Abstract
The expression of catabolic enzymes spermidine/spermine N1-acetyltransferase (SSAT) and spermine oxidase (SMO) increases after ischemic reperfusion injury. We hypothesized that polyamine catabolism is upregulated and that this increase in catabolic response contributes to tissue damage in endotoxin-induced acute kidney injury (AKI). SSAT mRNA expression peaked at threefold 24 h following LPS injection and returned to background levels by 48 h. The activity of SSAT correlated with its mRNA levels. The expression of SMO also increased in the kidney after LPS administration. Serum creatinine levels increased significantly at ∼15 h, peaking by 24 h, and returned to background levels by 72 h. To test the role of SSAT in endotoxin-induced AKI, we injected wild-type (SSAT-wt) and SSAT-deficient (SSAT-ko) mice with LPS. Compared with SSAT-wt mice, the SSAT-ko mice subjected to endotoxic-AKI had less severe kidney damage as indicated by better preservation of kidney function. The role of polyamine oxidation in the mediation of kidney injury was examined by comparing the severity of renal damage in SSAT-wt mice treated with MDL72527, an inhibitor of both polyamine oxidase and SMO. Animals treated with MDL72527 showed significant protection against endotoxin-induced AKI. We conclude that increased polyamine catabolism through generation of by-products of polyamine oxidation contributes to kidney damage and that modulation of polyamine catabolism may be a viable approach for the treatment of endotoxin-induced AKI.
Keywords: polyamine, polyamine catabolism, polyamine back conversion
endotoxin-induced acute kidney injury (AKI) resulting from sepsis is a major cause of morbidity and mortality in intensive care unit patients (27). Despite progress in understanding the pathophysiological processes and identification of factors involved in the mediation of endotoxin-induced AKI, the treatment options for this injury remain limited to supportive interventions. Identifying the molecules and pathways that contribute to tissue damage in endotoxin-induced AKI will lead to the development of therapeutic strategies aimed at ameliorating its adverse outcomes.
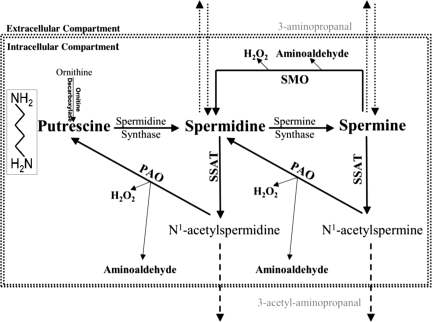
The role of polyamine catabolism in the etiology of endotoxin-induced AKI is not clear. Previous studies have indicated that the expression of spermidine/spermine N1-acetyltransferase (SSAT) and spermine oxidase (SMO), and as a result polyamine catabolism, increase in kidney and liver subjected to ischemia-reperfusion injuries (4, 34). The ablation of the SSAT gene reduces the severity of tissue damage resulting from ischemia-reperfusion injuries (33). Polyamines are positively charged molecules that interact with nucleic acids and proteins and play an important role in gene transcription and signal transduction (9, 11, 14, 21). Alterations in intracellular polyamine pools are known to adversely affect cell morphology, adhesion, and proliferation (8, 18, 26, 31, 32). Because of their physiological roles, cellular polyamine levels are tightly controlled through regulation of their transport, synthesis, and catabolism. Polyamine catabolism occurs via the SMO activity and/or through polyamine back conversion. As depicted in Fig. 1, the latter is a two-step process that involves the acetylation of spermine (Spm) or spermidine (Spd) by SSAT, followed by oxidation of the acetylated polyamines by polyamine oxidase (PAO). By contrast, SMO converts Spm to Spd directly. The degradation of polyamines by either system leads to the generation of toxic metabolites (i.e., hydrogen peroxide and aminoaldehydes). We have shown that increasing polyamine back conversion by SSAT overexpression in vitro results in the disruption of polyamine homeostasis and cell injury (17, 31). For example, increased expression of SSAT in HEK-293 cells leads to the depletion of Spd and Spm and increased putrescine (Put) pools (31). Cells engineered to overexpress SSAT are oxidatively stressed, exhibit altered morphology and reduced adhesion, have reduced rates of proliferation, exhibit high levels of DNA damage, and are G2 arrested (31, 32). Transgenic rodents that express high levels of SSAT have depleted Spd and Spm pools and increased levels of Put and acetylspermidine in most tissues, which results in organ-specific abnormalities (1–3, 22, 24). For example, overexpression of SSAT in transgenic rats led to the development of acute pancreatitis and an inability to initiate hepatic regeneration after partial hepatectomy (2, 3). SSAT transgenic mice also showed a dramatic reduction in white adipose tissue production and related functions (25). Conversely, ablation of SSAT in mice did not alter tissue polyamine pools; however, insulin resistance and lipogenesis were increased (15, 23). Based on the aforementioned maladaptive role of SSAT, we proposed that the expression of SSAT increases in kidneys subjected to endotoxic AKI and that increased polyamine back conversion and oxidation contribute to tubular damage in endotoxin-induced AKI. In these studies, we examined the renal expression and activity of SSAT and assessed the potential contribution of SSAT to renal damage in mice subjected to endotoxin-induced AKI.
Fig. 1.
Schematic presentation of the polyamine metabolic pathway. Spermidine (Spd) and spermine (Spm) are synthesized by the sequential addition of aminopropyl groups derived from decarboxylated S-adenosylmethionine onto putrescine (Put) and Spd to form Spd and Spm, respectively. Catabolism of polyamines proceeds via 2 distinct pathways: 1) the back conversion pathway [spermidine/spermine N1-acetyltransferase/polyamine oxidase (SSAT/PAO)] or 2) the oxidation of Spm by spermine oxidase (SMO). In the polyamine back conversion pathway, Spd and Spm are acetylated by SSAT and then oxidized by PAO, leading to generation of Put and catabolic by-products [i.e., hydrogen peroxide (H2O2), acetyl-3-aminopropionaldehyde]. The SMO oxidizes Spm directly and generates Spd, H2O2, and 3-aminopropanal.
MATERIALS AND METHODS
Endotoxin-induced AKI.
Care of the mice before and during the studies was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols had received prior approval by the Institutional Animal Care and Use Committees of the University of Cincinnati. Briefly, male wild-type (SSAT-wt) and SSAT-deficient (SSAT-ko) mice bred for more than 10 generations on the C57BL/6 background were subjected to endotoxin-induced AKI. Briefly, animals were given a single intraperitoneal injection of 10 mg/kg body wt of lipopolysaccharide (LPS) from Escherichia coli strain 0111:B4 (Sigma). Mice given an intraperitoneal injection of saline were used as controls. At timed intervals (3, 6, 15, 24, 48, and 72 h) following LPS or saline administration, animals were euthanized. Blood was collected by cardiac puncture and processed to prepare serum. Kidneys were harvested and either fixed in paraformaldehyde, embedded in paraffin, and processed for hematoxylin-eosin staining or snap-frozen in liquid nitrogen.
RNA extraction and Northern blot analysis.
Kidneys were processed for RNA extraction using the TRI Reagent method (5). Purified RNA was quantified and subjected to Northern blot analysis as previously described (31, 32). Briefly, 30 μg of each sample were size-fractionated, transferred to nitrocellulose filter, and probed with appropriate 32P-labeled probes to assess the expression of SSAT, SMO, and ICAM-1 transcripts.
Assessment of kidney function.
Renal function was assessed by comparing the serum creatinine levels of saline- and LPS-treated animals using a colorimetric assay (QuantiChrome creatinine assay kit; BioAssay Systems). In this assay, the change in absorbance over 30 min was measured. The change in absorbance of the experimental samples relative to standard samples was used to calculate the serum creatinine levels.
Measurement of tissue polyamine levels and SSAT activity.
Kidneys were harvested at timed intervals (3, 6, 15, 24, 48, and 72 h) after LPS or saline administration and snap-frozen. The frozen kidneys were extracted and analyzed for polyamine pools and SSAT activity as described previously (16, 17).
Genotyping of SSAT-wt and SSAT-ko animals.
Genotyping was accomplished by PCR analysis of genomic DNA obtained from tail snips. Briefly, isolated genomic DNA was PCR amplified (94°C for 3 min; 94°C for 30 s, 50°C for 30 s, and 72°C for 90 s for 35 cycles; and 72°C for 10 min) using 5′ SSAT (CTCCTCCTGCTGTTCAAGTA), 3′ SSAT (CAGTTCCTGGGGACGAC), and NEO 5′ SSAT (GCGCCCGGTTCTTTTTGTCA) primers. The amplified DNA was size-fractionated on a 1% agarose-Tris-acetate-EDTA (TAE) gel and analyzed for the presence of 408-bp (SSAT-wt) and 680-bp (SSAT-ko) products.
Effect of MDL72527 on the severity of endotoxin-induced AKI.
SSAT-wt mice were given intraperitoneal injection of LPS (10 mg/kg body wt) or saline. Animals were then (15 min after LPS administration) given a single intraperitoneal injection of MDL72527 (100 mg/kg) or vehicle (saline) (29, 30). At 24 h after LPS (10 mg/kg body wt) or saline administration, animals from both MDL72527- and vehicle-treated groups were euthanized, and blood was collected by cardiac puncture and processed to prepare serum. Serum creatinine was measured for assessment of renal function. Kidneys were harvested and snap-frozen, processed for analysis of tissue polyamine levels, and fixed for either histopathology or immunohistochemical studies.
Immunofluorescent detection of neutrophils and macrophages.
To assess the extent of neutrophil and macrophage infiltration, animals (n = 3/group) were given an overdose of pentobarbital sodium (75 mg/kg body wt) and then perfused through the ventricle with saline followed by 4% paraformaldehyde in PBS (pH 7.4). Kidneys were then harvested and fixed in paraformaldehyde, preserved in 30% sucrose in PBS, and embedded in Tissue-Tek OCT compound (Sakura Finetek USA, Torrance, CA). Kidney sections were cut (5 μm) and processed for detection of neutrophils or macrophages by using rat anti-mouse antibodies against neutrophil-alloantigen or F4/80 (AbD Serotech, Oxford, UK), respectively. Briefly, sections were subjected to antigen retrieval using 1% SDS in PBS for 10 min, followed by three PBS rinses at 25°C. After antigen retrieval and incubation with blocking reagent, sections were incubated with an appropriate dilution of primary antibody for 16 h at 4°C. Sections were washed three times in PBS, incubated with FITC-conjugated secondary antibody for 2 h at 25°C, and then washed with PBS (3 times) and water (1 time). To quantify the infiltrating cells, we counted 10 random fields (×20 magnification) per kidney section. The average and standard error of the mean (mean ± SE) for each group were determined and compared for statistical significance.
Measurement of tissue glutathione levels.
Total glutathione and oxidized glutathione (GSSG) levels were measured using a commercially available kit (Assay Design, Ann Arbor, MI). Total glutathione and GSSG levels were determined using the protocol outlined by the manufacturer. The glutathione levels were normalized, and the levels of reduced glutathione (GSH) were determined by subtracting the GSSG levels from total glutathione levels.
Data analyses.
Values are means ± SE. The significance of difference between mean values of multiple samples was examined using ANOVA. An unpaired t-test was used for comparison between two groups. A P value <0.05 was considered to be statistically significant.
RESULTS
Effect of LPS administration on kidney function.
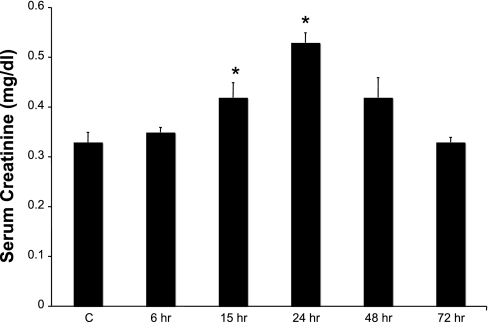
To assess the induction of kidney injury as a result of LPS administration, we compared serum creatinine levels of mice receiving saline injection (n = 6) with those of mice receiving LPS injection (n = 6) (Fig. 2). Significant (P ≤ 0.05) elevations in serum creatinine (0.42 ± 0.02 mg/dl) over the basal level (0.32 ± 0.02) were detectable as early as 15 h post-LPS injection. In LPS-treated animals over time, serum creatinine levels peaked by 24 h (0.54 ± 0.05 mg/dl, P ≤ 0.05) and by 72 h were similar to normal levels (0.33 ± 0.01 mg/dl). Thus creatinine levels indicate that LPS administration leads to the onset of mild AKI and renal dysfunction.
Fig. 2.
Effect of LPS administration on kidney function. The effect of LPS treatment (10 mg/kg ip) on renal function was examined by measuring the serum creatinine levels at timed intervals up to 72 h after LPS administration (n = 6/group). Data were analyzed for statistical significance using ANOVA and P values <0.05. *P ≤ 0.05.
Expression of SSAT in mice subjected to endotoxin-induced AKI.
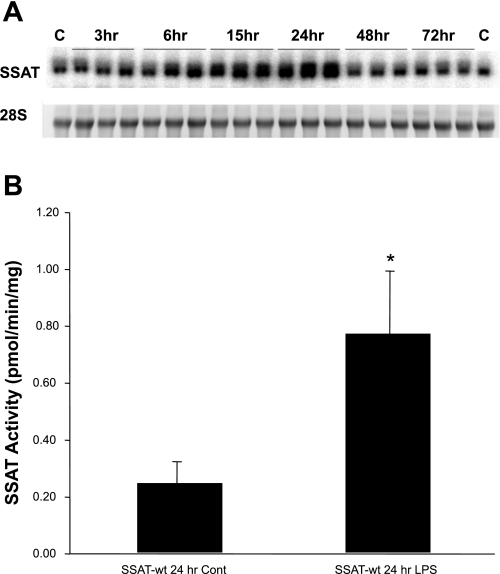
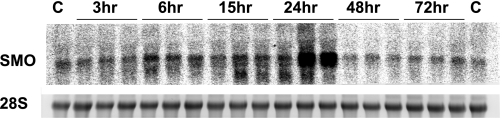
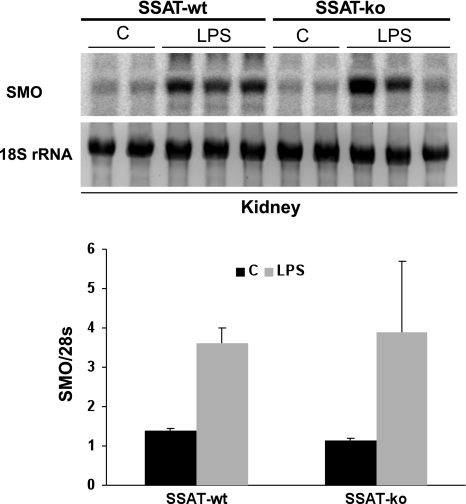
Mice subjected to injections of LPS (n = 3/time point) or saline (n = 2) showed elevated kidney expression of SSAT mRNA as early as 6 h after reperfusion (Fig. 3A). Renal SSAT mRNA levels were elevated at 15 h, peaked at 24 h, and returned to normal at 48 h after LPS administration. At 24 h, peak SSAT mRNA levels led to a significant (3-fold; P ≤ 0.05) increase in SSAT enzyme activity after LPS administration (Fig. 3B). Furthermore, we found the biosynthetic activity of ornithine decarboxylase (ODC) was lower in the animals treated with LPS compared with saline controls after 24 h of reperfusion (1.54 ± 0.39 vs. 9.09 ± 3.72 pmol·h−1·mg protein−1, respectively). These results indicate that whereas polyamine back conversion is induced by LPS, polyamine synthesis is not upregulated but is, in fact, reduced. The expression of kidney SMO mRNA in response to AKI was significantly upregulated at 15 h (Fig. 4) with kinetics similar to those of SSAT over the 72 h. These results indicate that the kidney expression and activity of SSAT and SMO increase in endotoxin-induced AKI.
Fig. 3.
Expression and activity of SSAT increase in SSAT-treated wild-type (SSAT-wt) mice subjected to endotoxin-induced acute kidney injury (AKI). A: total RNA (30 μg/well) was extracted from kidneys at timed intervals following LPS or saline treatment (n = 3/treatment time point and n = 2 for control animals), size-fractionated by gel electrophoresis, and subjected to Northern blot analysis using radiolabeled SSAT cDNA probe. Equal loading was confirmed by the examination of 28s rRNA bands (bottom). A single band of ∼1.3 kb was recognized by the SSAT probe (top). B: SSAT activity was measured in kidney extracts 24 h after saline or LPS injections (n = 3/group). Data are expressed as pmol·min−1·mg protein−1. Statistical significance was determined using P values <0.05. *P ≤ 0.05.
Fig. 4.
Expression of SMO increases in SSAT-wt mice subjected to endotoxin-induced AKI. Total RNA (30 μg/well) from kidneys of LPS- or saline-treated animals (n = 3) was size-fractionated and then subjected to Northern blot analysis using radiolabeled SMO cDNA probe. A single band was recognized by the SMO probe (top). Equal loading was confirmed by the examination of 28s rRNA bands (bottom).
Assessment of polyamine levels in the kidneys of mice subjected to endotoxin-induced AKI.
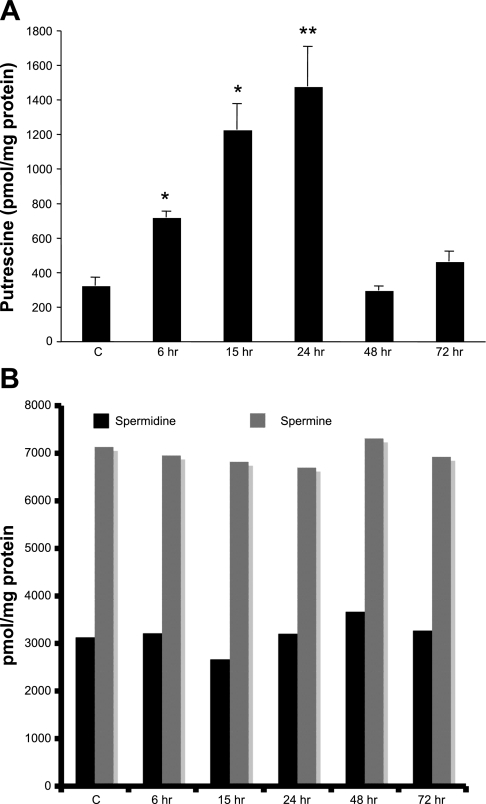
To ascertain the impact of SSAT upregulation and increased polyamine oxidation on the levels of cellular polyamines, we first examined tissue levels of Put, an end product of the polyamine back conversion pathway following LPS treatment (Fig. 5A). As early as 6 h after LPS administration, kidney Put levels increased by twofold. Put levels continued to rise in the kidneys, with the highest levels (5-fold increase) found between 15 and 24 h after LPS administration. Kidney Put levels returned to near control levels between 48 and 72 h post-LPS injection. The rise and fall of Put could theoretically result from changes in the biosynthetic enzyme ODC and/or the catabolic enzymes SSAT and PAO. Regarding biosynthesis, our results indicate that the activity of ODC was reduced after LPS administration (see data in Expression of SSAT in mice subjected to endotoxin-induced AKI). Thus we propose that increased kidney Put levels are mainly a reflection of enhanced SSAT-driven polyamine back conversion and not the activation of polyamine biosynthetic pathway, which also rules out the possibility of enhanced polyamine flux [i.e., concomitant onset of polyamine anabolism and catabolism (17)]. Examination of Spd and Spm pools in kidneys revealed similar tissue levels between the control and LPS-treated animals (Fig. 5B); furthermore, there was no accumulation of acetylated polyamines.
Fig. 5.
Measurement of polyamine levels in the kidneys of SSAT-wt mice subjected to endotoxin-induced AKI. Polyamine levels were measured in the kidneys of animals given intraperitoneal injection of saline or 10 mg/kg LPS (n = 3). Kidney polyamine contents were determined by HPLC. A: kidney Put levels were determined at timed intervals after treatment with LPS and compared with polyamine levels in the kidneys of saline-treated animals. *P ≤ 0.05; **P ≤ 0.01. B: Spd and Spm levels in the kidneys of saline- and LPS-treated animals. Polyamine levels are expressed as pmol/mg protein.
SSAT-ko mice are protected against endotoxin-induced AKI.
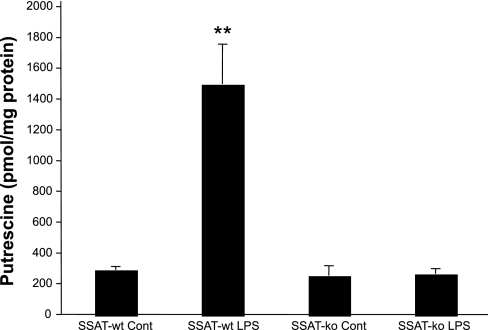
As the rate-limiting enzyme for polyamine back conversion, SSAT regulates a pathway that through acetylation and oxidation mediates the conversion of Spm into Spd and of Spd into Put, respectively. This process generates potentially cytotoxic by-products such as acetyl 3-aminopropanal, acrolein, and hydrogen peroxide. To determine the role of SSAT-driven polyamine back conversion in the etiology of endotoxin-induced AKI, we examined the effect of SSAT deficiency on the severity of renal injury. In these studies, male SSAT-ko and their male SSAT-wt littermates were given a single intraperitoneal injection of LPS or saline to examine the effects of LPS on kidney polyamine levels. Our results indicate that although there was a slight decrease in the kidney Spd levels in LPS-treated mice, the tissue levels of Spd and Spm were not significantly different between either genotype (data not shown). Comparison of Put levels (Fig. 6) in the kidneys of LPS-treated mice indicated that whereas the kidney content of this polyamine remained virtually unchanged in the SSAT-ko animals, it increased approximately fivefold in the SSAT-wt animals. The latter result indicates that the SSAT-dependent back conversion pathway, not active in SSAT-ko animals, is responsible for kidney Put production in LPS-treated SSAT-wt mice.
Fig. 6.
Comparison of Put levels in SSAT-wt and SSAT-deficient (SSAT-ko) mice in endotoxin-induced AKI. Kidney Put levels were measured in SSAT-wt and SSAT-ko animals (n = 3) 24 h after saline and LPS treatment. The Put contents in the kidneys of saline-treated (Cont) SSAT-wt and SSAT-ko animals were similar. Subsequent to LPS administration, Put levels increased by ∼4-fold in the kidneys of SSAT-wt animals compared with saline-treated animals of the same genotype. The kidney Put contents of the LPS-treated SSAT-ko animals were not significantly different from those of the saline-treated SSAT-ko and SSAT-wt mice. **P ≤ 0.01.
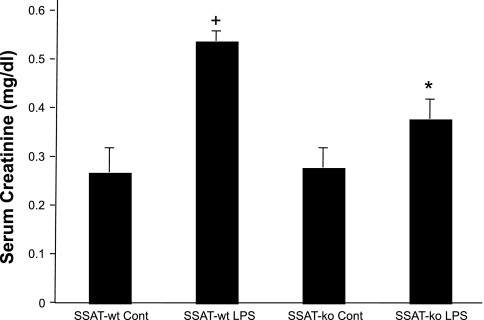
Next, the renal function of LPS- or saline-treated mice from both genotypes was compared (Fig. 7). Comparison of serum creatinine levels of saline-treated SSAT-wt and SSAT-ko animals (0.28 ± 0.04 vs. 0.29 ± 0.03, respectively) did not reveal a significant difference (P ≥ 0.05); however, after LPS administration, the serum creatinine levels in the SSAT-ko mice were significantly lower (P ≤ 0.05) compared with those of the SSAT-wt mice at 24 h (0.39 ± 0.04 vs. 0.55 ± 0.02, respectively), indicating that the kidney injury was less severe in SSAT-ko animals after LPS administration.
Fig. 7.
Comparison of renal function in SSAT-wt and SSAT ko mice after endotoxin-induced AKI. Serum creatinine levels were measured to assess the effect of SSAT deficiency on the severity of endotoxin-induced AKI. A colorimetric assay was utilized to obtain creatinine levels in the sera of vehicle- and LPS-treated SSAT-wt and SSAT-ko animals (n = 8/group) at 24 h posttreatment. Whereas serum creatinine levels of saline-treated SSAT-ko and SSAT-wt mice were similar, those of SSAT-ko animals subjected to LPS treatment were significantly (*P ≤ 0.05) lower than those of similarly treated SSAT-wt animals. Serum creatinine levels of LPS-treated SSAT-wt mice were significantly (+P ≤ 0.05) higher than those of saline-treated animals.
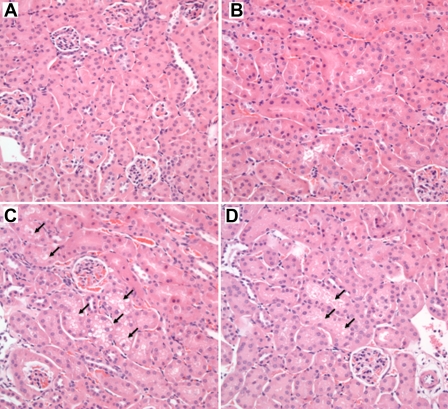
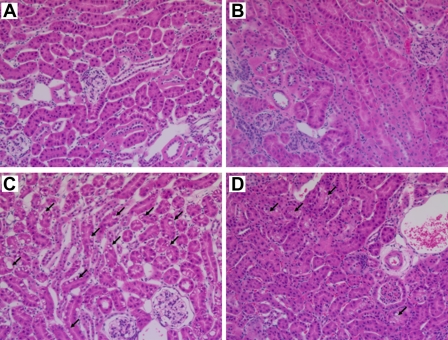
To confirm the results of serum creatinine measurements, we compared the renal histology of SSAT-wt and -ko mice subjected to endotoxic-AKI. Renal histology of both genotypes was comparable in saline-treated (control) animals (Fig. 8, A and B). Administration of LPS led to a mild injury in both SSAT-wt and -ko mice. Closer examination of the kidney histology of SSAT-wt and -ko animals revealed that the former had increased vacuolization of renal tubular epithelial cells (Fig. 8, C and D).
Fig. 8.
Comparison of renal histology of SSAT-wt and SSAT-ko mice after endotoxin-induced AKI. Renal histology of SSAT-wt and SSAT-ko mice were compared to assess the effect of SSAT deficiency on the severity of endotoxin-induced AKI. No histological differences were detected when kidneys of saline-treated SSAT-wt (A) and SSAT-ko (B) animals were compared. The tissue damage caused by endotoxin-induced AKI was not very severe; however, SSAT-wt (C) mice showed increased vacuolization in the affected tubular epithelium compared with SSAT-ko (D) littermates (arrows).
Our previous studies indicated that in vitro overexpression of SSAT leads to an increase in SMO mRNA levels (31). Also, comparison of SSAT-wt and SSAT-ko animals after liver and kidney ischemia-reperfusion injury indicated that the induction of SMO mRNA is modulated in the SSAT-ko animals (33). We therefore compared the induction of SMO mRNA in kidneys of SSAT-wt and SSAT-ko animals subjected to endotoxic-AKI. Our results (Fig. 9) suggest that the expression of SMO mRNA increases in both SSAT-wt and SSAT-ko mice after endotoxic AKI and that there are no significant differences in the induction levels of SMO between the two genotypes.
Fig. 9.
Comparison of SMO expression in the kidneys of SSAT-wt and SSAT-ko mice after endotoxin-induced AKI. Total RNA (10 μg/well) from kidneys of saline (n = 2/genotype)- or LPS-treated (n = 3/genotype) SSAT-wt and SSAT-ko animals was size-fractionated and then subjected to Northern blot analysis using radiolabeled SMO cDNA probe. Equal loading was confirmed by the examination of 18s rRNA bands (bottom). The intensity of SMO signals (top) was determined by densitometry and normalized against the intensity of the 18s rRNA band. The semiquantitative results are shown in the graph and indicate that SMO expression levels were not significantly affected by SSAT deletion in endotoxin-induced AKI. C, control (saline treatment).
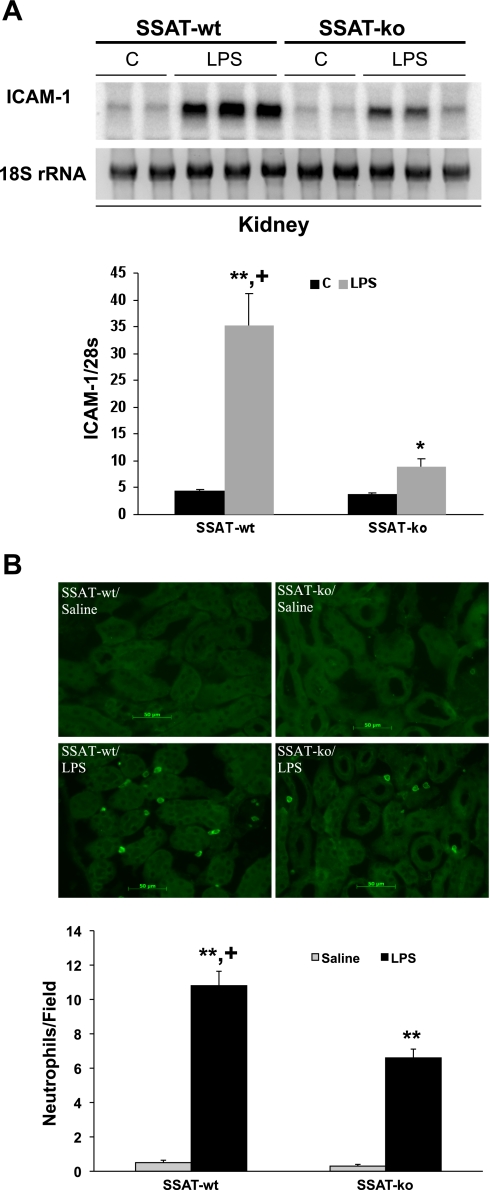
Leukocyte infiltration and inflammation play important roles in the mediation of tissue damage; therefore, we compared the induction of ICAM-1 and infiltration of neutrophils and macrophages in SSAT-wt and SSAT-ko animals subjected to endotoxic AKI (Fig. 10). Our results indicate that ICAM-1 expression levels are significantly (P ≤ 0.01) lower in the SSAT-ko animals (Fig. 10A). Furthermore, whereas there were no differences in the number of infiltrating neutrophils in the kidneys of saline-treated animals, we observed a significant (P ≤ 0.05) reduction in neutrophil infiltration in the injured kidneys of SSAT-ko (6.7 ± 0.51 per field) relative to SSAT-wt mice (10.8 ± 0.84 per field) subjected to endotoxin-induced AKI (Fig. 10B). Examination of infiltrating macrophages indicated that LPS treatment led to a significant increase in the number of macrophages in the peritubular space of both SSAT-wt and SSAT-ko mice compared with their saline-treated counterparts. However, the numbers of infiltrating macrophages were not significantly different in the kidneys of LPS-treated SSAT-wt and SSAT-ko mice (data not shown).
Fig. 10.
Comparison of the inflammatory response in the kidneys of SSAT-wt and SSAT-ko mice in endotoxin-induced AKI. A: comparison of ICAM-1 expression in the kidneys of SSAT-wt and SSAT-ko mice. Total RNA (10 μg/well) from kidneys of saline (n = 2/genotype)- or LPS-treated (n = 3/genotype) SSAT-wt and SSAT-ko animals was subjected to Northern blot analysis using radiolabeled ICAM-1 cDNA probe. Equal loading was confirmed by the examination of 18s rRNA bands (bottom). ICAM-1 signal strength was assessed by densitometry and normalized against the strength of the 18s rRNA band signal. The semiquantitative value of induction profiles of ICAM-1 are depicted in the graph. B: infiltration of neutrophils in kidneys of SSAT-wt and SSAT-ko mice was assessed at 24 h after saline or LPS injection by immunofluorescent microscopy. Infiltrating neutrophils were detected in the kidney (×20 magnification) using polyclonal rat anti-mouse neutrophil antibody. Quantitative comparison of the average number of infiltrating neutrophils per field (×20 magnification) in the kidney is shown in the graph. *P ≤ 0.05; **P ≤ 0.01, LPS- vs. saline-treated animals of the same genotype. +P ≤ 0.05, LPS-treated SSAT-wt vs. SSAT-ko mice.
PAO inhibitor MDL72527 reduces the severity of endotoxin-induced AKI.
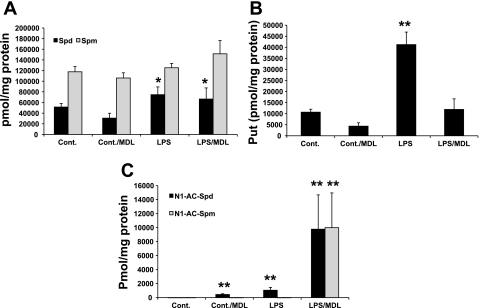
Increased expression of SSAT may contribute to the induction of tissue injury through generation of acetylated polyamines that are then oxidized by PAO to generate toxic molecules (Fig. 1). Oxidation of Spm by SMO also leads to the generation of toxic products (Fig. 1). To assess the role of PAOs in the mediation of endotoxin-induced AKI, we examined kidney function of saline- and LPS-treated animals given intraperitoneal injections of vehicle or MDL72527. SSAT-wt mice were injected intraperitoneally with LPS or saline, followed by an intraperitoneal injection of 100 mg/kg MDL72527, at a dose previously shown to inhibit the activity of PAOs and protect the brain against cerebral injury (6, 7), or vehicle (n = 8/group). To assess the efficacy of the dose of MDL72527 used for inhibition of PAOs, we first compared the kidney polyamine levels of animals in all four experimental groups (Fig. 11). The kidney Spm levels of saline/MDL72527-, LPS-, and LPS/MDL72527-treated groups were not significantly different from those of the mice injected with saline (Fig. 11A). Kidney Spd levels (Fig. 11A) were lower in saline- and saline/MDL72527-treated animals than comparably treated animals injected with LPS (P ≤ 0.05). Comparison of Put levels (Fig. 11B) in the kidneys of LPS-treated mice indicated that animals injected with MDL72527 had significantly lower (P ≤ 0.05) levels of Put than saline-treated animals. Animals treated with MDL72527 exhibited a significant increase in tissue accumulation of acetylated polyamines (P ≤ 0.01). This increase was of much greater magnitude in the LPS/MDL72527-treated mice (Fig. 11C), indicating that MDL72527 administration inhibits the activity of PAO. The increase in kidneys Spd levels in MDL72527-treated animals suggests that SMO activity is at best marginally affected.
Fig. 11.
Measurement of polyamine levels in the kidneys of saline- and MDL72527-treated control mice and mice subjected to endotoxin-induced AKI. Kidney polyamine contents were determined by HPLC and expressed as pmol/mg protein. A: comparison of Spd and Spm levels in the kidneys of mice given intraperitoneal injection of saline or LPS and then treated with vehicle or MDL72527. B: comparison of kidney Put levels in the same animals. C: comparison of kidney acetylated polyamine levels in the same animals. *P ≤ 0.05; **P ≤ 0.01, animals subjected to a particular treatment vs. saline-treated control animals.
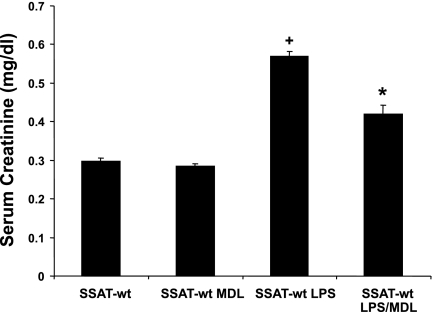
Next, the effect of inhibition of PAOs on the renal function of mice was determined by measuring their serum creatinine levels 24 h after induction of kidney injury and initiation of treatment (Fig. 12). Serum creatinine levels of control animals treated with vehicle or MDL72527 (0.30 ± 0.01 vs. 0.28 ± 0.01 mg/dl, respectively) were not significantly different (P ≥ 0.05). Comparison of the serum creatinine levels of LPS-treated animals that received injections of vehicle or MDL72527 (0.59 ± 0.013 vs. 0.44 ± 0.02 mg/dl, respectively) indicates that MDL72527-treated animals have significantly lower serum creatinine levels (P ≤ 0.05). The aforementioned results suggest that treatment with MDL72527 at the levels used in these studies inhibits the activity of PAO and reduces the severity of renal failure in endotoxin-induced AKI.
Fig. 12.
Effect of MDL72527 on renal function after endotoxin-induced AKI. The effect of PAO inhibition on the renal function of animals was determined 24 h post-LPS administration by measuring their serum creatinine levels. Animals given intraperitoneal injection of LPS or saline were treated with 100 mg/kg MDL72527 or vehicle (n = 8/group). Serum creatinine levels of saline-injected animals treated with MDL72527 or vehicle were not significantly different (P ≥ 0.05). Animals given intraperitoneal LPS injection and then treated with MDL72527 had significantly (*P ≤ 0.05) lower serum creatinine levels than LPS-treated animals receiving vehicle. Serum creatinine levels of the LPS-treated mice were significantly (+P ≤ 0.01) higher than those of saline-treated animals.
The results of serum creatinine measurements were confirmed by comparing the renal histology of SSAT-wt mice subjected to endotoxic AKI and treated with vehicle or MDL72527. The histology of the kidneys of vehicle- and MDL72527-treated control animals were normal (Fig. 13, A and B). Administration of LPS led to a mild tubular injury in both vehicle- and MDL72527-treated mice. However, the LPS-treated animals receiving vehicle injections had increased vacuolization of renal tubular epithelial cells compared with LPS-treated animals receiving MDL72527 (Fig. 13, C and D).
Fig. 13.
Comparison of renal histology in vehicle- or MDL72527-treated SSAT-wt mice after endotoxin-induced AKI. Renal histology of SSAT-wt mice treated with vehicle or 100 mg/kg MDL72527 were compared to assess the effect of inhibition of polyamine oxidation on the severity of endotoxin-induced AKI. No histological differences were detected when the kidneys of vehicle- (A) or MDL72527-treated (B) animals receiving saline injection (control) were compared. The tissue damage caused by endotoxin-induced AKI was not severe; however, vehicle-treated SSAT-wt (C) animals showed increased vacuolization in the affected tubular epithelium compared with MDL72527-treated SSAT-wt (D) animals (arrows).
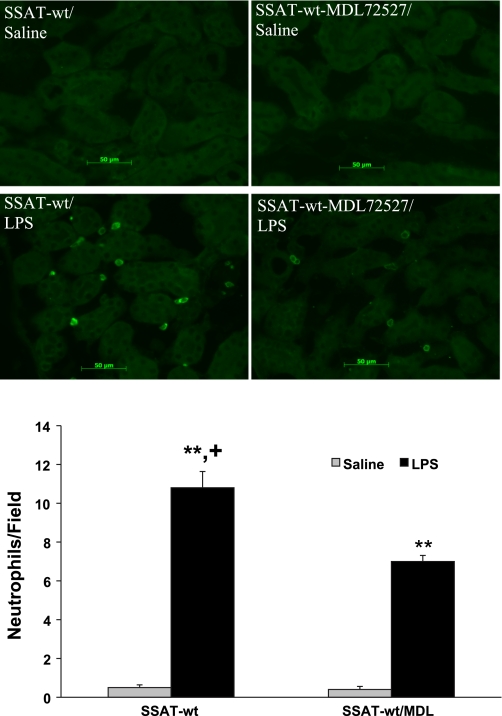
We next examined the effect of the inhibition of PAOs on the onset of inflammatory response in saline- and MDL72527-treated mice. Infiltration of neutrophils and macrophages into the kidneys of vehicle- or MDL72527-treated mice was examined at 24 h after administration of LPS or saline by using immunofluorescent microscopy (Fig. 14A). Our results indicate that there was a significant (P ≤ 0.05) reduction in neutrophil infiltration in the kidneys of MDL72527/LPS-treated (7.0 ± 0.31 per field) compared with vehicle/LPS-treated mice (10.8 ± 0.84 per field) (Fig. 14B). Vehicle- and MDL72527-treated animals had minimal (fewer than 0.5 ± 0.2 per field) neutrophil infiltration (Fig. 14B). The numbers of infiltrating macrophages were higher in MDL72527- and vehicle-treated animals subjected to endotoxic AKI than their saline-treated counterparts. Our results also indicate that the numbers of infiltrating macrophages were not significantly different in the kidneys of MDL72527- or vehicle-treated mice that were subjected to endotoxin-induced AKI (data not shown).
Fig. 14.
Effect of treatment with MDL72527 on neutrophil infiltration in LPS-induced AKI. Infiltration of neutrophils in kidneys of vehicle- and MDL72527-treated SSAT-wt mice was assessed at 24 h after saline or LPS injection by performing immunofluorescent microscopy. Top: infiltrating neutrophils were detected in the kidney (×20 magnification) using polyclonal rat anti-mouse neutrophil antibody. Bottom: quantitative comparison of the average number of infiltrating neutrophils per field (×20 magnification) in the kidney is presented in the graph. *P ≤ 0.05; **P ≤ 0.01, LPS- vs. saline-treated animals receiving vehicle or MDL72527 injections. +P ≤ 0.05 compared with LPS-treated mice receiving vehicle or MDL72527 injections.
Assessment of oxidative stress in SSAT-wt, SSAT-ko, and MDL72527-treated SSAT-wt animals after endotoxin-induced AKI.
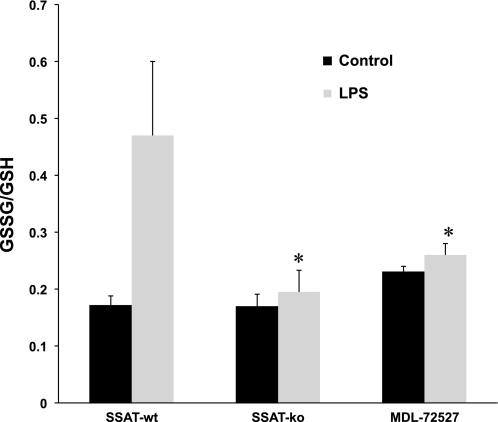
Since we had proposed that enhanced polyamine back conversion and oxidation lead to tubular injury through generation of toxic metabolites (H2O2, aminopropanals, and acrolein) and induction of oxidative injury, we next examined the onset of oxidative stress in the kidneys of SSAT-wt, SSAT-ko, and MDL72527-treated SSAT-wt (MDL/SSAT-wt) mice 24 h after administration of LPS or saline by determining the ratio of GSSG to GSH, a barometer of oxidative stress (Fig. 15). Our results indicate that the ratio of GSSG to GSH increased in all LPS-treated groups; however, ablation of SSAT or inhibition of PAOs significantly reduced the increase in GSSG-to-GSH ratios, suggesting that the aforementioned genetic modification or inhibition of polyamine oxidation reduces the severity of oxidative tissue injury in animals subjected to endotoxic AKI.
Fig. 15.
Comparison of GSSG-to-GSH ratio in the kidneys of SSAT-wt, SSAT-ko, and MDL72527-treated SSAT-wt (MDL/SSAT-wt) animals after endotoxin-induced AKI. The onset of oxidative stress in the kidneys of SSAT-wt, SSAT-ko, and MDL/SSAT-wt mice 24 h after administration of LPS or saline was examined by determining the ratio of GSSG to GSH (GSSG/GSH). GSSG/GSH increased in LPS-treated animals in all 3 groups; however, after LPS treatment, the deficiency of SSAT or inhibition of PAOs significantly (*P ≤ 0.05) dampened the increase in GSSG/GSH compared with LPS-treated SSAT-wt animals.
DISCUSSION
In the present study, we have addressed the role of polyamine back conversion and oxidation in the pathophysiology of endotoxin-induced AKI. Our data indicate that the expression of SSAT mRNA increased in the kidneys of mice within 6 h, reaching its peak by 24 h and returning to near background levels between 48 and 72 h after administration of one LPS injection (10 mg/kg ip) (Fig. 3A). The increase in the expression of SSAT mRNA closely correlated with the accumulation of Put, a product of polyamine back conversion (Fig. 5A). The increase in polyamine back conversion (6–24 h) preceded the disruption of renal function, as measured by serum creatinine levels, which was apparent between 15 and 48 h post-LPS administration (Fig. 2). The expression of SMO mRNA was also induced in the kidneys of animals after LPS injection (Fig. 4); however, its contribution to AKI may be secondary to the SSAT/PAO system. This is supported by the observations that 1) Put levels rose and fell in accordance with the changes in SSAT activity; 2) the levels of Spm and Spd were unchanged, indicating that oxidation by SMO is not robust enough to lower Spm and raise Spd levels (Fig. 5B); and 3) the protection afforded against LPS-induced AKI by SSAT deficiency and the inhibition of polyamine oxidation were of identical magnitude. On the basis of our results, we have proposed that increased SSAT expression and the attendant rise in polyamine back conversion and, to a lesser extent, elevated SMO levels contribute to tissue damage in endotoxin-induced AKI. This hypothesis was tested in two ways using genetically engineered SSAT-deficient animals or MDL72527, a PAO/SMO inhibitor. First, our results indicate that LPS-treated SSAT-ko animals have better preserved kidney function compared with SSAT-wt animals subjected to LPS treatment (Fig. 7). Furthermore, comparison of renal histopathology of SSAT-wt and SSAT-ko mice indicated that the latter have less severe renal injuries and reduced inflammatory involvement (Fig. 8). Oxidation of acetylated polyamines by PAO in back conversion or oxidation of Spm by SMO generates potentially cytotoxic compounds such as hydrogen peroxide and aminopropanal. Therefore, we examined the role of polyamine oxidation in the mediation of AKI tissue damage. LPS-treated animals receiving MDL72527 had less severe renal damage, as indicated by a blunted increase in serum creatinine levels, less severe tubular damage, and modulated inflammatory response, relative to animals that were treated with vehicle only (Figs. 12–14). Furthermore, our studies indicated that the elevation of GSSG/GSH, a barometer of oxidative stress, was significantly modulated in SSAT-ko and MDL/SSAT-wt compared with SSAT-wt animals (Fig. 15). The latter results suggest that ablation of SSAT or inhibition of polyamine oxidation reduces the renal damage in endotoxin-induced AKI by reducing the magnitude of oxidative tissue injury. In cell culture systems where polyamine analogs increase SSAT activity up to 1,000-fold, there is a rapid production and intracellular accumulation of acetylated polyamines (31). In contrast to the aforementioned in vitro systems, the threefold increase in SSAT activity observed in LPS-treated animals (Fig. 3B) was significant but modest by comparison. Furthermore, the low levels of acetylated polyamines in the kidneys of LPS-treated animals are presumably due to their oxidation and/or rapid export upon their production. Since Spd and Spm pools were similar in control and LPS-treated animals and acetyl-Spd levels were quite low in the latter, our results indicate that oxidative by-products generated by PAO contributed to the renal damage in endotoxin-induced-AKI.
These studies suggest that polyamine back-conversion and Spm oxidation are upregulated in mice treated with LPS. The results presented exclude an important role for polyamine flux (concomitant increase in polyamine synthesis and catabolism) in the mediation of kidney damage in endotoxin-induced AKI, since the activity of biosynthetic enzyme ODC, a hallmark feature of flux (17), was not increased in LPS-treated animals. Finally, our results demonstrating that the ablation of SSAT or inhibition of polyamine oxidation reduces the severity of renal damage provide compelling evidence that SSAT-dependent polyamine back conversion is an important mediator of endotoxin-induced AKI.
The contribution of SSAT and polyamine back conversion to the etiology of septic AKI has not been thoroughly examined. The role of SSAT in the mediation of tissue damage is supported by in vivo and in vitro evidence showing that its ablation reduces the severity of tissue damage and its overexpression leads to oxidative stress that is potentially detrimental to organ function (1–3, 22, 23, 28, 31, 33). Studies examining the effect of overexpression of SSAT in mice and rats indicate that this enzyme adversely affects the integrity and function of various tissues including skin, gastrointestinal tract, liver, pancreas, and fat (1–3, 22, 25). For example, SSAT transgenic animals show anomalous changes in the skin and reproductive tract. SSAT overexpression also leads to the onset of acute pancreatitis and impaired hepatic regeneration (1–3, 22). In addition, SSAT transgenic mice demonstrate a complete loss of white adipose tissue and decreases in lipogenesis by the loss of acetyl- and malonyl-CoA pools (15, 25). These results are complemented by in vitro observations indicating that SSAT expression leads to growth arrest (29, 30) and apoptosis (31, 32). For example, induced expression of SSAT by the Spm analog N1,N11-diethylnorspermine in L56Br-C1 and SK-MEL-28 cells resulted in the depletion of the cellular polyamine pools, inhibition of cell proliferation, induction of cytochrome c release from mitochondria, and the onset of caspase-dependent apoptosis (10). In addition, overexpression of SSAT in vitro has been shown to lead to oxidative stress by increasing the production of hydrogen peroxide (31, 32). Furthermore, cells overexpressing SSAT showed DNA damage, activation of a DNA damage checkpoint, and cell cycle (G2/M) arrest (32). These studies summarily demonstrate the detrimental impact of increased expression and activity of SSAT on cell growth and organ function.
In the absence of significant alterations in Spd and Spm levels in animals subjected to endotoxin-induced AKI, we predict that SSAT-enhanced back conversion by the SSAT/PAO system contributes to tissue damage through generation of toxic by-products of polyamine oxidation such as aldehydes and hydrogen peroxide. The latter is supported by the results, which demonstrate that the GSSG-to-GSH ratios are lower and, hence, oxidative stress levels are reduced as the result of ablation of SSAT or inhibition of PAOs (Fig. 15). The partial protection against renal dysfunction by MDL72527 treatment supports the hypothesis that polyamine oxidation, primarily through PAO, plays a maladaptive role in endotoxin-induced AKI. The by-products of polyamine oxidation, hydrogen peroxide, aminopropanal, and acrolein, can lead to tissue injury and adversely affect tissue integrity (13, 19, 20, 35). This idea is supported by studies in brain tissue demonstrating a role for aminopropanal in mediating brain injury, since its neutralization protects the brain against damage in cerebral ischemia-reperfusion injury (12, 13). Therefore, the role of polyamine oxidative by-products in endotoxin-induced AKI needs to be further examined. The significant accumulation of kidney Put in SSAT-wt but not SSAT-ko or MDL72527-treated animals during AKI (Figs. 6 and 11) also may be detrimental, since we previously demonstrated that high Put levels decrease cell viability in cultured cells (4). Interestingly, the deficiency of SSAT or inhibition of PAOs also led to decreased leukocyte infiltration (Figs. 12 and 14). The observed results suggest that the short-circuiting of polyamine catabolism may modulate the inflammatory response in endotoxic AKI, thereby further reducing the severity of tissue injury.
The data presented in this study indicate that the expression and activity of enzymes involved in polyamine catabolism increase in the kidneys of animals after endotoxin-induced AKI, which may contribute to tissue damage and loss of renal function. Protection from AKI was demonstrated by either ablation of SSAT or inhibition of polyamine oxidation. Taken together, this evidence supports the hypothesis that polyamine back conversion and oxidation play an important role in the pathophysiology of the endotoxin-induced AKI. These studies identify potentially novel therapeutic strategies (i.e., SSAT suppression and/or inhibition of polyamine oxidation) for treatment of endotoxin-induced AKI.
GRANTS
These studies were supported by a Merit Review Award (to M. Soleimani), a grant from Dialysis Clinic (to M. Soleimani), and National Institutes of Health Grants DK-62809 (to M. Soleimani), CA-22153, and CA-76428 (to C. W. Porter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Alhonen L, Karppinen A, Uusi-Oukari M, Vujcic S, Korhonen VP, Halmekyto M, Kramer DL, Hines R, Janne J, Porter CW. Correlation of polyamine and growth responses to N1,N11-diethylnorspermine in primary fetal fibroblasts derived from transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 273: 1964–1969, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Alhonen L, Parkkinen JJ, Keinanen T, Sinervirta R, Herzig KH, Janne J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc Natl Acad Sci USA 97: 8290–8295, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhonen L, Rasanen TL, Sinervirta R, Parkkinen JJ, Korhonen VP, Pietila M, Janne J. Polyamines are required for the initiation of rat liver regeneration. Biochem J 362: 149–153, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Dogan A, Rao AM, Baskaya MK, Hatcher J, Temiz C, Rao VL, Dempsey RJ. Contribution of polyamine oxidase to brain injury after trauma. J Neurosurg 90: 1078–1082, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Dogan A, Rao AM, Hatcher J, Rao VL, Baskaya MK, Dempsey RJ. Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J Neurochem 72: 765–770, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Hasan R, Alam MK, Ali R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Lett 368: 27–30, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Hegardt C, Johannsson OT, Oredsson SM. Rapid caspase-dependent cell death in cultured human breast cancer cells induced by the polyamine analogue N1,N11-diethylnorspermine. Eur J Biochem 269: 1033–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271: 559–564, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ivanova S, Batliwalla F, Mocco J, Kiss S, Huang J, Mack W, Coon A, Eaton JW, Al-Abed Y, Gregersen PK, Shohami E, Connolly ES, Jr, Tracey KJ. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc Natl Acad Sci USA 99: 5579–5584, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanova S, Botchkina GI, Al-Abed Y, Meistrell M, 3rd, Batliwalla F, Dubinsky JM, Indicial C, Wang H, Gregersen PK, Eaton JW, Tracey KJ. Cerebral ischemia enhances polyamine oxidation: identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J Exp Med 188: 327–340, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janne J, Alhonen L, Linemen P. Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Jell J, Merely S, Hansen ML, Mazurchuk R, Spernyak JA, Diegelman P, Kisiel ND, Barrero C, Deeb KK, Alhonen L, Patel MS, Porter CW. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem 282: 8404–8413, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kramer D, Stanek J, Diegelman P, Regenass U, Schneider P, Porter CW. Use of 4-fluoro-l-ornithine to monitor metabolic flux through the polyamine biosynthetic pathway. Biochem Pharmacol 50: 1433–1443, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem 283: 4241–4251, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kramer DL, Vujcic S, Diegelman P, Alderfer J, Miller JT, Black JD, Bergeron RJ, Porter CW. Polyamine analogue induction of the p53-p21WAF1/CIP1-Rb pathway and G1 arrest in human melanoma cells. Cancer Res 59: 1278–1286, 1999 [PubMed] [Google Scholar]
- 19.Kunduzova OR, Escourrou G, De La Farge F, Salvayre R, Seguelas MH, Leducq N, Bono F, Herbert JM, Parini A. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J Am Soc Nephrol 15: 2152–2160, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney Int 48: 1584–1591, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35: 55–91, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Min SH, Simmen RC, Alhonen L, Halmekyto M, Porter CW, Janne J, Simmen FA. Altered levels of growth-related and novel gene transcripts in reproductive and other tissues of female mice overexpressing spermidine/spermine N1-acetyltransferase (SSAT). J Biol Chem 277: 3647–3657, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, Suppola S, Pietila M, Uimari A, Laakso M, Alhonen L, Janne J. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med 10: 933–945, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pietila M, Alhonen L, Halmekyto M, Kanter P, Janne J, Porter CW. Activation of polyamine catabolism profoundly alters tissue polyamine pools and affects hair growth and female fertility in transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J Biol Chem 272: 18746–18751, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Pirinen E, Kuulasmaa T, Pietila M, Heikkinen S, Tusa M, Itkonen P, Boman S, Skommer J, Virkamaki A, Hohtola E, Kettunen M, Fatrai S, Kansanen E, Koota S, Niiranen K, Parkkinen J, Levonen AL, Yla-Herttuala S, Hiltunen JK, Alhonen L, Smith U, Janne J, Laakso M. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol 27: 4953–4967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao JN, Guo X, Liu L, Zou T, Murthy KS, Yuan JX, Wang JY. Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol 284: C848–C859, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Suppola S, Heikkinen S, Parkkinen JJ, Uusi-Oukari M, Korhonen VP, Keinanen T, Alhonen L, Janne J. Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N1-acetyltransferase further accelerates the catabolism of hepatic polyamines in transgenic mice. Biochem J 358: 343–348, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J 367: 665–675, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J 370: 19–28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, Casero RA, Soleimani M. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol 15: 1844–1852, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol 292: C1204–C1215, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zahedi K, Lentsch AB, Okaya T, Barone S, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Janne J, Porter CW, Soleimani M. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 296: G899–G909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 284: F1046–F1055, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc 39: 1332–1337, 2007. [DOI] [PubMed] [Google Scholar]