Abstract
Human ether-à-go-go-related gene (HERG) potassium channels play an important role in cardiac action potential repolarization, and HERG dysfunction can cause cardiac arrhythmias. However, recent evidence suggests a role for HERG in the proliferation and progression of multiple types of cancers, making it an attractive target for cancer therapy. Ceramide is an important second messenger of the sphingolipid family, which due to its proapoptotic properties has shown promising results in animal models as an anticancer agent. Yet the acute effects of ceramide on HERG potassium channels are not known. In the present study we examined the effects of cell-permeable C6-ceramide on HERG potassium channels stably expressed in HEK-293 cells. C6-ceramide (10 μM) reversibly inhibited HERG channel current (IHERG) by 36 ± 5%. Kinetically, ceramide induced a significant hyperpolarizing shift in the current-voltage relationship (ΔV1/2 = −8 ± 0.5 mV) and increased the deactivation rate (43 ± 3% for τfast and 51 ± 3% for τslow). Mechanistically, ceramide recruited HERG channels within caveolin-enriched lipid rafts. Cholesterol depletion and repletion experiments and mathematical modeling studies confirmed that inhibition and gating effects are mediated by separate mechanisms. The ceramide-induced hyperpolarizing gating shift (raft mediated) could offset the impact of inhibition (raft independent) during cardiac action potential repolarization, so together they may nullify any negative impact on cardiac rhythm. Our results provide new insights into the effects of C6-ceramide on HERG channels and suggest that C6-ceramide can be a promising therapeutic for cancers that overexpress HERG.
Keywords: human ether-à-go-go-related gene, activation kinetics, deactivation kinetics, cholesterol, inhibition
human ether-à-go-go-related gene (HERG) potassium channels play a crucial role as delayed rectifier channels controlling the rhythmic excitation of cardiac and endocrine cells (46, 53). The unique kinetics of HERG channel opening (activation), closing (deactivation), and inactivation implicates HERG as a critical ion channel in cardiac action potential (cAP) repolarization (14). HERG channels activate more slowly than many other potassium channels but inactivate very rapidly from the open state. This combination produces very small outward potassium currents during the plateau phase (phase 2) of the cAP (45), which helps to keep the cAP from repolarizing too soon. However, these channels also recover very rapidly from inactivation, but close relatively slowly, so that HERG channel current (IHERG) dominates during repolarization (phase III) of the cAP. Disruption of HERG potassium channel activity can lead to altered cardiac rhythm (arrhythmia) that may precipitate to fatal torsades de pointes (45).
Apart from its importance in cardiac repolarization, HERG is increasingly being recognized for its role in cancer. HERG is upregulated in many types of cancers, and HERG blockers have been shown to reduce tumor invasiveness and proliferation (2, 32, 35, 55). In nonexcitable cells, HERG channels show a low level of steady-state activity at resting membrane potential called a “window current.” This steady-state window current is thought to be important for the progression of cancers that overexpress HERG (6). Therefore, HERG blockers have been proposed as an adjuvant cancer therapy (6, 27, 35). The HERG blocker erythromycin has been shown to selectively suppress proliferation of the HERG-expressing human colon adenocarcenoma cell line HT-29 and showed synergistic effects with the anticancer agents vincristin and paclitaxel (17). Thus, HERG is increasingly recognized as a target for anticancer drugs (2, 27, 37). However, the block of cardiac HERG channels can result in cardiac arrhythmias (45), which complicates the use of such blockers in cancer therapy. Therefore, when studying drugs that affect HERG in other systems, such as cancer cells, it is important to consider their effect on the cardiac function.
Ceramide is a sphingolipid that is formed in the cell membrane from sphingomyelin as a result of various cellular stresses, including reactive oxygen species, radiation, ischemia reperfusion injury, or treatment with chemotherapeutic or proarrhythmic agents (23). Because of its proapoptotic properties, ceramide is under investigation for the treatment of multiple types of cancers (50, 51). Ceramide, apart from its biochemical role as an intracellular signaling molecule, also has biophysical effects through the formation/stabilization of lipid rafts affecting the membrane localization of ion channels and their function (9, 22, 25). Lipid rafts are membrane microdomains that are enriched with sphingolipids and cholesterol and are increasingly recognized to be important in the regulation of ion channel function (1, 58). Many ion channels such as Kv1.5 (31), shaker potassium channels (29), pacemaker channels (5), and cardiac sodium channels (60) are regulated by localization within lipid rafts, which in turn affect the gating kinetics of these ion channels. HERG channels have also been shown to localize to lipid rafts, which may have a significant impact on gating (4). In this study, we investigate the acute effects of cell-permeable C6-ceramide on HERG current (IHERG) and demonstrate that the ceramide effects on voltage-dependent gating parameters are mediated by translocation of HERG channels into lipid rafts, whereas ceramide-induced inhibition of IHERG is independent of localization into lipid rafts.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney (HEK)-293 cells stably expressing HERG (a gift from Dr. Eckhard Ficker; MetroHealth Medical Center, Cleveland, OH) were maintained in DMEM + Glutamax media (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 1× antibiotic-antimycotic, and the selection agent G418. For electrophysiology, cells were harvested using 0.25% trypsin-EDTA (Invitrogen) and plated into 35-mm culture dishes that served as the recording chamber (12). IHERG comprised >90% of the potassium current in these HEK-293 cells (21).
Electrophysiological recording.
Membrane currents were recorded in whole cell patch clamp mode at room temperature (∼23°C). Electrodes were pulled from Corning 8250 glass (ID 0.90 mm, OD 1.50 mm; Garner Glass, Claremont, CA) using a Flaming/Brown P-97 pipette puller (Sutter Instruments, Novato, CA). Electrodes typically had a resistance of 1–3 MΩ. Series resistance was compensated at ≥80%. Currents were amplified and filtered using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA) and digitized with an ITC-18 (Instrutech, Port Washington, NY) after analog filtering with the amplifier's four-pole low-pass Bessel filter.
Measurements of activation, inactivation, and recovery from inactivation.
The activation time course was determined using an envelope tail protocol where HERG channels were activated by steps to +60 mV for durations ranging from 60 to 1,800 ms, and the current was measured following repolarization to −100 mV. The peak tail currents were plotted against the step durations and fit with single exponential equation to obtain the activation τ. The HERG activation-voltage relationship was measured from peak tail currents (−40 mV) following 4-s voltage steps ranging from −60 to +40 mV (20-mV increments). Peak tail currents were plotted against step voltage and fit using a single Boltzmann equation to determine the half-activation voltage (V1/2) (21). The threshold for current activation was −20 mV, and maximum activation was achieved at +20 mV.
The development of inactivation was measured using a standard HERG inactivation protocol where voltage was stepped to +60 mV for 500 ms to inactivate channels, hyperpolarized to −100 mV for 5 ms to recover channels from inactivation, and returned to voltages ranging from −20 to +60 mV, which was where the inactivation τ was determined by fitting the current, using a single exponential equation (61). Reactivation kinetics were determined by fitting the initial rising phase of tail current with a single exponential equation at voltages ranging from +20 to −100 mV following a voltage step to +60 mV (44, 48, 63).
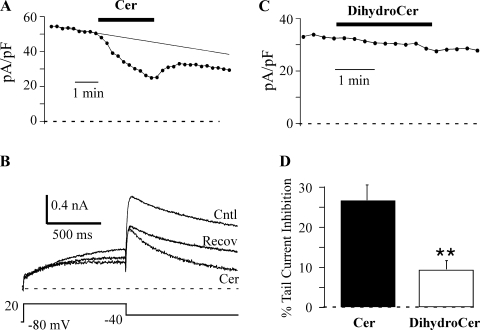
For examining the time course of ceramide effects, cells were pulsed to +20 mV (1-s duration) once every 20 s, and currents were measured from peak tail currents upon repolarization to −40 mV (the step time course protocol). Running this protocol under control conditions revealed a slow time-dependent loss of IHERG in most cells (rundown) (21), which resulted in an apparent incomplete recovery of current amplitude upon washout of ceramide. One method of addressing this problem was to set a constant duration for lipid application of 2.5 min for the time course measurements, which helped to ensure that clear recovery from inhibition could be observed. However, if uncompensated, rundown would still contribute to an overestimate of ceramide-induced inhibition. Since rundown cannot be independently measured during ceramide-induced inhibition, we estimated the effect of rundown using a linear regression fit to the control data point prior to the application of ceramide (Fig. 1). This method also allowed us to calculate the extent of recovery of IHERG during ceramide washout, which was typically incomplete.
Fig. 1.
Ceramide (Cer) inhibits human ether-à-go-go-related gene (HERG) current. A: HERG channel current (IHERG) was elicited once every 20 s by a 1-s depolarizing step to +20 mV. Peak tail current was measured 50 ms after repolarization to −40 mV and plotted against time. IHERG was allowed to stabilize in the control (Cntl) solution before a switch of the solution to 10 μM Cer for 2.5 min was made. The smooth line is a linear regression fit to the control data that was used to estimate control current amplitude at the time points used to measure Cer-induced inhibition. B: currents from the same cell as in A are shown before, during, and after recovery (Recov) from Cer treatment. Note the faster deactivation during the Cer treatment. C: the inactive analog dihydro-C6-ceramide (DihydroCer; 10 μM) fails to affect HERG current. This time course is from a different cell than that used in A. D: inhibition of peak tail current (as described in A) by Cer (n = 10) and DihydroCer (n = 7). **P < 0.01.
Solutions.
The internal solution contained (in mM) 120 KCl, 6 MgCl2, 10 N-methyl-d-glucamine (NMG)·HEPES, 5 NMG2·EGTA, 5 Tris2·ATP, and 0.3 Tris2·GTP. The pH was adjusted to 7.2 using NMG base, and the osmolarity was 304 mosM. The external solution contained (in mM) 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 NMG·HEPES, and 10 glucose. The pH was adjusted to 7.4 using NMG base, and the osmolarity was 313 mosM. Solutions were applied using a gravity-fed flow system with seven inputs and a single output. The exchange time for this system was 1–2 s. Cells were continuously perfused with the control solution before a switch to the solution containing ceramide was made.
The time constant for diffusion (τ) of intracellularly applied catalase and superoxide dismutase (SOD) was estimated on the basis of
where τ is the time constant of exchange (s), RS is series resistance (MΩ), M is molecular mass of the molecule (Da), and C is the capacitance of the cell (pF) (39). The values for τ varied between 2 and 5 min so that 9–20 min of intracellular perfusion with catalase or SOD (>3 × τ) was done to ensure steady-state conditions prior to ceramide application.
Data acquisition and analysis.
Data were acquired using S5 software (developed by Dr. Stephen Ikeda; National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD) on a Macintosh computer (Apple Computer, Cupertino, CA). IgorPro software (WaveMetrics, Lake Oswego, OR) was used to measure current amplitudes and to fit (Marquardt-Levenberg algorithm) equations to currents and data plots. P/4 was used for leak subtraction for step time-course protocols. For all other protocols, leak current was estimated by linear regression of the nearest current-voltage (I-V) relationship over voltages (−80 to −40 mV) that did not generate IHERG. Data were not used if the holding current differed by >10% between the I-V used to measure leak and the protocol for which that leak was to be used for subtraction. Student's t-test was used to compare the data, and data are represented as means ± SE.
Computer simulations.
Simulated currents were generated using Axovacs 3 (written by Stephen W. Jones, Case-Western Reserve University) running on a Dell Inspiron 640m computer (Dell Computer, Round Rock, TX). Voltage-dependent rate constants (kx) in the model were calculated from
where Ax is the rate constant at 0 mV, zx is the charge moved, V is voltage, and R, T, and F are the gas constant, absolute temperature, and Faraday's constant, respectively.
Drugs.
Catalase, terfenadine, NMG, KCl, MgCl2, HEPES, EGTA, Tris2·ATP, Tris2·GTP, NaCl, CaCl2, MgCl2, H2O2, and glucose were purchased from Sigma. C6-ceramide and dihydro-C6-ceramide were purchased from Biomol (Plymouth Meeting, PA). Ceramide and dihydroceramide stock solutions were prepared as published previously (20). Briefly, ceramide and dihydro-C6-ceramide were dried under nitrogen and dissolved in DMSO followed by conjugation with BSA (at 1:1 ratio). Control solutions had equivalent concentrations of DMSO and BSA. Physiological ceramides have longer side chains and are difficult to dissolve and deliver. Therefore, we used a cell-permeable C6-ceramide that is commonly used for studying the effects of ceramide (15).
Western blot analysis.
Western blot analyses were performed as described previously (10). Briefly, HEK-293 cells treated with C6-ceramide (10 μM) for different durations were washed in ice-cold Dulbecco's PBS solution, and lysis buffer [50 mmol/l HEPES, 137 mmol/l NaCl, 5 mmol/l NaF, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l Na3VO4, 1% NP-40, protease inhibitor cocktail (Roche, Indianapolis, IN)] was added. Cell lysates were centrifuged to clear the sediments, and the Bio-Rad DC protein assay was utilized to determine protein concentration. Typically, 20 μg of protein lysate per sample was separated on 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA) and transferred to Hybond nitrocellulose membranes (GE Healthcare). The membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h and then incubated with the primary anti-HERG antibodies (Alomone Laboratories, Jerusalem, Israel) overnight at 4°C (1:2,000 dilution in 5% nonfat milk TBST or 3% BSA in TBST). After incubation, the membranes were washed three times with TBST for 10 min each. The blots were then incubated with secondary horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology) antibody at a 1:5,000 dilution in 5% nonfat milk in TBST for 2 h at room temperature. The membranes were then washed three times with TBST. The bands were visualized by enhanced chemiluminescence and quantified using ImageQuant (Molecular Dynamics, Sunnyvale, CA) or GeneTools SynGene (K & R Technology, Frederick, MD) software.
Cell surface biotinylation assay.
Cell surface expression of HERG was assayed as described previously (41). HEK-293 cells stably transfected with HERG were treated with 10 μM C6-ceramide for varying durations. Cells were washed twice with PBS and then incubated with 0.3 mg/ml NHS-Sulfo-Biotin (Thermo Fisher Scientific, Waltham, MA) for 30 min at 4°C. Afterward, cells were incubated twice with 50 mM glycine (in TBS) at 4°C for 5 min each. Cells were then rinsed twice with PBS and then scraped into 150 μl of PBS + 1% Triton X-100 and incubated with end-over rocking for 30 min at 4°C. Nuclei were pelleted by spinning samples at 800 g for 10 min. The supernatant was transferred to a new tube, and a 25-μl sample was taken for protein assay. Lysates were incubated with Immunopure Streptavidin for 1 h at 4°C and subsequently washed with PBS + 1% Triton X-100 four times, followed by a wash with PBS. Equal protein was loaded on the gel for Western blot and probed for HERG using anti-HERG antibodies as described above.
Isolation of buoyant fractions by sucrose gradient centrifugation.
HEK-293 cells stably expressing HERG were grown in 100-mm cell culture dishes. After appropriate treatments at 37°C in the serum-free medium, lipid raft isolation was carried out at 4°C by a slightly modified, originally described detergent-free method (49). Briefly, HEK-293 cells were washed with ice-cold PBS twice followed by addition of 500 mM sodium carbonate buffer (pH = 11.0) containing 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mM β-glycerolphosphate, and a protease inhibitor tablet (Roche Applied Science). Cells were scraped and transferred to a loose-fitting Dounce glass homogenizer and homogenized with 10 strokes, followed by sonication with three 20-s bursts. The lysates were then mixed with equal volumes of 90% sucrose in MOPS-buffered saline (MBS) buffer (25 mM MES buffer, pH 6.5, 150 mM NaCl) to make a final concentration of 45% sucrose solution. Gradients were prepared by layering the ultracentrifuge tubes with 45% sucrose with the sample at the bottom and overlaid with 4 ml of 35% and 4 ml of 5% sucrose in MBS-carbonate buffer. Lipid raft separation was carried out by centrifuging the sample at 39,000 rpm for 20 h at 4°C using a Beckman swinging bucket rotor (SW41ti). Twelve 1-ml fractions were collected from top to bottom and used for Western blot analysis.
Cholesterol depletion and repletion studies.
Cholesterol depletion was carried out by treating the cells with 5 mM methyl-β-cyclodextrin (MβCD) dissolved in serum-free DMEM for 30 min. Control cells were treated with serum-free DMEM for the equivalent duration. Cholesterol repletion was carried out in the following way; 200 mg of MβCD dissolved in 2.2 ml of H2O and 6 mg of cholesterol dissolved in 80 μl of isopropanol was mixed to give a 6.8 mM stock of cholesterol in 70 mM MβCD. The solution mixture was maintained at 80°C until clear and used for cell treatment at appropriate concentrations by dissolving the stock solution in serum-free DMEM.
RESULTS
C6-ceramide inhibits IHERG.
We investigated the effects of exogenous C6-ceramide (referred to hereafter as ceramide) on IHERG. HERG channels were activated by a 1-s step to +20 mV, and the tail current was measured at −40 mV (Fig. 1). External application of 10 μM ceramide for 2.5 min resulted in a progressive inhibition of IHERG (26.4 ± 3.4%, n = 8) that had not reached steady state by the end of the application period. dihydro-C6-ceramide is a structurally similar molecule (with a trans double bond at sphingoid base position 4 to 5) that is commonly used as a lipid control for C6-ceramide (33). Application of dihydro-C6-ceramide (10 μM for 2.5 min) had no significant effect on current magnitude (Fig. 1), which supports ceramide as a selective modulator of HERG channel activity. Since the ceramide-induced inhibition was not complete after 2.5 min, longer applications were used (6.25–9 min, mean 7.5 min) to obtain an inhibition of 35.5 ± 4.5% (n = 4). The washout of ceramide appeared to be incomplete in many cells, resulting an average current loss of 14.2 ± 4.9% (n = 7). All of these inhibitory values were calculated assuming a linear rundown of current, which may be less valid for the later time points used to measure recovery of IHERG from ceramide-induced inhibition.
Ceramide left shifts HERG activation voltage dependence.
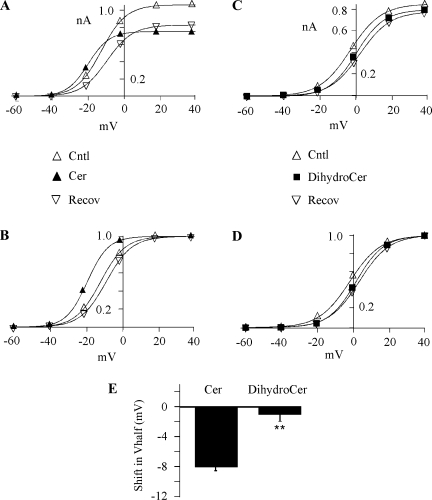
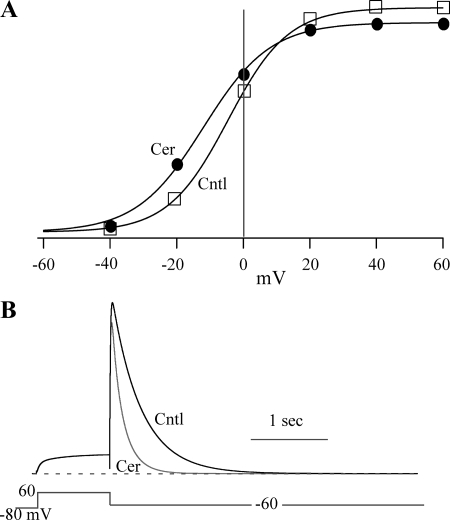
To examine the effect of ceramide on the I-V relationship, HERG channels were activated by stepping the voltage from −60 to +40 mV (20-mV increments), and tail currents were measured at −40 mV (Fig. 2). Repolarization to −40 mV induced the characteristically large tail currents that result from the rapid recovery from inactivation. A plot of peak tail current versus voltage showed that the threshold for current activation was −20 mV and that maximum activation was achieved at +20 mV (Fig. 2). Protocols to examine the I-V relationships were conducted before, during, and after ceramide application. A step-time course protocol similar to that shown in Fig. 1B was run to ensure that the recovery from ceramide had reached steady state prior to the I-V protocol being run. Thus, the total duration of ceramide application was 8–9 min for these experiments. Detailed examination of the I-V relationship in ceramide revealed that the ceramide effect was much more complex than simple inhibition of IHERG. As observed in Fig. 1, IHERG at +20 mV was inhibited by 10 μM ceramide, but surprisingly, the current at −20 mV was enhanced (Fig. 2A). This resulted from a left shift in HERG activation voltage dependence, which was quantified by fitting the activation versus voltage relationship using the Boltzmann equation. The ceramide-induced shift in ΔV1/2 was on average −8 ± 0.5 mV (n = 21), in contrast with dihydro-C6-ceramide, which did not alter HERG voltage dependence (ΔV1/2 = −0.4 ± 0.5 mV; n = 9) (Fig. 2, C and D). The ceramide-induced shift was verified using an I-V protocol with 10-mV increments with ΔV1/2 = −11.5 ± 2.5 mV (n = 3), which was not significantly different from the value obtained using the 20-mV increment I-V protocol (Fig. 2). Thus, ceramide induced two somewhat opposing effects, with HERG channels opening at more negative voltages and being inhibited at more depolarized voltages.
Fig. 2.
Cer hyperpolarizes HERG activation. A: activation-voltage relationships were recorded before, during, and after 10 μM Cer. Currents were measured upon repolarization to −40 mV following 5-s voltage steps ranging from −60 to 40 mV and are plotted versus the step voltage. The smooth lines represent single Boltzmann equation fits to generate half-activation voltage (V1/2) = −12, −19, and −10 mV; slope factor = 8, 8, and 8; and maximum current = 1.1, 0.8, and 0.8 nA for Cntl, Cer, and Recov, respectively. B: activation-voltage relationships shown in A were normalized to tail current generated after the +40-mV step to highlight the left shift in V1/2. The smooth lines are Boltzmann equation fits with V1/2 and slope, the same as those in A. C: activation-voltage relationship recorded before, during, and after 10 μM DihydroCer treatment. Fit parameters were V1/2 = −3, 0, and 2 mV; slope factor = 8, 8, and 8; and maximum current = 0.8, 0.8, and 0.8 nA for Cntl, DihydroCer, and Recov, respectively. D: normalized relationships (described in B) for the data shown in C. E: the negative shift in V1/2 induced by Cer. The change in V1/2 was calculated as the difference between test (either Cer, n = 21, or DihydroCer, n = 15) and Cntl, where Cntl was the average V1/2 measured before application and after Recov. **P < 0.01.
Ceramide alters HERG channel kinetics.
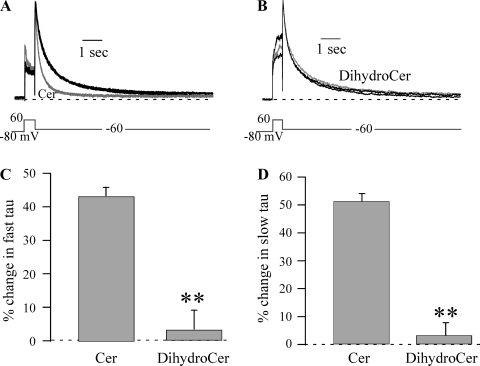
The currents illustrated in Fig. 1 demonstrate that acute application of ceramide also affects HERG channel kinetics. One effect was to increase the speed of HERG channel deactivation. To quantify this effect, we determined the deactivation time constant (τ) using double exponential fitting (τfast and τslow) of 10-s tail currents at −60 mV, which were sufficiently long to accurately measure the speed of HERG deactivation (≥3 times the largest deactivation τ) (Fig. 3). On average, deactivation τfast = 269 ± 20 ms and τslow = 1,452 ± 132 ms for control IHERG (n = 27), which was reduced to τfast = 138 ± 31 ms and τslow = 653 ± 146 ms by 10 μM ceramide (n = 20), respectively. Ceramide decreased the relative amplitude of τslow from 42 ± 2 to 34 ± 1% (n = 7, P < 0.05), which yielded an increase in relative amplitude of τfast from 58 ± 2 to 66 ± 1% (n = 7, P < 0.05). Deactivation kinetics at −100 mV were also affected, with τfast being decreased by 20 ± 5% (n = 6, P < 0.05), but no significant change was observed in τslow at −100 mV. However, this may have resulted from the very small amplitude of the slowly deactivating component, which comprised only 9% of the total deactivating current amplitude in control and was further reduced 40 ± 6% by ceramide (n = 6). dihydro-C6-ceramide did not significantly affect either the fast or slow component of deactivation (Fig. 3).
Fig. 3.
Cer speeds HERG deactivation. A: HERG current traces were normalized to peak tail current to highlight the faster deactivation induced by 10 μM Cer (gray curved line) compared with Cntl and Recov (black curved line). B: deactivation was not altered by DihydroCer (10 μM; gray curved line). C and D: %change in deactivation τ at −60 mV induced by Cer and DihydroCer for the fast (C) and slow (D) components. The %change was calculated using control deactivation τ that was the average of values measured before and after Recov from lipid application (either Cer, n = 27, or DihydroCer, n = 20). **P < 0.01.
We also examined the effect of ceramide on activation and inactivation kinetics of HERG, as described in materials and methods. Ceramide increased the speed of HERG activation by 25 ± 4% (control activation τ = 167 ± 25 vs. 129 ± 15 ms in ceramide at 60 mV, n = 5, P < 0.05). However, no effect of ceramide was observed on either the speed of (n = 3) or recovery from inactivation (n = 5; data not shown). It is possible that the altered gating kinetics are linked to the changes in V1/2 and/or IHERG inhibition.
Dose dependency of ceramide-altered HERG kinetics and IHERG inhibition.
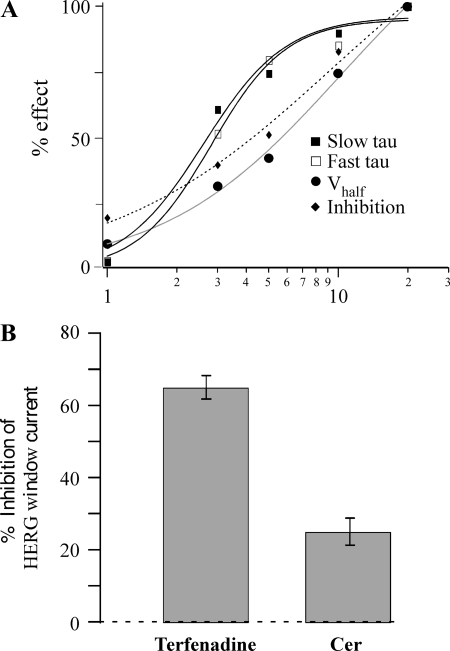
To begin to dissect the relationship(s) between the changes in kinetics, V1/2, and IHERG inhibition and also to get insights into the potency of ceramide effects on these parameters, we analyzed the dose dependency of ceramide upon these HERG channel parameters. The dose-response relationship was determined using concentrations of 1, 3, 5, 10, and 20 μM ceramide (Fig. 4A). All effects demonstrated a dose-dependent increase, but the EC50 varied among the different effects. The EC50 for the ceramide-induced decrease of deactivation τ was similar for both the fast (2.6 μM) and slow (2.8 μM) components, with a maximum effect at 10 μM. On the other hand, V1/2 and current inhibition showed that EC50 = 10.2 and 9.4 μM, respectively. However, a statistical test comparing the percent effects on V1/2 versus deactivation at 5 μM (where the EC50s appeared to show the greatest difference; Fig. 4A) showed that they were not significantly different, implying that the shift in V1/2 and deactivation τ could share the same mechanism (see below and Fig. 8).
Fig. 4.
IHERG is affected by physiological ceramide concentrations. A: dose-response relationships for the effect of Cer on slow and fast τ, V1/2, and inhibition. The %effect values were normalized to effect measured at 20 μM Cer. The smooth lines are fits to the data using the Hill equation to yield EC50 values of 2.6, 2.8, 10.2, and 9.4 μM, and Hill coefficient values were 2.4, 2.7, 1.1, and 0.9 for fast τ, slow τ, V1/2, and inhibition, respectively. The applied Cer concentrations were 1, 3, 5, 10, and 20 μM (n = 3, 3, 8, 8, and 6, respectively). B: Cer inhibits HERG window current. The holding potential was set to the voltage (usually −20 mV) generating maximum window current, which was measured before, during, and upon recovery from 10 μM Cer (n = 5). To confirm that HERG channels produced the window current, inhibition by a known HERG blocker, terfenadine (0.1 μM), is also presented (n = 5).
Fig. 8.
The model reproduces the kinetic effects of ceramide on IHERG. A: the left shift in the V1/2 and the IHERG inhibition (11%) induced by Cer model (●) are compared with values from the Cntl model (□). Currents were measured at −40 mV following 5-s steps to the indicated voltage. The smooth lines represent single Boltzmann equation fits to generate V1/2 = −4.5 and −10.2 mV and slope = 9 and 9 for Cntl and Cer, respectively. B: model reproduces the Cer-induced acceleration of HERG deactivation.
Ceramide inhibits HERG window current.
HERG activation and inactivation curves cross to reveal a voltage range over which HERG channels generate a steady-state current, termed window current. This steady efflux of potassium is thought to be responsible for the proliferative properties of tumors that overexpress HERG, and inhibition of the window current is thought to be the mechanism by which HERG blockers synergistically enhance the effect of anticancer drugs (17). Given the dual effect of ceramide on HERG gating (e.g., left-shifted activation vs. inhibition), it was unclear how the window current would be affected. Activation and inactivation voltage protocols were run to determine the voltage generating maximum window current for each cell, and the effect of ceramide was tested with the holding potential set to that voltage (typically −20 mV). Application of 10 μM ceramide inhibited window current by 25 ± 4% (n = 5; Fig. 4B). Additional experiments demonstrated that the voltage generating peak window current was not altered by 10 μM ceramide (−20 mV in both control and in ceramide, n = 4; not shown). To confirm that HERG channels produced the window current, we applied the HERG blocker terfenadine (0.1 μM) (21, 42), which inhibited 65 ± 3% of the window current. Thus, the inhibitory effect of ceramide appears to dominate the gating effect (ΔV1/2) at steady-state to block HERG window current.
Ceramide-induced inhibition is not mediated by changes in HERG protein levels or reactive oxygen species.
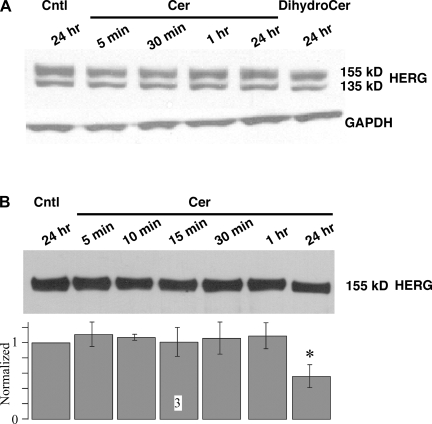
As a first step toward understanding the mechanism by which ceramide affects IHERG, we examined HERG protein levels. Ceramide has previously been shown to induce ubiquitylation of HERG, leading to its downregulation (15). We measured HERG protein levels by Western blot after different durations (5 min to 24 h) of 10 μM ceramide treatment (Fig. 5A). HERG protein was visualized as a fully glycosylated 155-kDa band that is present in the cell membrane and an immature core glycosylated 135-kDa band (62). The bands were quantified and normalized for equal loading using GAPDH levels. Surprisingly, ceramide treatment did not significantly impact the HERG protein levels. Since Western blots from whole cell lysates cannot distinguish between HERG expressed on the cell surface versus internalized HERG, we investigated the effect of ceramide treatment (5 min to 24 h) on HERG channels expressed on the cell surface using a biotinylation assay (Fig. 5B). Treatment with ceramide failed to alter HERG surface expression even after a 1-h application, but there was a 44% reduction in cell surface HERG after the 24-h treatment (n = 3; Fig. 5B). To confirm that our method isolated only surface proteins, we probed the membrane for intracellular protein GAPDH using anti-GAPDH antibodies, which demonstrated that there was no detectible GAPDH protein (data not shown). Thus, a reduction in HERG protein level cannot explain the inhibitory effect of acute ceramide treatment.
Fig. 5.
Acute Cer treatment does not affect HERG protein levels. A: HEK-293 cells were treated with either 10 μM Cer or DihydroCer for the durations indicated. Densitometric values of the mature 155-kDa band were normalized with GAPDH levels and are shown below a representative gel (n = 3). B: HEK-293 cells stably expressing HERG were treated with 10 μM Cer for the durations indicated. Cells were then treated with biotin to bind proteins expressed on the cell surface, followed by treatment with streptavidine beads. Protein bound to streptavidine beads was separated, and equal protein was loaded on the gel and probed for HERG. A representative blot is shown above, with normalized and averaged densitometer values shown in the bar graph below (n = 3). All data were normalized to the Cntl value for that run, and only the value at 24-h Cer treatment was statistically different from Cntl. *P < 0.05.
Ceramide treatment can produce hydrogen peroxide, and treatment with H2O2 has been shown to affect HERG kinetics by speeding deactivation and accelerating activation, and these effects of H2O2 could be abrogated by intracellular application of catalase (7, 40). To examine the potential involvement of H2O2 generation in ceramide modulation of HERG channel gating, we determined the ceramide effect in the presence of 1,000 U of catalase intracellularly applied via the pipette. The diffusion time for catalase to move from the pipette into the cell was calculated as described in materials and methods. The intracellular application of catalase failed to prevent the ceramide-induced changes in HERG deactivation, left shift in V1/2, and inhibition (P > 0.05, n = 6; data not shown). To confirm that the catalase was active, we carried out experiments in which we exogenously applied H2O2 (in a similar manner as ceramide) with or without 1,000 U of catalase in the pipette. In the absence of catalase, 1 mM H2O2 induced a significant increase in step current (9.1 ± 4.1%, n = 6, P < 0.05) that was prevented by the inclusion of catalase in the pipette (0.7 ± 0.3%, n = 4, P < 0.05 vs. H2O2 alone). Several additional effects of H2O2 were also observed, which included a significant left shift in V1/2 (−5.1 ± 1.3 mV, n = 6) (7) and a significant increase in deactivation τslow (7.4 ± 2.5%, n = 6), with no significant change in τfast. H2O2 failed to significantly affect τslow (0.7 ± 5.9%, n = 4), with catalase in the pipette and the V1/2 being smaller, but still shifted significantly (ΔV1/2 = −3.1 ± 0.3 mV, n = 4). Thus the inclusion of catalase in the pipette can prevent H2O2 effects on HERG. The H2O2-induced increase of step current amplitude and slowing of HERG deactivation are particularly noteworthy since they are opposite of the effects induced by ceramide, which along with our catalase results demonstrate that H2O2 does not mediate the ceramide effects on HERG gating. The effect on deactivation was unexpected, since previous work had demonstrated a significant speeding of deactivation (both the fast and slow components) by 1 mM H2O2 (7). Although we do not understand the reasons for this discrepancy, it is clear that the acute effects of ceramide on HERG channel gating are not mediated by the generation of H2O2.
Bai et al. (3) have reported that prolonged exposure (10 h) to C2-ceramide resulted in the inhibition of IHERG with an IC50 = 19.5 μM and no effect on the HERG kinetics. The inhibition was attributed to the generation of reactive oxygen species (ROS). However, these authors found that acute application of C2-ceramide had no effect on IHERG. We examined the role of ROS in the C6-ceramide-mediated effects on HERG by intracellular application of SOD (100 U) via the pipette. Following sufficient time for the enzyme to diffuse into the cell (see above), we examined the ceramide effects on IHERG. SOD failed affect either the ceramide-induced inhibition of IHERG or gating changes, suggesting that the effects are independent of ROS generation (P > 0.05, n = 6; data not shown).
Ceramide increases translocation of HERG to lipid rafts.
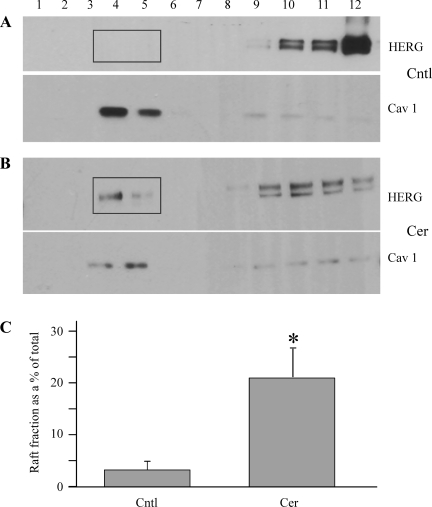
Given that the specificity and dose dependency of ceramide effects upon IHERG were not due to changes in HERG protein levels, H2O2, or ROS, we next investigated biophysical mechanisms underlying ceramide-induced HERG modulation. Previous reports have suggested that recruitment or sequestration of ion channels within lipid rafts affects channel kinetics (5, 38). We have shown previously that exogenous ceramide accumulates within lipid rafts and affects localization and activity of signaling proteins (20). Thus, we utilized the nondetergent sucrose gradient technique to isolate lipid rafts after ceramide treatment, as described in materials and methods (54). Lipid-rich microdomains, being buoyant, float up to the interface of 5 and 35% sucrose in the gradient after high-speed ultracentrifugation and can be visualized by Western blot (Fig. 6). The levels of the mature HERG fraction (155 kDa) were quantified using densitometry measurements, and the lipid raft fraction was calculated as a percentage of the total HERG protein (the sum of all 12 fractions). Cells were treated with ceramide for 10 min to mimic the electrophysiological measurements (8–9 min application for I-V measurements; see above). Ceramide treatment significantly increased the relative levels of HERG protein in the lipid raft fractions (Fig. 6B). However, the localization of caveolin-1, which is a commonly used lipid raft marker, was not significantly altered by ceramide treatment (raft fraction in control = 74 ± 19 vs. 62 ± 10 in ceramide; n = 3, P > 0.05), which is consistent with our previous findings (20). Thus, ceramide increases HERG channel localization within lipid rafts, which could alter the voltage dependence and kinetics of HERG channel gating.
Fig. 6.
Cer localizes HERG channels to lipid rafts. HERG expressing HEK-293 cells was treated with either Cntl solution (A) or 10 μM Cer (B) for 10 min, and lipid microdomains were isolated by detergent-free sucrose gradient method (highlighted by rectangles). The 12 fractions from top to bottom were probed for HERG and the lipid raft marker caveolin 1 (Cav1) with anti-HERG and anti-Cav1 antibodies. C: lipid raft fractions (fractions 4 and 5) were quantified and expressed as %total protein in the 12 fractions (n = 3). *P < 0.05.
Cholesterol depletion abrogates ceramide effect on HERG.
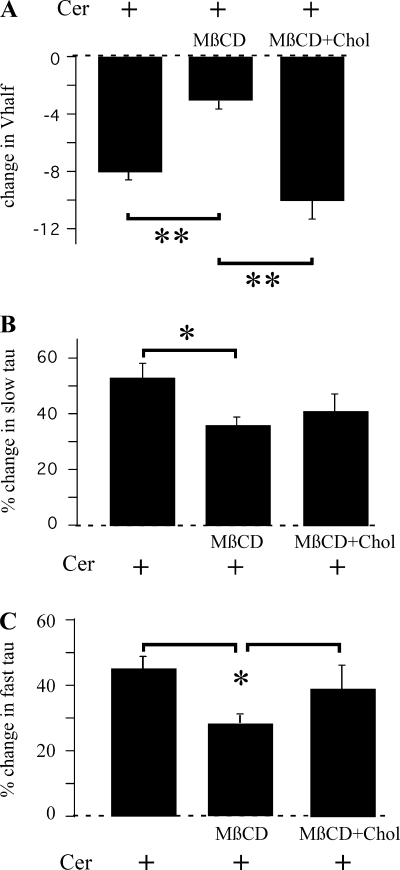
To further study the involvement of lipid rafts in the ceramide-induced effects on HERG, we disrupted lipid rafts by depleting cellular cholesterol content. Cholesterol can associate with the hydrophobic side chains of sphingolipids to form ordered membrane domains (28, 30). Depletion of cholesterol with MβCD disrupts the formation of membrane microdomains (9, 11) and alters the membrane localization of ion channels such as Kv1.3 and Shaker-like potassium channels (9, 29). We used MβCD to test whether membrane cholesterol is necessary for the ceramide-mediated effects on the HERG channels. Acute application of 5 mM MβCD had no appreciable effect on HERG kinetics (data not shown). However, a 30-min pretreatment with 5 mM MβCD significantly accelerated channel closing, as reported previously (4), but unlike the previous report, V1/2 was not significantly affected. At −60 mV, τfast was decreased from 195 ± 24 (n = 9) to 72 ± 13 ms (P < 0.05, n = 5), whereas τslow was decreased from 978 ± 136 to 349 ± 43 ms (P < 0.05) by the 30-min treatment with 5 mM MβCD. These changes are in line with those reported previously (4). On the other hand, V1/2 values calculated from a single Boltzmann equation fit to activation-voltage relationships were −4 ± 2 mV (n = 22) for control vs. −7 ± 3 mV (n = 9) for 5 mM MβCD (not significantly different). The Boltzmann slope factor was also not impacted by pretreatment with MβCD. When the cells were pretreated with MβCD, some of the effects of ceramide were abrogated. Preincubation with MβCD significantly decreased both the ceramide-induced left shift in V1/2 (P < 0.01; Fig. 7A) and the reduction of both τfast and τslow (Fig. 7, B and C), suggesting that functional lipid rafts are necessary for the effects of ceramide upon HERG kinetics. To further confirm that the effects are due to cholesterol depletion, we conjugated MβCD with cholesterol, which not only prevents cholesterol depletion from cells but is also shown to enrich the membrane with cholesterol (52). This treatment failed to alter either activation V1/2 or the Boltzmann slope factor but significantly slowed HERG channel deactivation, with τslow increasing from 1,320 ± 156 ms (n = 4) in control to 2,041 ± 133 ms (P < 0.05, n = 7) and τfast increasing from 232 ± 44 to 315 ± 44 ms (P < 0.05) with 30-min treatment in cholesterol-conjugated MβCD. This effect is opposite that induced by MβCD alone, which suggests modulation of HERG channel deactivation by cholesterol without changes in V1/2. Cholesterol-conjugated MβCD fully restored the ceramide-induced left shift in V1/2 and partially restored the decrease in deactivation τ (Fig. 7). However, pretreatment with either MβCD or cholesterol-conjugated MβCD failed to impact the ceramide-induced inhibition of IHERG (25 ± 3 and 25 ± 3% in MβCD and cholesterol-conjugated MβCD-pretreated cells, respectively, compared with 26 ± 3% in control cells; not significant). These results suggest that cholesterol depletion, which is known to disrupt formation of lipid rafts, reduces ceramide-induced kinetic changes, whereas ceramide-induced IHERG inhibition results from a separate mechanism.
Fig. 7.
Cholesterol depletion abrogates the effect of Cer on HERG kinetics. HERG-expressing cells were pretreated for 30 min with either 5 mM methyl-β-cyclodextrin (MβCD; n = 5) or 5 mM MβCD conjugated with cholesterol (Chol; n = 6) prior to Cer application. Cer-induced changes in V1/2 (A), fast deactivation τ (B), and slow deactivation τ (C) are depicted. *P < 0.05; **P < 0.01.
Modeling supports multiple ceramide effects on HERG.
Our evidence supports two separate effects of ceramide on HERG channels (i.e., current inhibition and kinetic changes). This potential mechanistic dichotomy was investigated further, using a mathematical modeling approach to determine whether the gating changes could induce the observed IHERG inhibition. The model (scheme 1) was based on Wang et al. (56), as described previously (21).
| Scheme 1 |
The rate constant (A, s−1) and charge moved (z) for each transition are given in Table 1. C1–C3, O, and I represent closed, open, and inactivated states, respectively.
Table 1.
Rate parameters for scheme 1
| Control |
Ceramide |
||
|---|---|---|---|
| Rate Constants | A | z | A |
| Forward | |||
| k1 | 2 | 0.6 | |
| k2 | 10 | ||
| k3 | 14 | 1.5 | 56 |
| k4 | 90 | 0.6 | |
| Backward | |||
| k−1 | 50 | −0.6 | |
| k−2 | 10 | ||
| k−3 | 0.1 | −1.5 | 0.3 |
| k−4 | 20 | −0.6 | |
A (s−1) is the rate constant at 0 mV, and z is the charge moved.
Our results support ceramide-induced acceleration of activation and deactivation gating without an effect on inactivation kinetics, which focused our attention on the transition rates along the open/closed pathway. Increasing only the forward C3→O rate to mimic the ∼8-mV hyperpolarizing shift in V1/2 accelerated deactivation kinetics, but the magnitude of that change was too small compared with our experimental results. Increasing only the backward O→C3 rate to match the enhanced deactivation speed observed in the data resulted in a depolarizing shift in V1/2. However, increasing both the forward and backward C3↔O rates was sufficient to reproduce the ceramide-induced left shift in the V1/2 (Fig. 8A), as well as the acceleration of deactivation kinetics (Fig. 8B), without affecting either inactivation or recovery from inactivation (data not shown). This demonstrates that the hyperpolarizing shift in V1/2 likely results from the combined effects of ceramide on the opening and closing rate constants. However, the model produced only a modest reduction in IHERG (11%). Therefore, consistent with our experimental evidence, the majority of IHERG inhibition (∼25%) appears to result from a separate ceramide effect.
DISCUSSION
In this study, we demonstrate that ceramide regulates the kinetics of HERG channels expressed in HEK-293 cells, consistent with localization of the HERG channels within lipid rafts (also called structured membrane microdomains). We used an external application of cell-permeable C6-ceramide that is a synthetic analog shown to mimic many effects of endogenous ceramides and is under investigation as an anticancer agent due to its proapoptotic properties (15). We investigated the dose-response relationship of the effects of C6-ceramide on HERG gating and inhibition and used 10 μM for detailed investigation because it produced maximal effects and also mimicked the endogenous ceramide levels reached during physiological or pathophysiological stimuli (23). Treatment with C6-ceramide induced two effects, IHERG inhibition and HERG gating changes (hyperpolarizing shift in V1/2, speeding of activation and deactivation). These effects were specific to C6-ceramide, since the structurally similar ceramide analog dihydro-C6-ceramide did not affect HERG channel activity. Furthermore, the acute ceramide effects were not mediated by generation of H2O2, ROS, or changes in protein levels. Ceramide treatment increased the levels of mature HERG protein within caveolin-enriched lipid rafts. Disruption of these lipid rafts with MβCD abrogated the ceramide-induced gating changes but not IHERG inhibition. Taken together, localization of HERG channels within lipid rafts may provide a biophysical mechanism for ceramide-induced changes in HERG kinetics, with the inhibition occurring through an independent mechanism.
Comparisons with previous work: ceramide effects on HERG.
Our work corroborates and extends many results from previous investigations into the effects of ceramide (3, 15) and lipid rafts (4) on IHERG. However, there are also significant differences. As with the present work, these studies used 10 μM ceramide and HEK-293 cells stably expressing HERG channels, so ceramide concentration and cell type are unlikely sources for the observed differences. As we have shown using C6-ceramide, Bai et al. (3) found that C2-ceramide inhibited IHERG, but they concluded that the inhibition was mediated by ceramide-generated ROS. The ROS-mediated inhibition required long-term C2-ceramide exposure (10 h) since acute application failed to alter IHERG. Thus, it is possible that our failure to detect a role for ROS in our C6-ceramide-induced inhibition is that our longest exposure time was 10 min. In addition, it has been reported that exogenously applied C2-ceramide can be metabolized to other ceramide metabolites (47) that could have their own effects on HERG channels and would not be generated from C6-ceramide. Therefore, it is possible that differences in our data from those of Bai et al. (3) could result from the ceramide isoforms used (C2- vs. C6-ceramide).
Chapman et al. (15) demonstrated that C6-ceramide accelerates HERG deactivation kinetics and inhibits IHERG but concluded that the inhibition results from a downregulation of functional HERG channels. These authors failed to observe a hyperpolarizing shift in V1/2 (15), but given across-cell variability, the −8-mV change that we have reported using within-cell comparisons may not be detectable when different cells are used for test versus control. The 23–30% inhibition reported by Chapman et al. (15) is in line with the maximal 36% inhibition observed in our study, but their application times required ≥30 min to reach maximal inhibition. We postulated our observed ceramide-induced inhibition, and in particular, the apparent incomplete recovery (14%) from that inhibition could result from HERG channel downregulation. However, unlike Chapman et al. (15), we failed to detect a reduction in total HERG channel levels even after 24 h of ceramide treatment. In addition, we could detect no change in plasma membrane HERG levels with ceramide applications of ≤1 h but found a 44% reduction after 24-h exposure to ceramide. Since ceramide application was limited to 10 min in our electrophysiological studies, HERG channel downregulation cannot explain the inhibition observed here (see below).
Our results lead us to conclude that the ceramide-induced gating changes result from the translocation of HERG channels into lipid rafts. Some of the supporting evidence comes from the abrogation of their gating changes by MβCD. However, as reported by Balijepalli et al. (4), we observe a significant acceleration of HERG deactivation kinetics in MβCD-pretreated cells, which presents the possibility that the MβCD effect precluded an additional effect of ceramide on deactivation (i.e., tail current could not be accelerated further). However, ceramide also significantly accelerated the fast component of deactivation at more hyperpolarized voltages, where the rates are significantly larger (τfast = 39 ms at −100 mV vs. 138 ms at −60 mV). Thus, a limiting rate cannot explain abrogation of the ceramide effect on deactivation by MβCD. However, it appears that cholesterol is an important modulator of HERG gating since we found that cholesterol-conjugated MβCD significantly slowed HERG deactivation. We believe this effect of cholesterol to be independent of raft localization since increased cholesterol levels induced an effect on deactivation opposite of that induced by ceramide, which we conclude to be mediated by lipid raft localization. It is interesting that these MβCD-induced gating changes failed to alter the voltage dependence of HERG channel activation (V1/2 and Boltzmann slope factor), which differs from the previous report of a depolarizing shift in V1/2 (4). Surprisingly, this shift was observed with both MβCD and cholesterol-conjugated MβCD (4), suggesting that the positive shift is an effect of MβCD on HERG gating and, unlike deactivation, is unrelated to membrane cholesterol content. Our studies utilized 5 mM MβCD instead of the 10 mM used by Balijepalli et al. (4), which could explain why we failed to observe the shift in V1/2.
Comparisons with previous work: lipid rafts and HERG.
In response to ceramide application, we observed a significant increase of HERG channels localized to lipid rafts, which we concluded alters the gating of those channels. As noted above, we found only a very small fraction (∼5%) of HERG channels localized to rafts under control conditions. This contrasts with previous results from Balijepalli et al. (4), who showed that 40–100% of HERG channels were raft localized. However, we used whole cell preparations for our work, whereas Balijepalli et al. (4) utilized only the membrane fraction. This difference likely explains the larger fraction of raft-localized HERG channels in the Balijepalli et al. (4) study. However, it is interesting that the level of HERG channels localized to rafts was markedly different in that study, depending on the method (4). When the detergent-sucrose gradient method was used, ∼40% of HERG channels were found in the raft-containing gradient fractions, whereas nearly 100% of HERG channels were raft localized when the OptiPrep method was used. Regardless of the absolute numbers, our results support a smaller fraction of raft-associated HERG channels in HEK-293 cells under control conditions that is increased significantly by ceramide. This translocation is associated with a significant acceleration of HERG activation and deactivation kinetics.
A separate mechanism for ceramide-induced inhibition.
To better understand the kinetic transitions of HERG gating induced by ceramide, we developed a mathematical model. Our intention was to determine the minimal changes to the HERG gating model that could be reproduced with ceramide (scheme 1; Table 1 and Fig. 8). We found that an increase of the rate constants into and out of the open state was able to reproduce the effect of ceramide on activation and deactivation kinetics, which produced the experimentally observed hyperpolarizing shift in V1/2. Our model generated only a slight inhibition (11%) of maximal IHERG, which was not sufficient to explain the experimentally measured 36% maximal inhibition. This correlated nicely with our cholesterol depletion results, which also failed to affect the ceramide-induced inhibition of IHERG. Therefore, ceramide-induced inhibition of IHERG likely occurs via a separate mechanism, such as direct binding of ceramide to the pore to block the channel, ceramide-activated second messenger systems to reduce HERG activity (20), or altering the solvation state of the channel pore to reduce potassium flux. A recent study by Wyatt et al. (59) showed that ionic flux in reconstituted gramicidin channels was lower in presence of ceramide compared with sphingomyelin. This was attributed to the altered hydrogen bond dynamics in the presence of ceramide, resulting in the reduced proton flux in ceramide compared with sphingomyelin. Further studies are needed to determine whether a similar mechanism mediates ceramide-induced inhibition of IHERG. Inhibition of IHERG is known to prolong the cAP, leading to cardiac arrhythmias (45). However, the gating changes that mediate the hyperpolarizing shift in V1/2 could increase HERG activity during cAP repolarization to shorten the QT interval. Therefore, it is possible that the combined effects on HERG channels could cancel each other to maintain normal cAP duration and cardiac rhythm in the presence of increased ceramide.
Physiological implications.
Localization of ion channels into lipid rafts results in compartmentalization that is thought to be involved in the regulation of cardiac rhythm. Pacemaker hyperpolarization-activated, cyclic nucleotide-gated-4 (HCN4) channels localize to caveolae in both HEK expression systems and rabbit cardiomyocytes (5). Cardiac sodium channels are also localized to caveolar domains of rat cardiomyocytes (60). Lipid membrane microdomains may also serve as platforms for assembling different proteins involved in regulation of ion channels. Our evidence suggests that one such lipid mediator, ceramide, induces translocation of HERG channels to lipid microdomains, which may serve as a “hysteresis rectifier,” dampening changes in the cAP (induced by inhibition of IHERG) by speeding the movement of channel into and out of the open state.
Increased potassium channel expression has been observed in a variety of cancer cell types, and the evidence supports a role for these channels in proliferation and metastasis. This includes Kv1.3, K2p9.1, EAG1, and HERG (35, 57). HERG channel activity is known to be associated with tumor progression in several cancers, such as human endometrial cancer, primary human acute myeloid leukemias, and neuroblastomas (8, 18, 35, 36). Steady-state IHERG has been predicted to regulate many mechanisms like cell volume, calcium influx, and signaling cascades that drive tumor proliferation/metastasis (13), and inhibition of steady-state IHERG (also called HERG window current) has been shown to provide synergistic effects with other anticancer drugs to suppress tumor growth (17). Ceramide is involved in multiple signaling cascades and is commonly released in the cell membrane in response to multiple stress stimuli (24). Treatment with exogenous ceramide or the generation of endogenous ceramide has been shown to induce apoptosis through the activation of downstream signaling molecules such as protein phosphatases, Bcl2 proteins, and cathepsin D (43), which has led to the suggestion of ceramide as an anticancer therapeutic (16, 26, 50, 51). Due to its central role in apoptosis, manipulation of cellular ceramide is being considered as a cancer therapy either alone or in conjunction with other drugs (34). Our results demonstrate the ability of C6-ceramide to inhibit HERG window current. The −8-mV shift in V1/2 during C6-ceramide treatment in our study results from substantial speeding of both activation and deactivation kinetics by ceramide. The combinatorial decreases in IHERG current and HERG kinetic parameters mediate the resultant changes in HERG window current. A 25% block of the HERG window current when combined with the multiple proapoptotic effects of ceramide upon prosurvival signaling cascades such as Akt could significantly enhance the ability of ceramide to selectively induce cytotoxicity in cancer cells that overexpress HERG. Given the potential compensatory effects of lipid raft-dependent HERG gating effects to maintain cAP duration, ceramide-induced HERG inhibition could selectively impact cancer proliferation without serious cardiovascular complications. Much work is still needed to investigate these predictions. However, our data provide new insights into the effects of C6-ceramide on HERG channel activity and demonstrate that these properties could make ceramide particularly effective in treating cancers that overexpress HERG.
GRANTS
This work was supported in part by an American Heart Association predoctoral fellowship (0715336U) to S. B. Ganapathi, National Heart, Lung, and Blood Institute Grants RO1-HL-066371 and RO1-HL-076789 to M. Kester, and a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds to K. S. Elmslie.
DISCLOSURES
The Pennsylvania Department of Health specifically disclaims responsibility for analyses, interpretations, and conclusions presented here.
ACKNOWLEDGMENTS
We thank Dr. Eckhard Ficker (MetroHealth Medical Center, Cleveland, OH) for the gift of HEK-293 cell line stably expressing HERG channels.
Present address of K. S. Elmslie: Department of Pharmacology, Kirksville College of Osteopathic Medicine, AT Still University, Kirksville, MO 63501.
REFERENCES
- 1.Abi-Char J, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel JL, Nattel S, Hatem SN. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol 582: 1205–1217, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp 266: 225–232, discussion 232–224, 2005 [PubMed] [Google Scholar]
- 3.Bai Y, Wang J, Shan H, Lu Y, Zhang Y, Luo X, Yang B, Wang Z. Sphingolipid metabolite ceramide causes metabolic perturbation contributing to HERG K+ channel dysfunction. Cell Physiol Biochem 20: 429–440, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Balijepalli RC, Delisle BP, Balijepalli SY, Foell JD, Slind JK, Kamp TJ, January CT. Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels (Austin) 1: 263–272, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res 94: 1325–1331, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Becchetti A, Arcangeli A. A comment on ion channels as pharmacological targets in oncology. J Gen Physiol 132: 313–314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berube J, Caouette D, Daleau P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J Pharmacol Exp Ther 297: 96–102, 2001 [PubMed] [Google Scholar]
- 8.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res 58: 815–822, 1998 [PubMed] [Google Scholar]
- 9.Bock J, Szabó I, Gamper N, Adams C, Gulbins E. Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem Biophys Res Commun 305: 890–897, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt Is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem 277: 3286–3292, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275: 17221–17224, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Buraei Z, Schofield G, Elmslie KS. Roscovitine differentially affects CaV2 and Kv channels by binding to the open state. Neuropharmacology 52: 883–894, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Camacho J. Ether a go-go potassium channels and cancer. Cancer Lett 233: 1–9, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Casis O, Olesen SP, Sanguinetti MC. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol 69: 658–665, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, Pasternack M, Tornquist K. Downregulation of the HERG (KCNH2) K+ channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci 118: 5325–5334, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Charles R, Sandirasegarane L, Yun J, Bourbon N, Wilson R, Rothstein RP, Levison SW, Kester M. Ceramide-coated balloon catheters limit neointimal hyperplasia after stretch injury in carotid arteries. Circ Res 87: 282–288, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Chen SZ, Jiang M, Zhen YS. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother Pharmacol 56: 212–220, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L, Noci I, Borri P, Borrani E, Giachi M, Becchetti A, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG potassium channels are more frequently expressed in human endometrial cancer compared with non-cancerous endometrium. Br J Cancer 83: 1722–1729, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmslie KS, Werz MA, Overholt JL, Jones SW. Intracellular ATP and GTP are both required to preserve modulation of N-type calcium channel current by norepinephrine. Pflugers Arch 423: 472–479, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282: 12450–12457, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ganapathi SB, Kester M, Elmslie KS. State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy. Am J Physiol Cell Physiol 296: C701–C710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulbins E, Kolesnick R. Measurement of sphingomyelinase activity. Methods Enzymol 322: 382–388, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 274: 1855–1859, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Holopainen JM, Subramanian M, Kinnunen PK. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry 37: 17562–17570, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 110: 3–8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenzo ED, Pillozzi S, Masselli M, Crociani O, Becchetti A, Arcangeli A. Potassium Channels as Novel Pharmacological Targets in Acute Myeloid Leukemia. Blood (ASH Annual Meeting Abstracts) 112, 2008 [Google Scholar]
- 28.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res 69: 798–807, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of shaker-like potassium channels to lipid rafts. J Biol Chem 275: 7443–7446, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Martens JR, O'Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci 25: 16–21, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J Biol Chem 276: 8409–8414, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Masi A, Becchetti A, Restano-Cassulini R, Polvani S, Hofmann G, Buccoliero AM, Paglierani M, Pollo B, Taddei GL, Gallina P, Di Lorenzo N, Franceschetti S, Wanke E, Arcangeli A. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer 93: 781–792, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277: 25843–25846, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Modrak DE. Measurement of ceramide and sphingolipid metabolism in tumors: potential modulation of chemotherapy. Methods Mol Med 111: 183–194, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol 205: 115–124, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, Bartolozzi B, Becchetti A, Wanke E, Bernabei PA, Olivotto M, Pegoraro L, Arcangeli A. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 16: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V, Boddi V, Pegoraro L, Becchetti A, Arcangeli A. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 110: 1238–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Pottosin II, Valencia-Cruz G, Bonales-Alatorre E, Shabala SN, Dobrovinskaya OR. Methyl-beta-cyclodextrin reversibly alters the gating of lipid rafts-associated Kv1.3 channels in Jurkat T lymphocytes. Pflugers Arch 454: 235–244, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch 411: 204–211, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem 272: 21388–21395, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, Wood TL. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem 282: 22513–22524, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Roy M, Dumaine R, Brown AM. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation 94: 817–823, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem 49: 413–440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature 440: 463–469, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Schwarz JR, Bauer CK. Functions of erg K+ channels in excitable cells. J Cell Mol Med 8: 22–30, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shayman JA, Abe A, Hiraoka M. A turn in the road: How studies on the pharmacology of glucosylceramide synthase inhibitors led to the identification of a lysosomal phospholipase A2 with ceramide transacylase activity. Glycoconj J 20: 25–32, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol 387: 227–250, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song KS, Li Shengwen, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther 307: 468–475, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res 11: 3465–3474, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Tikku S, Epshtein Y, Collins H, Travis AJ, Rothblat GH, Levitan I. Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. Am J Physiol Cell Physiol 293: C440–C450, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci 22: 240–246, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279: 44945–44954, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wadhwa S, Wadhwa P, Dinda AK, Gupta NP. Differential expression of potassium ion channels in human renal cell carcinoma. Int Urol Nephrol 41: 251–257, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol 502: 45–60, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol 154: 91–107, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Wong W, Schlichter LC. Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95. J Biol Chem 279: 444–452, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Wyatt DL, de Godoy CM, Cukierman S. Enhancement of proton transfer in ion channels by membrane phosphate headgroups. J Phys Chem B 113: 6725–6731, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 90: 443–449, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Zhang S, Kehl SJ, Fedida D. Modulation of human ether-à-go-go-related K+ (HERG) channel inactivation by Cs+ and K+. J Physiol 548: 691–702, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem 273: 21061–21066, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J 74: 230–241, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]