Abstract
The hydrolysis of phosphate diesters is one of the most difficult reactions known. Here we show that in acetone or cyclohexane, at 25 °C, phosphodiesters undergo hydrolysis 5 × 105 and 2 × 109-fold more rapidly than in water, respectively, and that this rate enhancement is achieved by lowering the enthalpy of activation.
The hydrolysis of unactivated alkyl phosphodiester monoanions, such as those present in the backbone of DNA, proceeds with a half-life of 3 × 107 years at 25 °C in the absence of a catalyst.1 The restriction enzyme EcoRV reduces that half-life to 0.24 ms.2 Among the catalytic effects that may be responsible for the rate enhancements produced by enzymes is desolvation of the substrate,3 and recent experiments have shown that the extraction of a phosphate monoester dianion from water into cyclohexane increases its second order rate constant for hydrolysis by a factor of ~1012.4 The extent to which nonpolar surroundings might also accelerate phosphodiester hydrolysis is unclear. The hydrolysis of a phosphate diester with a weakly basic leaving group, bis(4-nitrophenyl) phosphate, has been shown to be accelerated ~15-fold in DMSO,5 but little experimental information appears to be available about solvent effects on the hydrolysis of unactivated phosphodiesters. We therefore decided to examine the hydrolysis of dineopentyl phosphate (DNP) in nonpolar solvents, for comparison with its uncatalyzed hydrolysis in water.1 In this molecule, steric hindrance precludes nucleophilic attack at carbon. Here, we show that the tetrabutylammonium (TBA+) salt of DNP− enters wet cyclohexane (containing 4.2 × 10−3 M water at saturation)6 at concentrations sufficient to permit measurement of its rate of hydrolysis. The results indicate that DNP− hydrolysis proceeds much more rapidly as the solvent becomes more nonpolar, and that at 25 °C, the second order rate constant for hydrolysis is enhanced by a factor of 2 × 109 when the reaction is transferred from water to wet cyclohexane.
To determine the distribution coefficient of DNP from water to cyclohexane, a small volume (0.1 mL) of an aqueous solution of DNP−:TBA+ (0.05 M DNP−Na+, titrated to pH 9 with TBA+OH−) was first extracted with a large volume (10 mL) of cyclohexane with vigorous stirring overnight, after which equilibrium was found to have been established. The concentrations of DNP− and TBA+ in the cyclohexane phase were then determined by back-extracting the clear cyclohexane layer into D2O (0.6 mL) for 1H NMR analysis (see ESI† for additional experimental details). A second extraction of the cyclohexane layer yielded no additional TBA+ or DNP−. The resulting equilibrium constant for DNP− transfer from water to cyclohexane was 3.9 (±0.4) × 10−5 at 25 °C, and TBA+ and DNP− were present in the cyclohexane layer at equimolar concentrations. The observed distribution coefficient was unaffected by varying the concentration of DNP− in the aqueous phase, indicating that the DNP was fully dissociated in the cyclohexane phase (ESI†).7 DNP−:TBA+ transfer became more favorable as temperature was raised from 10 and 50 °C, yielding a linear van’t Hoff plot (ESI†) that was used to estimate the thermodynamic changes associated with transfer of DNP−:TBA+ from water to cyclohexane (Table 1).
Table 1.
Rates and thermodynamics of NP and DNP hydrolysis in water, acetone, and cyclohexane
| Observed rate constantsd |
Catalysis by desolvatione |
Transfer of substratef |
Phase transfer rateg |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG‡ | ΔH‡ | TΔS‡ | k25/s−1 | ΔG‡ | ΔH‡ | TΔS‡ | k25/s−1 M−1 | ΔG | ΔH | TΔS | Keq | ΔG‡ | ΔH‡ | TΔS‡ | kpt/s−1 | ||
| DNP− | H2Oa | 38.1 | 29.5 | −8.6 | 7.0 × 10−16 | 40.5 | 29.5 | −11.0 | 1.3 × 10−17 | 0 | 0 | 0 | 1 | 38.1 | 29.5 | −8.6 | 7.0 × 10−16 |
| Acetone | 32.9 | 22.6 | −10.3 | 3.9 × 10−12 | 32.6 | 22.6 | −10.0 | 7.0 × 10−12 | — | — | — | — | — | — | — | — | |
| CHX | 31.0 | 19.6 | −11.4 | 1.0 × 10−10 | 27.8 | 19.6 | −8.2 | 2.4 × 10−8 | 6.0 | 32.4 | 26.4 | 3.9 × 10−5 | 37.4 | 52 | 14.6 | 3.9 × 10−15 | |
| NP2− | H2Ob | 44.3 | 47.0 | 2.7 | 2.0 × 10−20 | 47.7 | 47.0 | −0.7 | 3.6 × 10−22 | 0 | 0 | 0 | 1 | 44.3 | 47.0 | 2.7 | 2.0 × 10−20 |
| CHXc | 32.9 | 47.6 | 14.7 | 3.8 × 10−12 | 29.7 | 47.6 | 17.9 | 9.0 × 10−10 | 7.1 | 11.3 | 4.2 | 7.0 × 10−6 | 40.0 | 59.2 | 19.3 | 2.4 × 10−17 | |
From ref. 1.
From ref. 14.
From ref. 4.
Obtained from Arrhenius plots constructed with experimentally measured rates.
Obtained from Arrhenius plots constructed with second order rate constants obtained by dividing experimentally measured rate constants by the concentration of water present in the reaction medium (Fig. 1).
Transfer coefficients measured for the extraction of the substrate from water to cyclohexane. Values obtained from van’t Hoff plots constructed with experimentally measured transfer coefficients (ESI†).
Obtained as described in ref. 4. This value represents the combination of substrate extraction from water (“transfer of substrate” column) and phosphate ester hydrolysis (“observed rate constants” column).
To determine the rate of DNP− hydrolysis in wet cyclo-hexane, portions (10 mL) of the cyclohexane layer (prepared as described above) were incubated in Teflon-lined acid digestion bombs (Parr Instruments Co. #276AC) for various time intervals ranging from 16 hours to 21 days at temperatures ranging from 130–210 °C. After heating, the cyclohexane layer was back-extracted with H2O (1 mL), the aqueous layer was evaporated to dryness (the volatile neopentanol was removed during that process), and the residue was dissolved in D2O for analysis by 1H NMR using added dioxan as an internal integration standard. The extent of hydrolysis was determined by monitoring the decomposition of the diester using the integrated intensities of the peaks arising from DNP, and separate experiments showed that reaction followed first order kinetics in both cyclohexane and acetone at several temperatures. The activation energies are such that hydrolysis of the monoester (neopentyl phosphate, NP) in cyclohexane4 occurs almost instantaneously in the temperature range (130–210 °C) over which DNP− hydrolysis was monitored in the present experiments (Fig. 1B). Identical rate constants were obtained when the extent of reaction was measured either by the disappearance of DNP using proton NMR, or by the release of inorganic phosphate determined spectrophotometrically using an acid molybdate procedure.8
Fig. 1.
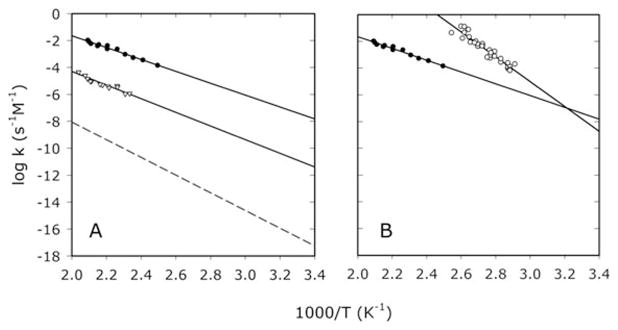
A. Arrhenius plots constructed with the second order rate constants for the hydrolysis of DNP−in water1 (dashed line), acetone (open triangles), and cyclohexane (closed circles). B. Arrhenius plots showing the hydrolyses of NP2− (open circles)4 and DNP− (closed circles) in cyclohexane. The Arrhenius plots intersect at 47.6 °C.
To determine the rate of DNP− hydrolysis in acetone, DNP−:TBA+ was dissolved (0.01 M) in acetone containing 1% water (v/v), sealed in quartz tubes under vacuum, and incubated for varying time periods ranging from 11 hours to 3 days at temperatures ranging from 155 to 230 °C. After incubation, reaction mixtures were diluted in DMSO-d6 for analysis by 1H NMR. The same results were obtained when the rate of reaction was determined by monitoring either the decrease of DNP− or the increase of neopentyl alcohol (two equivalents), indicating that monoester hydrolysis was more rapid than diester hydrolysis in acetone as had also been observed in cyclohexane (see above).
First order rate constants for DNP− hydrolysis in wet cyclohexane and acetone yielded linear Arrhenius plots, from which first order rate constants at 25 °C were estimated by extrapolation (Fig. 1, Table 1). The hydrolysis of DNP− was found to proceed much more rapidly in wet cyclohexane (k = 1.0 × 10−10 s−1 at 25 °C) than in water (7 × 10−16 s−1).1 In acetone, the rate of DNP− hydrolysis (3.9 × 10−12 s−1) fell between the values observed in water and cyclohexane. After correction for the relative concentrations of reactant water in cyclohexane (4.2 × 10−3 M),6 in acetone (0.56 M), and in water (55.5 M), the second order rate constant for DNP− hydrolysis increased 1.8 × 109-fold upon transfer from water to cyclohexane9 and 5.4 × 105-fold upon transfer from water acetone. The temperature dependence of these second order rate constants showed that in both cyclohexane and acetone the rate enhancement was achieved by a major decrease in ΔH‡, accompanied by a minor increase in the value of TΔS‡ (Table 1). These results are consistent with H2O attack on DNP−, or with the kinetically indistinguishable attack by OH− on neutral DNPH.10 Recent simulations suggest that those mechanisms have similar ΔG‡ values in water.11
When ethanol (4 × 10−2 M) was added to reaction mixtures as an alternate acceptor in wet cyclohexane, formation of ethyl neopentyl phosphate was observed by 1H NMR. The second order rate constant for DNP− ethanolysis was found to be larger than the second order rate constant for hydrolysis by a factor of ~5. Thus (in contrast to the susceptibility of phosphate monoesters and ATP to nucleophilic attack), the second order rate constant for decomposition of DNP− varies with the reactivity of the nucleophile.12 For that reason, and because the value of the entropy of activation is substantial and negative in both cyclohexane and water (Table 1), it seems probable that DNP− undergoes hydrolysis by an associative mechanism in cyclohexane, as has been proposed for the reaction of unactivated phosphate diesters in water.11
The activation parameters observed for hydrolysis of DNP− in cyclohexane differ from those observed in water in a manner that seems compatible with the expected effects of changing solvation. In water, hydrogen bonds between the charged substrate and solvent water, which stabilize the ground state, weaken as the substrate approaches the transition state in which charge is less localized. Those hydrogen bonds are absent in the ground state for hydrolysis in cyclohexane, reducing the value of ΔH‡. The effect of acetone on the rate of DNP− hydrolysis is similar to the effect of cyclohexane, although it is somewhat less pronounced.
These effects of cyclohexane on the thermodynamics of activation for DNP− hydrolysis stand in sharp contrast to those observed for hydrolysis of the phosphate monoester NP2−, for which transfer to cyclohexane increases TΔS‡ for hydrolysis by 12 kcal mol−1 while ΔH‡ remains almost unchanged (Table 1). It is clear that the mechanisms of hydrolysis of monoester dianions and diester monoanions differ in several respects. First, the free energies of solvation of DNP− and NP2− are very different in the ground state, as implied by their differing thermodynamics of transfer from water to cyclohexane (Table 1). Thus, the value of TΔS for transfer of the phosphate diester monoanion from water to cyclohexane is ~21 kcal mol−1 more positive than the value for transfer of the monoester dianion. Second, there is considerably more bond formation in the associative transition state for phosphate diester hydrolysis11 (relative to the ground state) than in the dissociative transition state for phosphate monoester hydrolysis, in which the bond to the leaving group is almost completely broken and the bond to the nucleophile has just begun to form.13 These differences in net charge in the ground state, and in the extent of delocalization of charge in the transition state, would be expected to result in significant differences in the extent of solvent reorganization during monoester and diester hydrolysis; and the magnitudes of those differences would be expected to change with transfer of these reactions to wet cyclohexane. The extent of bond making and breaking in the transition state may also change, at least to some degree, when these reactions are transferred from water to cyclohexane.
Monoester and diester hydrolysis differ in mechanism, and also in the thermodynamic expression of their responses to desolvation: entropy-based in the case of monoesters and enthalpy-based in the case of diesters. Yet both reactions are accelerated greatly by transfer to cyclohexane, illustrating the versatility of desolvation as a means of substrate activation. The 109-fold acceleration of the rate of phosphodiester hydrolysis is large enough to suggest that physical transfer of the substrate from water to less polar surroundings may be one factor that contributes to the rate enhancement produced by phosphodiesterases.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (Grant No. GM-18325). We thank Dr Nicholas Williams (University of Sheffield) for the generous gift of the neopentyl phosphate diester.
Footnotes
Electronic supplementary information (ESI) available: Detailed experimental methods; van’t Hoff analysis of equilibria for transfer of DNP− from water to cyclohexane; effect of ester concentration on distribution coefficients observed for transfer of DNP− from water to cyclohexane; detail of Arrhenius plots for DNP− hydrolysis in cyclo-hexane and acetone.
Notes and references
- 1.Schroeder GK, Lad C, Wyman P, Williams NH, Wolfenden R. Proc Natl Acad Sci U S A. 2006;103:4052–4055. doi: 10.1073/pnas.0510879103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells RD, Klein RD, Singleton CK. In: The Enzymes. 3. Boyer PD, editor. Vol. 14. Academic Press; New York: 1981. pp. 157–191. [Google Scholar]
- 3.Cohen SG, Vaidya VM, Schultz RM. Proc Natl Acad Sci U S A. 1970;66:249–256. doi: 10.1073/pnas.66.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kemp DS, Paul K. J Am Chem Soc. 1970;92:2553–2554. doi: 10.1021/ja00711a062. [DOI] [PubMed] [Google Scholar]; Crosby J, Stone R, Lienhard GE. J Am Chem Soc. 1970;92:2891–2900. doi: 10.1021/ja00712a048. [DOI] [PubMed] [Google Scholar]
- 4.Stockbridge RB, Wolfenden R. J Am Chem Soc. 2009;131:18248–18249. doi: 10.1021/ja907967y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Tagle P, Vargas-Zuniga I, Taran O, Yatsimirsky AK. J Org Chem. 2006;71:9713–9722. doi: 10.1021/jo061780i. [DOI] [PubMed] [Google Scholar]
- 6.Kertes AS, editor. Solubility Data Series. Vol. 37. Pergamon Press; Oxford: 1989. pp. 225–229. [Google Scholar]
- 7.Gustavii K. Acta Pharm Suec. 1967;4:233–246. [PubMed] [Google Scholar]
- 8.Chen PS, Toribara TY, Warner H. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 9.At 25 °C, the enhancement of the first order rate constant for DNP− hydrolysis by transfer to cyclohexane (1.4 × 105-fold) roughly compensates for the unfavorable transfer of DNP− from water to cyclohexane (Kd = 3.9 × 10−5). Thus, extraction into cyclohexane results in a small “phase transfer rate enhancement” (5.5-fold at 25 °C). That value increases to 34-fold at 37 °C and 4200-fold at 81 °C—the boiling point of cyclohexane. Those values are considerably surpassed by the values observed earlier for hydrolysis of the corresponding monoester, neopentyl phosphate (ref. 4).
- 10.We cannot rule out the possibility that hydroxide ion acts as the nucleophile in cyclohexane. However, transfer of TBA+:OH− to cyclohexane was not observed, based on a lower limit of detection of ~5 × 10−8 M. Thus, the concentration of H2O in cyclohexane (4 × 10−3 M) exceeded the concentration of OH− by a factor of at least 105.
- 11.Kamerlin SC, Williams NH, Warshel A. J Org Chem. 2008;73:6960–6969. doi: 10.1021/jo801207q. [DOI] [PubMed] [Google Scholar]
- 12.Ethanol is a better nucleophile than water by a factor of ~10 in reactions involving the addition of a nucleophile to a carbonyl group ( Sander EG, Jencks WP. J Am Chem Soc. 1968;90:6154–6162.). The results may be contrasted with the results obtained for ATP ( Admiraal SJ, Herschlag D. Chem Biol. 1995;2:729–739. doi: 10.1016/1074-5521(95)90101-9.) and phosphate monoesters ( Kirby AJ, Varvoglis AG. J Am Chem Soc. 1967;89:415–423.), which are thought to proceed through dissociative mechanisms and are insensitive to the nature of the attacking nucleophile.
- 13.Hengge AC. In: Comprehensive Biological Catalysis: A Mechanistic Reference. Sinnott M, editor. Vol. 1. Academic; New York: 1998. pp. 517–541. [Google Scholar]; Thatcher GRJ, Kluger R. Adv Phys Org Chem. 1989;25:99–265. [Google Scholar]
- 14.Lad C, Williams NH, Wolfenden R. Proc Natl Acad Sci U S A. 2003;100:5607–5610. doi: 10.1073/pnas.0631607100. [DOI] [PMC free article] [PubMed] [Google Scholar]


