Abstract
An efficient protocol for elaboration of the 5,6-fused 2-pyridone ring system, exploiting the tandem condensation of propiolamide and cyclic β-ketomethyl esters in water, followed by acid or base promoted intramolecular ring closure and decarboxylation, has been developed.
Keywords: Heterocycles, condensation, fused-ring systems, Tandem reactions, Michael additions
The 2-pyridone ring system comprises the central structural element in numerous important bioactive natural products and related congeners, including the camptothecins,1 drug candidates active against leukemia,1b Huperzine A,2 a potent acetylcholine esterase inhibitor,2b and Merck’s L-697,661,3 an HIV-1 reverse trancriptase inhibitor. In addition, the similarity of 2-pyridone ring system to nucleoside bases, in conjunction with the aromatic nature permitting π-π stacking interactions, as well as the availability of two tautomeric forms capable of participating in multidirectional hydrogen bonds raises the 2-pyridone ring systems to that of a “privileged structure”4 in the context of drug discovery and development.
To date most protocols for the construction and/or annulation of the 2-pyridone ring by enlarge are limited to the conversion of 2-pyrones employing ammonia,5 the oxidation of pyridines6 and the Guareschi-Thorpe7 condensation of acyclic precursors such as cyanoacetamide with 1,3-diketones. There is thus a need for alternative, efficient synthetic tactics to construct the pyridone ring systems.
In conjuction with our recently achieved enantiospecific syntheses of (+)-lyconadin A and (−)-lyconadin B8 (Scheme 1), we envisioned late stage installation of the requisite 2-pyridone ring employing advanced amino ketone 1. We initially selected a one-pot tactic, employed to great advantage by Kozkowski and coworkers in their syntheses of huperzine A and analogs,9 which entails the condensation of methyl propiolate and ammonia with a ketone or β-ketoester. The poor Michael acceptor ability of the unsaturated amide in ouir case however precluded addition, resulting in the recovery of starting materials. Increasing the reactivity of the ketone by conversion to the β-ketoester 2, employing acrylonitrile, a tactic similar to that used in the Mulzer synthesis of huperzine A,10 followed by somewhat modified conditions for cyclization and decarboxylation (cf. Me4NOAc, MeCN) permitted completion of the total synthesis of (+)-lyconadin A (4).8
Scheme 1.
Installation of the 2-pyridone ring in the total synthesis of (+)-lyconadin A.
In this letter we report the culmination of a study to explore the scope and utility of this tactic as a general protocol for the construction of 5,6-fused 2-pyridones. We first examined the Michael addition of α-carbomethoxy-cyclopentanone with propiolamide (Table 1). When the lyconadins’ conditions8 (Cs2CO3 in DMSO) were employed, no reaction was observed at room temperature (entry 1); elevated temperatures were also not profitable leading to extensive polymerization (entry 2). Given the low electrophilicity as well as their inability to stabilize effectively the resulting carbanion acetylenic amides are poor Michael acceptors.11 Indeed, to the best of our knowledge, there is only one report where in a propiolamide has proven to be a competent Michael acceptor.12 Not withstanding this lack of litereature precedent, screening a variety of conditions quickly revealed that Na2CO3 in water at room temperature proved optimal.13 Use of, K2CO3 in acetone at 50 °C also provided the requisite addition product, albeit in somewhat lower yield.
Table 1.
Conditions examined to effect Michael addition
 | |||||
|---|---|---|---|---|---|
| Base |
Solvent |
Time (h) |
T (°C) |
Result |
|
| 1 | Cs2CO3 | DMSO | 12 | rt | No reaction |
| 2 | Cs2CO3 | DMSO | 2 | 80 | Polymerization |
| 3 | NaH | DMF | 12 | rt | No reaction |
| 4 | NaH | DMF | 2 | 60 | Polymerization |
| 5 | NaOH | MeOH | 12 | rt | Polymerization |
| 6 | K2CO3 | Acetone | 1 | 50 | 76 % |
| 7 | Na2CO3 | H2O | 1 | rt | 88 % |
Having achieved an effective Michael addition process, we turned to the development of a one-pot protocol to generate the 2-pyridone ring. Here we called upon a tandem reaction sequence, involving cyclization, ester hydrolysis and decarboxylation (Scheme 2).
Scheme 2.
Proposed formation of fused 2-pyridones
In our total synthesis of (+)-lyconadin A, tetrabutylammoniumacetate proved to be the optimal reagent since stronger nucleophilic reagents such as sodium cyanide or thiophenol resulted in the conjugate addition to the initially derived Michael adduct to furnish 4-substituted 2-pyridones.8 However, when this reagent was applied to 5 (Table 2) only polymerization was observed. Examination of other Krapcho decarboxylation conditions14 exploring a variety of substrates, including 6- and 7-membered rings posessing ethyl esters revealed the sensitivity of these substrates to nucleophilic attack. Of particular note, at elevated temperatures complex mixtures consisting of hydrolyzed and decarboxylated substrates, spirobicyclic and 3-substituted glutarimides and polymers in conjugation with decomposition products were observed. We reasoned that use of the acidic conditions might supress these alternative pathways, in particular intramolecular attack of amide nitrogen with the ester to yield the spiro glutarimides15 as well as aldol reactions leading to polymeric byproducts. Weakly acidic conditions proved somewhat promising, albeit providing the desired 2-pyridone 7 in low yield (18 %) along with the recovered starting material and enamide 6. Strong acidic conditions (cf. conc. HCl) on the other hand not only accelarated the reaction but pleasingly led to the desired pyridone 7 in nearly quantitative yield (Table 2).16
Table 2.
Optimization of the cyclization conditions
 | ||||
|---|---|---|---|---|
| Reagent |
Solvent |
t |
T °C |
Result |
| Me4NOAc | MeCN | 3 h | 130 | no desired product |
| Me4NOAc | MeCN | 12 h | 130 | polymerization |
| NaOH | H2O/DMSO | 12 h | 130 | polymerization |
| LiI | DMF | 12 h | 120 | no desired product |
| TBAI, LiI | DMF/H2O | 12 h | 130 | Polymerization |
| HCl(1M) | H2O | 24 h | 130 | 24% 6, 18% 7 |
| HCl (conc) | H2O | 6 h | 130 | 99% 7 |
Having optimized the tandem cyclization/decarboxylation reaction sequence with 5, we next applied the conditions to substrates possesing different ring sizes (Table 3). Substrates posessing 5 and 6 membered rings furnished good yields upon addition of pro-piolamide, whereas increasing the ring size reduced the yields with accompanying polymerization. Best results were obtianed with 5,6 and 7 membered ring ketones while 8 membered ring did not lead to the desired product. Strong base11 was however was effective in furnishing 2-pyridone 16.
Table 3.
Effect of ring size
| Starting Material |
Addition Product |
% |
Final Product |
% |
|---|---|---|---|---|
 5 |
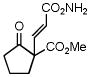 6 |
88 |
 8 |
99 |
 9 |
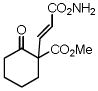 10 |
85 |
 11 |
99 |
 12 |
 13 |
67 |
 14 |
76 |
 15 |
 16 |
65 |
 17 |
44 |
We next explored the effect of α-substitution on the 6-membered ring. Both electron withdrawing and donating substituents not surprisingly led to lower yields in the Michael addition step, possibly due to steric hinderance. In particularly noticable is the drop in the yield of 20 most likely due a Favorskii reaction leading to polymerization (Table 4). Ring closure, hydrolysis and decarboxylation leading to the 5,6-substituted 2-pyridones than proceeded in good to excellent yield, similar to the un-substituted system.
Table 4.
Effect of α-substitution 6-membered rings
| Starting Material |
Addition Product |
% |
Final Product |
% |
|---|---|---|---|---|
 9 |
 10 |
85 |
 11 |
99 |
 18 |
 19 |
75 |
 20 |
82 |
 21 |
 22 |
44 |
 23 |
68 |
 24 |
 25 |
62 |
 26 |
71 |
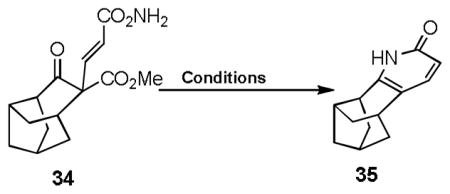
We also examined systems having different distal ring fusions (Table 5). Substrates leading to structure with higher ring strains not surprisingly provided somewhat lower yields in both Michael addition and subsequent annulation and decarboxylation processes. Basic and neutral (NH4OAc) conditions were also tested with 32, a substrate lacking ability to enolize (Table 6). Although the acidic protocol provided 34 in 69% yield, basic and neutral conditions also delivered the product in modest yield, thus providing alternative protocols for substrates with base or acid sensitive functionalities.
Table 5.
Substrates having different ring fusions
| Starting Material |
Addition Product |
% |
Final Product |
% |
|---|---|---|---|---|
 27 |
 28 |
71 |
 29 |
76 |
 30 |
 31 |
42 |
 32 |
44 |
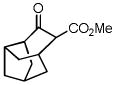 33 |
 34 |
52 |
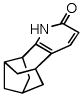 35 |
69 |
Table 6.
 | ||||
|---|---|---|---|---|
| Reagent |
Solvent |
Time |
T °C |
Result |
| Me4NOAc | MeCN | 40 h | 150 | 42 % |
| NaOH | H2O/DMSO | 18 h | 150 | 50 % |
| HCl (conc) | H2O | 6 h | 130 | 69% |
In summary, an effective, versatile protocol comprising the tandem condensation of propiolamide with cyclic β-ketomethylesters followed by cyclization, dehydration and decarboxylation to furnish 5,6-fused 2-pyridones has been developed. To demonstrate the utility of the protocol, a small library of fused 2-pyridones was prepared and submitted to the NIH Molecular Libraries Small Molecule Repository (MLSMR).
Supplementary Material
Acknowledgments
Support for this project was provided by the National Institutes of Health through Grant GM-081253, under the Pilot-Scale Libraries for HTS Program.
References
- 1.(a) Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. J Am Chem Soc. 1966;88:3888. [Google Scholar]; (b) Wall ME. Med Res Rev. 1998;18:299. doi: 10.1002/(sici)1098-1128(199809)18:5<299::aid-med2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kozikowski AP, Campiani G, Sun LQ, Wang S, Saxena A, Doctor BP. J Am Chem Soc. 1996;118:11357. [Google Scholar]; (b) Campiani G, Kozikowski AP, Shaomeng W, Liu M, Nacci V, Saxena A, Doctor BP. Bioorg Med Chem Lett. 1998;8(11):1413. doi: 10.1016/s0960-894x(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 3.Parreira RLT, Abrahao O, Galembeck SE. Tetrahedron. 2001;57:3243. [Google Scholar]
- 4.For a discussion of the term “privileged structure (scaffold)” see: Evans BE, Rittle KE, Bock MG, DiPar-do RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. J Med Chem. 1988;31:2235. doi: 10.1021/jm00120a002.
- 5.Torres M, Gil S, Parra M. Curr Org Chem. 2005;9:1757. [Google Scholar]
- 6.Baron H, Renfry FGP, Thorpe JF. J Chem Soc. 1904;85:1726. [Google Scholar]
- 7.Decker H. Ber Dtsch Chem Ges. 1892;25:443. [Google Scholar]
- 8.Beshore D, Smith AB., III J Am Chem Soc. 2007;129:4148. doi: 10.1021/ja070336+. [DOI] [PubMed] [Google Scholar]
- 9.Kozikowski AP, Reddy ER, Miller CP. J Chem Soc Perkin Trans I. 1990:195. [Google Scholar]
- 10.Högenauer K, Baumann K, Enz A, Mulzer J. Bioorg Med Chem Lett. 2001;11:262. doi: 10.1016/s0960-894x(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 11.Nagata W, Yoshioka M. Org React. 1977;25:255. [Google Scholar]
- 12.xx
- 13.Representative procedure for Michael addition: To a solution of propiolamide (325 mg, 4.70 mmol) and sodiumcarbonate (270 mg, 2.75 mmol) in water (5 mL) at 0 °C methyl-2-oxocyclopentanecarboxylate (3) (390 mg, 2.75 mmol) was added dropwise. The reaction mixture was warmed to room temperature over a 2 h. and than extracted with CH2Cl2 (3×20 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. Purification by flash column chromatography (eluent 1:1 ethyl acetate/hexanes r.f. 0.2) afforded 13 as a white solid (511 mg, 88% yield).
- 14.Krapcho AP. Synthesis. 1982;805:893. [Google Scholar]
- 15.Popović-D̵orđević JB, Ivanović MD, Kiricojević VD. Tetrahedron Lett. 2005;46:2611. [Google Scholar]
- 16.Representative procedure for annulation: A thick walled tube containing a stirbar and methyl 1-(3-amino-3 oxoprop-1-enyl)2-oxocyclopentanecarboxylate (154.1 mg, 0.730 mmol) (13) was dissolved in concentrated HCl (2 mL). The tube was sealed tightly and heated to 130 °C in an oil bath. After 6h the reaction mixture was allowed to cool to room temperature, the tube carefully opened and the reaction mixture poured onto ice (5g). The pH was adjusted to 7 by dropwise addition of saturated aqueous NaHCO3 and then the mixture extracted with ethylacetate. The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to furnish a white solid. Purification through a short pad of silica gel (eluent 100% ethyl acetate) afforded 23 as a white amorphous solid (97.4 mg, 99% yield).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




