Abstract
The anion exchanger Pendrin, which is encoded by SLC26A4 (human)/Slc26a4 (mouse) gene, is localized on the apical membrane of non-acid-secreting intercalated (IC) cells in the kidney cortical collecting duct (CCD). To examine its role in the mediation of bicarbonate secretion in vivo and the apical Cl−/HCO3− exchanger in the kidney CCD, mice with genetic deletion of pendrin were generated. The mutant mice show the complete absence of pendrin expression in their kidneys as assessed by Northern blot hybridization, Western blot, and immunofluorescence labeling. Pendrin knockout (KO) mice display significantly acidic urine at baseline [pH 5.20 in KO vs. 6.01 in wild type (WT); P < 0.0001] along with elevated serum HCO3− concentration (27.4 vs. 24 meq/l in KO vs. WT, respectively; P < 0.02), consistent with decreased bicarbonate secretion in vivo. The urine chloride excretion was comparable in WT and KO mice. For functional studies, CCDs were microperfused and IC cells were identified by their ability to trap the pH fluorescent dye BCECF. The apical Cl−/HCO3− exchanger activity in B-IC and non-A, non-B-IC cells, as assessed by intracellular pH monitoring, was significantly reduced in pendrin-null mice. The basolateral Cl−/HCO3− exchanger activity in A-IC cells and in non-A, non-B-IC cells, was not different in pendrin KO mice relative to WT animals. Urine NH4+ (ammonium) excretion increased significantly, consistent with increased trapping of NH3 in the collecting duct in pendrin KO mice. We conclude that Slc26a4 (pendrin) deletion impairs the secretion of bicarbonate in vivo and reduces apical Cl−/HCO3− exchanger activity in B-IC and non-A, non-B-IC cells in CCD. Additional apical Cl−/HCO3− exchanger(s) is (are) present in the CCD.
Keywords: bicarbonate excretion, metabolic alkalosis, chloride excretion, cortical collecting duct
the kidney cortical collecting duct (CCD) plays a major role in systemic acid-base homeostasis by secretion of acid or bicarbonate via specialized intercalated (IC) cells (1, 5, 8, 21–23, 26, 37). The intracellular bicarbonate is generated along with H+ (acid) in CCD IC cells by the dissociation of carbonic acid under the action of cytoplasmic carbonic anhydrase II (CAII) (1, 8, 21, 23). The generated acid is extruded via H+-extruding transporters, and the bicarbonate is transported via the Cl−/HCO3− exchanger (21–23). The membrane distribution of acid-base transporters, apical vs. basolateral, differs in acid- or bicarbonate-secreting cells. The acid secretion occurs in A-IC cells via apical H+-ATPase and H+-K+-ATPase; bicarbonate is transported across the basolateral membrane via Cl−/HCO3− exchange (1, 5, 8, 21–23, 26, 37). The bicarbonate secretion occurs in non-A IC cells via apical Cl−/HCO3− exchange (1, 8, 21, 23, 37), and acid is transported via the basolateral H+-ATPase. Functional and molecular studies have identified two populations of non-A IC cells, which are referred to as B-IC and non-A, non-B-IC cells (5, 8). In B-IC cells, the Cl−/HCO3− exchanger activity is limited to the apical membrane, whereas in non-A, non-B-IC cells, Cl−/HCO3− exchange activity is detected on both the apical and basolateral membranes (1, 8, 21, 23, 37).
SLC26 (human)/Slc26 (mouse) isoforms are members of a conserved family of anion transporters that display tissue-specific patterns of expression in epithelial cells (4, 9, 10, 14, 15, 19, 25, 27, 32, 42). Several SLC26 members can function as Cl−/HCO3− exchangers. These include SLC26A3 (DRA), SLC26A4 (pendrin), SLC26A6 (PAT1 or CFEX), SLC26A7 (PAT2), and SLC26A9 (PAT4) (6, 24, 28, 35, 39–41). SLC26A7 and SLC26A9 can also function as chloride channels (6, 7, 11, 41). The above SLC26 isoforms differ from one another on the basis of their tissue distribution and their functional characteristics. DRA is predominantly expressed in the intestine but not the kidney, whereas pendrin is expressed in the kidney but not the intestine. SLC26A6 (PAT1), SLC26A7 (PAT2), and SLC26A9 (PAT4) are expressed in both the kidney and gastrointestinal tract (19, 27). The expression of SLC26A6, SLC26A7, and SLC26A9 in the gastrointestinal tract or kidney are distinct from each other (19, 27, 35).
SLC26A4/Slc26a4 is predominantly expressed in the thyroid, inner ear, and kidney (9, 28). In the kidney, Slc26a4 is expressed on the apical membrane of B-IC and non-A, non-B-IC cells in the CCD (15, 22, 32, 33, 36, 37). Studies in genetically engineered mice indicate that pendrin plays an important role in chloride reabsorption in the presence of aldosterone excess or salt depletion (30, 33, 34); however, its role in the mediation of apical Cl−/HCO3− exchange in the CCD is not well studied. To answer these questions, mice with genetic deletion of pendrin were generated. Our results demonstrate that while pendrin is the major mediator of bicarbonate secretion in the CCD, other apical Cl−/HCO3− exchangers may be present in this nephron segment.
EXPERIMENTAL PROCEDURES
Preparation of Slc26a4-null targeting construct and vector, embryonic stem cell electroporation, chimeric mouse generation, germ line transmission, and generation of Slc26a4-null mice.
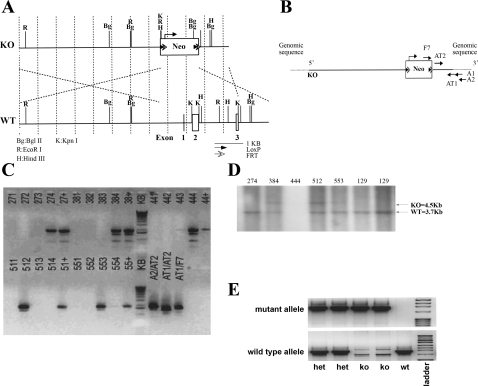
Our strategy was to delete the Slc26a4 gene by knocking out exons 1–3, which encode 101 amino acids, including the ATG initiation site in exon 2. Toward this end, a ∼13.3-kb region used to construct the targeting vector was first subcloned from a positively identified C57BL/6 bacterial artificial chromosome (BAC) clone with a homologous recombination-based technique. The construct was designed with the short homology arm (SA) extending 1.6 kb 3′ to exon 3. The long homology arm (LA) is located on the 5′ side of exon 1 and is ∼7.6 kb long. The Neocassette replaces 4.2 kb of the gene including exons 1–3. The schematic diagram in Fig. 1A depicts the targeting construct used to generate the Slc26a7 knockout (KO) mouse, with location of the Neocassette indicated. The schematic in Fig. 1B depicts the targeting vector, and Fig. 1C demonstrates the identification of recombinant clones by PCR. The sequence for each primer used in PCR screening is as follows: A1: 5′-GGG TGC CGC TAT TTT AGA GCT AGC-3′; A2: 5′-GGCAGGCAAGCATTCTACCACTAAG-3′; AT1: 5′-GGC TCT CTT TCT TGA GCA ACT GTC-3′; AT2: 5′-CCA CGC ATC AGT GCT CAC TCT C-3′; F7: 5′-GGA ACT TCG CTA GAC TAG TAC GCG TG-3′. Parameters for PCR reaction were as follows: initial denaturation step at 99°C for 10 min, 1 cycle; followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 2 min 30 s. This was followed by a final elongation step of 72°C for 7 min. The samples were then stored at 4°C. More than 100 surviving colonies were screened. Individual clones were screened with A2/F7 primers. Recombinant clones were identified by a 1.9-kb PCR fragment. The positive controls were positive pooled samples (indicated by + in Fig. 1C). PCR reaction controls were done with screening and internal primers. Several embryonic stem cell (ES) clones including clones 384 and 512 showed homologous recombination as shown by the presence of a 1.9-kb PCR fragment. DNAs from clones 384 and 512, as well as several other clones, were further examined by Southern blotting (Fig. 1D).
Fig. 1.
Generation of Slc26a4−/− mutant mice. A: schematic diagram of the Slc26a4 targeting construct. The strategy was to delete the Slc26a4 gene by knocking out exons 1–3, which encode 101 amino acids including the ATG initiation site in exon 2. The construct was designed with the short homology arm (SA) extending 1.6 kb 3′ to exon 3 and the long homology arm (LA) located on the 5′ side of exon 1, with ∼7.6-kb length. The Neocassette (Neo) replaces 4.2 kb of the gene including exons 1–3. The targeting vector was confirmed by restriction analysis after each modification step and by sequencing using primers designed to read from the selection cassette into the 3′ end of the SA (N1) and the 5′ end of the LA (N2) or primers that anneal to the vector sequence, P6 and T7, and read into the 5′ and 3′ ends of the bacterial artificial chromosome (BAC) subclone. KO, knockout; WT, wild type. B: Slc26a4 targeting allele and delineation of locations of primers. Ten micrograms of the targeting vector was linearized by NotI and then transfected by electroporation of C57BL/6 × 129/SvEv hybrid embryonic stem cells (ES). After selection in G418 antibiotic, surviving clones were expanded for PCR analysis to identify recombinant ES clones. Primers A1 and A2 were designed downstream (3′) to the SA outside the region used to generate the targeting construct. C: identification of homologous recombinant clones. PCR reactions using A1 and A2 with the F7 primer at the 3′ end of the Neocassette amplify 1.6- and 1.9-kb fragments, respectively. The control PCR reaction was done with primers AT1 and AT2, which are at the 3′ and 5′ ends of the SA, respectively, inside the region used to create the targeting construct. This amplifies a band of 1.5 kb. PCR analysis of DNA isolated from >100 surviving colonies identified several individual clones that showed homologous recombination as determined by the presence of a 1.9-kb fragment. The positive control was performed with primers AT1/N1, which gave the expected fragment size of 1.9 kb. D: Southern blot analysis. For Southern blotting, the fragment encoded by EcoR1-EcoRI, which spans from the 5′ end of the Neocassette to the 3′ end of the SA (A) was amplified for verification of homologous recombination. Two ES clones, 384 and 512, that were identified by PCR screening (C) were further examined by Southern blotting and showed homologous recombination as shown by the presence of a 4.5-kb fragment. E: generation of Slc26a4+/+ and Slc26a4−/− mice. Tail DNA genotyping identified Slc26a7+/+ (wt), +/− (het), and −/− (ko) mice. Band at top shows the detection of the mutant allele, and band at bottom represents the WT allele.
Animals.
Mice were cared for in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Cincinnati. All animal handlers are IACUC trained. Animals had access to food and water ad libitum, were housed in humidity-, temperature-, and light/dark-controlled rooms, and were inspected daily. Animals were euthanized with the use of excess anesthetics (pentobarbital sodium) according to institutional guidelines and approved protocols.
In vitro microperfusion of kidney cortical collecting duct and measurement of apical and basolateral Cl−/HCO3− exchanger activity in intercalated cells.
Kidneys from WT and pendrin-null mice were removed after euthanasia and placed in ice-cold dissection medium. Thin coronal slices (∼1 mm) were cut and transferred to the dissection chamber. CCDs were obtained by hand dissection and were quickly transferred to the temperature-controlled specimen chamber mounted on an inverted Zeiss Axiovert S-100 microscope (Carl Zeiss, Thornwood, NY). Tubules were perfused according to the method of Atkins and Burg as previously described (3). Solutions were delivered by gravity flow at a rate of 4 ml/min. All the solutions were bubbled with 95% O2-5% CO2 and maintained at 37°C.
For intracellular pH (pHi) measurement, tubules were microperfused for 5–10 min with 5 μM pH-sensitive dye BCECF-AM, which is taken up by IC, but not principal, cells when perfused from the luminal side (1, 8, 16, 21, 37). Fluorescent measurements were done with the Zeiss Axiovert S-100 inverted microscope equipped with a xenon arc lamp (Sutter Instruments), an ORCA-R2 cooled charge-coupled device (CCD) camera (Hamamatsu), and excitation and emission filter wheels (Sutter Instruments), and SlideBook software (Intelligent Imaging Innovations) was used for image acquisition and ratiometric measurements (16–18). Excitation wavelengths were alternated between 488 and 440 nm, and emission was recorded at 520 nm. Only one tubule per animal was examined. Intracellular calibration was performed by using the high-K+-nigericin method at the end of each experiment (16, 29, 36). The activity of Cl−/HCO3− exchanger was determined as the initial rate of pHi changes over time (dpHi/dt) following exposure of cells to either bath or lumen chloride-free solution and was expressed as pH units per minute.
The Cl−/HCO3− exchanger activity was identified as the intracellular alkalinization following the apical (perfusate) or basolateral (bath) chloride removal, which also indicated the polarity of the exchanger. After pHi stabilization in Cl−-free medium, the perfusate or bath solution was switched back to a Cl−-containing solution, resulting in the recovery of pHi to baseline levels via Cl−/HCO3− exchange (8, 16). The chloride-containing solution was identical to Burg's solution, which has (in mM) 115 NaCl, 25 NaHCO3, 2.5 K2HPO4, 1.2 MgSO4, 5.5d-glucose, 1 Na citrate, 4 Na lactate, 6l-alanine, and 2 CaCl2. In chloride-free solution, the NaCl was replaced with Na gluconate. Solutions containing HCO3− were continuously bubbled with 95% O2-5% CO2. Solutions had a pH of ≈7.4 at 37°C and osmolality of 290 ± 3 mosmol/kgH2O. Solutions containing gluconate salts had 4 mM Ca acetate to account for complexion of Ca2+ with gluconate.
Acid-secreting A-IC cells were identified by the presence of Cl−/HCO3− exchanger activity on the basolateral, but not the apical, membrane. Bicarbonate-secreting B-IC cells were identified by the presence of Cl−/HCO3− exchanger activity on the luminal, but not the basolateral, membrane, as well as by the intracellular acidification upon basolateral Cl− removal. The basolateral Cl− removal facilitates basolateral Cl− exit, increasing the driving force for the apical Cl−/HCO3−exchanger and apical HCO3− exit, thus resulting in intracellular acidification. A subset of IC cells exhibited the presence of Cl−/HCO3− exchanger activity on their luminal as well as basolateral membrane, similar to what we have previously observed in the rat kidney (16). This subset of IC cells may represent non-A, non-B cells.
Immunofluorescence labeling and Western blotting.
Immunofluorescence labeling was performed on kidney sections from WT and pendrin KO mice as described previously (16, 18) with pendrin- or Na+-HCO3− cotransporter (NBC)1-specific antibodies. Western blot analysis was performed on kidney microsomal membrane proteins (at 60 μg/lane) with pendrin-, phosphate-dependent glutaminase (GA)-, or glutamine dehydrogenase (GDH)-specific antibodies. Pendrin antibody was generated in our laboratory (16). Phosphate-dependent GA antibody was a generous gift from Dr. Norman Curthoys at Colorado State University, and GDH antibody was purchased from commercial sources (Rockland Immunochemicals, Boyertown, PA).
RNA isolation and Northern blot hybridization.
Total cellular RNA was extracted from kidney cortex according to established methods, quantitated spectrophotometrically, and stored at −80°C. Hybridization was performed according to established protocols. The following DNA fragments were used as specific probes for Northern blot hybridization: for pendrin, a fragment encoding nucleotides 1883-2217 from a mouse cDNA; for phosphate-dependent GA, a fragment corresponding to nucleotides 984-2026 from a rat cDNA (accession no. NM_012569); and for GDH, a fragment corresponding to nucleotides 491-1541 from a rat cDNA (accession no. NM_012570). Each Northern blot hybridization was performed on four separate samples from four different animals.
Blood and urine electrolyte analysis.
Mice were housed in metabolic cages and had free access to rodent chow and water. Food intake, water intake, and urine volume were measured daily. Urine was collected under mineral oil. Serum and urine chloride concentration were measured with a digital chloridometer (HBI Haake Buchler Instruments). Ammonium (NH4+) excretion was measured with a phenol/sodium hypochlorite method described by Berthelot and previously used in our laboratory (2). Serum concentrations of Na+, K+, Ca2+, and HCO3− were measured in blood with an i-STATR-1 analyzer and i-STAT EG7+ cartridges (Abbott Laboratories, Abbott Park, IL).
Statistical analysis.
Northern hybridization band densities was determined by densitometry with ImageQuaNT software (Molecular Dynamics, Sunnyvale, CA). The results are presented as means ± SE. Statistical significance between WT and pendrin KO mice was determined by Student's unpaired t-test, and P < 0.05 was considered significant.
RESULTS
Schematic diagrams for the pendrin KO construct and the targeting vector are shown in Fig. 1, A and B, respectively. Figure 1C is a PCR analysis of DNA isolated from ES cells after electroporation with the targeting construct and selection with G418. As indicated, several recombinant clones were identified by the presence of a 1.9-kb mutant fragment.
For Southern blotting, the fragment encoded by EcoR1-EcoRI, which spans from the 5′ end of the Neocassette to the 3′ end of the SA, was amplified for verification of homologous recombination. The expected KO recombinant fragment is 4.5 kb, whereas the WT fragment is 3.7 kb long. The sequences of the two primers adjacent to the 3′ EcoRI were 5′-GTCCCTCACTTTATAGGTAGCCTG-3′ and 5′-CTCTGCTTATCCTCAGGGCC-3′. Figure 1D is a Southern blot analysis based on this strategy and demonstrates homologous recombination for clones 384 and 512.
ES cells from clone 384 or 512 were expanded and used for microinjection into C57BL/6J blastocysts. Chimeric mice were bred to obtain germ line transmission. Intercrossing of Slc26a4 heterozygotes (+/−) generated Slc26a4 KO mice. Tail DNA genotyping was performed with the A2/N1 primer set (see above). Figure 1E is a representative tail DNA genotyping and demonstrates the generation of Slc26a4+/+, Slc26a4+/−, and Slc26a4−/− mice.
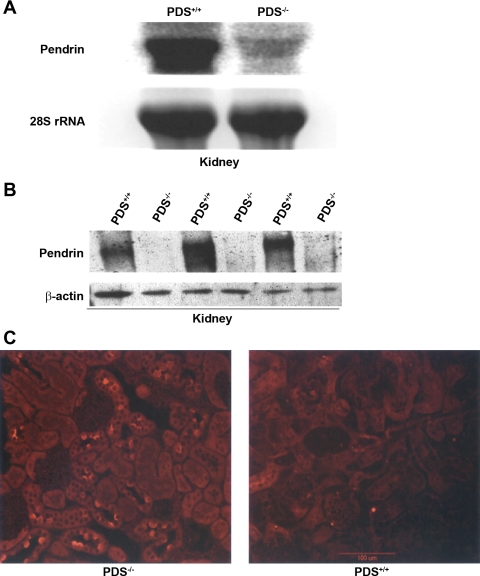
Northern blot hybridizations demonstrated the complete absence of pendrin mRNA in the kidney of Slc26a4-null mice (Fig. 2A). The very faint band with larger molecular weight in pendrin KO kidney represents mutant mRNA that contains the Neocassette (Fig. 2A). Figure 2B is a Western blot analysis and demonstrates the complete absence of pendrin in microsomal membrane proteins isolated from kidneys of pendrin-null mice. Pendrin KO mice look healthy, have normal appetite, and have no apparent phenotype.
Fig. 2.
Generation of Slc26a4−/− mutant mice. A: expression of Slc26a4 in the kidney. Crossing of male and female heterozygote mice (+/−) resulted in the generation of Slc26a4 KO (−/−) mice. Northern blot hybridization on RNA isolated from kidneys of Slc26a4 +/+ and −/− mice indicated that the expression of Slc26a4 is completely absent in the Slc26a4-null mouse. The faint larger band in KO mice represents the mutant RNA. PDS, pendrin. B: immunoblot analysis of pendrin. Microsomal membrane proteins from kidneys of Slc26a4+/+ and Slc26a4−/− mice were isolated, resolved on a gel, and blotted against pendrin antibody. Bottom: β-actin expression as a control for loading. Top: results demonstrate the complete ablation of pendrin in kidneys of KO mice. C: immunofluorescence labeling. Immunofluorescence labeling was performed on frozen kidney sections as previously described (18). As noted, the expression of pendrin, which is located on the apical membrane of a subset of cortical collecting duct (CCD) cells (right) was completely abolished in pendrin KO mice (left).
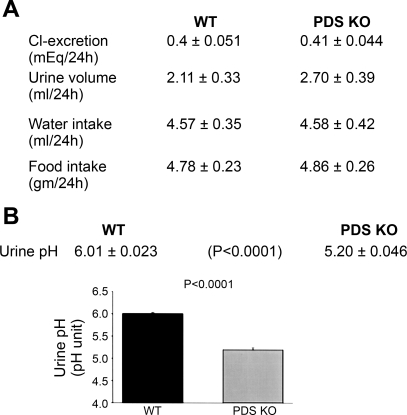
Immunofluorescence labeling with pendrin antibody verified the complete absence of pendrin expression in kidneys of null mice (Fig. 2C). Figure 3A demonstrates balanced studies in WT and mutant mice in metabolic cages. As shown, the 24-h urine chloride as well as urine volume and water intake are not different in pendrin-null mice relative to WT animals. Figure 3B depicts urine pH measurements and demonstrates a highly significant reduction in urine pH in pendrin KO mice.
Fig. 3.
Balanced studies in pendrin KO and WT mice. A: balanced studies and urine chemical analysis in WT and mutant mice. Animals were placed in metabolic cages and allowed free access to food and water for 72 h before the experiments started. Urine output, water intake, food intake, and urine chloride concentration were measured. The 24-h urine chloride as well as urine volume and water intake are not different in pendrin-null mice relative to WT animals. B: urine pH measurements in pendrin KO and WT mice. Urine pH was measured with a pH-sensitive microelectrode from samples collected in animals placed in metabolic cages. As shown, urine pH was significantly decreased in pendrin KO mice.
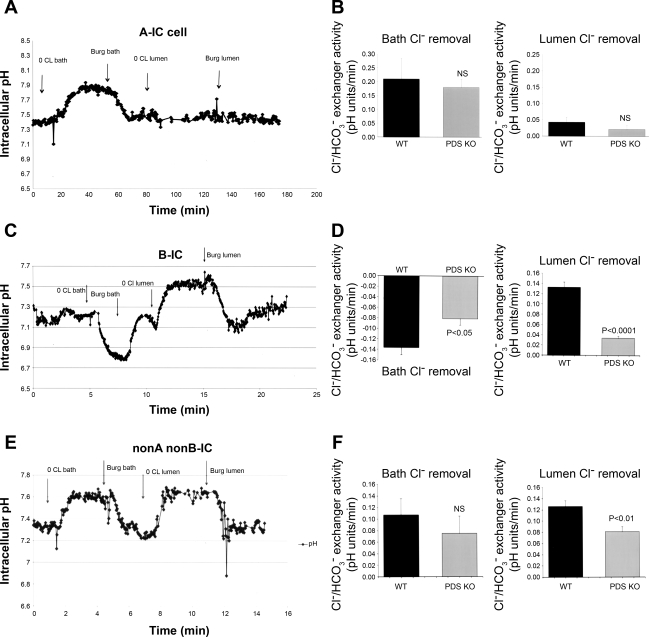
The reduction in urine pH is consistent with decreased secretion of bicarbonate in the collecting duct in pendrin-null mice. Such an assumption is plausible only if pendrin significantly contributes to bicarbonate section via apical Cl−/HCO3− exchange. To ascertain the role of pendrin in apical Cl−/HCO3− exchange in the CCD, tubules were microperfused and pHi was monitored as described in experimental procedures. Three different IC cell types were identified based on their response to luminal or bath chloride removal. The first and most abundant were cells that showed intracellular alkalinization in response to bath chloride removal with no significant changes in pHi in response to luminal chloride removal (representative tracings in Fig. 4A). These cells represent A-IC cells, as manifested by basolateral Cl−/HCO3− exchange, which is predominantly mediated via anion exchanger (AE)1 in the CCD and by AE1 and Slc26a7 in the outer medullary collecting duct (OMCD). Figure 4B is the summation of several studies in at least four WT and four pendrin KO mice. As demonstrated in Fig. 4B, cells classified as A-IC cells showed comparable rates for basolateral Cl−/HCO3− exchanger activity in pendrin KO mice relative to WT littermates. The apical chloride removal in A-IC cells did not elicit a significant pHi alteration in WT or pendrin KO mice (Fig. 4B).
Fig. 4.
Intracellular pH (pHi) monitoring in intercalated (IC) cells in microperfused CCD. A: representative pHi tracings in A-IC cells in Slc26a4+/+ mouse. CCDs of WT or KO animals were microperfused in vitro. A-IC cells were identified by the presence of Cl−/HCO3− exchange limited to their basolateral membrane. Removal of bath chloride causes intracellular alkalinization, whereas the removal of luminal chloride has no significant effect on pHi. Burg solution refers to the chloride-containing solution originally used in Dr. Burg's laboratory (see experimental proceduresand Ref. 3). B: pHi summary results in A-IC cells. There was no significant difference in WT and pendrin KO mice. The results of experiments comparing the basolateral Cl−/HCO3− exchanger activity did not show any significant difference in WT and KO mice. NS, not significant. C: representative pHi tracings in B-IC cells in Slc26a4+/+ mouse. CCDs of WT or KO animals were microperfused in vitro. B-IC cells were identified by the presence of Cl−/HCO3− exchange limited to their apical membrane. Removal of luminal chloride causes intracellular alkalinization, whereas the removal of bath chloride causes acidification. D: pHi summary results in B-IC cells. The results of experiments comparing the apical Cl−/HCO3− exchanger activity showed significant reduction in B-IC cells in pendrin KO mice. E: representative pHi tracings in non-A, non-B-IC cells in Slc26a4+/+ mouse. CCD of WT or KO animals were microperfused in vitro. Non-A, non-B-IC cells were identified by the presence of Cl−/HCO3− exchange to both apical and basolateral membranes. Removal of bath or luminal chloride causes intracellular alkalinization. F: pHi summary results in non-A, non-B-IC cells. The results of experiments comparing the apical Cl−/HCO3− exchanger activity showed significant reduction in non-A, non-B-IC cells in pendrin KO mice. The basolateral Cl−/HCO3− exchanger activity was not significantly different in non-A, non-B-IC cells in pendrin KO mice.
The second most abundant cell type shows intracellular alkalinization in response to luminal chloride removal but intracellular acidification in response to bath chloride removal (representative tracings in Fig. 4C). These cells represent B-IC cells, as manifested by Cl−/HCO3− exchange on the apical membrane but no anion exchange on the basolateral membrane. The intracellular acidosis in response to bath Cl− removal reflects apical Cl−/HCO3− exchanger activation subsequent to reduction in cytoplasmic chloride secondary to the exit of chloride across the basolateral membrane down its gradient. Figure 4D is the summation of several studies in at least four WT and four pendrin KO mice. As shown in Fig. 4D, cells classified as non-A, non-B-IC showed mild decrease in their apical and basolateral Cl−/HCO3− exchanger activity in pendrin KO mice, with only the apical anion exchanger activity achieving statistically significant reduction levels relative to WT mice.
The third cell type shows intracellular alkalinization in response to either luminal or bath chloride removal, consistent with bipolar Cl−/HCO3− exchange (representative tracings in Fig. 4E). Baseline pHi measurements in IC cells in WT and KO mice were as follows: Baseline pHi in A-IC cells was 7.300 ± 0.012 and 7.263 ± 0.036 in WT and KO mice, respectively (P > 0.05, n = 5). Baseline pHi in B-IC cells was 7.399 ± 0.033 and 7.264 ± 0.044 in WT and KO mice, respectively (P > 0.05, n = 5). Baseline pHi in non-A, non-B-IC cells was 7.364 ± 0.043 and 7.445 ± 0.025 in WT and KO mice, respectively (P > 0.05, n = 5). Figure 4F is the summation of several studies in WT and pendrin KO mice. Figure 4F indicates that cells classified as B-IC cells demonstrate significant reduction in their apical Cl−/HCO3− exchanger activity. Interestingly, the intracellular acidification in response to bath chloride removal in B-IC cells, which is an indirect index of apical anion exchanger activity, was moderately reduced in pendrin KO mice (Fig. 4F).
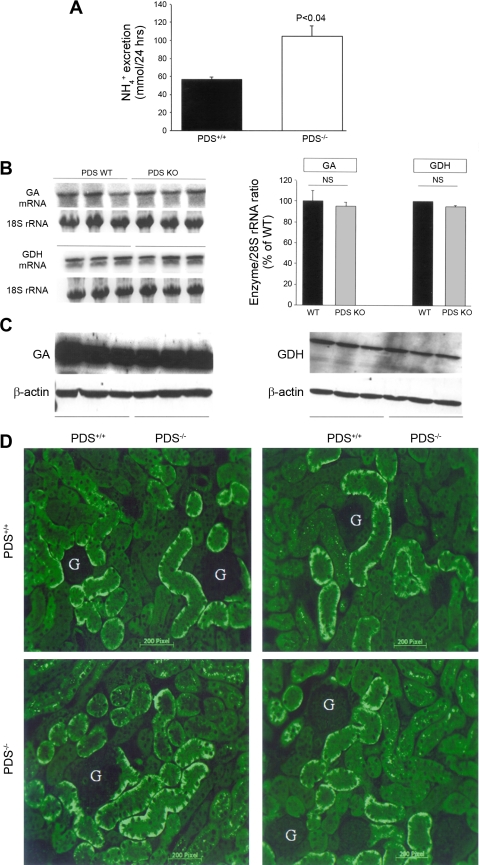
Figure 5 indicates that urinary ammonium excretion is enhanced in pendrin-null animals (Fig. 5A). To determine whether enhanced ammonium excretion is due to increased ammonium synthesis, the mRNA expression of GDH and phosphate-dependent GA, two key enzymes responsible for ammonium synthesis in the proximal tubule, were examined. Densitometric analysis of the GA and GDH mRNA expression levels from Northern blot hybridization studies demonstrated that the expression of GDH and GA remained unchanged in pendrin KO mice (Fig. 5B). The protein abundance of phosphate-dependent GA and GDH was examined by Western blot. As demonstrated, the abundance of GA and GDH was comparable in WT and pendrin KO mice (Fig. 5C, left and right, respectively).
Fig. 5.
Renal NH4+ excretion in pendrin KO and WT mice. A: 24-h urine NH4+ excretion. Twenty-four-hour urinary excretion of NH4+ was significantly increased in pendrin KO mice. B: expression of glutamine dehydrogenase (GDH) and phosphate-dependent glutaminase (GA). Left: mRNA expression of GDH and GA. Right: quantitation of GDH and GA mRNA expression levels in WT and KO mice. C: protein abundance of GDH and phosphate-dependent GA. Western blot analysis demonstrated that the protein abundance of GDH and GA remained unchanged in pendrin KO mice relative to WT littermates. D: immunofluorescence labeling of the Na+-HCO3− cotransporter NBC1. Expression of kNBC1 in the kidney proximal tubule was examined by immunofluorescence labeling in pendrin KO mice (bottom) and WT littermates (top). As shown, NBC1 expression did not show any significant difference in the kidneys of pendrin KO animals relative to WT mice. G, glomerulus.
In the last series of experiments we examined the expression of NBC1 in kidneys of WT and pendrin KO mice. Our immunofluorescence labeling studies with NBC1 antibody indicate comparable expression pattern in WT and pendrin KO mice, suggesting that other proteins in other nephron segments (in this case proximal tubule) appear normal in pendrin-null mice (Fig. 5D).
Given the reduction in urine pH and increased excretion of NH4+ in pendrin KO mice, we examined the systemic acid-base parameters by measuring serum HCO3− in WT and KO mice (experimental procedures). As shown in Table 1, serum bicarbonate was significantly elevated in pendrin KO mice relative to WT animals. Serum chloride, sodium, and potassium concentrations are also shown in Table 1 and do not demonstrate any significant alterations in pendrin KO mice.
Table 1.
Serum chloride, sodium, and potassium concentrations
| Na+ | K+ | Cl− | HCO3− | Ca2+ | |
|---|---|---|---|---|---|
| WT | 137 ± 1.55 | 4.7 ± 0.10 | 102 ± 3.84 | 24 ± 0.98 | 1.04 ± 0.0.4 |
| PDS KO | 137 ± 1.56 | 4.42 ± 0.21 | 102 ± 1.20 | 27.4 ± 0.53 | 1.056 ± 0.053 |
| P | NS | NS | NS | 0.014 | NS |
Data (in meq/l) are means ± SE concentrations for pendrin knockout (PDS KO) mice and their wild-type (WT) littermates; n = 4 or 5 mice in each group. NS, not significant.
DISCUSSION
SLC26A4/Slc26a4 was originally identified by linkage analysis studies in patients with Pendred syndrome, an autosomal recessive disorder manifested by goiter and deafness (9). The original studies indicated a faint expression of its mRNA in the kidney, and follow-up studies demonstrated its presence in CCD cells (9, 28). Functional expression studies demonstrated that pendrin can mediate Cl−/HCO3− exchange (28). On the basis of its localization and its ability to mediate Cl−/HCO3− exchange, it was postulated that pendrin is the bicarbonate-secreting molecule in the CCD (13, 20, 28–30, 33, 34). However, no studies have directly examined the role of pendrin in the mediation of apical Cl−/HCO3− exchanger activity in CCD.
The present studies are the first to directly examine the role of pendrin in the mediation of apical Cl−/HCO3− exchanger activity in the CCD. This was accomplished by utilizing microperfusion technique and pHi measurement in B-IC and non-A, non-B-IC cells (Fig. 4). Previous studies, on the other hand, relied on measures such as renal bicarbonate secretion in the bicarbonate-loaded state in WT and mutant animals to determine the role of pendrin in bicarbonate secretion (20). Our results in microperfused kidney CCD indicate that pendrin is the dominant apical Cl−/HCO3− exchanger in B-IC cells; the majority of the apical Cl−/HCO3− exchanger activity was abolished in pendrin-null mice, although significant residual anion exchanger activity persisted. Interestingly, the intracellular acidification in response to bath chloride removal, which is an indirect measure of apical Cl−/HCO3− exchange, was only partially reduced in pendrin KO mice (Fig. 4). Whether or not the cell pH reduction in response to bath chloride removal is exclusively due to apical anion exchanger activation or reflects the contribution of other transporters remains to be determined.
Our experiments further demonstrate that the apical Cl−/HCO3− exchanger activity in non-A, non-B-IC cells decreased mildly and the basolateral Cl−/HCO3− exchanger activity in these cells was not significantly reduced in pendrin-null mice. These results strongly suggest that pendrin accounts for a fraction of the apical Cl−/HCO3− exchanger activity in non-A, non-B-IC cells. It is worth mentioning that the molecular identity of the basolateral Cl−/HCO3− exchanger in non-A, non-B-IC cells remains unknown. Furthermore, the identity of the molecule(s) mediating the remaining majority of apical Cl−/HCO3− exchange in non-A, non-B-IC cells remains speculative.
The bicarbonate-secreting cells in the CCD have been identified by various investigators based on either molecular markers or functional properties (1, 5, 8, 21, 26, 37). On the basis of several elegant studies published so far, it is clear that non-acid-secreting IC cells are not homogenous: while some cells express H+-ATPase on their apical or basolateral membrane, some cells express this transporter on both apical and basolateral membranes. Functional studies on the identification of subtypes of bicarbonate-secreting cells in rabbit are conflicting: while some demonstrate that B-IC cells have bipolar distribution of Cl−/HCO3− exchange, others do not (8, 38).
In detailed studies in various experimental models, changes in pendrin protein were detected in animals with altered renal chloride transport (29). The results further suggested that factors regulating distal chloride delivery play an important role in altering pendrin expression in the connecting tubule and CCD (29). In our studies the urine pH was found to be significantly decreased in pendrin KO mice (Fig. 3), supporting the studies in microperfused kidney CCDs (Fig. 4) and strongly suggesting an important role for this exchanger in bicarbonate secretion into the lumen of the collecting duct in vivo. An earlier study examining the role of pendrin in chloride absorption measured urine pH in animals subjected to a low-chloride diet and showed that pendrin-deficient animals excrete more acidic urine (31). We conclude that pendrin-null mice excrete an acidic urine pH both at basal state (Fig. 3) and in response to chloride-deficient diet (31).
Our biochemical analysis measurements demonstrate that urinary ammonium excretion was increased in pendrin-null mice (Fig. 5), likely due to trapping of NH3 by the excess H+ in the acidic urine. The decreased HCO3− secretion in the collecting duct along with increased NH4+ excretion are the two likely reasons that serum HCO3− is elevated, albeit mildly, in pendrin KO mice. The present studies are the first report indicating that deletion of pendrin causes a mild metabolic alkalosis. An earlier study did not find any alterations in acid-base balance and urinary ammonium excretion in pendrin-deficient animals (12). Whether differences in methods for measurement of NH4+ excretion could be responsible for these conflicting reports remains speculative.
The observation that the generation of acidic urine in pendrin-null mice occurs without any alteration in the urine chloride excretion (Fig. 3) warrants discussion. As widely accepted, bicarbonate secretion into the lumen of CCD via pendrin is coupled to chloride absorption. As such, disrupting pendrin function should not only decrease bicarbonate secretion but also increase chloride excretion. The fact that urine chloride remains normal but urine pH is reduced indicates complex adaptation at the level of collecting duct or other nephron segments in pendrin-deficient mice. One such possibility is adaptive (or maladaptive) regulation of apical chloride-absorbing or -secreting transporters in the collecting duct or other nephron segments in pendrin-deficient mice. Additional studies are needed to better define the nature of this adaptation.
In conclusion, deletion of Slc26a4 (pendrin) impairs the secretion of bicarbonate in vivo and reduces the apical Cl−/HCO3− exchanger activity in B-IC and non-A, non-B-IC cells in CCD. The significant residual apical anion exchanger activity indicates that additional apical Cl−/HCO3− exchanger(s) is (are) present in the CCD. Pendrin is active at basal state and plays an essential role in bicarbonate secretion in CCD, thus preventing the generation of metabolic alkalosis.
GRANTS
These studies were supported by a Merit Review grant from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-62809 (to M. Soleimani) and by grants from the DCA dialysis care group (to M. Soleimani).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amlal H, Sheriff S, Faroqui S, Ma L, Barone S, Petrovic S, Soleimani M. Regulation of acid-base transporters by vasopressin in the kidney collecting duct of Brattleboro rat. Am J Nephrol 26: 194–205, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Atkins JL, Burg MB. Bicarbonate transport by isolated perfused rat collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 249: F485–F489, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem 269: 3017–3021, 1994 [PubMed] [Google Scholar]
- 5.Brown D, Breton S. H+V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J Exp Biol 203: 137–145, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chang MH, Plata C, Zandi-Nejad K, Sindiæ A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9—anion exchanger, channel and Na+ transporter. J Membr Biol 228: 125–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol 584: 333–345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17: 411–422, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BS, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell 78: 1073–1087, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280: 6463–6470, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70: 102–112, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem 277: 14246–14254, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Petrovic S, Wang Z, Ma L, Soleimani M. Regulation of the apical Cl−/HCO3− exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol 284: F103–F112, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Petrovic S, Spicer Z, Greeley T, Shull GE, Soleimani M. Novel Schering and ouabain-insensitive potassium-dependent proton secretion in the mouse cortical collecting duct. Am J Physiol Renal Physiol 282: F133–F143, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Petrovic S, Ma L, Wang Z, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in rat kidney proximal tubule. Am J Physiol Cell Physiol 285: C608–C617, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Romero MF, Chang MH, Plata C, Zandi-Nejad K, Mercado A, Broumand V, Sussman CR, Mount DB. Physiology of electrogenic SLC26 paralogues. Novartis Found Symp 273: 126–138, 2006 [PubMed] [Google Scholar]
- 20.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol 55: 267–288, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Satlin LM, Bergmann JE. Fluorescent characterization of collecting duct cells: a second H+-secreting type. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1003–F1014, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA 90: 4166–4170, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Soleimani M. Expression, regulation and the role of SLC26 Cl−/HCO3− exchangers in kidney and gastrointestinal tract. Novartis Found Symp 273: 91–102, 2006 [PubMed] [Google Scholar]
- 28.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol 280: F356–F364, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17: 2153–2163, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 179: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM. Dietary Cl− restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Vincourt JB, Jullien D, Amalric F, Girard JP. Molecular and functional characterization of SLC26A11, a sodium-independent sulfate transporter from high endothelial venules. FASEB J 17: 890–892, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 982–987, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestine transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of inner stripe of rabbit outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F406–F415, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Weiner ID, Weill AE, New AR. Distribution of Cl−/HCO3− exchange and intercalated cells in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F952–F964, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M. Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284: 29470–29479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci USA 105: 17955–17960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155, 2000. [DOI] [PubMed] [Google Scholar]