Abstract
Branching morphogenesis is a fundamental process in the development of the kidney. This process gives rise to a network of ducts, which form the collecting system. Defective branching can lead to a multitude of kidney disorders including agenesis and reduced nephron number. The formation of branching tubules involves changes in cell shape, cell motility, and reorganization of the cytoskeleton. However, the exact intracellular mechanisms involved are far from understood. We have used the three-dimensional (3D) Madin-Darby canine kidney (MDCK) cell culture system to study how p21-activated kinase 1 (Pak1), which is an important regulator of the cytoskeleton, modulates branching. Our data reveal that Pak1 plays a crucial role in regulating branching morphogenesis. Expression of a dominant-negative Pak1 mutant (DN-Pak1) in MDCK cysts resulted in the spontaneous formation of extensions and branching tubules. Cellular contractility and levels of phosphorylated myosin light chain (pMLC) were increased in DN-Pak1 cells in collagen. Expression of a DN-Pak1 mutant that does not bind to PIX (DN-Pak1-ΔPIX) failed to form extensions in collagen and did not have increased contractility. This shows that the DN-Pak1 mutant requires PIX binding to generate extensions and increased contractility in 3D culture. Furthermore, a β1-integrin function-blocking antibody (AIIB2) inhibited the formation of branches and blocked the increased contractility in DN-Pak1 cysts. Taken together, our work shows that DN-Pak1-induced branching morphogenesis requires PIX binding and β1-integrin signaling.
Keywords: cyst, tubulogenesis, myosin II, contractility
the proper morphogenesis and maintenance of renal epithelial tubules are of central importance to the development and function of the kidney. Branching morphogenesis or tubulogenesis is a process that gives rise to a network of epithelial cell-lined tubules and ducts, which form the collecting system. Defective branching can lead to a number of kidney disorders including agenesis, reduced nephron number, and polycystic kidney disease (46). The maintenance of renal epithelial tubule structure is critical in response to injury and repair. Much is known about the growth factors and membrane receptors that regulate branching from in vivo studies (52). Many of the signaling pathways that control branching morphogenesis affect the actomyosin cytoskeleton, which plays an essential role in regulating the cellular processes involved in the morphogenesis and maintenance of epithelial tubules including cellular motility, adhesion, and contractility. However, the exact molecular mechanisms involved in regulating the cytoskeleton in renal epithelial tubules and how changes in cytoskeleton dynamics affect the morphogenesis, maintenance, and repair of renal tubules are unclear.
Three-dimensional (3D) cell culture is a powerful approach to investigate the molecular mechanisms that regulate the cytoskeleton during the morphogenesis and maintenance of renal epithelial tubules. The 3D Madin-Darby Canine Kidney (MDCK) cell culture system is a well-established model system for studying renal epithelial branching morphogenesis and tubulogenesis (30, 49, 69). In 3D cell culture, single cells are embedded in an extracellular matrix such as collagen I, and these cells proliferate and organize into 3D structures like cysts or tubules. Cysts are spherical, hollow structures composed of a monolayer of epithelial cells surrounding a central lumen. Treatment of cysts with hepatocyte growth factor (HGF) activates several signaling pathways, which in concert, induce a highly complex series of morphogenetic events that start with formation of basal membrane extensions, followed by formation of cords of cells and eventually end with the formation of branching tubules (42, 69). Whereas the molecular mechanisms that regulate and coordinate these events are still incompletely understood, it has become clear that several subprograms control distinct stages of tubulogenesis. For instance, activation of the ERK pathway is required for the early stages, whereas activation of matrix metalloproteases is uniquely required for later stages (33). Other HGF-induced signaling pathways, such as activation of phosphatidyl inositol-3-kinase (PI3K), are likely required for all stages of tubulogenesis (65).
In addition to soluble growth factors such as HGF, the ECM is an important regulator of cystogenesis and tubulogenesis. Specific ECM components either stimulate or inhibit tubulogenesis in MDCK cells (50) and control cell polarity and lumen formation during cystogenesis (32, 64). These effects likely are mediated via integrin heterodimers, which serve as ECM receptors. In particular, α2β1- and/or α3β1-integrin are required for cystogenesis and tubulogenesis in MDCK and in vitro organ culture (20, 45, 64, 71). Integrins cluster in focal adhesions upon ECM binding, and recruitment of specific components into these structures is critical for tubulogenesis (19).
The Rho family of GTPases Rho, Rac, and cdc42 are activated by HGF (40, 43) and in response to integrin-mediated cell-matrix adhesion in 3D culture (64). Given their well-established roles in the regulation of the actin cytoskeleton, cell motility, and cell adhesion (15), and findings that Rac and cdc42 are required for HGF-induced cell scattering (40, 43), Rho GTPases are likely to be important regulators of cystogenesis and branching morphogenesis. Indeed, studies with dominant-negative Rac1 showed that inhibition of Rac1 blocks normal cell polarization, lumen formation, and tubulogenesis in 3D cyst culture (32, 41). Furthermore, similar studies in tubule formation using a collagen overlay model showed that both Rac1 and RhoA regulate tubulogenesis in MDCK cells (12, 13). Whereas these studies indicate important roles for Rac1 in cystogenesis and tubulogenesis, the downstream pathways are still unclear. Dominant-negative versions of Rho-GTPases act by sequestering upstream guanine exchange factors, which often are able to activate different Rho-GTPases (58). Thus these mutants can potentially inhibit signaling by several Rho GTPases and their downstream effector molecules.
To investigate specific pathways downstream of Rac1, we analyzed the role of p21-activated kinase 1 (Pak1) in cystogenesis. Pak1 is one of the most well-established Rac and cdc42 effector molecules and a key regulator of cytoskeletal organization (1, 67, 74). Consistent with being a downstream effector of Rac1, Pak1 is activated through β1-integrins (10, 76) and by HGF in many cell types, including MDCK (2, 43). Pak1 can act both by phosphorylating downstream substrates but also has kinase-independent functions, by serving as a scaffold (16). Pak1 has potential roles in regulating actin-myosin contractility and may activate myosin II by phosphorylating myosin light chain (MLC) (23, 51). On the other hand, Pak1 was also reported to prevent MLC phosphorylation by negatively regulating myosin light chain kinase (MLCK) (47). Functions of Pak1 that are at least partially kinase independent include the recruitment of key signaling proteins to the membrane (16) and the regulation of cell motility and focal adhesion turnover (25, 51, 60, 68).
Here we show that expression of a DN-Pak1 mutant in MDCK cysts resulted in the spontaneous formation of cellular extensions and branching tubules. These changes in cyst morphology involve alterations to the cytoskeleton including changes in cell shape, increased contractility, and myosin II activity. The morphogenetic effects of the DN-Pak1 depended on its interaction with βPIX and the function of β1-integrins. Together, our results suggest that Pak1 plays an important role in regulating branching and myosin II activity during renal epithelial morphogenesis.
METHODS
Cell lines and cell culture.
The DN-Pak1 (Pak1-K299R) and Pak1-R193A, P194A, K299R (hereafter called DN-Pak1-ΔPIX) cell lines that inducibly express these mutant proteins using the Tet-off system have been described previously (68). Cells were grown in MEM with l-glutamine, supplemented with 5% fetal calf serum, and penicillin-streptomycin in a humidified atmosphere at 37°C in 5% CO2, and 95% air. To suppress transgene expression, cells were maintained in growth medium with 20 ng/ml of doxycycline.
3D culture of cysts.
Cyst culture was performed according to previously established methods with slight modifications (34). Cells were subcultured 1:5 the day before being plated in collagen or a basement membrane extract (BME). For plating in collagen or BME, cells were rinsed one time in PBS, incubated for 10 min in PBS at 37°C, and then trypsinized with 0.25% trypsin-EDTA for an additional 10 min at 37°C to generate a single cell suspension. Cells were resuspended in a collagen I solution containing 2.1 mg/ml of collagen I (PureCol, Advanced Biomatrix, San Diego, CA), 0.23% NaHCO3 wt/vol, 20 mM HEPES (pH 7.6), and growth medium (with or without doxycycline). The cell-collagen mixture (150 µl) was plated on Nunc Anopore membrane inserts with a 0.02-μm pore size and 10-mm diameter (Nalge Nunc International, Rochester, NY) in a 24-well tissue culture plate. For some experiments, cells were resuspended in BME (Trevigen, Gaithersburg, MD) and plated on Nunc inserts as above. The cell-collagen mixture, or cell-BME mixture, was allowed to gel for 40 min at 37°C, and then growth medium was added to the culture. Transgene expression was induced on the day of plating in collagen by adding growth medium to the gels without doxycycline. Cysts were fed with growth medium every 3–4 days in culture. Cysts for phase-contrast microscopy and immunofluorescence experiments were plated in collagen gel (or BME) at a concentration of 2 × 104 cells/ml. For imaging live cysts several days, cells were resuspended in collagen as stated earlier, and 150 μl of the cell-collagen mixture was plated directly into one well of a 48-well tissue culture plate. To monitor individual cysts, a gridded coverslip (Electron Microscopy Sciences, Hatfield, PA) was attached to the bottom of the plate under the corresponding well. The numbered grid was used as a reference to follow specific cysts over several days. Cysts were photographed daily from day 7 to day 10 by using phase-contrast microscopy. For biochemistry experiments, cells were plated at a concentration of 5 × 104 cells/ml in collagen on top of an additional cell-free collagen gel. For treatment of cysts with the β1-integrin function-blocking rat monoclonal antibody AIIB2, the antibody was added directly to the culture medium from day 5 to day 8 in culture at a final concentration of 8 μg/ml as described previously (64).
Antibodies and other reagents.
The rat anti-E-cadherin antibody was obtained from Sigma-Aldrich. Polyclonal rabbit anti-phospho-Thr18,Ser19-MLC2 (hereafter called pMLC) antibody was from Cell Signaling Technology. The mouse monoclonal anti-GAPDH antibody was from Biodesign International. The rabbit β-catenin antibody was from Santa Cruz Biotechnology. Rat anti-ZO1 was obtained via the Developmental Studies Hybridoma Bank at the University of Iowa. All other antibodies were as described previously (64). F-actin was stained with phalloidin-Alexa Fluor 488 (Invitrogen). Secondary antibodies comprised Alexa Fluor 488 donkey anti-mouse IgG (H+L), anti-rat, and anti-rabbit conjugates and Alexa Fluor 633 goat anti-rat IgG (H+L), anti-mouse, and anti-rabbit conjugates (Invitrogen). (−)-Blebbistatin was obtained from Sigma-Aldrich.
Immunofluorescence confocal microscopy.
The protocol for immunofluorescence staining of cysts was previously described in detail (34). Samples were rinsed once in PBS plus Ca2+ and Mg2+ and then fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Next, gels were washed in PBS, quenched in 50 mM NH4Cl in PBS, and permeabilized in PBS with 0.1% Triton-X100 (TX-100). Primary antibodies were added at a 1:100 dilution in 5% normal donkey serum in PBS/TX-100 overnight rotating at 4°C. The following day the samples were washed extensively in PBS/TX-100, and then secondary antibodies were added at a 1:200 dilution overnight rotating at 4°C. The final day, the gels were washed in PBS/TX-100, rinsed in PBS, followed by dH2O, and then mounted on glass slides in Fluorosave (Calbiochem) with 10 μg/ml 4′-6-diamidino-2-phenylindole (DAPI) to stain nuclei. To stain F-actin, gels were fixed and permeabilized as above and treated with phalloidin-Alexa-Fluor 488 (Invitrogen) overnight at 4°C rotating and washed and mounted the following day. Cysts were imaged on a Zeiss 510 LSM confocal microscope with an Axiovert 200M microscope and a C-Apochromat ×63/1.2W Corr lens. Images were adjusted for brightness with Adobe Photoshop CS version 9.0.
Western blot analysis.
To prepare protein lysates of cysts, collagen gels containing cysts were transferred directly into 40 μl of 4× Laemmli buffer, mixed, and boiled for 5 min. Equal volumes of lysates were loaded onto 8% [for hemagglutin (HA) and myc detection] or 12% (for pMLC detection) SDS-PAA gels and transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore). For comparison of pMLC levels, phosphatase inhibitors [1 mM sodium-vanadate (Na3VO4) and 1 mM NaF] were added to the Laemmli buffer, and pMLC signals were normalized to GAPDH after quantitative Western blot analysis using an Odyssey detector (LI-COR, Lincoln, NE). Samples for the analysis of pMLC levels were collected from cysts at 7 days in culture. Secondary antibodies for Odyssey detection comprised Alexa Fluor 680-conjugated donkey anti mouse and donkey anti-rabbit IgG (Invitrogen).
3D collagen gel contractility assays.
Contractility assays were done essentially as described previously (39), with some modifications. Cells were induced to express DN-Pak1 or DN-Pak1-ΔPIX by culturing cells in the absence of doxycycline for 3 days before being plated in collagen gels. Cells were trypsinized as above to generate single cell suspensions and then mixed in a collagen I solution containing 1.5 mg/ml of collagen I, 0.23% NaHCO3, and 20 mM HEPES (pH 7.6) in growth medium (with or without doxycycline). Single 60-μl drops were plated onto nontissue culture-treated six-well plates (Fisher Scientific). The collagen gels were allowed to solidify for 20 min at 37°C, and then growth medium was added to the wells. The gels were gently lifted off the plate using a cell scraper, and gels were photographed using a Canon SD300 digital camera. A small ruler was placed in the image field for scale. The diameter of the gels was determined at 0, 12, and 16 h by measuring the diameters using Adobe Photoshop. Actomyosin-generated contraction forces of cells cause the gel to decrease in diameter with time. Contractility assays were performed with at least triplicate samples, and for at least three experiments. l-Blebbistatin (Sigma) was added directly to culture medium at 50 μM final concentration to inhibit myosin II activity. DMSO was used as a vehicle control for the (−)-blebbistatin-treated cells.
Statistical analyses.
Data are means ± SD for at least three experiments. Differences between two groups were examined using an unpaired t-test. Statistical significance was assumed at P < 0.05.
RESULTS
Expression of a DN-Pak1 mutant in 3D MDCK cell culture induces branching morphogenesis.
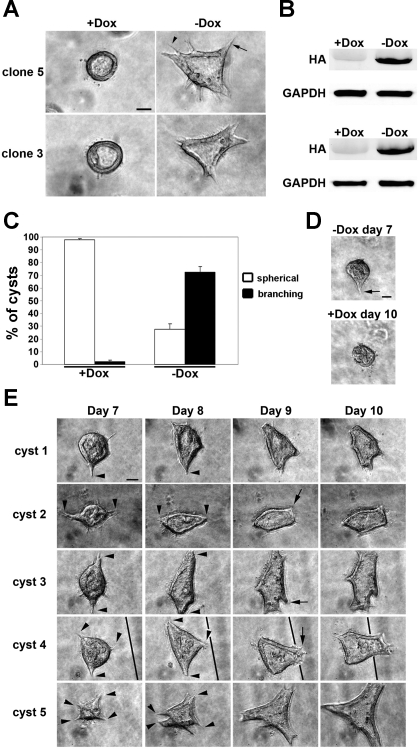
Since Rac1 was previously shown to be essential in the proper orientation of epithelial polarity in 3D MDCK cyst culture (32), and Pak1 is a major downstream effector of Rac1 (1), we wanted to determine whether Pak1 has a function in epithelial morphogenesis in 3D cell culture. For this, we used a previously established MDCK cell line stably expressing a DN-Pak1 mutant Pak1-K299R, which is a full-length, kinase-dead mutant (68). This mutant protein is expressed under the control of the tetracycline-regulated transactivator using the Tet-off system (14). In the presence of doxycycline, the DN-Pak1 mutant protein is not expressed in these cells. Cells were plated in collagen I gels and monitored by phase-contrast microscopy for cyst morphology. Control cells were plated in collagen I in the presence of doxycycline (+Dox), and cells were plated in collagen in the absence of doxycycline (−Dox) to induce DN-Pak1 expression. After 8 days in culture, control cells formed cysts, which were spherical in shape and contained a hollow lumen (Fig. 1A). However, DN-Pak1-expressing cysts had an abnormal morphology, with many relatively narrow and broad cellular processes arising from the basal surface of cysts. We refer to this morphology as “branching,” because it resembles the cellular processes that form during HGF-induced branching morphogenesis (35, 62). These cellular processes include basal membrane extensions and lumen-containing tubules. Here, we refer to these simply as branches. To confirm that this phenotype was not due to a clonal variation, we tested a second DN-Pak1 clone (clone 3) that showed the same branching phenotype in 3D culture (Fig. 1A). The branching phenotype of DN-Pak1 mutant cysts was observed as early as 5 days in culture but was strongest around 10 days in culture. Induction of expression of the HA-tagged DN-Pak1 mutant protein was verified in 3D cyst cultures by Western blot (Fig. 1B). We quantified the morphology of the DN-Pak1 cysts by direct observation of cysts using phase-contrast microscopy. Of the control cysts, 98% had a normal, spherical morphology, whereas only 28% of DN-Pak1 cysts had a spherical morphology (Fig. 1C). The remaining 72% of DN-Pak1 cysts had the branching phenotype. DN-Pak1 cysts also had a reduced capacity to form clear lumens compared with control cysts (data not shown). However, the lumen phenotype appeared independent of the branching phenotype because there were many DN-Pak1 cysts that had a strong branching phenotype and a clear lumen. These results show that expression of the DN-Pak1 induces branching morphogenesis in 3D MDCK cell culture.
Fig. 1.
Expression of DN-Pak1 in Madin-Darby canine kidney (MDCK) cells in three-dimensional (3D) culture results in a spontaneous branching phenotype. A: in the presence of doxycycline (+Dox), dominant-negative Pak1 mutant (DN-Pak1) clones 5 and 3 form cysts with a normal spherical morphology and central lumen (left) at 8 days in culture. In the absence of doxycycline (−Dox), clones 5 and 3 form cysts with many narrow (arrowhead) and broad (arrow) branches. B: expression of the hemagglutin (HA)-tagged DN-Pak1 mutant protein in cysts was verified by Western blot. The HA-tagged DN-Pak1 protein was expressed at high levels only in the −Dox treated cysts in both clone 5 and 3. GAPDH was used as a loading control. C: quantitation of morphology of cysts. n = 3. Error bars represent SD. D: DN-Pak1 (−Dox) branching cyst at day 7 (top), arrow marks a single branch arising from this cyst. Bottom: branch has retracted in this cyst after the addition of Dox from day 7 to day 10. E: analysis of DN-Pak1 (−Dox) cyst morphology from days 7 to 10 in culture. Five representative branching cysts are shown. Arrowheads mark branches that broaden and/or elongate from day 7 to day 8. Arrows mark the formation of new branches at day 9. The line in images for cyst 4 marks a grid line at the bottom of the dish. Scale bars in A, D, and E are 30 μm.
To determine whether continuous expression of DN-Pak1 was required for the maintenance of branches, we grew DN-Pak1 cysts in the absence of Dox for 7 days and photographed branching cysts. Then Dox was added to the culture medium to repress expression of DN-Pak1, and cysts were rephotographed 3 days later. To do this, we examined live cysts in collagen gels plated over gridded coverslips, which allowed us to follow individual cysts over time. The addition of Dox from days 7 to 10 caused branches to retract in DN-Pak1 cysts (Fig. 1D). This result demonstrates that DN-Pak1 expression is required for the maintenance of branches once they initiated in these cysts.
To better understand the mechanism of branching in DN-Pak1 cysts, we examined how cyst morphology changes over several days. To do this, we again examined cysts plated over gridded coverslips. We photographed cysts daily starting at day 7 and ending at day 10. Five representative DN-Pak1 cysts are shown in Fig. 1E. In DN-Pak1 cysts, branching begins with the formation of one or more relatively short and narrow branches (Fig. 1, arrowheads day 7). In general, these branches become broader and elongate over several days (arrowheads day 8). Note that the development of narrow branches into broad branches is relatively rapid and occurs over a time course of 24 h (compare day 7 and 8 for cysts 1, 3, and 5) but that the broad branches were less dynamic. In some cysts, new branches continue to form off of established branches, showing that this is a dynamic process (arrows day 9 in cyst 2, 3, and 4). In some cases, the position of the cyst changes slightly relative to the grid from day 7 to day 10 (Fig. 1E, cyst 4), suggesting that increased motility of DN-Pak1 cells in collagen plays an important role in branching morphogenesis of these cysts.
DN-Pak1-induced branches maintain apical-basolateral polarity and cell junctions and are not associated with alterations in laminin deposition.
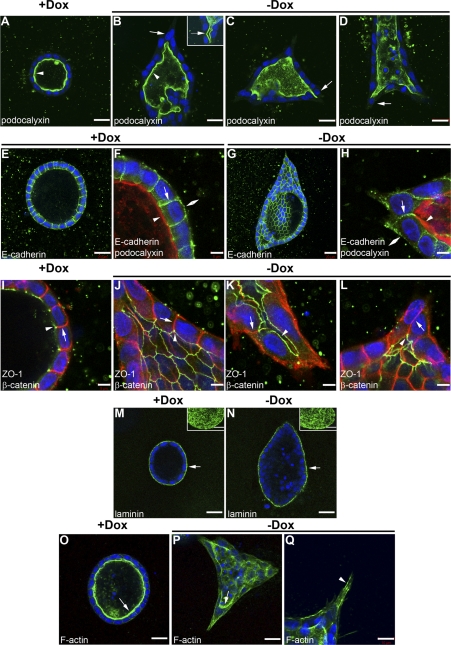
Since inhibition of Rac1 activity was previously implicated in the proper orientation of apical-basal polarity and laminin organization in 3D MDCK cysts (32), we determined whether Pak1 also played a role in these processes. We used immunofluorescence confocal microscopy to analyze the localization of several markers of apical-basal polarity in the DN-Pak1 branching cysts. Analysis of the apical membrane marker podocalyxin/gp135 (5, 28) showed no difference in localization between control and DN-Pak1 expressing branching cysts; in both cases, podocalyxin/gp135 was localized to the apical/lumenal surface of cysts (Fig. 2, A and B). Closer examination of podocalyxin/gp135 staining also revealed that the broader branches in DN-Pak1 cysts contained lumens that were continuous with the cyst lumen (inset in Fig. 2B). In other words, DN-Pak1-expressing cysts spontaneously form tubules. Furthermore, cells that are initiating branching maintained contact with the lumen (arrows in Fig. 2, B–D). E-cadherin, which is a marker for the basolateral membrane, was localized to the basolateral surface of both control and DN-Pak1 cysts (Fig. 2, E–H). E-cadherin also localized to the basolateral surface of branching cells in DN-Pak1 cysts (Fig. 2H). To examine polarity of DN-Pak1 cysts in more detail, we analyzed the basolateral marker β-catenin and the tight junction marker ZO-1. In both control and DN-Pak1 cysts, β-catenin was localized to the basolateral surface and ZO-1 was localized to the tight junctions near the apical surface (Fig. 2, I and J). The localization of β-catenin and ZO-1 also did not appear to be disrupted in DN-Pak1 cells that were initiating branching (Fig. 2, K and L). Together, these results indicated that apical-basal polarity was maintained during DN-Pak1-induced branching.
Fig. 2.
DN-Pak1 does not induce loss of apical-basal polarity or junctional integrity. A: control cyst showing podocalyxin (green) is localized to the apical surface (arrowhead). B: DN-Pak1 (−Dox) cyst showing localization of podocalyxin to the apical surface (arrowhead). Arrow shows that cells initiating branching maintain contact with the lumen. Inset in B shows a branch in a DN-Pak1 cyst that forms a tubule and contains a lumen (arrow), which is continuous with the cyst lumen. C and D: podocalyxin staining (green) in two additional examples of DN-Pak1 cysts. Arrows show that cells that initiate branching maintain contact with the lumen. E: low magnification of control cyst stained for E-cadherin (green). F: high magnification of cross section of control cyst wall showing E-cadherin (green) is localized to the basal membrane (double arrowhead) and lateral membrane (arrow). Podocalyxin is shown in red (arrowhead) at the apical surface. G: low magnification of DN-Pak1 cyst (partially through the cyst lumen) stained for E-cadherin (green). H: high magnification of DN-Pak1 cyst and cells initiating branching with E-cadherin (green) at the basal (double arrowhead) and lateral (arrow) membranes, and podocalyxin (red) at the apical membrane (arrowhead). Scale bars are 20 μm in E and G, and 5 μm in F and H. I: control cyst showing localization of ZO-1 (green) to the tight junctions near the apical surface (arrowhead), and β-catenin (red) to the lateral membrane (arrow). J–L: localization of β-catenin (red) and ZO-1 (green) to the basal membrane (arrow) and tight junctions (arrowhead) in DN-Pak1 nonbranching (J) and branching cells (K and L). β-Catenin and ZO-1 colocalization is shown in yellow. Scale bars are 5 μm. M and N: laminin (green) is deposited at the basal surface (arrows) of both control (M) and DN-Pak1 (N) cysts. Insets show projection images of serial confocal sections of the basal surface of cysts. Scale bars are 20 μm. O and P: F-actin staining (green) strongly labels the apical microvilli in control and DN-Pak1 cysts (arrows). Q: staining for F-actin in a DN-Pak1 cyst extension showing F-actin-rich stress fibers (arrowhead). Scale bar is 10 μm. Nuclei in A–Q were stained with DAPI in blue.
MDCK cells secrete laminin from their basolateral surface (4) and abnormal laminin deposition in response to inhibition of β1-integrins or Rac1 results in defects in cyst morphogenesis (64). To examine laminin deposition in DN-Pak1 cysts, we performed immunofluorescence confocal microscopy using polyclonal antibodies raised against laminin-111. This antibody recognizes the β1- and γ1-laminin chains and most likely stains laminin-511 in MDCK cells (32, 64). Laminin was detected at the basal cell surface of both control and DN-Pak1 branching cysts, and no obvious difference in laminin organization was observed (Fig. 2, M and N). This suggests that the formation of branches in the DN-Pak1 cysts does not result from abnormal laminin deposition. We also examined proliferation and apoptosis by staining cysts for Ki67 and cleaved caspase-3, respectively. Analysis by immunofluorescence confocal microscopy showed no significant difference in the percentage of Ki67-positive cells or cells that stained for cleaved caspase-3 between control cysts and DN-Pak1 cysts at 10 days in culture (data not shown).
Finally, we analyzed the organization of the actin cytoskeleton in DN-Pak1-induced branches by staining for F-actin with phalloidin. Strong apical staining, representing apical microvilli, was seen at the lumenal surface in both control and DN-Pak1 cysts (Fig. 2, Q and P). We also found strong labeling of F-actin in bundles within extensions of DN-Pak1 cysts (Fig. 2Q). This suggests that stress fibers form in these extensions. Stress fibers are bundles of F-actin and myosin II filaments and are characteristic of cells under increased contractility.
DN-Pak1 increases cellular contractility in 3D MDCK cell culture.
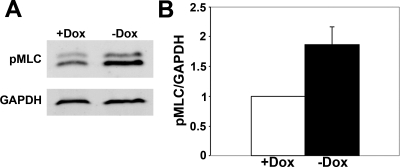
Actomyosin dynamics play an important role in branching morphogenesis, and the initial stages of branching morphogenesis of MDCK cysts are dependent on actin polymerization (65). Actin dynamics are regulated by myosin II, which bundles and moves actin filaments and generates tension forces that are essential in cell motility and contractility (8). Nonmuscle myosin II activity is regulated by phosophorylation of the regulatory MLC (53). We hypothesized that DN-Pak1 cells have increased contractility in 3D culture based on the following observations. First, in contrast to control gels, collagen gels containing DN-Pak1 cysts were contracted (had reduced gel diameter) and detached from cell culture inserts by 12 days in culture (data not shown). Second, the branching morphology of the DN-Pak1 mutant cysts suggested that these cysts had major changes in actomyosin dynamics and were under increased tension. Third, DN-Pak1 cysts form cellular extensions with F-actin-rich stress fibers as shown above (Fig. 2Q). Finally, Pak has previously been implicated in the regulation of myosin II activity, although the exact way in which Pak affects myosin II activity is not clear (6, 47, 54, 70). To examine DN-Pak1 cysts for further molecular evidence of increased contractility, we analyzed the levels of phosphorylated MLC (pMLC). The formation of stress fibers results from increased myosin II activity and increased levels of pMLC, which is used as a standard readout of myosin II activity. Western blot analysis showed that diphosphorylated pMLC (Ser18,Thr19) was increased in DN-Pak1 cysts compared with controls (Fig. 3, A and B). These results indicated that the DN-Pak1 cysts had increased myosin II activity and increased cellular contractility.
Fig. 3.
Expression of DN-Pak1 in cysts results in increased phosphorylated myosin light chain (pMLC) levels. A: Western blot showing levels of pMLC in control (+Dox) and DN-Pak1 (−Dox) cysts. This anti-pMLC antibody recognizes a doublet in MDCK cells (59), which probably represents multiple isoforms of pMLC. GAPDH is used as a loading control. B: quantitation of pMLC levels in control and DN-Pak1 cysts. pMLC levels were normalized to GAPDH. n = 4. Error bar represents SD. P < 0.05.
To directly examine whether DN-Pak1 expression also resulted in increased cellular contractility in 3D culture, we performed collagen gel contractility assays. Control and DN-Pak1 cells were resuspended in floating collagen I gels, photographed (0 h), and then incubated for 12 to 16 h. After the incubation, gels were photographed again to measure gel diameter. We found that collagen gels containing control cells were reduced in diameter by only 28% after 12 h, whereas gels containing DN-Pak1 cells were reduced in diameter by 45% (Fig. 4, A and B). By 16 h, gels containing control cells were reduced in diameter by 37%, and gels containing DN-Pak1 cells were reduced in diameter by 53%. Similar results were obtained with two different DN-Pak1 clones (data not shown). Together, our results show that DN-Pak1 greatly increased cellular contractility. The increased contractility was dependent on myosin II activity: treatment of cells with a specific myosin II inhibitor blebbistatin (55) significantly reduced contractility in control cells and completely blocked the increased contractility in DN-Pak1 cells compared with controls (Fig. 4, A and B). Blebbistatin treatment for 16 h did not inhibit expression of the DN-Pak1 protein (Fig. 4C).
Fig. 4.
DN-Pak1 cells have increased contractility in collagen gel. A: images of floating collagen I gels containing control and DN-Pak1 cells treated with DMSO (vehicle) or blebbistatin (50 μM) at 0, 12, and 16 h of incubation. Scale bar is 1 mm. B: measurements of gel diameters (in mm) for collagen gels at 0, 12, and 16 h. n = 3. Error bars represent SD. *P < 0.05. C: Western blot analysis for HA-tagged DN-PAK1 in control and DN-Pak1 expressing cells in collagen gels at 16 h. GAPDH was used as a loading control.
DN-Pak1 mutant protein requires binding to PIX to generate branches and increased contractility in 3D culture.
Our finding that Pak1 has a role in myosin II-dependent cell contractility is consistent with previous studies that have implicated Pak1 in the regulation of this process. Recently, Coniglio et al. (7) showed that the Pak isoforms Pak1 and Pak2 have distinct effects on MLC phosphorylation. This study also demonstrated that both Pak isoforms differentially affected the morphology and dynamic behavior of focal adhesions. Since the substrate specificity of both Pak isoforms is virtually identical (38), it is likely that specific inclusion of Paks into signaling complexes at focal adhesions is a determining factor in the regulation of MLC (7). We therefore investigated whether inclusion of Pak into signaling complexes at focal adhesions was required for the DN-Pak1 phenotype. Pak forms a complex with the Pak-interacting Rac guanine-nucleotide exchange factor PIX, which recruits Pak to focal adhesions (26). Furthermore, many studies have shown that the Pak-PIX complex has important roles in regulating cytoskeletal dynamics and focal adhesion turnover (reviewed in Ref. 67).
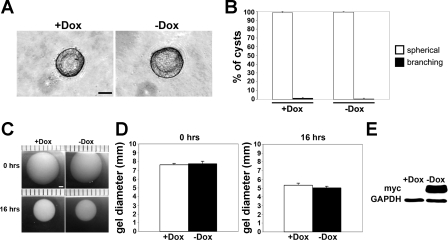
To investigate whether the DN-Pak1 protein requires binding to PIX to generate branches in 3D culture, we used an MDCK cell line that stably expressed a DN-Pak1 mutant protein that does not bind βPIX (68). Expression of the DN-Pak1 mutant protein with two point mutations that prevent PIX binding (Pak1-R193A,P194A,K299R) referred to as DN-Pak1-ΔPIX was regulated by the Tet-off system. We found that cysts expressing DN-Pak1-ΔPIX had a normal, spherical morphology (Fig. 5A), and this was confirmed for two different DN-Pak1-ΔPIX clones (data not shown). Ninety-nine percent of control (+Dox) cysts had a spherical morphology, and similarly 99% of DN-Pak1-ΔPIX cysts (−Dox) had a spherical morphology (Fig. 5B). These results show that the DN-Pak1 mutant protein requires binding to PIX to generate branches in 3D culture.
Fig. 5.
Cells expressing a DN-Pak1 protein that cannot bind PIX (DN-Pak1-ΔPIX) do not form branching cysts or have increased contractility in collagen. A: control (+Dox) and DN-Pak1-ΔPIX expressing (−Dox) cysts both have a normal spherical morphology. Scale bar is 30 μm. B: quantitation of control and DN-Pak1-ΔPIX cyst morphology. Data are represented as the mean of four independent experiments. Error bars represent SD. C: images of control and DN-Pak1-ΔPIX cysts at 0 and 16 h of incubation. Scale bar is 1 mm. D: measurement of gel diameters (in mm) at 0 and 16 h of incubation. n = 3. Error bars represent SD. E: Western blot analysis was used to confirm the expression of the myc-tagged DN-Pak1-ΔPIX protein in DN-Pak1-ΔPIX cells in collagen. GAPDH was used as a loading control.
We also determined whether DN-Pak1 binding to PIX was required for the increased contractility of DN-Pak1 cells in 3D culture. For this, we tested the DN-Pak1-ΔPIX cells in 3D collagen I contractility assays. At 16 h, there was no difference in the reduction in gel diameters between control and DN-Pak1-ΔPIX cells (Fig. 5, C and D). Expression of the myc-tagged DN-Pak1-ΔPIX mutant protein was confirmed by Western blot analysis (Fig. 5E). This result shows that the DN-Pak1 mutant protein requires interaction with βPIX to generate increased cellular contractility in 3D collagen gel culture.
DN-Pak1-induced branching and cellular contractility depends on the ECM composition and requires β1-integrin signaling.
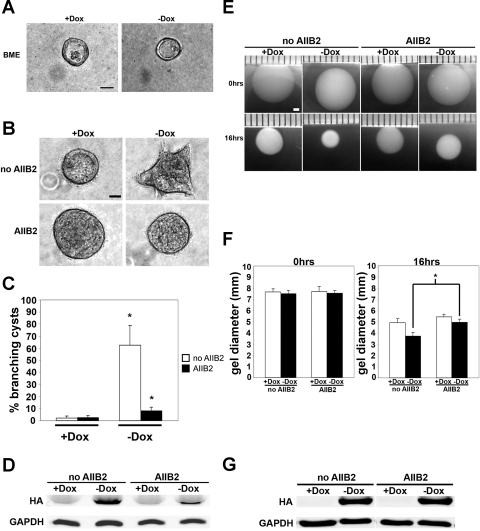
Whereas HGF induces the formation of branching tubular structures in MDCK cells cultured in collagen type I gels, these cells fail to form these structures upon HGF treatment when cultured in a BME (50). This indicates that specific cell-ECM interactions are important for regulating branching morphogenesis. To determine whether DN-Pak1 cysts form branches in other ECM besides collagen I, we plated DN-Pak1 cells in a growth factor-reduced BME and examined cyst morphology. We found that BME completely blocked the ability of DN-Pak1 cysts to form branches as analyzed by phase-contrast microscopy (Fig. 6A). This result shows that the composition of the extracellular matrix plays an important role in the DN-Pak1 branching phenotype. In other words, the branching of DN-Pak1 cysts is not cell-autonomous but requires specific cell-ECM signaling.
Fig. 6.
DN-PAK1 mutant protein requires β1-integrin signaling to generate branches and increase cellular contractility in 3D culture. A: control and DN-Pak1 cysts in BME. Scale bar is 30 μm. B: control and DN-Pak1 control cysts (no AIIB2) or cysts treated with AIIB2 from days 5 to 8 in culture. Scale bar is 30 μm. C: quantitation of branching cysts in control and DN-Pak1 no AIIB2-treated and AIIB2-treated cysts. n = 3. Error bars represent SD. *P < 0.05. D: Western blot for the HA-tagged DN-Pak1 protein in control (no AIIB2) and AIIB2-treated cysts. GAPDH was used as a loading control. E: images of collagen gels containing control and DN-Pak1 cells no AIIB2-treated or AIIB2-treated for 0 and 16 h of incubation. Scale bar is 1 mm. F: measurement of gel diameters (in mm) at 0 and 16 h. n = 3. Error bars represent SD. *P < 0.05. G: Western blot for HA-tagged DN-Pak1 protein in control and DN-Pak1 cells in collagen gels at 16 h incubation. GAPDH was used as a loading control.
To further investigate the role of cell-ECM signaling, we examined the role of integrins in the DN-Pak1 phenotype. When engaged in ECM adhesion, integrins cluster in focal contacts. As mentioned above, the Pak-PIX complex plays important roles in the stabilization and turnover of these structures. Most integrins in MDCK cells comprise β1-integrin-containing heterodimers, which are receptors for collagen I (27). To determine whether β1-integrin signaling has a role in the formation of branches in DN-Pak1 cysts, we treated these cysts with the β1-integrin function-blocking antibody AIIB2 (56) and analyzed the effect on extension formation. It was previously shown that treatment of MDCK cells in collagen with the AIIB2 antibody resulted in inverted polarity of cysts only when cells were treated during the first 3 days in culture, and that cells become refractory to the polarity-reversing effect of AIIB2 after this time window (64, 66). Since DN-Pak1 cysts begin to form branches at day 5 in culture (not shown), we treated cysts with AIIB2 from day 5 to day 8, after polarity was established, to determine whether β1-integrin is required for branch formation of DN-Pak1 cysts. Under these conditions, inhibition of β1-integrins resulted in a significant block in branch formation (Fig. 6, B and C). Specifically, we found that 63% of DN-Pak1 cysts had branches when cultured without AIIB2, which decreased to 8% in AIIB2-treated cysts (Fig. 6C). Interestingly, DN-Pak1 expression was somewhat decreased in DN-Pak1 cysts after 3 days of AIIB2 treatment (Fig. 6D). This result may indicate that β1-integrin functions to stabilize the DN-Pak1 protein in 3D culture. It is possible that β1-integrin is required for the establishment and/or maintenance of focal contacts in 3D culture, so in the presence of AIIB2, focal contacts are lost and the DN-Pak1 protein is destabilized.
To examine whether β1-integrin signaling was also essential for the increased cellular contractility seen in DN-Pak1 cells in 3D, we treated these cells with the AIIB2 antibody in collagen gel contractility assays. Control cells and DN-Pak1 mutant cells were resuspended in collagen gels and incubated for 16 h as before and plated in the presence or absence of the AIIB2 antibody. Inhibition of β1-integrin signaling resulted in a reduction of contractility in both the control and DN-Pak1 cells at 16 h and blocked the increased contractility of DN-Pak1 cells compared with controls (Fig. 6, E and F). Thus β1-integrin signaling is essential for the increased contractility of the DN-Pak1 cells in collagen. We found that treatment of cells with the AIIB2 antibody during this assay did not inhibit expression of the DN-Pak1 protein (Fig. 6G). It is therefore unlikely that the highly reduced branching we observed in the AIIB2-treated cysts is due to reduced expression levels of DN-Pak1 under these conditions.
DISCUSSION
We show here that expression of DN-Pak1 induces branching tubulogenesis from MDCK cysts grown in collagen I. HGF-induced tubulogenesis from MDCK cysts is a widely used model system to study the basic epithelial morphogenic mechanisms that control the formation of branching tubules (42, 46, 69). In the classic four-stage model, tubulogenesis proceeds over several days in four distinct stages, named extensions, chains, cords, and tubules (35). In the extension stage, some cells form a basal extension but retain an apical surface that is continuous with the cyst wall. In contrast, apical polarity is lost upon formation of chains and cords, when chains of single cells or cords of 2–3 cells in diameter extend from the cyst body into the collagen, while maintaining cell-cell contact via adherens and tight junctions. Next, cells within cords will form apical surfaces surrounding small nascent lumens, which then merge and eventually become continuous with the central lumen. Another quite different mechanism for HGF-induced tubulogenesis in MDCK was reported by Williams and Clark (62). In this model, HGF also induces basal extensions. However, this is followed by two of more neighboring cells moving into the collagen, which go on to form a tubule by epithelial sheet deformation, where a continuous sheet of polarized cells evaginates into the collagen to form a tubule in which all individual cells remain polarized and retain contact with the central lumen at all times. Consistent with earlier findings (3, 50), tubulogenesis by this mechanism is also inhibited by BME, suggesting the involvement of specific cell-matrix interactions. Furthermore, as this type of tubulogenesis is accompanied by accumulation of actin fibers in basal extensions and lacks an obvious involvement of increased cell proliferation, it was concluded that increased migration is the main underlying mechanism of tubulogenesis (62).
Whereas in our hands MDCK cells behave according to the classic four-stage model upon addition of HGF [(65) and data not shown], tubulogenesis induced by DN-Pak1 was similar to the mechanism as described by Williams and Clark. Thus cells never lost apical-basolateral polarization and maintained tight junctions at all times. Extensions were also rich in actin fibers, and no major changes in proliferation or apoptosis were observed. In this respect, this type of tubulogenesis more closely resembles branching of the ureteric bud, which also branches with a continuous lumen and is not accompanied by a loss of cellular polarization (29). In the MDCK-HGF model, HGF can induce many different signaling pathways, which collectively drive branching tubulogenesis. Our results indicate that Pak1 plays a role in some, but not all, of these pathways.
We previously characterized DN-Pak1-expressing MDCK cells in two-dimensional culture and found that DN-Pak1 mutants sequestered βPIX in large focal contacts (68). Similar results were obtained in many other cell types, in which inhibition of Pak results in an increased number of large focal contacts (23, 43, 75). In MDCK cells, DN-Pak1 also increases cell migration and abolishes contact inhibition by a process that does not affect cell-cell junctions (24, 68). Instead, the phenotype is due to dysregulation of Pak-PIX-dependent focal adhesion turnover, which results in increased cell-matrix-dependent PI3K signaling (24). These findings likely are significant for the role of Pak1 in tubulogenesis we show here. We previously reported that early stages of tubulogenesis are similar to the mechanisms that drive cell migration. Specifically, both processes rely on PI3K signaling and actin remodeling but do not depend on cell proliferation, which is only required at later stages (65). PI3K is activated in MDCK cysts in response to HGF (3) and lipid products of PI3K specifically accumulate at tips of extensions in early HGF-induced tubulogenesis (65). PI3K activity is also required for HGF-induced tubulogenesis in MDCK and other renal epithelial cells (11, 65) and sufficient to induce tubulogenesis in the absence of HGF (22). It is therefore possible that increased PI3K signaling at focal adhesions in DN-Pak1-expressing cells is at least part of the mechanism that underlies the branching phenotype in these cells. DN-Pak1-induced tubulogenesis also relies on β1-integrins and on the interaction of DN-Pak1 with PIX, which recruits Pak1 to focal adhesions. Taken together, these observations suggest that Pak1 is involved in the early steps of tubulogenesis; in particular those that rely on cell migration. Specifically, we propose that expression of DN-Pak1 in 3D culture results in stabilized focal contacts, which depends on an interaction of DN-Pak1 with βPIX, and which promotes increased cell motility and a branching phenotype.
Although the DN-Pak1 cysts have a phenotype that resembles HGF-induced branching morphogenesis, these cysts do not form the complex branching tubular networks, which are generated after HGF treatment for several days. The DN-Pak1 cysts can spontaneously initiate branching morphogenesis and form extensions and mostly relatively short tubules. This could be due to a disruption in focal contact turnover. Branching morphogenesis in 3D culture requires proper assembly and disassembly of focal contacts. Two proteins that play key roles in focal contact dynamics, besides the Pak-PIX complex, include the docking protein paxillin and focal adhesion kinase (FAK). Paxillin phosphorylation results in formation of a complex between paxillin and FAK, which regulates focal contact turnover. Expression of a paxillin mutant in renal epithelial cells in 3D culture results in a failure of paxillin to bind to FAK and disassemble focal contacts (18). Cysts expressing this paxillin mutant form relatively short basal processes and not a branching tubular network as in control cells expressing wild-type paxillin. FAK also has an important role in regulating HGF-induced branching morphogenesis of MDCK cells. Overexpression of wild-type FAK in MDCK cells in collagen increased branching morphogenesis by making cells more sensitive to the effects of HGF, whereas cells that expressed dominant negative FAK-related nonkinase do not survive (61). These results show that focal contact dynamics have crucial roles in regulating branching morphogenesis.
Interestingly, inhibition of protein kinase C (PKC) by staurosporine or downregulation of PKC by long-term treatment with phorbol 12-myristate 13-acetate (PMA) induces a branching phenotype similar to DN-Pak1-induced branching (48). Serine/threonine kinases other then PKC may also inhibit branching (48). Since PKC has been implicated in the turnover of focal adhesions via a mechanism that involves Rac-dependent phosphorylation of paxillin (31), it is possible that inhibition of PKC induces branching by a mechanism that depends on regulation of cell-matrix adhesion. Consistent with this are reports that staurosporine affects focal adhesion turnover (21) and stimulates endothelial tubulogenesis in 3D culture by a mechanism that depends on FAK (57). It is possible that these processes may involve downstream regulation via the Pak-PIX complex. Alternatively, direct or indirect effects on Pak kinases cannot be excluded since staurosporine is a broad-spectrum kinase inhibitor (44).
Inhibition of β1-integrin with the function-blocking antibody AIIB2 inhibits DN-Pak1-induced branching. As Pak is activated in response to integrin-mediated adhesion (17, 36, 76), this suggests that DN-Pak1 acts via a β1-integrin-dependent signaling pathway. Inhibition of β1-integrin with AIIB2 or by small interfering RNA (siRNA)-mediated knockdown, or inhibition of Rac with DN-Rac results in inverted polarity via abrogation of Rac-mediated downregulation of Rho (32, 64, 66). However, β1-integrin is not required for maintenance of polarity, as cysts are refractory to polarity-reversing effects of the AIIB2 antibody after 3 days in culture. Even though Pak1 is downstream of β1-integrin and Rac1, the cyst phenotypes are very different between DN-Rac1 cysts and DN-Pak1 cysts. Instead of reverting cell polarity, expression of DN-Pak1 resulted in cysts with normal apical-basal polarity, which branch spontaneously. We did not find activation of Rho in DN-Pak1 cells in 3D culture (data not shown), which suggests that Pak1 is not involved in regulating the orientation of polarity downstream of β1-integrin and Rac. Rather, Pak1 may regulate other pathways downstream of β1-integrin that are involved in cell migration and tubulogenesis. Two independent studies recently analyzed the role of β1-integrin in kidney development. For this, β1-integrin was conditionally deleted in the ureteric bud by crossing HoxB7Cre mice with integrin β1flox/flox mice (63, 73). Both studies reported that conditional knockdown of β1-integrin resulted in kidneys with reduced nephron number and dilation of tubules, suggestive of defects in branching morphogenesis. In contrast, no effects of cell polarization were observed upon knockdown of β1-integrin in vivo (63, 73). Our finding that DN-Pak1-induced branching in MDCK cysts depends on β1-integrin is therefore consistent with the roles of this integrin in branching morphogenesis in the kidney in vivo. The fact that cell polarization crucially depends on the presence of β1-integrin in MDCK but not in vivo indicates that the β1-integrin-dependent signaling pathways that control polarity or tubulogenesis, respectively, in MDCK cysts are mechanistically distinct.
Finally, we show that expression of DN-Pak1 increased contractility in a βPIX- and β1-integrin-dependent manner. It was previously shown that diphosphorylated pMLC is involved in the spatial organization of stress fiber contraction and assembly in MDCK cells (59). In addition, pMLC localized to the basal membrane in cysts and accumulated at the tips of cellular extensions during the early stages of HGF-induced tubulogenesis (65). Similarly, pMLC accumulated at the leading edge of lamellipodia that initiate tubulogenesis induced by collagen overlay (12). This suggests that pMLC has an important role in regulating actin filament dynamics at the basal membrane during branching morphogenesis. Pak1 has an essential role in human fibroblast-mediated contraction of floating collagen matrices, although in this context, contractility was dependent on cell ruffling activity rather than protrusion and retraction of cellular extensions. (39). Activation of Pak also promoted dendritic spine formation in neurons by activating MLC and myosin II in a PIX-dependent manner (72). Since we find that DN-Pak1 induces increased contractility in 3D MDCK cell culture in a PIX-dependent manner, our results strongly suggest that the Pak-PIX complex regulates myosin II activity of MDCK cells in collagen matrices. Stabilized focal contacts, as induced by DN-Pak1, could initiate a signaling process at the basal membrane that leads to extension formation and increased motility and probably involves actin polymerization and increased myosin II activity to stabilize actin filaments. Indeed, we previously showed an increased association of DN-Pak1 with the cytoskeleton compared with wild-type-Pak1 (68), and we show here that a continuous presence of DN-Pak1 is required to maintain the branches. Others showed evidence indicating that myosin activity is required for ureteric bud branching (9). An additional or alternative mechanism by which activation of myosin II may induce tubulogenesis, is by promoting “purse-string” constriction of the apical cytoskeleton, which forces cells into a triangular shape, thereby promoting evagination (29). Such mechanism may involve ezrin, which links the actin cytoskeleton to the plasma membrane, and which has been indicated in both ureteric bud branching (29) and HGF-induced branching in MDCK cells (37).
In conclusion, our results suggest that Pak1 plays an important role in regulating the dynamics of the cytoskeleton and focal contacts during renal epithelial morphogenesis in 3D MDCK cell culture and does so through βPIX and β1-integrin-dependent mechanisms. Our hypothesis is that the DN-Pak1 protein, in a complex with βPIX, is trapped at focal contacts in 3D culture, which is similar to the effect of this mutant in 2D culture (24, 68). The trapping of DN-Pak1 likely stabilizes focal adhesions in 3D culture, and this is dependent on cell-ECM interactions through β1-integrin. The DN-Pak1-PIX stabilized focal adhesions may result in an increase in PI3K signaling, which could initiate branching, and can also result in stress fiber formation and increased myosin II activity and cellular contractility. Collectively, this results in the formation of stable extensions from the basal surface of DN-Pak1 cysts. Inhibiting the DN-Pak1-PIX interaction (DN-Pak1-ΔPIX) or β1-integrin signaling (AIIB2 antibody) results in a failure to localize DN-Pak1 to the membrane and form stabilized focal adhesions, which are required for spontaneous extension formation. Therefore, these results suggest that Pak1 may regulate cytoskeleton and focal contact dynamics during renal epithelial morphogenesis.
GRANTS
M. M. Zegers was supported by funds of the Concern Foundation and the National Institutes of Health (R01 GM-076363).
ACKNOWLEDGMENTS
We thank Nisha Sipes and Yi Zheng for advice on contractility assays. We thank Martin ter Beest for advice and critical reading of the manuscript.
REFERENCES
- 1.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cell Signal 21: 1738–1747, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Cantley LG, Barros EJ, Gandhi M, Rauchman M, Nigam SK. Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 267: F271–F280, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. Dependence on pH of polarized sorting of secreted proteins. Nature 329: 632–635, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Cheng HY, Lin YY, Yu CY, Chen JY, Shen KF, Lin WL, Liao HK, Chen YJ, Liu CH, Pang VF, Jou TS. Molecular identification of canine podocalyxin-like protein 1 as a renal tubulogenic regulator. J Am Soc Nephrol 16: 1612–1622, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 19: 839–854, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol 28: 4162–4172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci 121: 11–18, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Davies J. Intracellular and extracellular regulation of ureteric bud morphogenesis. J Anat 198: 257–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J 19: 2008–2014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derman M, Cunha M, Barros E, Nigam S, Cantley L. HGF-mediated chemotaxis and tubulogenesis require activation of the phosphatidylinositol 3-kinase. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1211–F1217, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Eisen R, Ratcliffe DR, Ojakian GK. Modulation of epithelial tubule formation by Rho kinase. Am J Physiol Cell Physiol 286: C857–C866, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Eisen R, Walid S, Ratcliffe DR, Ojakian GK. Regulation of epithelial tubule formation by Rho family GTPases. Am J Physiol Cell Physiol 290: C1297–C1309, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89: 5547–5551, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall A. Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol 10: 1356–1364, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Howe AK. Cell adhesion regulates the interaction between Nck and p21-activated kinase. J Biol Chem 276: 14541–14544, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell 16: 257–267, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell 12: 1275–1285, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jiang ST, Chiu SJ, Chen HC, Chuang WJ, Tang MJ. Role of alpha(3)beta(1) integrin in tubulogenesis of Madin-Darby canine kidney cells. Kidney Int 59: 1770–1778, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Kabir J, Lobo M, Zachary I. Staurosporine induces endothelial cell apoptosis via focal adhesion kinase dephosphorylation and focal adhesion disassembly independent of focal adhesion kinase proteolysis. Biochem J 367: 145–155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khwaja A, Lehmann K, Marte BM, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem 273: 18793–18801, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol 147: 831–844, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Jia L, Thompson-Baine A, Puglise JM, ter Beest MB, Zegers MM. Cadherins and Pak1 control contact inhibition of proliferation by Pak1-βPIX-complex dependent regulation of cell-matrix signaling. Mol Cell Biol. 30: 1971–1983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manser E, Huang H, Loo T, Chen X, Dong J, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol 17: 1129–1143, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Matlin KS, Haus B, Zuk A. Integrins in epithelial cell polarity: using antibodies to analyze adhesive function and morphogenesis. Methods 30: 235–246, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Meder D, Shevchenko A, Simons K, Fullekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol 168: 303–313, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol 275: 44–67, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67: 901–908, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Nomura N, Nomura M, Mizuki N, Hamada J. Rac1 mediates phorbol 12-myristate 13-acetate-induced migration of glioblastoma cells via paxillin. Oncol Rep 20: 705–711, 2008 [PubMed] [Google Scholar]
- 32.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838, 2001 [DOI] [PubMed] [Google Scholar]
- 33.O'Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell 7: 21–32, 2004 [DOI] [PubMed] [Google Scholar]
- 34.O'Brien LE, Yu W, Tang K, Jou TS, Zegers MM, Mostov KE. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol 406: 676–691, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol 204: 64–79, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Price L, Leng J, Schwartz M, Bokoch G. Activation of rac and cdc42 by integrins mediates cell spreading. Mol Biol Cell 9: 1863–1871, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell 14: 2181–2191, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem 282: 15667–15678, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol 172: 423–432, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley A, Comoglio P, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol 15: 1110–1122, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers KK, Jou TS, Guo W, Lipschutz JH. The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int 63: 1632–1644, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol 13: 328–335, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell 11: 1709–1725, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 10: 218–220, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Saelman EU, Keely PJ, Santoro SA. Loss of MDCK cell alpha 2 beta 1 integrin expression results in reduced cyst formation, failure of hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J Cell Sci 108: 3531–3540, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Sakurai H, Nigam SK. In vitro branching tubulogenesis: implications for developmental and cystic disorders, nephron number, renal repair, and nephron engineering. Kidney Int 54: 14–26, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283: 2083–2085, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Santos O, Moura L, Rosen E, Nigam S. Modulation of HGF-induced tubulogenesis and branching by multiple phosphorylation mechanisms. Dev Biol 159: 535–548, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Santos OF, Barros EJ, Yang XM, Matsumoto K, Nakamura T, Park M, Nigam SK. Involvement of hepatocyte growth factor in kidney development. Dev Biol 163: 525–529, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol 160: 293–302, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol 145: 837–849, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development 131: 1449–1462, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem 279: 46621–46630, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299: 1743–1747, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem 268: 17597–17601, 1993 [PubMed] [Google Scholar]
- 57.Tarzami ST, Hsieh SS, Esterman MA, Singh JP. Staurosporine promotes endothelial cell assembly and FAK phosphorylation during in vitro angiogenesis. J Cardiovasc Pharmacol 45: 22–29, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol 17: 58–64, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Watanabe T, Hosoya H, Yonemura S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol Biol Cell 18: 605–616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb BA, Eves R, Crawley SW, Zhou S, Cote GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol 289: C898–C907, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Wei WC, Kopec AK, Tang MJ. Requirement of focal adhesion kinase in branching tubulogenesis. J Biomed Sci 16: 5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams MJ, Clark P. Microscopic analysis of the cellular events during scatter factor/hepatocyte growth factor-induced epithelial tubulogenesis. J Anat 203: 483–503, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu W, Kitamura S, Truong DM, Rieg T, Vallon V, Sakurai H, Bush KT, Vera DR, Ross RS, Nigam SK. Beta1-integrin is required for kidney collecting duct morphogenesis and maintenance of renal function. Am J Physiol Renal Physiol 297: F210–F217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. β1-Integrin Orients Epithelial Polarity via Rac1 and Laminin. Mol Biol Cell 16: 433–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu W, O'Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell 14: 748–763, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep 9: 923–929, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zegers M. Roles of p21-activated kinases and associated proteins in epithelial wound healing. Int Rev Cell Mol Biol 267: 253–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zegers MM, Forget MA, Chernoff J, Mostov KE, ter Beest MB, Hansen SH. Pak1 and PIX regulate contact inhibition during epithelial wound healing. EMBO J 22: 4155–4165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 13: 169–176, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Côté G, Wysolmerski R. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci 113: 471–482, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol 238: 289–302, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci 25: 3379–3388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fassler R, Yurchenco P, Pozzi A, Zent R. beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136: 3357–3366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao ZS, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J 386: 201–214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol 20: 6354–6363, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem 280: 10624–10635, 2005. [DOI] [PubMed] [Google Scholar]