Abstract
Repeated bouts of exercise promote the biogenesis of mitochondria by multiple steps in the gene expression patterning. The role of mRNA stability in controlling the expression of mitochondrial proteins is relatively unexplored. To induce mitochondrial biogenesis, we chronically stimulated (10 Hz; 3 or 6 h/day) rat muscle for 7 days. Chronic contractile activity (CCA) increased the protein expression of PGC-1α, c-myc, and mitochondrial transcription factor A (Tfam) by 1.6-, 1.7- and 2.0-fold, respectively. To determine mRNA stability, we incubated total RNA with cytosolic extracts using an in vitro cell-free system. We found that the intrinsic mRNA half-lives (t1/2) were variable within control muscle. Peroxisome proliferator-activated receptor-γ, coactivator-1α (PGC-1α) and Tfam mRNAs decayed more rapidly (t1/2 = 22.7 and 31.4 min) than c-myc mRNA (t1/2 = 99.7 min). Furthermore, CCA resulted in a differential response in degradation kinetics. After CCA, PGC-1α and Tfam mRNA half-lives decreased by 48% and 44%, respectively, whereas c-myc mRNA half-life was unchanged. CCA induced an elevation of both the cytosolic RNA-stabilizing human antigen R (HuR) and destabilizing AUF1 (total) by 2.4- and 1.8-fold, respectively. Increases in the p37AUF1, p40AUF1, and p45AUF1 isoforms were most evident. Thus these data indicate that CCA results in accelerated turnover rates of mRNAs encoding important mitochondrial biogenesis regulators in skeletal muscle. This adaptation is likely beneficial in permitting more rapid phenotypic plasticity in response to subsequent contractile activity.
Keywords: mitochondrial biogenesis, PGC-1α, exercise, mitochondrial transcription factor A, AU-rich element
skeletal muscle exhibits dynamic plasticity in response to functional demands. With chronic contractile activity (CCA), muscles undergo a wide variety of biochemical and physiological adaptations (21, 39). For example, regular endurance exercise undertaken over a number of weeks leads to the biogenesis of mitochondria, eliciting a sequential phenotypic fiber type transition from glycolytic to oxidative metabolism (4, 21, 22). This process is orchestrated, in part, by the well-characterized key regulatory factor, peroxisome proliferator-activated receptor-γ, coactivator-1α (PGC-1α) (3a, 21, 61). Specifically, PGC-1α coactivates transcription factors to induce the expression of mitochondrial transcription factor A (Tfam), a nuclear gene product that is involved in the transcription and replication of mitochondrial DNA (mtDNA) (51, 52).
In response to exercise, mRNA and protein levels of PGC-1α and Tfam are elevated (2, 17, 43–45, 54). Increases in steady-state mRNA levels as a result of a stimulus such as exercise are the product of both synthesis (transcription) and degradation (mRNA stability). Thus the relative contribution of both of these processes plays an important role in phenotypic adaptations (16). However, the impact of changes in mRNA stability for muscle mitochondrial adaptations has been relatively unexplored.
When compared with other biological molecules, the distinguishing property of mRNA is its high rate of turnover, which is tissue specific (8). Of particular interest is the class of mRNA bearing AU-rich elements (AREs) in the 3′-untranslated region (−UTR) (6, 7, 11, 12). The physical interaction of AREs with specialized RNA-binding proteins (RBPs) is a strong determinant of mRNA stability and/or translation, and hence the subsequent level of protein expression. In muscle, a pair of RBPs with reciprocal functions can act independently and synchronously to regulate the expression of labile genes (42, 46, 55, 60). Human antigen R (HuR) and the four isoforms of ARE-RNA binding factor 1 (AUF1) (p37AUF1, p40AUF1, p42AUF1, and p45AUF1) are primarily localized to the nucleus (10, 31, 64). Their export to the cytoplasm has been associated with the stabilization or destabilization of mRNA transcripts, respectively (10, 31). During muscle differentiation, HuR accumulates in the cytoplasm and interacts with mRNAs encoding MyoD, myogenin (62, 63), and acetylcholinesterase (13, 14). In contrast, the AUF1 proteins have been shown to bind to and destabilize the mRNAs encoding human angiotensin 1 receptor (47), sarcoendoplasmic reticulum calcium ATPase2a (5), and the calcitonin receptor (69). Furthermore, HuR and AUF1 have common targets, and growing evidence has demonstrated that homomultimerization of HuR and AUF1 can bind simultaneously and competitively to a single ARE (32, 47, 69). Interestingly, both HuR and the AUF1 proteins target the ARE in the c-myc 3′-UTR (18, 31). c-Myc is a transcription factor that has also been implicated in the regulation of mitochondrial biogenesis via the control of Tfam expression (29, 34).
The effect of contractile activity on the stability of mRNAs that regulate mitochondrial biogenesis has never been investigated. Thus the purposes of our study were 1) to characterize the mRNA stability of PGC-1α, c-myc, and Tfam in relation to steady-state mRNA and protein levels; 2) to investigate the presence of relevant AREs within the 3′-UTRs of PGC-1α, c-myc, and Tfam regulatory genes; and 3) to determine the effect of CCA on the expression of mRNA destabilizing/destabilizing proteins along with coincident changes in mRNA half-lives.
MATERIALS AND METHODS
Animals and in vivo chronic contractile activity protocol.
All animals were housed in a temperature-controlled room (22.5 ± 0.5°C) with 12 h light (19:00–7:00) and 12 h dark (7:00–19:00) cycles and were allowed food and water ad libitum. Male Sprague-Dawley rats (300–400 g; Charles River, St. Constant, QC, Canada) were anesthetized with a ketamine-xylazine combination dose via intraperitoneal injection (0.2 ml/100 g body wt). The tibialis anterior (TA) muscles were removed from a subset of animals for total RNA isolation. In another set of animals (300–325 g) used for chronic contractile activity (CCA), two stimulating electrodes (Medwire, Leico Industries, New York, NY) were passed subcutaneously from the thigh and exteriorized at the back of the neck under aseptic conditions. A portable stimulator unit was placed in a plastic housing connected to the exteriorized electrode wires at the back of the neck and fastened to the back of the animal with cloth tape, as done previously (1, 36). Care was taken to ensure that the procedure did not restrict animal movement, cause discomfort, or restrict breathing. The electrodes were sutured to the underlying muscle ∼1–2 mm on either side of the common peroneal nerve. The overlying hamstring muscle was sutured, the skin was stapled, and sterile ampicillin (Penbritin, Ayerst, Montreal, Canada) was injected to minimize risks of infection. In all animals, the contralateral limb was used as a nonstimulated internal control. Stimulation was adjusted at the time of electrode implantation to result in palpable contractions of the TA and extensor digitorum longus (EDL) muscles. When animals had regained ≥100% of their initial body weight and had recovered for a minimum of 1 wk, chronic stimulation (10 Hz, 0.1-ms duration) was begun for 3 h or 6 h/day for 7 days. Animals were observed to move freely about their cages during the experimental periods. After the final recovery period (21 or 18 h) on the eighth day, animals were then anesthetized with ketamine-xylazine as described above, and the EDL muscles were extracted and frozen in liquid nitrogen. All animals were then immediately euthanized via thoracotomy and removal of the heart. All samples were frozen at −80°C and pulverized at the temperature of liquid nitrogen for subsequent RNA and protein analyses. All procedures involving animals were approved by the York University Animal Care Committee, in accordance with the Canadian Council on Animal Care.
Cytochrome c oxidase activity.
Cytochrome c oxidase (COX) activity was measured as previously described in detail (9). Briefly, muscle powder homogenates were added to a buffered test solution containing fully reduced cytochrome c. Enzyme activity was determined as the maximal rate of oxidation of fully reduced cytochrome c measured by the change in absorbance at 550 nm in a Synergy HT microplate reader at 30°C.
In vitro RNA isolation.
Total RNA was isolated using Tri-reagent (Invitrogen) as recommended by the manufacturer, with slight modifications (16). Frozen muscle powders (30 or 200 mg) and Tri-reagent (Invitrogen) were homogenized (Ultra Turrax 7-mm probe) at 30% power output for 30 s and subsequently transferred to a sterile tube. After 5 min of incubation at room temperature, 200 μl of chloroform were added to the homogenate that was then shaken vigorously for 15 s. A subsequent color change was observed from clear pink to milky pink, followed by centrifugation (Eppendorf, 5415 D) at 12,000 g at 4°C (15 min). After the upper aqueous phase was transferred to a separate RNAse-free tube, 500 μl of isopropanol were added, and the supernate was vigorously shaken. To precipitate total RNA, the supernate was incubated overnight at −20°C. The RNA pellet was collected by subjecting the supernate to centrifugation at 16,100 g at 4°C (10 min). The isopropanol was discarded and the pellet was washed with 75% ethanol. After a final spin at 16,100 g at 4°C (1 min), the RNA pellet was resuspended in sterile water. With the use of 995 μl of sterile water and 5 μl of RNA, total RNA concentration and purity were determined by ultraviolet photometry at 260 and 280 nm, respectively. RNA quality was verified by separation of the 28S and 18S rRNA on denaturing formaldehyde-1% agarose gels.
In vitro cytosolic protein extraction.
EDL muscle powders (50 mg) were homogenized in sterile homogenization buffer (25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20 mM HEPES pH 7.9, 0.5 mM 1,4-dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and RNAse-free water) at 40% power output for 3× 10 s. The homogenates were centrifuged at 5,000 g at 4°C (15 min). The supernatant fractions were then subjected to further centrifugation at 15,000 g at 4°C (15 min) to remove mitochondria. The resultant supernatant representing a crude cytosolic fraction (S15) was transferred to a sterile tube. Protein concentrations of the S15 fractions were determined by the Bradford colorimetric assay.
In vitro RNA decay assay.
Analysis of RNA degradation was performed as previously described, with slight modifications (16). Total RNA (30 μg) from TA muscles from control animals was incubated with S15 protein extract from EDL muscles (20 μg) in a 100-μl reaction volume at 37°C. Separate aliquots were removed at 5, 10, 20, and 30 min. Total RNA was reisolated with the phenol-chloroform extraction procedure and precipitated at −20°C overnight. Total RNA was subsequently washed, pelleted, and resuspended in 20 μl of sterile ddH2O. RNA concentration and purity were determined by measuring absorbance at 260 and 280 nm, respectively. The quality of total RNA was validated by separation of the 28S and 18S rRNA on denaturing formaldehyde-1% agarose gels.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA (2 μg) was reverse transcribed to cDNA using SuperScript III reverse transcriptase as recommended by the manufacturer (Invitrogen). cDNA was amplified by PCR using GoTaq Flexi DNA polymerase (Promega), using the manufacturer's recommendations. Sequence-specific primers listed in Table 1 for PGC-1α, c-myc, Tfam, or S12 were added in a 50-μl volume PCR reaction containing 2 μl of cDNA. PCR products (40 μl each) were separated on 1.8% agarose gels and visualized by ethidium bromide (EtBr) staining.
Table 1.
List of primers used in PCR analyses
| Gene | Forward Primer | Reverse Primer | Size,(nt), bp |
|---|---|---|---|
| PGC-1α | 5′-GAC CAC AAA CGA TGA CCC TCC-3′ | 5′-CCT GAG AGA GAC TTT GGA GGC-3′ | 635 |
| c-myc | 5′-TCA AGA GGC CAC AGC AAA C-3′ | 5′-AAA AGC TAC GCT TCA GCT CG-3′ | 274 |
| Tfam | 5′-ATG GCG CTG TTC CGG GGA ATG TGG G-3′ | 5′-TTA ATT CTC AGA GAT GTC TCC CGG G-3′ | 735 |
| S12 | 5′-GGA AGG CAT AGC TGC TGG-3′ | 5′-CCT CGA TGA CAT CCT TGG-3′ | 638 |
PGC-1α, peroxisome proliferator-activated receptor-γ, coactivator-1α; Tfam, mitochondrial transcription factor A.
Western blot analysis.
Total cytosolic protein extracts were isolated from muscle powders as described above. Briefly, total protein (50 μg/lane) was electrophoresed through one-dimensional 12 or 15% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. Blots were blocked (1 h) at room temperature with 5% milk in 1× TBS-Tween20 (Tris-buffered saline/Tween-20; 25 mM Tris·HCl, pH 7.5, 1 mM NaCl, and 0.1% Tween-20), followed by overnight incubation with antibodies diluted in 5% blocking buffer directed toward PGC-1α (1:500; Caymen Chemicals), Tfam [1:750; (17)], c-myc (1:500; Santa Cruz Biotechnology), HuR 3A2 (1:2,000; Santa Cruz Biotechnology), AUF1 (1:500; Upstate/Millipore), aciculin (1:200; DSHB University of Iowa), and GAPDH (1:40,000; Abcam). After 3 × 5 min washes with TBS-Tween20, blots were incubated at room temperature for 1 h with the appropriate secondary antibody conjugated to horseradish peroxidase. Blots were washed again 3× 5 min with TBS-Tween20, developed with enhanced chemiluminescence, and quantified via densitometric analysis of the intensity of the signal with SigmaScanPro v.5 software (Jandel Scientific, San Rafael, CA).
Statistical analyses.
Data were analyzed with GraphPad 4.0 software, and values are reported as means ± SE. Where indicated, Student's paired t-test, one- or two-way analyses of variance followed by Bonferroni post hoc tests were used to determine individual differences between conditions. Results were considered to be statistically significant if P < 0.05 was achieved. The nonlinear regression equation 50 = 100e(−kx) was used to determine mRNA half-lives.
Computational analysis.
To identify putative HuR and AUF1 binding motifs in PGC-1α 3′-UTRs of mammals, we deployed sequence comparison of six mammalian species against human using the software ClustalW2 (33). A pairwise score was calculated as the number of identities in the best alignment divided by the number of residues compared. Gap positions were excluded. Both of these scores were initially calculated as percent identity scores and were converted to distances by dividing by 100 and subtracting from 1.0 to give number of differences per site (33).
RESULTS
Effect of CCA on whole muscle mitochondrial biogenesis.
To evaluate the effect of CCA on mitochondrial content, we measured COX activity in stimulated and contralateral nonstimulated EDL muscle. COX activity in nonstimulated EDL muscle was 6.06 ± 0.56 U/g muscle. Seven days of stimulation resulted in an equal elevation in animals subjected to both 3 and 6 h/day conditions (unpublished data). Therefore, the results were pooled to show a 1.5-fold elevation of COX activity (Fig. 1A; P < 0.05). Thus our chronic stimulation protocol was effective in inducing mitochondrial biogenesis in whole muscle.
Fig. 1.
Effect of chronic contractile activity (CCA) on peroxisome proliferator-activated receptor- coactivator-1α (PGC-1α) protein and mRNA levels. Animals were subjected to CCA for 7 days. A: cytochrome c oxidase (COX) enzyme activity of whole muscle. The EDL muscles of animals were subjected to CCA for 3 or 6 h/day for 7 days [n = 18; *P < 0.05, control (CTRL) vs. stimulation (STIM)]. B: PGC-1α protein, along with a loading control (aciculin), were measured in extracts from CTRL or STIM EDL muscles (n = 6; *P < 0.05, CTRL vs. STIM). C: PGC-1α and S12 mRNA transcripts were measured by RT-PCR from total RNA isolated from CTRL or STIM EDL muscles. A representative ethidium bromide (EtBr)-stained agarose gel is illustrated along with a graphical representation of the data (n = 10).
Effect of CCA on PGC-1α protein content, steady-state mRNA level, and mRNA stability.
CCA resulted in an increase of PGC-1α protein content by 1.6-fold when compared with nonstimulated control muscle (Fig. 1B; P < 0.05). In contrast, the levels of PGC-1α mRNA were not affected by the chronic stimulation paradigm (Fig. 1C). To evaluate PGC-1α mRNA stability, we employed a cell-free mRNA decay assay, as done previously (16). PGC-1α mRNA was degraded by cytosolic ribonucleases, as incubation of total RNA in the absence of cytosolic proteins (Fig. 2A) and in the presence of a ribonuclease inhibitor (Fig. 2B) attenuated the mRNA decay. The half-life (t1/2) of PGC-1α mRNA was 24.5 min (Fig. 2C) in the presence of cytosolic proteins isolated from control muscle. In contrast, PGC-1α mRNA stability decreased by 48% in the cytosol isolated from chronically stimulated muscle (Fig. 2C; P < 0.05). S12 mRNA, used as an internal control, was more stable (t1/2 = 58.6 min) than PGC-1α mRNA in the cytosol from control, nonstimulated muscle. No effect of chronic stimulation on S12 mRNA was observed (unpublished data). Therefore, these data for S12 mRNA were combined (Fig. 2C).
Fig. 2.
Effect of CCA on PGC-1α mRNA stability in skeletal muscle. A: total RNA (12 μg) from the tibualis anterior (TA) muscle was incubated with RNase-free isolation buffer alone for 0, 10, 20, and 40 min. PGC-1α and S12 mRNA transcripts were examined by RT-PCR and EtBr-stained agarose gel electrophoresis. B: total RNA (30 μg) was incubated with 20 μg of cytosolic proteins for 0, 5, 10, 20, and 30 min in the presence or absence of a ribonuclease inhibitor. Reactions in the absence of RNA or cDNA are also shown as negative controls. C: total RNA isolated from TA muscle was incubated with cytosolic proteins from CTRL or STIM EDL muscles of animals subjected to CCA for 7 days. After each time point, total RNA was reisolated and PGC-1α (n = 5; *P < 0.05, CTRL vs. STIM) along with an internal control S12 (n = 10) were examined by RT-PCR. A representative EtBr gel is illustrated along with a graphical representation of the data. Reactions in the absence of cytosolic proteins, as well as the absence of RNA or cDNA, are shown as positive and negative controls, respectively.
Effect of CCA on c-myc protein content, steady-state mRNA level, and mRNA stability.
CCA resulted in an increase of c-myc protein content by 1.7-fold (Fig. 3A; P < 0.05). In contrast, both c-myc steady-state mRNA level and mRNA stability were unchanged in response to chronic stimulation (Fig. 3, B and C). The basal half-lives of c-myc (82.9 min) and S12 (52.6 min) mRNAs were not significantly different from each other.
Fig. 3.
Effect of CCA on c-myc steady-state protein, mRNA levels, and mRNA stability. Animals were subjected to CCA for 7 days. A: c-myc protein, along with a loading control (aciculin), were measured in extracts from CTRL or STIM EDL muscles (n = 8; *P < 0.05, CTRL vs. STIM). B: c-myc and S12 mRNA transcripts were measured by RT-PCR from total RNA isolated from CTRL or STIM EDL muscles. A representative EtBr-stained agarose gel is illustrated along with a graphical representation of the data (n = 10). C: total RNA isolated from TA muscle was incubated with cytosolic proteins from CTRL or STIM EDL muscles of animals subjected to CCA for 7 days. After each time point, total RNA was reisolated, and c-myc (n = 4) along with an internal control S12 (n = 10) were examined by RT-PCR. A representative EtBr gel is illustrated along with a graphical representation of the data. Reactions in the absence of cytosolic proteins, as well as the absence of RNA or cDNA, are shown as positive and negative controls, respectively.
Effect of CCA on Tfam protein content, steady-state mRNA level, and mRNA stability.
CCA led to an increase of Tfam protein content by 2.0-fold (Fig. 4A; P < 0.05) but not steady-state mRNA levels (Fig. 4B). In the presence of cytosolic proteins isolated from control muscle, Tfam mRNA half-life was 31.2 min, and this was reduced by 44% to 13.6 min in chronically stimulated muscle compared with the control condition (Fig. 4C; P < 0.05).
Fig. 4.
Effect of CCA on mitochondrial transcription factor A (Tfam) steady-state protein and mRNA levels and mRNA stability. Animals were subjected to CCA for 7 days. A: Tfam protein, along with a loading control (GAPDH), were measured in extracts from CTRL or STIM EDL muscles (n = 8; *P < 0.05, CTRL vs. STIM). B: Tfam and S12 mRNA transcripts were measured by RT-PCR from total RNA isolated from CTRL or STIM EDL muscles. A representative EtBr-stained agarose gel is illustrated along with a graphical representation of the data (n = 8). C: total RNA isolated from TA muscle was incubated with cytosolic proteins from CTRL or STIM EDL muscles of animals subjected to CCA for 7 days. After each time point, total RNA was reisolated and Tfam (n = 7, *P < 0.05 CTRL vs. STIM) along with an internal control S12 (n = 10) were examined by RT-PCR. A representative EtBr gel is illustrated along with a graphical representation of the data. Reactions in the absence of cytosolic proteins as well as the absence of RNA or cDNA are shown as positive and negative controls, respectively.
Effect of CCA on RNA-binding proteins HuR and AUF1.
Analysis of each AUF1 isoform revealed that the most abundant isoform was p45AUF1, whereas p42AUF1 expression was low in fast-twitch EDL muscle. CCA resulted in the increase of stabilizing HuR (Fig. 5A; P < 0.05) as well as destabilizing total AUF1 proteins (Fig. 5B; P < 0.05) by 2.4- and 1.9-fold, respectively. p37AUF1, p40AUF1, and p45AUF1 isoforms increased in response to CCA by 2.3-, 1.7-, and 1.5-fold, respectively (Fig. 5C; P < 0.05), when compared with the contralateral control muscle.
Fig. 5.
Effect of CCA on RNA-binding proteins, human antigen R (HuR), and ARE-RNA binding factor 1 (AUF1). Animals were subjected to CCA for 7 days. A: HuR, along with a loading control aciculin, were measured in extracts from CTRL or STIM EDL muscles (n = 8; *P < 0.05, CTRL vs. STIM). B: analyses and graphical representations of protein expression of p37AUF1, p40AUF1, p42AUF1, p45AUF1, and aciculin, the loading control are shown (n = 6, *P < 0.05, CTRL vs. STIM). C: distribution of various isoforms are shown. (n = 6; *P < 0.05 CTRL vs. STIM).
Effect of CCA on mRNA stability.
A summary of the half-lives of the transcripts measured in our study is shown in Fig. 6. These half-lives are not intended to represent the decay rates as found in vivo, since they are a product of the conditions employed in the in vitro decay assay (i.e., the ratio of cytosolic protein to total RNA). However, since the assay conditions used were identical for all transcripts evaluated, as well as in control and stimulated muscle, this information is valuable to show 1) the variability in the basal half-lives of mRNAs and 2) the effect of chronic contractile activity. The intrinsic mRNA stabilities of PGC-1α and Tfam were less than that of c-myc and S12 (Fig. 6, P < 0.05). In addition, CCA induced a differential response on mRNA stability, which was reduced in PGC-1α and Tfam, but no significant effect of stimulation was observed for S12 or c-myc (Fig. 6).
Fig. 6.
Effect of CCA on mRNA stability in skeletal muscle. Total RNA isolated from TA muscle was incubated with cytosolic proteins from CTRL or STIM EDL muscles of animals subjected to CCA for 7 days. After each time point, total RNA was reisolated and PGC-1α, c-myc, and Tfam along with an internal control S12 (n = 4–10) were examined by RT-PCR. * and †, Statistical significance from respective CTRLs and a main effect for all CTRL mRNAs, respectively.
DISCUSSION
Repeated contractions of skeletal muscle over several weeks promote the biogenesis of mitochondria. The resulting increase in mitochondrial content in skeletal muscle leads to a preferential shift in energy utilization toward a greater dependence on oxidation phosphorylation for ATP provision. A consequence of this adaptation is that the muscle become more fatigue resistant, a characteristic favorable for health in both young and old individuals (37, 39). Therefore, understanding the molecular basis of mitochondrial biogenesis is important for our comprehension of how exercise can improve the quality of life.
Mitochondrial biogenesis requires the coordinated regulation of two distinct genomes localized in separate cellular compartments. Three important biogenesis regulators investigated in our study were PGC-1α, c-myc, and Tfam. PGC-1α coactivates genes encoding a number of transcription factors to induce the mRNA expression of NUGEMPs (21, 22, 28). One of the most important NUGEMPs is Tfam, which regulates the expression and copy number of mtDNA. The transcription factor c-myc is also involved in the regulation of Tfam (29, 34). Once Tfam is translated into protein, it is shuttled across the mitochondrial membranes to its functional destination. Inside the matrix, Tfam activates the transcription of the 13 mitochondrial proteins that are essential for oxidative phosphorylation (for review, see Ref. 52). There is an increasing body of evidence that describes the changes in protein and mRNA expression of both PGC-1α and Tfam in skeletal muscle. However, the regulation of these transcriptional activators during exercise requires greater understanding. Studies in the past have focused largely on transcriptional mechanisms, whereas the significance of mRNA stability as an important regulator of gene expression in skeletal muscle is just beginning to earn recognition (7, 62). Given the significance of mRNA stability in gene expression, we 1) compared the intrinsic mRNA stabilities of PGC-1α, c-myc, and Tfam in skeletal muscle and 2) examined effect of CCA on the stability of these mRNA species using an in vitro cell-free system.
First, we showed that the basal half-lives of these regulatory mRNAs were inherently different. PGC-1α and Tfam mRNAs were significantly less stable than the mRNAs of c-myc and S12 (Fig. 6), supporting previous in vivo evidence that the intrinsic half-lives of transcripts in muscle can vary from minutes (Egr-1) to hours (−UTRophin) (19, 26, 53). Generally, the half-life of mRNA species negatively correlates with regulatory importance. For example, mRNAs encoding transcription factors are short-lived compared with mRNAs of genes related to metabolism and cell structure (53). c-Myc mRNA is well known for its short half-life (20–30 min) in vivo (48, 52). Our in vitro analyses of mRNA stability revealed that the turnover rates of PGC-1α and Tfam were greater than c-myc, suggesting that they encode proteins of greater regulatory importance, at least in skeletal muscle.
In an effort to understand the role of mRNA stability in exercise-induced mitochondrial biogenesis, we employed a chronic, low-frequency electrical stimulation model to simulate chronic exercise. Although not locomotory exercise, this protocol provides a potent stimulus for the induction of mitochondrial biogenesis in skeletal muscle (1, 16, 35, 36, 56), and it therefore serves as a good experimental model to study the underlying biochemical and physiological adaptations involved in organelle synthesis. Our stimulation conditions were effective at inducing mitochondrial biogenesis, as COX activity increased, and the magnitude of upregulation was similar to values reported previously (17, 36). Contractile activity provoked increases in PGC-1α and Tfam protein expression, consistent with published findings from our laboratory (17, 25), and modestly less than the changes produced by other forms of exercise (58, 68). In addition, we also showed that CCA induced an increase in c-myc protein expression in skeletal muscle. The extent of the induction of these proteins was typical of that observed with this chronic stimulation model (16, 23, 36, 55). However, the increases in protein expression were not accompanied by concomitant elevations in the corresponding steady-state mRNA levels. This is likely due to the transient nature of mRNA expression changes as a result of exercise. For example, using the identical CCA model as the present study, Gordon et al. (17) found that Tfam mRNA expression peaked on day 4 but was reduced to resting muscle levels by day 14, whereas protein levels were elevated by ∼1.5-fold. Other studies have reported that PGC-1a mRNA expression increases during, and immediately after, an acute bout of exercise (2, 44–46, 54) and returns to resting levels within hours postexercise (2, 42). Similarly, c-myc mRNA expression also increases after an acute bout of exercise in mice (24) and in humans (40, 60). Together, these findings suggest that the mRNA responses of PGC-1α, c-myc, and Tfam are time dependent, postexercise. They increase transiently after each period of contractile activity, contribute to the accumulation of protein, and then decline thereafter.
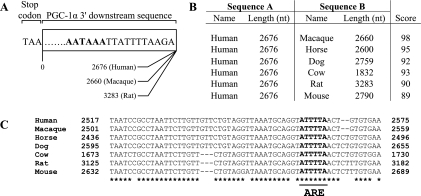
The present study is the first to demonstrate a differential response in mRNA degradation kinetics as a result of CCA. In the presence of cytosolic proteins isolated from stimulated muscle, the half-lives of PGC-1α and Tfam mRNAs were significantly reduced compared with the control, nonstimulated condition. In contrast, neither c-myc nor S12 mRNA stability was significantly altered following CCA. In a previous study employing the identical chronic stimulation protocol as the present study, Freyssenet et al. (13) showed that CCA resulted in the increase of cytochrome c mRNA abundance, which was attributed, in part, to an increased mRNA stability in the early stages (days 2 and 3) of the CCA paradigm. Later, by day 7, mRNA stability had returned to control levels (16). Conversely, Irrcher et al. (26) showed that contractile activity resulted in reduced Egr-1 mRNA stability in cultured myotubes. These divergent data are clearly indicative of transcript-specific adaptations to CCA. This depends on the presence or absence of mRNA-specific sequences, which support the interaction with stabilizing/destabilizing RBPs. We speculate that the short half-lives of PGC-1α and Tfam mRNAs are caused by one or more potent instability sequences, within the 3′-UTR of each transcript. The 3′-UTR of Tfam is known to contain AREs, which may serve as destabilizing cis-elements. Similarly, AREs may exist in the PGC-1α 3′-UTR, the full-length of which has yet to be completely identified empirically. Multiple AREs covering a stretch of ∼600 nucleotides are found within ∼3 kb downstream of the PGC-1α coding sequence, followed by a consensus poly(A) signal (Fig. 7A). With the use of ClustalW2 software (33), sequence alignment of the human PGC-1α sequences against six mammalian species, revealed scores ≥89 (Fig. 7, B and C), suggesting that they are evolutionary conserved and thus may dictate the mRNA turnover rate of this regulatory protein.
Fig. 7.
PGC-1α 3′ downstream sequences contain AU-rich elements. A: schematic representation of the aligned sequence beginning from the stop codon (TAA) up to the consensus poly(A) signal (bold) and immediate downstream residues. For some species, this stretch of sequence is an amalgamation of the end and the beginning of two adjacent contigs, where bases may be missing. B: tabular result of PGC-1α sequence between the stop codon and the poly(A) signal comparing human (column A) against six mammals (column B) using the software ClustalW2. A pairwise score was calculated as the number of identities in the best alignment divided by the number of residues compared. Gap positions were excluded (30). C: sequence alignments of one of several putative HuR and AUF1 binding sites designated ARE (bold) within the PGC-1α 3′ downstream sequences suggest that this region is evolutionarily conserved.
Since mRNA stability is determined by the interaction between intrinsic cis-elements and extrinsic trans-regulatory factors, we investigated the expression of ARE-regulatory RBPs, stabilizing HuR, and destabilizing AUF1. AUF1 consists of four isoforms with variable concentrations. The most abundant of which is p45AUF1 in EDL muscle. HuR and AUF1 are established regulators of c-myc mRNA and they may also be involved in PGC-1α and Tfam mRNA expression. We demonstrate for the first time that CCA resulted in elevated cytosolic levels of HuR, p37AUF1, p40AUF1, and p45AUF1. This suggests that the upregulation or the cytoplasmic redistribution of HuR and AUF1 are in synchronization. This supports previous evidence that shows a strong positive correlation between HuR and AUF1 expression in all tissues and during various stages of development (10, 18, 31, 41). However, the concomitant induction of both proteins does not necessarily indicate parallel cellular consequences, which depend on the starting concentration of each protein isoform within the cell, as well as the relative affinity of each isoform for the mRNA binding site. The affinities of HuR and AUF1 isoforms for the ARE are likely important in determining the final rate of mRNA decay, since these proteins compete with each other for binding in the 3′-UTR (10, 32, 59). Future analyses of RNA-protein binding using electromobility shift or pull-down assays will be used to investigate this. Affinity is determined, in part, by phosphorylation, and it has been shown that HuR is a target of p38 mitogen-activated protein kinase and 5′-AMP-activated protein kinase (AMPK) (11, 30, 65, 66). Both of these kinases are activated by contractile activity (38). Thus the alterations in mRNA stability observed with CCA may stem, in part, from kinase-induced phosphorylation of RBPs on the AREs of target mRNAs.
Our data allow us to speculate that, in contrast to chronic exercise, inactivity may lead to the opposite adaptive response of transcriptional regulator mRNA stability in skeletal muscle. For example, immobilization, denervation, cancer cachexia, diabetes, and renal failure (3, 50) result in reduced steady-state PGC-1α mRNA levels. Under such conditions, transcription rates of PGC-1α are likely reduced, and mRNA stability may be enhanced in compensatory fashion. These ideas require experimental verification using muscle disuse models in the future.
In summary, our data show that the half-lives of mRNAs encoding transcription factors are variable in skeletal muscle. With respect to PGC-1α, we have revealed that the 3′-UTR may harbor functional regulatory sequences. Furthermore, the responsiveness of each mRNA species to extracellular stimuli was variable. CCA selectively increased the mRNA degradation rate of both PGC-1α and Tfam, two important regulatory proteins involved in mitochondrial biogenesis. We speculate that this characteristic is beneficial because, when combined with a parallel increase in transcription, this permits a higher turnover rate, and a more rapid adaptive response in the face of an imposed stress, such as acute contractile activity. In support of this, Pilegaard et al. (48) illustrated that PGC-1α transcription and mRNA expression reached higher levels following exercise in the trained condition, suggesting that the mechanisms regulating the exercise-induced activation of PGC-1α in muscle became more sensitive with chronic exercise. Furthermore, the dynamics of the mRNA reservoir may be fine-tuned by the induction of, and balance between, specific RBPs such as HuR and AUF1. Collectively, our study deepens the understanding of mitochondrial adaptive responses to CCA at the posttranscriptional level. Further investigations are needed to dissect the interplay among mRNAs and their binding proteins, with the potential of generating interventions to enhance exercise-induced benefits in healthy, aged, or diseased muscle.
GRANTS
This work was supported by funding from the National Sciences and Engineering Research Council of Canada (NSERC) to David A. Hood. Ruanne Y. J. Lai was supported by an Ontario Graduate Scholarship. Vladimir Ljubicic was supported by a Doctoral Fellowship provided by the Heart and Stroke Foundation of Canada. D. A. Hood is the holder of a Canada Research Chair in Cell Physiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors are grateful for the technical assistance of Olga Ostojic.
REFERENCES
- 1.Adhihetty PJ, Ljubicic V, Hood DA. Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am J Physiol Endocrinol Metab 292: E748–E755, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab 298: E799–E806, 2010 [DOI] [PubMed] [Google Scholar]
- 3a.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev 18: 426–434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Blum JL, Samarel AM, Mestril R. Phosphorylation and binding of AUF1 to the 3′-untranslated region of cardiomyocyte SERCA2a mRNA. Am J Physiol Heart Circ Physiol 289: H2543–H2550, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 58: 266–277, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakkalakal JV, Miura P, Belanger G, Michel RN, Jasmin BJ. Modulation of -UTRophin A mRNA stability in fast versus slow muscles via an AU-rich element and calcineurin signaling. Nucleic Acids Res 36: 826–838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor MK, Takahashi M, Hood DA. Tissue-specific stability of nuclear- and mitochondrially encoded mRNAs. Arch Biochem Biophys 333: 103–108, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Cooperstein SJ, Lazarow A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem 189: 665–670, 1951 [PubMed] [Google Scholar]
- 10.David PS, Tanveer R, Port JD. FRET-detectable interactions between the ARE binding proteins, HuR and p37AUF1. RNA 13: 1453–1468, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal 16: 1113–1121, 2004 [DOI] [PubMed] [Google Scholar]
- 12.DeMaria CT, Brewer G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J Biol Chem 271: 12179–12184, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Deschênes-Furry J, Angus LM, Belanger G, Mwanjewe J, Jasmin BJ. Role of ELAV-like RNA-binding proteins HuD and HuR in the post-transcriptional regulation of acetylcholinesterase in neurons and skeletal muscle cells. Chem Biol Interact 157–158: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Deschênes-Furry J, Belanger G, Mwanjewe J, Lunde JA, Parks RJ, Perrone-Bizzozero N, Jasmin BJ. The RNA-binding protein HuR binds to acetylcholinesterase transcripts and regulates their expression in differentiating skeletal muscle cells. J Biol Chem 280: 25361–25368, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic perioxsome proliferator-activated receptor-gamma coactivator-1 alpha expression. Diabetes 58: 1499–508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freyssenet D, Connor MK, Takahashi M, Hood DA. Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am J Physiol Endocrinol Metab 277: E26–E32, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gordon JW, Rungi AA, Inagaki H, Hood DA. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 90: 389–396, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Gouble A, Morello D. Synchronous and regulated expression of two AU-binding proteins, AUF1 and HuR, throughout murine development. Oncogene 19: 5377–5384, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gramolini AO, Jasmin BJ. Expression of the -UTRophin gene during myogenic differentiation. Nucleic Acids Res 27: 3603–3609, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol N-UTR Metab 34: 465–472, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209: 2265–2275, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hood DA, Zak R, Pette D. Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits. Eur J Biochem 179: 275–280, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. J Appl Physiol 101: 151–163, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 284: C1669–C1677, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Irrcher I, Hood DA. Regulation of Egr-1, SRF, and Sp1 mRNA expression in contracting skeletal muscle cells. J Appl Physiol 97: 2207–2213, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Irrcher I, Walkinshaw DR, Sheehan TE, Hood DA. Thyroid Homrone (T3) rapidly activates p38 and AMPK in skeletal muscle in vivo. J Appl Physiol 104: 175–185, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Joseph AM, Pilegaard H, Litvintsev A, Leick L, Hood DA. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem 42: 13–29, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PLoS One 3: e1798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafarga V, Cuadrado A, Lopez dS I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol 29: 4341–4351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafon I, Carballes F, Brewer G, Poiret M, Morello D. Developmental expression of AUF1 and HuR, two c-myc mRNA binding proteins. Oncogene 16: 3413–3421, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23: 3092–3102, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 25: 6225–6234, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ljubicic V, Adhihetty PJ, Hood DA. Application of animal models: chronic electrical stimulation-induced contractile activity. Can J Appl Physiol 30: 625–643, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ljubicic V, Adhihetty PJ, Hood DA. Role of UCP3 in state 4 respiration during contractile activity-induced mitochondrial biogenesis. J Appl Physiol 97: 976–983, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell 8: 394–404, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Ljubicic V, Hood DA. Specific attenuation of protein kinase phosphorylation in muscle with a high mitochondrial content. Am J Physiol Endocrinol Metab 297: E749–E758, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Ljubicic V, Joseph AM, Saleem A, Uguccioni G, Collu-Marchese M, Lai RY, Nguyen LM, Hood DA. Transcriptional and post-transcriptional regulation of mitochondrial biogenesis in skeletal muscle: effects of exercise and aging. Biochim Biophys Acta 2009 [DOI] [PubMed] [Google Scholar]
- 40.Mahoney DJ, Safdar A, Parise G, Melov S, Fu M, MacNeil L, Kaczor J, Payne ET, Tarnopolsky MA. Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am J Physiol Regul Integr Comp Physiol 294: R1901–R1910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda K, Marasa B, Martindale JL, Halushka MK, Gorospe M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging 1: 681–698 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1alpha mRNA and protein in human skeletal muscle. J Appl Physiol 105: 1098–1105, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Misquitta CM, Chen T, Grover AK. Control of protein expression through mRNA stability in calcium signalling. Cell Calcium 40: 329–346, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 148: 3441–3448, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Mortensen OH, Plomgaard P, Fischer CP, Hansen AK, Pilegaard H, Pedersen BK. PGC-1beta is downregulated by training in human skeletal muscle: no effect of training twice every second day vs. once daily on expression of the PGC-1 family. J Appl Physiol 103: 1536–1542, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 96: 189–194, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Pende A, Contini L, Sallo R, Passalacqua M, Tanveer R, Port JD, Lotti G. Characterization of RNA-binding proteins possibly involved in modulating human AT 1 receptor mRNA stability. Cell Biochem Funct 26: 493–501, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabbitts PH, Watson JV, Lamond A, Forster A, Stinson MA, Evan G, Fischer W, Atherton E, Sheppard R, Rabbitts TH. Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J 4: 2009–2015, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. Suppressing Fox03 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem 97: 673–683, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS. Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res 16: 45–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spangenburg EE, Brown DA, Johnson MS, Moore RL. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem 332: 225–231., 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi M, Hood DA. Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol 74: 934–941, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Takahashi M, McCurdy DT, Essig DA, Hood DA. delta-Aminolaevulinate synthase expression in muscle after contractions and recovery. Biochem J 291: 219–223, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang K, Breen EC, Wagner PD. Hu protein R-mediated ponscriptional regulation of VEGF expression in rat gastrocnemius muscle. Am J Physiol Heart Circ Physiol 283: H1497–H1504, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1α protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab 286: E208–E216, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Topisirovic I, Siddiqui N, Orolicki S, Skrabanek LA, Tremblay M, Hoang T, Borden KL. Stability of eukaryotic translation initiation factor 4E mRNA is regulated by HuR, and this activity is dysregulated in cancer. Mol Cell Biol 29: 1152–1162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol 102: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 3: 333–341, 2006 [DOI] [PubMed] [Google Scholar]
- 62.van der Giessen K, Di-Marco S, Clair E, Gallouzi IE. RNAi-mediated HuR depletion leads to the inhibition of muscle cell differentiation. J Biol Chem 278: 47119–47128, 2003 [DOI] [PubMed] [Google Scholar]
- 63.van der GK, Gallouzi IE. Involvement of transportin 2-mediated HuR import in muscle cell differentiation. Mol Biol Cell 18: 2619–2629, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner BJ, DeMaria CT, Sun Y, Wilson GM, Brewer G. Structure and genomic organization of the human AUF1 gene: alternative pre-mRNA splicing generates four protein isoforms. Genomics 48: 195–202, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Fan J, Yang X, Furer-Galban S, Lopez dS I, von KC, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol 22: 3425–3436, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Yang X, Kawai T, Lopez dS I, Mazan-Mamczarz K, Chen P, Chook YM, Quensel C, Kohler M, Gorospe M. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR. J Biol Chem 279: 48376–48388, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Wright DC, Han DH, Garcia-Roves P, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem 282: 194–199, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Yasuda S, Wada S, Arao Y, Kogawa M, Kayama F, Katayama S. Interaction between 3′ untranslated region of calcitonin receptor messenger ribonucleic acid (RNA) and adenylate/uridylate (AU)-rich element binding proteins (AU-rich RNA-binding factor 1 and Hu antigen R). Endocrinology 145: 1730–1738, 2004. [DOI] [PubMed] [Google Scholar]