surg. 2000;191(3):238–243. doi: 10.1016/s1072-7515(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 43.Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Jr, Fagerstrom RM. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N. Engl. J. Med. 1981;305(14):795–799. doi: 10.1056/NEJM198110013051404. [DOI] [PubMed] [Google Scholar]
- 44.Lewis RT, Allan CM, Goodall RG, et al. Cefamandole in gastroduodenal surgery: a controlled, prospective, randomized, double-blind study. Can. J. Surg. 1982;25(5):561–563. [PubMed] [Google Scholar]
- 45.Kreter B, Woods M. Antibiotic prophylaxis for cardiothoracic operations. Meta-analysis of thirty years of clinical trials. J. Thorac. Cardiovasc. Surg. 1992;104(3):590–599. [PubMed] [Google Scholar]
- 46.Meijer WS, Schmitz PI, Jeekel J. Meta-analysis of randomized, controlled clinical trials of antibiotic prophylaxis in biliary tract surgery. Br. J. Surg. 1990;77(3):283–290. doi: 10.1002/bjs.1800770315. [DOI] [PubMed] [Google Scholar]
- 47.Barker FG., 2nd Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35(3):484–490. doi: 10.1227/00006123-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin. Infect. Dis. 2004;38(12):1706–1715. doi: 10.1086/421095. [DOI] [PubMed] [Google Scholar]
- 49.Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115(4):1048–1057. doi: 10.1542/peds.2004-1276. [DOI] [PubMed] [Google Scholar]
- 50.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20(4):593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker AP, Nichols RL, Wilson RF, et al. Efficacy of a β-lactamase inhibitor combination for serious intraabdominal infections. Ann. Surg. 1993;217(2):115–121. doi: 10.1097/00000658-199302000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohn SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann. Surg. 2000;232(2):254–262. doi: 10.1097/00000658-200008000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teppler H, McCarroll K, Gesser RM, Woods GL. Surgical infections with enterococcus: outcome in patients treated with ertapenem versus piperacillintazobactam. Surg. Infect. (Larchmt) 2002;3(4):337–349. doi: 10.1089/109629602762539553. [DOI] [PubMed] [Google Scholar]
- 54.Burnett RJ, Haverstock DC, Dellinger EP, et al. Definition of the role of enterococcus in intraabdominal infection: analysis of a prospective randomized trial. Surgery. 1995;118(4):716–721. doi: 10.1016/s0039-6060(05)80040-6. [DOI] [PubMed] [Google Scholar]
- 55.Ohlin B, Cederberg A, Forssell H, Solhaug JH, Tveit E. Piperacillin/tazobactam compared with cefuroxime/metronidazole in the treatment of intra-abdominal infections. Eur. J. Surg. 1999;165(9):875–884. doi: 10.1080/11024159950189393. [DOI] [PubMed] [Google Scholar]
- 56.Bochicchio GV, Baquero F, Hsueh PR, et al. In vitro susceptibilities of Escherichia coli isolated from patients with intra-abdominal infections worldwide in 2002–2004: results from SMART (Study for Monitoring Antimicrobial Resistance Trends) Surg. Infect. (Larchmt) 2006;7(6):537–545. doi: 10.1089/sur.2006.7.537. [DOI] [PubMed] [Google Scholar]
- 57.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1998–2007. J. Antimicrob. Chemother. 2009;64(1):169–174. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baquero F, Hsueh PR, Paterson DL, et al. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2005 results from Study for Monitoring Antimicrobial Resistance Trends (SMART) Surg. Infect. (Larchmt) 2009;10(2):99–104. doi: 10.1089/sur.2008.0020. [DOI] [PubMed] [Google Scholar]
- 59.Alexander JW, Rahn R, Goodman HR. Prevention of surgical site infections by an infusion of topical antibiotics in morbidly obese patients. Surg. Infect. (Larchmt) 2009;10(1):53–57. doi: 10.1089/sur.2008.038. [DOI] [PubMed] [Google Scholar]
- 60.Alexander JW, Rahn R. Prevention of deep wound infection in morbidly obese patients by infusion of an antibiotic into the subcutaneous space at the time of wound closure. Obes. Surg. 2004;14(7):970–974. doi: 10.1381/0960892041719680. [DOI] [PubMed] [Google Scholar]
- 61. Pai MP, Bearden DT. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy. 2007;27(8):1081–1091. doi: 10.1592/phco.27.8.1081.. • Review article aimed at providing an overview of the antimicrobial dosing considerations and data in obese adult patients.
- 62.Pories WJ, van Rij AM, Burlingham BT, Fulghum RS, Meelheim D. Prophylactic cefazolin in gastric bypass surgery. Surgery. 1981;90(2):426–432. [PubMed] [Google Scholar]
- 63.Chiba K, Tsuchiya M, Kato J, Ochi K, Kawa Z, Ishizaki T. Cefotiam disposition in markedly obese athlete patients, Japanese sumo wrestlers. Antimicrob. Agents Chemother. 1989;33(8):1188–1192. doi: 10.1128/aac.33.8.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yost RL, Derendorf H. Disposition of cefotaxime and its desacetyl metabolite in morbidly obese male and female subjects. Ther. Drug Monit. 1986;8(2):189–194. doi: 10.1097/00007691-198606000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Newman D, Scheetz MH, Adeyemi OA, et al. Serum piperacillin/tazobactam pharmacokinetics in a morbidly obese individual. Ann. Pharmacother. 2007;41(10):1734–1739. doi: 10.1345/aph.1K256. [DOI] [PubMed] [Google Scholar]
- 66.Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS., Jr Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob. Agents Chemother. 2006;50(4):1222–1227. doi: 10.1128/AAC.50.4.1222-1227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bearden DT, Earle SB, McConnell DB, Belle DJ, Kohlhepp SJ. Pharmacokinetics of meropenem in extreme obesity; Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; 16–19 December (2005); Washington, DC, USA. [Google Scholar]
- 68.Korsager S. Administration of gentamicin to obese patients. Int. J. Clin. Pharmacol. Ther. Toxicol. 1980;18(12):549–553. [PubMed] [Google Scholar]
- 69.Sketris I, Lesar T, Zaske DE, Cipolle RJ. Effect of obesity on gentamicin pharmacokinetics. J. Clin. Pharmacol. 1981;21(7):288–293. doi: 10.1002/j.1552-4604.1981.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 70.Bauer LA, Edwards WA, Dellinger EP, Simonowitz DA. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur. J. Clin. Pharmacol. 1983;24(5):643–647. doi: 10.1007/BF00542215. [DOI] [PubMed] [Google Scholar]
- 71.Traynor AM, Nafziger AN, Bertino JS., Jr Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob. Agents Chemother. 1995;39(2):545–548. doi: 10.1128/aac.39.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leader WG, Tsubaki T, Chandler MH. Creatinine-clearance estimates for predicting gentamicin pharmacokinetic values in obese patients. Am. J. Hosp. Pharm. 1994;51(17):2125–2130. [PubMed] [Google Scholar]
- 73.Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO., Jr Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob. Agents Chemother. 1982;21(4):575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allard S, Kinzig M, Boivin G, Sorgel F, LeBel M. Intravenous ciprofloxacin disposition in obesity. Clin. Pharmacol. Ther. 1993;54(4):368–373. doi: 10.1038/clpt.1993.162. [DOI] [PubMed] [Google Scholar]
- 75.Caldwell JB, Nilsen AK. Intravenous ciprofloxacin dosing in a morbidly obese patient. Ann. Pharmacother. 1994;28(6):806. doi: 10.1177/106002809402800622. [DOI] [PubMed] [Google Scholar]
- 76.Hollenstein UM, Brunner M, Schmid R, Muller M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. Relat. Metab. Disord. 2001;25(3):354–358. doi: 10.1038/sj.ijo.0801555. [DOI] [PubMed] [Google Scholar]
- 77.Mann HJ, Buchwald H. Cefamandole distribution in serum, adipose tissue, and wound drainage in morbidly obese patients. Drug Intell. Clin. Pharm. 1986;20(11):869–873. doi: 10.1177/106002808602001109. [DOI] [PubMed] [Google Scholar]
- 78.Forse RA, Karam B, MacLean LD, Christou NV. Antibiotic prophylaxis for surgery in morbidly obese patients. Surgery. 1989;106(4):750–756. [PubMed] [Google Scholar]
- 79.Vance-Bryan K, Guay DR, Gilliland SS, Rodvold KA, Rotschafer JC. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37(3):436–440. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther. Drug Monit. 1994;16(5):513–518. doi: 10.1097/00007691-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur. J. Clin. Pharmacol. 1998;54(8):621–625. doi: 10.1007/s002280050524. [DOI] [PubMed] [Google Scholar]
- 82.Penzak SR, Gubbins PO, Rodvold KA, Hickerson SL. Therapeutic drug monitoring of vancomycin in a morbidly obese patient. Ther. Drug Monit. 1998;20(3):261–265. doi: 10.1097/00007691-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Rybak MJ, Lomaestro BM, Rotscahfer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009;49(3):325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 84. Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005;189(4):395–404. doi: 10.1016/j.amjsurg.2005.01.015.. •• Advisory statement that provides an overview of recommendations from the National Surgical Infection Prevention Project and from current guidelines on antimicrobial prophylaxis.
- 85.Steinberg JP, Braun BI, Hellinger WC, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann. Surg. 2009;250(1):10–16. doi: 10.1097/SLA.0b013e3181ad5fca. [DOI] [PubMed] [Google Scholar]
- 86.Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch. Surg. 1996;131(11):1165–1171. doi: 10.1001/archsurg.1996.01430230047009. [DOI] [PubMed] [Google Scholar]
- 87.St Jacques P, Sanders N, Patel N, Talbot TR, Deshpande JK, Higgins M. Improving timely surgical antibiotic prophylaxis redosing administration using computerized record prompts. Surg Infect. (Larchmt) 2005;6(2):215–221. doi: 10.1089/sur.2005.6.215. [DOI] [PubMed] [Google Scholar]
- 88.Trussell J, Gerkin R, Coates B, et al. Impact of a patient care pathway protocol on surgical site infection rates in cardiothoracic surgery patients. Am. J. Surg. 2008;196(6):883–889. doi: 10.1016/j.amjsurg.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 89.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–880. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 90.Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann. Pharmacother. 2009;43:304–315. doi: 10.1345/aph.1L486. [DOI] [PubMed] [Google Scholar]
- 91.LEVAQUIN®. Product monograph. Ortho-McNeil-Janssen Pharmaceuticals, Inc. US5053407. 2009. [Google Scholar]
- 92.AVELOX®. Product monograph. Bayer HealthCare Pharmaceuticals Inc. US81532312. 2008. [Google Scholar]