Abstract
Background. Kidney function declines with age, but a substantial portion of this decline has been attributed to the higher prevalence of risk factors for kidney disease at older ages. The effect of age on kidney function has not been well described in a healthy population across a wide age spectrum.
Methods. The authors pooled individual-level cross-sectional data from 18 253 persons aged 28–100 years in four studies: the Cardiovascular Health Study; the Health, Aging and Body Composition Study; the Multi-Ethnic Study of Atherosclerosis and the Prevention of Renal and Vascular End-Stage Disease cohort. Kidney function was measured by cystatin C. Clinical risk factors for kidney disease included diabetes, hypertension, obesity, smoking, coronary heart disease, cerebrovascular disease, peripheral arterial disease and heart failure.
Results. Across the age range, there was a strong, non-linear association of age with cystatin C concentration. This association was substantial, even among participants free of clinical risk factors for kidney disease; mean cystatin C levels were 46% higher in participants 80 and older compared with those <40 years (1.06 versus 0.72 mg/L, P < 0.001). Participants with one or more risk factors had higher cystatin C concentrations for a given age, and the age association was slightly stronger (P < 0.001 for age and risk factor interaction).
Conclusions. There is a strong, non-linear association of age with kidney function, even in healthy individuals. An important area for research will be to investigate the mechanisms that lead to deterioration of kidney function in apparently healthy persons.
Keywords: ageing, chronic kidney disease, cystatin C, epidemiology
Introduction
Kidney disease disproportionately affects older persons; the incidence of end-stage renal disease increases with age and the majority of subjects who initiate renal replacement therapy are over 60 years of age [1,2]. Although the strong association between age and kidney function is well established [3], it is unclear what portion of this decline is due to the higher prevalence of risk factors for kidney disease at older ages, such as hypertension, diabetes and vascular disease [4]. The inclusion of persons with these comorbid conditions in prior descriptive studies of kidney function may have inaccurately attributed the risk factor-related decline in kidney function to ageing [5]. A greater understanding of age-related decline in kidney function in a clinically healthy population will inform studies aimed at prevention of kidney disease and its related morbidity in the elderly.
Adding to the complexity of describing age-associated changes in kidney function has been the use of creatinine-based measures in most prior epidemiologic studies. Serum creatinine concentrations are influenced by several factors other than glomerular filtration rate (GFR) including sex, body composition, activity, dietary intake and health status [6]. Older persons may have normal serum creatinine concentrations, despite the presence of kidney dysfunction [7]. Cystatin C is an alternative measure of kidney function that is a better estimate of GFR compared with serum creatinine in the elderly [8–10]. The present study combined cross-sectional data from four studies: the Cardiovascular Health Study (CHS); the Health, Aging and Body Composition Study (Health ABC); the Multi-Ethnic Study of Atherosclerosis (MESA) and the Prevention of Renal and Vascular End-Stage Disease cohort (PREVEND).
The recent availability of cystatin C measurements in several large studies has afforded the opportunity to characterize cystatin C concentrations in ∼18 000 individuals aged 28–100 years. The goal of this study was to examine cystatin C concentrations across adulthood to characterize the association of age with kidney function in a healthy population and to compare this association to that in persons with clinical risk factors for kidney disease.
Subjects and methods
Study population
The CHS aimed to evaluate risk factors for the development and progression of cardiovascular disease in the elderly [11]. The study recruited persons from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania in 1989–1990 for the original cohort and 1992–1993 for a supplementary enrolment period designed to increase the number of African American participants. To be considered eligible for the study, persons had to meet the following criteria: (1) age ≥65 years; (2) not institutionalized; (3) expected to remain in the current community for 3 years or longer; (4) not under active treatment for cancer; and (5) able to give informed consent without requiring a proxy respondent.
The Health ABC study was designed to examine the relation between age-related changes of health and body composition and incident functional limitations in initially well-functioning black and white adults aged 70–79 years. Each of the two study sites, Pittsburgh, PA and Memphis, TN, recruited participants from a list of Medicare beneficiaries between April 1997 and June 1998. Inclusion criteria were (1) reported ability to walk one-quarter mile, climb ten steps and perform basic activities of daily living without difficulty; (2) absence of life-threatening illness; and (3) plan to remain in the geographic area for at least 3 years.
MESA is a cohort of men and women, aged 45–84 years, and free of clinical cardiovascular disease at baseline, designed to examine progression from subclinical to clinical cardiovascular disease in adults [12]. The cohort is ∼38% white, 28% African American, 23% Hispanic and 11% Asian (of Chinese descent). Participants were recruited from six field centres (New York, NY; Baltimore, MD; Chicago, IL; Los Angeles, CA; Minneapolis/St Paul, MN and Winston Salem, NC) with a variety of population-based approaches from 2000 to 2002.
The PREVEND study is a prospective cohort that includes inhabitants aged 28–75 of the city of Groningen, The Netherlands in 1997–1998. The study was designed to investigate the natural course of urinary albumin excretion and its impact on renal and cardiovascular disease; for this purpose, the PREVEND cohort was enriched for subjects with higher concentrations of albuminuria. However, the present study includes only a sub-sample that is representative of the general population of Groningen; these methods have been previously described [13,14].
The present study and parent studies have been approved by all relevant institutional review boards.
Kidney function
Three of the four studies (CHS, Heath ABC and MESA) had cystatin C measured by the same protocol and laboratory at the University of Vermont, and the PREVEND study used the same method at a different site. Cystatin-C was measured in all studies by a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that utilizes a particle-enhanced immunonepholometric assay (N Latex Cystatin-C) [15]. The assay range is 0.195–7.330 mg/L, with the reference range for young, healthy individuals reported as 0.53–0.95 mg/L (cystatin C in mg/L may be converted to nmol/L by multiplying by 74.9). Intra-assay coefficients of variation (CVs) range from 2.0 to 2.8% and inter-assay CVs range from 2.3 to 3.1%. Samples were measured from frozen plasma (CHS and Health ABC) or serum (MESA and PREVEND) and stored at −70°C (CHS, Health ABC, MESA) or −20°C (PREVEND). Studies have shown that cystatin C is stable when frozen, and can withstand several freeze/thaw cycles [16]. To ensure the comparability of the University of Vermont and PREVEND laboratories, we ran a comparison study of 101 samples. The correlation between the two sets was 0.97, the CV was 6% and the mean difference was 0.055 mg/L, which was not significantly associated with the mean concentration.
We calculated estimated GFR (eGFR) based on the cystatin C equation (eGFR = cystatin C−1.19 × 76.7) developed by the CKD-EPI group [17].
Other variables
Age, sex and race were determined by self-report. Participants were considered healthy if they were free of clinical risk factors for kidney disease including diabetes [self-reported history or fasting glucose ≥126 mg/dL or oral glucose tolerance test ≥200 mg/dL (glucose in mg/dL may be converted to mmol/L by multiplying 0.0555)], hypertension (self-reported history or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg), obesity (body mass index >30 kg/m2), smoking (former or current), coronary heart disease (history of coronary heart disease, myocardial infarction, coronary arterial bypass graft, angioplasty, angina), cerebrovascular disease (history of stroke, transient ischaemic attack, carotid endarterectomy), peripheral arterial disease (history of revascularization, claudication) and heart failure. Plasma glucose values in PREVEND were transformed to whole blood values using an internally validated correction factor [18,19]. We limited our definition of clinical risk factors for kidney disease to those conditions that were potentially modifiable or preventable and could be identified in a clinical setting.
Statistical analysis
Baseline characteristics of participants were summarized across study populations. To characterize the shape of the relationship between age and cystatin C, we plotted the variables using a locally weighted regression (Stata function–lowess) in persons with and without risk factors for kidney disease. We next calculated the age-specific mean and standard deviations (SD) of cystatin C by decade of age (<40, 40–50, 50–60, 60–70, 70–80, 80+ years). These analyses were repeated in sex strata among persons free of risk factors for kidney disease. Clinical risk factor, sex and race interaction terms with age were tested based on linear regression models in healthy participants. To account for the non-linearity of the age and cystatin C relationship, age was modelled as the best-fit polynomial function including up to a fifth order term, based on a data-adaptive learning algorithm [20].
To compare the distribution of cystatin C across the age spectrum, we plotted the kernel density estimates (R function–density) of cystatin C concentrations by decade of age among participants without risk factors for kidney disease. We used a smoothing bandwidth of 1.5 times the SD of the smoothing kernel.
As a complementary analysis, we calculated the prevalence and standard error of eGFR <60 ml/min/1.73m2 (cystatin C >1.24 mg/L) by decade of age.
To estimate the period (calendar year) and birth cohort (birth year) effects on cystatin C concentrations, we restricted the age range to the overlap present across all four studies, 69–75 years (n = 6053). We included age, study and birth year or period year in a linear regression model with cystatin C as the outcome. We also examined if there were systematic differences in cystatin C concentration by study (CHS, Health ABC, MESA and PREVEND). We included age, sex, race and study indicator variables in a linear regression model with cystatin C as the outcome. We restricted this analysis to persons free of clinical risk factors for kidney disease to account for differences in the risk factors across the studies.
All analyses were conducted using Stata 9.0 (StataCorp., College Station, TX) and R (The R Foundation, Vienna, Austria).
Results
CHS and Health ABC included older aged participants, MESA included both middle and older aged persons and PREVEND sampled individuals across adulthood (Table 1). All of the cohorts were more than half female. CHS, Health ABC and MESA included black participants, whereas only 1% of PREVEND participants were self-indentified as black. PREVEND participants had a lower prevalence of obesity and diabetes, and lower mean blood glucose. Systolic blood pressure levels were higher in the two older cohorts, whereas diastolic blood pressure levels were similar across the cohorts. The prevalence of coronary heart disease, cerebrovascular disease, peripheral arterial disease and heart failure varied across the cohorts; by design, MESA participants were free of these conditions. The participants without clinical risk factors for kidney disease comprised 36% of the participants under 40 years, 25% of 40–49 year olds, 20% of 50–59 year olds, 13% of 60–69 year olds, 11% of 70–79 year olds and 10% of those over 80 years.
Table 1.
Participant characteristics by study
| CHS (n = 5177) | Health ABC (n = 3044) | MESA (n = 6756) | PREVEND (n = 3276) | |
|---|---|---|---|---|
| Mean (SD) or n (%) | ||||
| Characteristic | ||||
| Visit year | 1989–1992 | 1997–1998 | 2000–2002 | 1997–1998 |
| Age (years) | 72.5 (5.4) | 73.6 (2.9) | 62.1 (10.2) | 48.7 (12.4) |
| Age range (years) | 65–100 | 69–80 | 45–84 | 29–75 |
| Female | 3156 (61%) | 1567 (51%) | 3564 (53%) | 1807 (55%) |
| Black | 856 (17%) | 1265 (42%) | 1868 (28%) | 30 (1%) |
| Obese | 1047 (20%) | 778 (26%) | 2170 (32%) | 439 (14%) |
| Smoking current | 627 (12%) | 314 (10%) | 877 (13%) | 1009 (31%) |
| Former | 2127 (41%) | 1397 (46%) | 2464 (37%) | 1193 (37%) |
| Never | 2418 (47%) | 1328 (44%) | 3393 (50%) | 1059 (32%) |
| Cystatin C (mg/L) | 1.06 (0.34) | 1.05 (0.34) | 0.90 (0.24) | 0.79 (0.20) |
| Fasting glucose (mg/dL) | 111 (38) | 105 (35) | 104 (31) | 83 (16) |
| Systolic blood pressure (mmHg) | 136 (22) | 136 (21) | 127 (21) | 126 (18) |
| Diastolic blood pressure (mmHg) | 71 (11) | 71 (12) | 72 (10) | 73 (9) |
| History of diabetes | 737 (14%) | 419 (14%) | 721 (11%) | 56 (2%) |
| History of hypertension | 3055 (59%) | 1179 (38%) | 1681 (25%) | 668 (20%) |
| Coronary heart disease | 1042 (20%) | 618 (20%) | 0 | 182 (6%) |
| Cerebrovascular disease | 352 (7%) | 244 (8%) | 0 | 23 (1%) |
| Peripheral arterial disease | 144 (3%) | 152 (5%) | 0 | 25 (1%) |
| Heart failure | 94 (3%) | 240 (5%) | 0 | N/A |
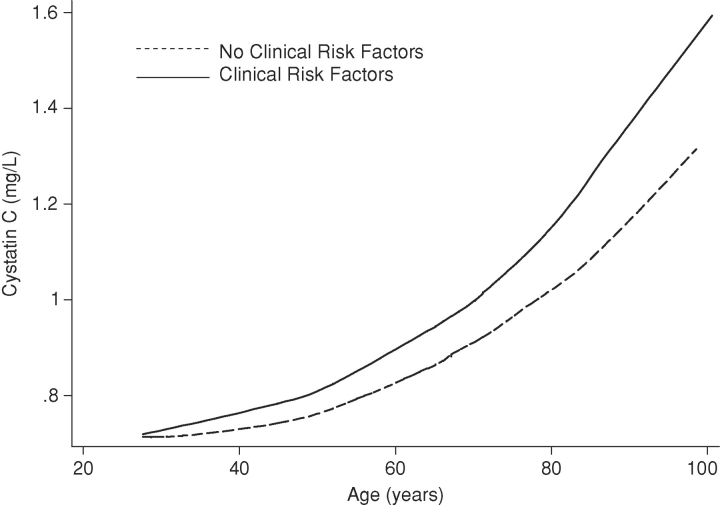
Across the age range, we observed a strong, non-linear association of age with cystatin C concentrations in participants with and without clinical risk factors for kidney disease (Figure 1). The rate of increase in cystatin C concentrations appeared to accelerate with age particularly among persons with clinical risk factors for kidney disease compared to those with no risk factors.
Fig. 1.
Smoothed plot of age and cystatin C across the age range among participants with and without clinical risk factors for kidney disease. Clinical risk factors included diabetes, hypertension, obesity, smoking, coronary heart disease, cerebrovascular disease, peripheral arterial disease and heart failure.
Overall, and among participants with and without clinical risk factors, the mean cystatin C concentration was higher in each ascending decade of life, with an accelerated effect (Table 2). In persons less than 40 years, the mean cystatin C was only about 6% higher (0.04 mg/L) among those with clinical risk factors for kidney disease, but this difference increased with age to 16% (0.17 mg/L) in participants over 80 years. These differences in cystatin C concentrations between participants with and without clinical risk factors in any decade were substantially smaller than the differences between the youngest (<40 years) and oldest (≥80 years) age groups [62% (0.47 mg/L) in participants with clinical risk factors and 47% (0.34 mg/L) in participants without]. The association of age with cystatin C concentration was slightly stronger in women compared with men (0.003 mg/L/decade, P = 0.03), although men had a higher concentration of cystatin C for a given age (Table 2). The association of age with cystatin C concentration did not significantly differ between blacks and whites (P = 0.56).
Table 2.
Mean cystatin C concentrations by decade of age among participants with and without clinical risk factors for kidney disease
| Age (years)* | ||||||
|---|---|---|---|---|---|---|
| <40 (n = 911) | 40–49 (n = 1831) | 50–59 (n = 2677) | 60–69 (n = 4564) | 70–79 (n = 7367) | 80+ (n = 903) | |
| Cystatin C (mg/L) mean (SD) | ||||||
| Risk factors† | 0.76 (0.21) | 0.78 (0.16) | 0.84 (0.17) | 0.95 (0.28) | 1.05 (0.34) | 1.23 (0.38) |
| (n = 15 407) | (n = 584) | (n = 1369) | (n = 2135) | (n = 3950) | (n = 6556) | (n = 813) |
| No risk factors | ||||||
| All | 0.72 (0.17) | 0.75 (0.17) | 0.79 (0.13) | 0.85 (0.14) | 0.96 (0.19) | 1.06 (0.19) |
| (n = 2846) | (n = 327) | (n = 462) | (n = 542) | (n = 614) | (n = 811) | (n = 90) |
| Men‡ | 0.76 (0.21) | 0.80 (0.17) | 0.83 (0.11) | 0.87 (0.13) | 0.99 (0.20) | 1.08 (0.17) |
| (n = 1049) | (n = 131) | (n = 210) | (n = 210) | (n = 197) | (n = 268) | (n = 33) |
| Women | 0.69 (0.14) | 0.70 (0.16) | 0.76 (0.14) | 0.84 (0.15) | 0.94 (0.19) | 1.05 (0.21) |
| (n = 1797) | (n = 196) | (n = 252) | (n = 332) | (n = 417) | (n = 543) | (n = 57) |
*P-value for trend across decades <0.001 for all groups.
†P-value for age and risk factor interaction <0.001.
‡P-value for age and sex interaction = 0.03.
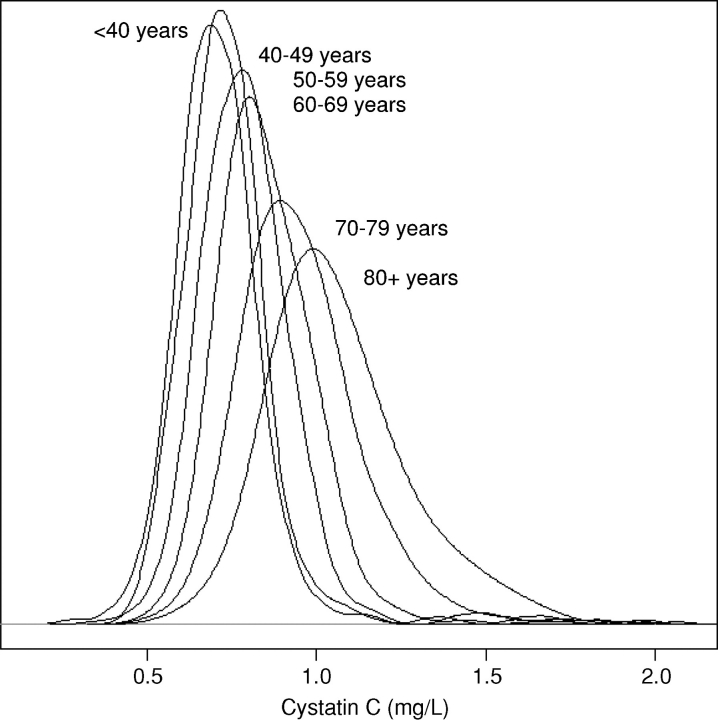
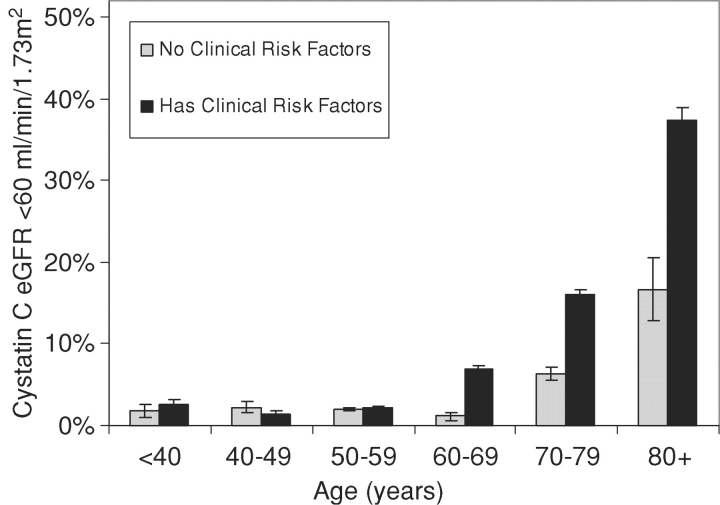
We observed a strong association of age with cystatin C across the distribution of cystatin C concentrations in persons without clinical risk factors for kidney disease (Figure 2). Both the mean cystatin C concentration and the variance were greater in each increasing decade of higher age, with a near normal distribution. When we used a threshold to identify those persons at the high end of the distribution (cystatin C based eGFR <60 ml/min/1.73 m2, equal to a cystatin C concentration of 1.24 mg/L), we found the proportion of persons with worse kidney function increased more with age among persons with clinical risk factors compared to those without risk factors (Figure 3). The prevalence was similar in those with and without risk factors under 60 years, but in participants over 60 years, the presence of clinical risk factors was associated with an over 2-fold risk of eGFR <60 ml/min/1.73 m2 (14% versus 5%).
Fig. 2.
The plot illustrates the distribution of cystatin C concentrations by decade of age in participants without risk factors for kidney disease. The mean and variance are greater with each ascending decade of age.
Fig. 3.
The prevalence of cystatin C eGFR<60 ml/min/1.73m2 (equivalent to cystatin C > 1.24 mg/L) by decade of age is shown in persons with and without clinical risk factors for kidney disease. Risk factors include the following: diabetes, hypertension, obesity, smoking, coronary heart disease, cerebrovascular disease, peripheral arterial disease and heart failure.
Cohort and period effects were not significant when included in a model with age (P = 0.27 for both). There were small study effects of the Health ABC cohort and CHS cohorts compared with PREVEND and MESA. After adjustment for age, sex and race, both CHS and Health ABC cystatin C concentrations were 0.05 mg/L higher compared with PREVEND (P < 0.001 for both) among participants without clinical risk factors for kidney disease. MESA cystatin C concentrations were 0.02 mg/L higher compared with PREVEND, although this difference did not reach statistical significance (P = 0.08).
Discussion
We observed a strong, non-linear association of age with cystatin C concentrations even in participants without clinical risk factors for kidney disease. These results suggest that kidney function worsens considerably with age, even among apparently healthy persons. If validated, these findings could impact our understanding of ageing of the kidney. Unknown risk factors or subclinical levels of known risk factors likely have significant deleterious effects on kidney function. Furthermore, some decline in kidney function in older persons may represent usual ageing.
The strong association of age with higher cystatin C concentrations among apparently healthy persons raises the question of what causes age-related declines in kidney function. The ageing of the kidney may represent cumulative exposure to subclinical levels of risk factors and microvascular disease that could affect kidney function without crossing the threshold of clinical disease [21]. In addition to risk factor exposure, there are biological processes that encompass ageing [4]. One possibility is that age-associated loss of kidney function is due to a progressive loss of nephron units and a decline or alteration in cellular function over time. As kidney function declines, there is increased stress on the remaining nephrons, which likely leads to an acceleration in their decline [22]. It has also been hypothesized that lower energy expenditure impacts GFR, which may influence kidney function in older adults [23–25]. Finally, laboratory studies have observed a number of biochemical decrements in ageing kidney cells, including fewer mitochondria, lower enzyme concentrations and ATPase activity, decreased sodium transport and oxygen consumption and lower tubular transport [26].
The hypothesis that some kidney function decline represents usual ageing is consistent with studies that have described normal age-related declines in function in other physiologic domains. Cardiac output, maximum oxygen consumption, muscle strength, lung function, immune function, metabolic rate and some hormone concentrations are all observed to decline with age, even in healthy elderly [27]. In the current study, we identified healthy study participants as those free of clinical risk factors for kidney disease: non-smoker, not obese, and no history of diabetes, hypertension, cardiovascular disease or heart failure. Restriction of the healthy subpopulation to persons without subclinical disease may have further attenuated the age effect. However, a more rigorous definition of risk factors may be a difficult target goal for health in the general population, as in the present study, healthy persons were only about 10% of those over 70 years of age.
Our findings are consistent with prior studies of ageing of the kidneys, although some included persons with comorbid conditions known to affect kidney function [5] [4]. In one of the earliest cross-sectional reports, Davies and Shock described a non-linear decrease in GFR after 40 years; however, the study included persons with comorbid diseases. In another seminal report, Wesson illustrated the progressive decline of GFR with age by combination of data from 27 cross-sectional studies [28]. However, persons with comorbidities were included and the data were sparse on persons over age 60. Investigators from the Baltimore longitudinal studies of ageing were among the first to longitudinally study kidney function in a healthy subpopulation, with follow-up time up to 24 years. Consistent with our findings, they reported a progressive linear decline in creatinine clearance among 548 individuals free of diabetes, coronary heart disease, abnormal urinalysis or cerebrovascular disease [29]. In addition, a recently published study on cystatin C concentrations in the USA reported a strong and independent association of age with cystatin C concentrations in persons over 60 years, even after adjustment for chronic health conditions [30]. Our study used a stricter definition of healthy compared to prior studies and it represents the largest collaborative effort to describe the age-related change in cystatin C concentrations.
Several studies have reported that cystatin C is a better marker for measured GFR compared with creatinine [8]. To increase the interpretability of cystatin C concentration into a clinically meaningful metric, researchers have developed equations to transform cystatin C into estimated GFR [17]. These equations can be used to estimate kidney function, but they were developed in a relatively young population with established chronic kidney disease and have not yet been validated in the general population. The mean cystatin C in the largest equation study, conducted by the CKD-EPI group, was 1.8 (SD = 0.8) mg/L (17), whereas the mean cystatin C level in the present study was 0.95 (0.30) mg/L. Application of a regression equation outside of the range of cystatin C values in which it was developed may introduce bias into the estimates; therefore, we chose to present untransformed cystatin C instead of eGFR for the majority of analyses. Because the cystatin C eGFR analysis is a cutpoint analysis (equivalent to the prevalence of cystatin C > 1.24 mg/L), the validity of the equation in the present study population is not essential to make inferences.
While a longitudinal study design is preferred in studies of ageing, few studies have measured kidney function across decades of follow-up. Instead, cross-sectional designs have been used in most studies of ageing and kidney function [28,29,31], as in other studies of age trajectories of growth and decline [32–34]. The primary limitation of cross-sectional data is that each observation includes not only an age effect, but also period and cohort effects [35], which can lead to discordant results from cross-sectional and longitudinal studies. However, if the period and cohort effects are small, the effect of age can be accurately estimated. Our findings suggest that the period and cohort effects were insignificant in the study population.
Our study has limitations that should be considered when interpreting the findings. Primarily, none of the included studies measured GFR; therefore, we cannot measure the extent to which cystatin C concentrations are influenced by factors other than GFR. In addition, the relation of cystatin C with GFR has not been well studied in a population of older adults with near normal kidney function. By the use of cross-sectional data, we are unable to characterize definitively the period or birth cohort effects, although there was little evidence of period or birth cohort effects among participants in the age range that overlapped across the four cohorts (69–75 years). Selection bias is a potential limitation of our study; however, since age and poor kidney function are both associated with an increased risk of mortality, this would most likely bias the age association towards null. In addition, the present study did not include historical data on the severity of risk factors over the life course or information on medication use. Finally, there may be some misclassification of risk factor status.
In conclusion, cystatin C concentrations increase substantially with age, even in the absence of clinical risk factors for kidney disease. Studies aimed at prevention of kidney disease should consider that some age-related decline in kidney function is likely, even in healthy individuals. An important area for research will be to investigate the mechanisms that lead to deterioration in function in apparently healthy persons. Future studies should also examine the extent to which treatment of clinical risk factors may prevent the progression to kidney disease and its related adverse consequences. To that end, accurate assessment of kidney function is important for the clinical care of elderly persons who are disproportionately affected by kidney disease.
Acknowledgments
CHS was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Health ABC was supported through the National Institute on Aging contracts N01-AG-6-2101, N01-AG-6-2103 and N01-AG-6-2106 and in part by the Intramural Research Program of the NIH, National Institute on Aging. MESA was supported by grant R01-HL-63963-01A1 and by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung and Blood Institute. PREVEND is supported by grant E013 of the Dutch Kidney Foundation, Bussum, The Netherlands, and grants T32DK07791 and DK52866 from the National Institute of Diabetes and Digestive, and Kidney Diseases. This project was also supported by grant R01AG027002 from the National Institutes on Aging. Dr Odden is supported by an institutional Health Resources & Services Administration National Research Service Award (T32HP19025). Dr Shlipak was supported by the American Federation for Aging Research and National Institute on Aging (Paul Beeson Scholars Program), by the Robert Wood Johnson Foundation (Generalist Faculty Scholars Program), and the American Heart Association.
Conflict of interest statement. None declared.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. USRDS 2008. 2007 Annual Data Report. Atlas of End-Stage Renal Disease in the United States.
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Wesson LG. Renal hemodynamics in physiological states. In: Wesson LG, editor. Physiology of the Human Kidney. New York: Grune and Stratton; 1969. pp. 96–108. [Google Scholar]
- 4.Zhou XJ, Rakheja D, Yu X, et al. The aging kidney. Kidney Int. 2008;74:710–720. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 5.Fliser D. Ren sanus in corpore sano: the myth of the inexorable decline of renal function with senescence. Nephrol Dial Transplant. 2005;20:482–485. doi: 10.1093/ndt/gfh710. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 7.Lamb EJ, O’Riordan SE, Delaney MP. Kidney function in older people: pathology, assessment and management. Clin Chim Acta. 2003;334:25–40. doi: 10.1016/s0009-8981(03)00246-8. [DOI] [PubMed] [Google Scholar]
- 8.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens. 2006;15:270–275. doi: 10.1097/01.mnh.0000222694.07336.92. [DOI] [PubMed] [Google Scholar]
- 10.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Gansevoort RT, Verhave JC, Hillege HL, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl. 2005;94:S28–S35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 15.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 16.Finney H, Newman DJ, Gruber W, et al. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43(6 Pt 1):1016–1022. [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brantsma AH, Bakker SJ, Hillege HL, et al. Urinary albumin excretion and its relation with C-reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care. 2005;28:2525–2530. doi: 10.2337/diacare.28.10.2525. [DOI] [PubMed] [Google Scholar]
- 19.Burnett RW, D’Orazio P, Fogh-Andersen N, et al. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001;307:205–209. doi: 10.1016/s0009-8981(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 20.Sinisi SE, Van Der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Stat Appl Genet Mol Biol. 2004;3:Article18. doi: 10.2202/1544-6115.1069. [DOI] [PubMed] [Google Scholar]
- 21.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46:214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Lewis J, Greene T, Appel L, et al. A comparison of iothalamate-GFR and serum creatinine-based outcomes: acceleration in the rate of GFR decline in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol. 2004;15:3175–3183. doi: 10.1097/01.ASN.0000146688.74084.A3. [DOI] [PubMed] [Google Scholar]
- 23.Daugirdas JT, Levin NW, Kotanko P, et al. Comparison of proposed alternative methods for rescaling dialysis dose: resting energy expenditure, high metabolic rate organ mass, liver size, and body surface area. Semin Dial. 2008;21:377–384. doi: 10.1111/j.1525-139X.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kachadorian WA, Johnson RE. Renal responses to various rates of exercise. J Appl Physiol. 1970;28:748–752. doi: 10.1152/jappl.1970.28.6.748. [DOI] [PubMed] [Google Scholar]
- 25.Singer MA. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis. 2001;37:164–178. doi: 10.1016/s0272-6386(01)80073-1. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman RD. Overview: renal physiology and pathophysiology of aging. Am J Kidney Dis. 1990;16:275–282. doi: 10.1016/s0272-6386(12)80002-3. [DOI] [PubMed] [Google Scholar]
- 27.Timiras PS. Physiological Basis of Aging and Geriatrics. 4th ed. New York: Informa Healthcare; 2007. [Google Scholar]
- 28.Wesson L. Physiology of the Human Kidney. New York, NY: Grune and Stratton; 1969. Renal hemodynamics in physiolgical states; pp. 96–108. [Google Scholar]
- 29.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 30.Kottgen A, Selvin E, Stevens LA, et al. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tager IB, Segal MR, Speizer FE, et al. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am Rev Respir Dis. 1988;138:837–849. doi: 10.1164/ajrccm/138.4.837. [DOI] [PubMed] [Google Scholar]
- 33.Tanner JM. Fetus into Man. Boston, MA: Harvard University Press; 1978. [Google Scholar]
- 34.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Laird N, Dockery DW, et al. Age, period, and cohort effects on pulmonary function in a 24-year longitudinal study. Am J Epidemiol. 1995;141:554–566. doi: 10.1093/oxfordjournals.aje.a117471. [DOI] [PubMed] [Google Scholar]